Development of an Indexed Score to Identify the Most Suitable Biological Material to Assess SARS-CoV-2
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
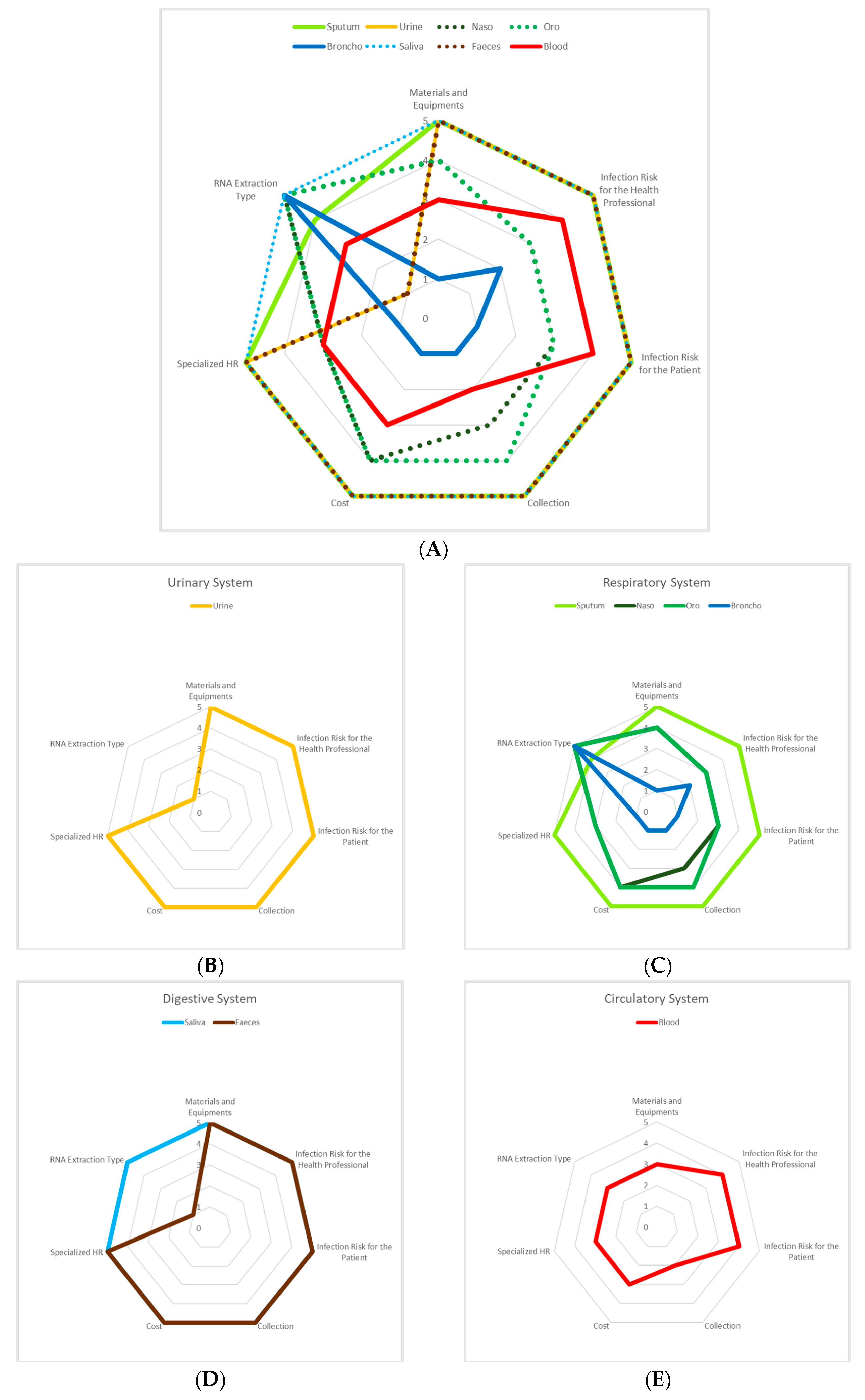
3.1. Urinary System
3.2. Respiratory System
3.3. Digestive System
3.4. Circulatory System
3.5. Viral Load
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Full name’s | Acronym |
| Angiotensin-converting enzyme II | ACE2 |
| Bronchoalveolar | BAL |
| Chemiluminescent immunoassay | CLIA |
| Clustered regularly interspaced short palindromic repeats | CRISPR |
| Confidence interval | CI |
| Coronavirus Disease 2019 | COVID-19 |
| Cycle threshold | CT |
| Dithiothreitol | DTT |
| droplet digital polymerase chain reaction | ddPCR |
| Enzyme-linked immunosorbent assay | ELISA |
| Lateral flow assay | LFA |
| Nacetyl-L-cysteine | NALC |
| Nasopharyngeal | NPS |
| Negative percent agreement | NPA |
| Not mentioned | NM |
| Oropharyngeal | OPS |
| Personal protective equipment | PPE |
| Positive percent agreement | PPA |
| Proteinase K | PK |
| Quantitative reverse transcription PCR | RT-qPCR |
| Rapid antigen tests | RATs |
| Respiratory syncytial virus | RSV |
| Reverse-transcription loop-mediated isothermal amplification | RT-LAMP |
| Ribonucleic acid | RNA |
| Severe Acute Respiratory Syndrome Coronavirus 2 | SARS-CoV-2 |
| World Health Organization | WHO |
References
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 January 2021).
- López-Martínez, B.; Guzmán-Ortiz, A.L.; Nevárez-Ramírez, A.J.; Parra-Ortega, I.; Olivar-López, V.B.; Ángeles-Floriano, T.; Vilchis-Ordoñez, A.; Quezada, H. Saliva as a Promising Biofluid for SARS-CoV-2 Detection during the Early Stages of Infection. Bol. Med. Hosp. Infant. Mex. 2020, 77, 228–233. [Google Scholar] [CrossRef] [PubMed]
- NIH: National Institute on Aging. Why COVID-19 Testing Is the Key to Getting Back to Normal. Available online: http://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal (accessed on 16 April 2021).
- Corman, V.; Bleicker, T.; Brünink, S.; Drosten, C.; Landt, O.; Koopmans, M.; Mc, E.; Zambon, M. Diagnostic Detection of 2019-nCoV by Real-Time RT-PCR; World Health Organization: Berlin, Germany, 2020; p. 13. [Google Scholar]
- Bhattacharya, D.; Parai, D.; Rout, U.K.; Dash, P.; Nanda, R.R.; Dash, G.C.; Kanungo, S.; Giri, S.; Choudhary, H.R. Saliva for Diagnosis of SARS-CoV-2: First Report from India. J. Med. Virol. 2021, 93, 2529–2533. [Google Scholar] [CrossRef]
- Senok, A.; Alsuwaidi, H.; Atrah, Y.; Al Ayedi, O.; Al Zahid, J.; Han, A.; Al Marzooqi, A.; Al Heialy, S.; Altrabulsi, B.; AbdelWareth, L.; et al. Saliva as an Alternative Specimen for Molecular COVID-19 Testing in Community Settings and Population-Based Screening. Infect. Drug Resist. 2020, 13, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Silva, R.; Jardim, A.C.G.; Siqueira, W.L. Coronavirus COVID-19 Impacts to Dentistry and Potential Salivary Diagnosis. Clin. Oral. Investig. 2020, 24, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, M.; Zhang, Y.; Wen, J.; Wang, Y.; Wang, L.; Guo, J.; Liu, C.; Li, D.; Wang, Y.; et al. The Yield and Consistency of the Detection of SARS-CoV-2 in Multiple Respiratory Specimens. Open Forum Infect. Dis. 2020, 7, ofaa379. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS). Available online: https://apps.who.int/iris/bitstream/handle/10665/70863/WHO_CDS_CSR_GAR_2003.11_eng.pdf?sequence=1&isAllowed=y (accessed on 2 January 2021).
- Khurshid, Z.; Asiri, F.Y.I.; Al Wadaani, H. Human Saliva: Non-Invasive Fluid for Detecting Novel Coronavirus (2019-nCoV). Int. J. Environ. Res. Public Health 2020, 17, 2225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, R.-H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L.; et al. Molecular and Serological Investigation of 2019-nCoV Infected Patients: Implication of Multiple Shedding Routes. Emerg. Microbes Infect 2020, 9, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Azghandi, M.; Kerachian, M.A. Detection of Novel Coronavirus (SARS-CoV-2) RNA in Peripheral Blood Specimens. J. Transl. Med. 2020, 18, 412. [Google Scholar] [CrossRef] [PubMed]
- Jayamohan, H.; Lambert, C.J.; Sant, H.J.; Jafek, A.; Patel, D.; Feng, H.; Beeman, M.; Mahmood, T.; Nze, U.; Gale, B.K. SARS-CoV-2 Pandemic: A Review of Molecular Diagnostic Tools Including Sample Collection and Commercial Response with Associated Advantages and Limitations. Anal. Bioanal. Chem. 2020, 413, 49–71. [Google Scholar] [CrossRef]
- Lai, T.; Xiang, F.; Zeng, J.; Huang, Y.; Jia, L.; Chen, H.; Wu, J.; Xie, J.; Liu, S.; Deng, W.; et al. Reliability of Induced Sputum Test Is Greater than That of Throat Swab Test for Detecting SARS-CoV-2 in Patients with COVID-19: A Multi-Center Cross-Sectional Study. Virulence 2020, 11, 1394–1401. [Google Scholar] [CrossRef]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal–Oral Transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Song, Y.; Zhu, S.; Wang, D.; Zhang, H.; Han, G.; Weng, Y.; Xu, J.; Xu, J.; et al. Excretion of SARS-CoV-2 through Faecal Specimens. Emerg. Microbes Infect. 2020, 9, 2501–2508. [Google Scholar] [CrossRef]
- Yang, X.; Jin, Y.; Li, R.; Zhang, Z.; Sun, R.; Chen, D. Prevalence and Impact of Acute Renal Impairment on COVID-19: A Systematic Review and Meta-Analysis. Crit. Care 2020, 24, 356. [Google Scholar] [CrossRef] [PubMed]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Hingrat, Q.L.; et al. Clinical and Virological Data of the First Cases of COVID-19 in Europe: A Case Series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef]
- de Souza, S.P.; Silveira, M.A.D.; de Freitas Souza, B.S.; Nonaka, C.K.V.; de Melo, E.; Cabral, J.; Coelho, F.; da Hora Passos, R. Evaluation of Urine SARS-CoV-2 RT-PCR as a Predictor of Acute Kidney Injury and Disease Severity in Critical COVID-19 Patients. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- BIOKÉ. NucleoSpin Dx Virus. Available online: https://www.bioke.com/webshop/mn/740895.html (accessed on 26 March 2021).
- bioMérieux. NucliSens easyMag—User Manual. Marcy l’Etoile—France 2009. Available online: https://www.manualslib.com/manual/1377074/Biomerieux-Nuclisens-Easymag.html#manual (accessed on 26 March 2021).
- Skobe, C. The Basics of Specimen Collection and Handling of Urine Testing. Available online: https://www.bd.com/en-us/offerings/capabilities/specimen-collection/vacutainer-educational-services-and-materials/labnotes/labnotes-14-2-2004 (accessed on 26 March 2021).
- Murphy, K. SARS CoV-2 Detection from Upper and Lower Respiratory Tract Specimens. Chest 2020, 158, 1804–1805. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 10 February 2021).
- Di Maio, P.; Iocca, O.; Cavallero, A.; Giudice, M. Performing the Nasopharyngeal and Oropharyngeal Swab for 2019-novel Coronavirus (SARS-CoV-2) Safely: How to Dress, Undress, and Technical Notes. Head Neck 2020, 42, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, J.; Jia, R.; Xia, S.; Wang, X.; Cao, L.; Zeng, M.; Xu, J. Dynamic Surveillance of SARS-CoV-2 Shedding and Neutralizing Antibody in Children with COVID-19. Emerg. Microbes Infect. 2020, 9, 1254–1258. [Google Scholar] [CrossRef]
- Gupta, K.; Bellino, P.M.; Charness, M.E. Adverse Effects of Nasopharyngeal Swabs: Three-Dimensional Printed versus Commercial Swabs. Infect. Control Hosp. Epidemiol. 2020, 42, 641–642. [Google Scholar] [CrossRef]
- Fabbris, C.; Cestaro, W.; Menegaldo, A.; Spinato, G.; Frezza, D.; Vijendren, A.; Borsetto, D.; Boscolo-Rizzo, P. Is Oro/Nasopharyngeal Swab for SARS-CoV-2 Detection a Safe Procedure? Complications Observed among a Case Series of 4876 Consecutive Swabs. Am. J. Otolaryngol. 2021, 42, 102758. [Google Scholar] [CrossRef]
- Cleveland Clinic. Nosebleeds (Epistaxis). Available online: https://my.clevelandclinic.org/health/diseases/13464-nosebleed-epistaxis (accessed on 10 February 2021).
- Wang, H.; Liu, Q.; Hu, J.; Zhou, M.; Yu, M.; Li, K.; Xu, D.; Xiao, Y.; Yang, J.; Lu, Y.; et al. Nasopharyngeal Swabs Are More Sensitive Than Oropharyngeal Swabs for COVID-19 Diagnosis and Monitoring the SARS-CoV-2 Load. Front. Med. 2020, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Xiang, J.; Yan, M.; Li, H.; Huang, S. Comparison of Throat Swabs and Sputum Specimens for Viral Nucleic Acid 2 Detection in 52 Cases of Novel Coronavirus (SARS-Cov-2) Infected 3 Pneumonia (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Thwe, P.M.; Ren, P. Analysis of Sputum/Tracheal Aspirate and Nasopharyngeal Samples for SARS-CoV-2 Detection by Laboratory-Developed Test and Panther Fusion System. Diagn. Microbiol. Infect. Dis. 2021, 99, 115228. [Google Scholar] [CrossRef] [PubMed]
- Gualano, G.; Musso, M.; Mosti, S.; Mencarini, P.; Mastrobattista, A.; Pareo, C.; Zaccarelli, M.; Migliorisi, P.; Vittozzi, P.; Zumla, A.; et al. Usefulness of Bronchoalveolar Lavage in the Management of Patients Presenting with Lung Infiltrates and Suspect COVID-19-Associated Pneumonia: A Case Report. Int. J. Infect. Dis. 2020, 97, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Luo, Q.; Mo, F.; Long, L.; Zheng, W. SARS-CoV-2 RNA More Readily Detected in Induced Sputum than in Throat Swabs of Convalescent COVID-19 Patients. Lancet Infect. Dis. 2020, 20, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Hyun, M.; Lee, J.Y.; Park, S.; Ryoo, N.; Kwon, Y.S.; Park, J.S.; Kim, J.Y.; Jeon, J.C.; Peck, K.R. Detection of SARS-CoV-2 in Nasal Swabs: Comparison with Nasopharyngeal Swabs. J. Infect. Dev. Ctries. 2020, 14, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lessmann, A.; Plaza, V. Multidisciplinary Consensus on Sputum Induction Biosafety during the COVID-19 Pandemic. Allergy 2020, 76, 2407–2419. [Google Scholar] [CrossRef] [PubMed]
- Luvira, V.; Jittmittraphap, A.; Muangnoicharoen, S.; Chantawat, N.; Janwitthayanan, W.; Leaungwutiwong, P. Temporal Change of SARS-CoV-2 in Clinical Specimens of COVID-19 Pneumonia Patients. Am. J. Trop. Med. Hyg. 2020, 103, 1204–1206. [Google Scholar] [CrossRef]
- Ceron, J.J.; Lamy, E.; Martinez-Subiela, S.; Lopez-Jornet, P.; Capela-Silva, F.; Eckersall, P.D.; Tvarijonaviciute, A. Use of Saliva for Diagnosis and Monitoring the SARS-CoV-2: A General Perspective. J. Clin. Med. 2020, 9, 1491. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Processing of Sputum Specimens for Nucleic Acid Extraction 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 26 March 2021).
- Peng, J.; Lu, Y.; Song, J.; Vallance, B.A.; Jacobson, K.; Yu, H.B.; Sun, Z. Direct Clinical Evidence Recommending the Use of Proteinase K or Dithiothreitol to Pretreat Sputum for Detection of SARS-CoV-2. Front. Med. 2020, 7, 549860. [Google Scholar] [CrossRef]
- Guedes, F.; Boléo-Tomé, J.P.; Rodrigues, L.V.; Bastos, H.N.; Campainha, S.; de Santis, M.; Mota, L.; Bugalho, A. Recommendations for Interventional Pulmonology during COVID-19 Outbreak: A Consensus Statement from the Portuguese Pulmonology Society. Pulmonology 2020, 26, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Bugalho, A.; Guedes, F.; Bastos, H.N.; Mota, L.; Rodrigues, L.V.; Boléo-Tomé, J.P.; Campainha, S. Recomendações Da SPP Para a Realização de Broncoscopias Durante o Surto de COVID-19; Sociedade Portuguesa de Pneumologia: Lisbon, Portugal, 2020. [Google Scholar]
- Direção-Geral da Saúde. Norma n.o 015/2020 de 23 de Março 2020; Da Direção-Geral Da Saúde: Lisbon, Portugal, 2020. [Google Scholar]
- COVID-19 Resources Center Bronchoalveolar Lavage (BAL)|How to Collect a Sample for COVID-19 Testing. Available online: https://covid19.aischannel.com/video-tutorials-diagnosis/videos/bronchoalveolar-lavage-bal-how-to-collect-a-sample-for-covid-19-testing-lower-respiratory-tract (accessed on 10 February 2021).
- World Health Organization. Guidelines for the Collection of Clinical Specimens during Field Investigation of Outbreaks; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Wong, M.C.; Huang, J.; Lai, C.; Ng, R.; Chan, F.K.L.; Chan, P.K.S. Detection of SARS-CoV-2 RNA in Fecal Specimens of Patients with Confirmed COVID-19: A Meta-Analysis. J. Infect. 2020, 81, e31–e38. [Google Scholar] [CrossRef]
- QIAGEN. QIAamp Viral RNA Mini Handbook. Available online: https://labettor.com/uploads/products/protocols/4992.pdf (accessed on 26 March 2021).
- Lo, I.L.; Lio, C.F.; Cheong, H.H.; Lei, C.I.; Cheong, T.H.; Zhong, X.; Tian, Y.; Sin, N.N. Evaluation of SARS-CoV-2 RNA Shedding in Clinical Specimens and Clinical Characteristics of 10 Patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020, 16, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- QIAGEN. QIAamp Viral RNA Mini Kit. Available online: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/sample-processing/qiaamp-viral-rna-mini-kit/?clear=true#orderinginformation (accessed on 26 March 2021).
- Kim, J.-M.; Kim, H.M.; Lee, E.J.; Jo, H.J.; Yoon, Y.; Lee, N.-J.; Son, J.; Lee, Y.-J.; Kim, M.S.; Lee, Y.-P.; et al. Detection and Isolation of SARS-CoV-2 in Serum, Urine, and Stool Specimens of COVID-19 Patients from the Republic of Korea. Osong Public Health Res. Perspect. 2020, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, J.; Peng, J.; Li, X.; Deng, X.; Geng, Z.; Shen, Z.; Guo, F.; Zhang, Q.; Jin, Y.; et al. Detection of SARS-CoV-2 in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. Cell Prolif. 2020, 53, e12923. [Google Scholar] [CrossRef] [PubMed]
- Abasiyanik, M.F.; Flood, B.; Lin, J.; Ozcan, S.; Rouhani, S.J.; Pyzer, A.; Trujillo, J.; Zhen, C. Sensitive Detection and Quantification of SARS-CoV-2 in Saliva. medRxiv 2020. [Google Scholar] [CrossRef]
- Williams, E.; Isles, N.; Chong, B.; Bond, K.; Yoga, Y.; Druce, J.; Catton, M.; Ballard, S.A. Detection of SARS-CoV-2 in Saliva: Implications for Specimen Transport and Storage | Microbiology Society. J. Med. Microbiol. 2020, 70, 001285. [Google Scholar]
- Barat, B.; Das, S.; Giorgi, V.D.; Henderson, D.K.; Kopka, S.; Lau, A.F.; Miller, T.; Moriarty, T.; Palmore, T.N.; Sawney, S.; et al. Pooled Saliva Specimens for SARS-CoV-2 Testing. J. Clin. Microbiol. 2021, 59, e02486-20. [Google Scholar] [CrossRef]
- Kandel, C.; Zheng, J.; McCready, J.; Serbanescu, M.A.; Racher, H.; Desaulnier, M.; Powis, J.E.; Vojdani, K.; Finlay, L.; Sheldrake, E.; et al. Detection of SARS-CoV-2 from Saliva as Compared to Nasopharyngeal Swabs in Outpatients. Viruses 2020, 12, 1314. [Google Scholar] [CrossRef]
- Sakanashi, D.; Asai, N.; Nakamura, A.; Miyazaki, N.; Kawamoto, Y.; Ohno, T.; Yamada, A.; Koita, I.; Suematsu, H.; Hagihara, M.; et al. Comparative Evaluation of Nasopharyngeal Swab and Saliva Specimens for the Molecular Detection of SARS-CoV-2 RNA in Japanese Patients with COVID-19. J. Infect. Chemother. 2021, 27, 126–129. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Ishii, Y.; Aoki, K.; Yagi, S.; Maeda, T.; Miyazaki, T.; Yoshizawa, S.; Aoyagi, K.; Tateda, K. Immunochromatographic Test for the Detection of SARS-CoV-2 in Saliva. J. Infect. Chemother. 2021, 27, 384–386. [Google Scholar] [CrossRef]
- Williams, E.; Bond, K.; Zhang, B.; Putland, M.; Williamson, D.A. Saliva as a Noninvasive Specimen for Detection of SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00776-20. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.-K.; Yip, C.C.-Y.; Poon, R.W.-S.; Chan, K.-H.; Cheng, V.C.-C.; Hung, I.F.-N.; Chan, J.F.-W.; Yuen, K.-Y.; To, K.K.-W. Evaluating the Use of Posterior Oropharyngeal Saliva in a Point-of-Care Assay for the Detection of SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, C.; Zeng, J.; Luo, C.; Hu, S.; Peng, Y.; Li, W.; Xie, Z.; Ling, Y.; Zhang, X.; et al. Analysis of Viral Load in Different Specimen Types and Serum Antibody Levels of COVID-19 Patients. J. Transl. Med. 2021, 19, 30. [Google Scholar] [CrossRef]
- Sui, Z.; Zhang, Y.; Tu, S.; Xie, J.; Huang, W.; Peng, T.; Dong, L.; Yang, J.; Ouyang, Y.; Liu, S.; et al. Evaluation of Saliva as an Alternative Diagnostic Specimen Source for SARS-CoV-2 Detection by RT-dPCR. J. Infect. 2021, 82, e38–e40. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Kim, S.-M.; Kim, H.-S.; Kim, Y.-I.; Kim, J.H.; Cho, J.Y.; Kim, S.; Kang, H.; Kim, S.-G.; Park, S.-J.; et al. Viable SARS-CoV-2 in Various Specimens from COVID-19 Patients. Clin. Microbiol. Infect. 2020, 26, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R.; et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin. Infect. Dis. 2020, 71, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta-Analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Sakai-Tagawa, Y.; Koga, M.; Akasaka, O.; Nakachi, I.; Koh, H.; Maeda, K.; Adachi, E.; Saito, M.; Nagai, H.; et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar] [CrossRef]
- Böger, B.; Fachi, M.M.; Vilhena, R.O.; Cobre, A.F.; Tonin, F.S.; Pontarolo, R. Systematic Review with Meta-Analysis of the Accuracy of Diagnostic Tests for COVID-19. Am. J. Infect. Control 2021, 49, 21–29. [Google Scholar] [CrossRef]
- Alhamid, G.; Tombuloglu, H.; Rabaan, A.A.; Al-Suhaimi, E. SARS-CoV-2 Detection Methods: A Comprehensive Review. Saudi J. Biol. Sci. 2022, 29, 103465. [Google Scholar] [CrossRef] [PubMed]
- Manzar, S.; Kazmi, F.; Shahzad, H.B.; Qureshi, F.A.; Shahbaz, M.; Rashid, S. Estimation of the Risk of COVID-19 Transmission through Aerosol-Generating Procedures. Dent. Med. Probl. 2022, 59, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, H.R.; Yu, H. Viral Respiratory Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 284–288. ISBN 978-0-323-55512-8. [Google Scholar]
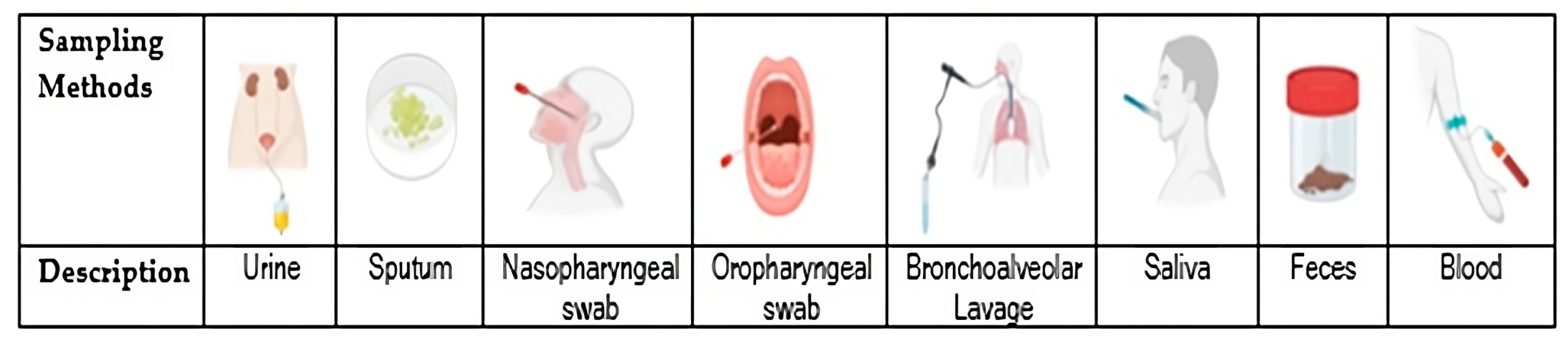
| Sampling Methods | Criteria Application |
|---|---|
| Urine | The urine specimen is vulnerable to tampering through dilution or adulteration. Urine samples pose a minimal risk of infection, although there is a possibility of sample spillage resulting in insufficient volume. It is advised to wear gloves during the handling of urine samples. They have the potential to be self-collected, which presents an advantage as it eliminates the need for a trained health professional to carry out the sampling. Low cost. Medium accessibility. |
| Sputum | Clinical diagnostic sputum tests are designed to identify the underlying causes of lower respiratory tract infections and various other diseases. Moreover, these tests serve as an effective means of assessing the efficacy of clinical treatment. When a patient presents with pneumonia, performing a sputum culture is imperative. However, one major drawback of relying solely on sputum smears for diagnosis is that a significant number of pulmonary cases may go undetected. The effectiveness of sputum smears in detecting disease is more pronounced in cases of cavitary pulmonary disease among patients with a forceful cough. It is worth noting that the cough and spit method may lead to slight discomfort due to repeated coughing. Once more, sputum samples have the potential to be self-collected, which eliminates the need for a trained health professional for sampling. Low cost. Medium accessibility. |
| Nasopharyngeal swab | The nasopharyngeal swab is commonly employed for the identification of several viruses and bacterial infections. It is generally regarded as a safe and well-tolerated technique, although a multitude of complications have been frequently documented, including but not limited to retained swabs, epistaxis, and cerebrospinal fluid leakage. These complications are often linked with high-risk factors such as severe septal deviations, pre-existing defects in the skull base, and previous sinus or transsphenoidal pituitary surgery. Professionals must possess adequate anatomical knowledge and employ appropriate techniques to carry out nasopharyngeal COVID-19 testing. Healthcare professionals must conduct these tests in a dedicated and sterilized room to prevent the transmission of the virus. Healthcare providers conducting the nasopharyngeal swab test must adhere to the prescribed personal protective measures, encompassing an N95 mask, disposable cap, goggles, gown, latex gloves, and shoe covers. Despite potential discomfort and extended result waiting periods, this method remains paramount for its heightened sensitivity and accuracy owing to the elevated virus concentration in the nasopharyngeal region. Medium cost. Medium accessibility. |
| Oropharyngeal swab | Oropharyngeal (OPS) sampling is considered a more practical method to use. Before being extracted, the swab is gently twisted a few times and directed towards the posterior wall of the oropharynx. A broader variety of swab items can be used to get OPS samples, and while self-collection may be possible, it is advised that a healthcare professional collect OP swabs. Performing throat cultures on individuals who have an irritated epiglottis is not recommended. Acute respiratory obstruction and bacterial infection can both be exacerbated by swabbing the epiglottis. Healthcare professionals must conduct these tests in a dedicated and sterilized room to prevent the transmission of the virus. All healthcare professionals administering the oropharyngeal swab test are required to don the recommended personal protective equipment (PPE), which should include an N95 mask, disposable cap, goggles, gown, latex gloves, and shoe covers. Medium cost. Medium accessibility. |
| Bronchoalveolar Lavage | Bronchoalveolar lavage fluid (BAL) samples may be considered as a viable option due to their characteristics as a highly effective specimen with a notable rate of detection. However, in the collection process, a bronchoscope is passed through the mouth or nose into an appropriate airway in the lungs, with a measured amount of fluid introduced and then collected for examination. This sample is typically obtained from patients who are severely ill or undergoing mechanical ventilation. Bronchoalveolar lavage serves as a valuable and secure procedure for sampling the cellular components of the lung. BAL, as a diagnostic tool, can be employed to accurately diagnose various infections and acquire material for culture and sensitivity analysis. The most prevalent risks associated with this procedure are akin to those observed in flexible bronchoscopy, including transient hypoxemia, post-BAL fever (which can be seen in up to 30% of patients), bronchospasm, and, very rarely, pneumothorax. This procedure can only be performed by a trained physician, and it is required to wear the recommended personal protective equipment (PPE), which should include an N95 mask, disposable cap, goggles, gown, latex gloves, and shoe cover. Very high cost. Low accessibility. |
| Saliva | Saliva testing and collection may be a simple, low-cost sample technique that causes the user as little discomfort as possible. It is also a reliable, user-friendly, portable, and scalable platform for the diagnosis of diseases. The type of saliva sampled—whole saliva or saliva generated by certain glands—as well as whether the sample was taken following stimulation will determine the outcome. The amount of spit and the device’s capacity to retrieve the biomarkers determine which one is best. Moreover, the salivary composition can be influenced by several factors, including the flow rate, pH, temperature, food, and age (with infants, children, and the elderly exhibiting the most fluctuation). However, since new, extremely sensitive technologies have been developed, saliva has a lower level of analytes. Additionally, the potential to be self-collected eliminates the need for a trained health professional for sampling. Low cost. High accessibility. |
| Faeces | SARS-CoV-2 is frequently detected in faecal specimens even after negative results from throat swabs during the post-symptomatic phase. The presence of the virus in the stool seems to be comparable among patients with and without gastrointestinal symptoms. It is crucial to acknowledge that the stool sample might contain infectious pathogens, which can be transmitted to others. Consequently, it is mandatory for all healthcare professionals handling the samples to wear the prescribed personal protective equipment (PPE), including an N95 mask, disposable cap, goggles, gown, latex gloves, and shoe covers. The performance of a faecal culture testing does not entail any associated risks and can be self-collected. Low cost. Medium accessibility. |
| Blood | In certain scenarios, such as when dealing with patients in a comatose state, the act of sampling through the oropharyngeal or nasopharyngeal route is rarely achievable, thus making blood sampling a viable alternative. Furthermore, the utilization of blood tests may pose fewer risks for the medical personnel carrying out the sampling compared to the use of respiratory swabs. It is important to acknowledge that the identification of viral RNA-aemia might not indicate an active infection, but rather signify a post-viral infection period. As this approach involves an invasive methodology, there exists the potential for adverse effects such as pain, swelling, bruising, discoloration of tissue, or scarring around the vein from which the blood sample is drawn. It is plausible that some of these tissue alterations and scarring could persist permanently. Additionally, these collection procedures must be conducted exclusively by licensed technicians with the proper collection material including collection tubes, needles, blood collection chairs and sterilization materials. Every healthcare professional must use the specified personal protective equipment (PPE), comprising an N95 mask, disposable cap, goggles, gown, latex gloves, and shoe covers. High cost. High accessibility. |
| Human Body Systems | ||||||||
|---|---|---|---|---|---|---|---|---|
| Urinary | Respiratory | Digestive | Circulatory | |||||
| Urine | Sputum | NPS | OPSs | BAL | Saliva | Faeces | Blood | |
| Materials and Equipment | 5 | 5 | 4 | 4 | 1 | 5 | 5 | 3 |
| Infection Risk for the Health Professional | 5 | 5 | 3 | 3 | 2 | 5 | 5 | 4 |
| Infection Risk for the Patient | 5 | 5 | 3 | 3 | 1 | 5 | 5 | 4 |
| Collection | 5 | 5 | 3 | 4 | 1 | 5 | 5 | 2 |
| Cost | 5 | 5 | 4 | 4 | 1 | 5 | 5 | 3 |
| Specialized HR | 5 | 5 | 3 | 3 | 1 | 5 | 5 | 3 |
| RNA Extraction Type | 1 | 4 | 5 | 5 | 5 | 5 | 1 | 3 |
| 4.4 | 4.9 | 3.6 | 3.7 | 1.7 | 5.0 | 4.4 | 3.1 | |
| N Total. Saliva Positive | Sensitivity (Positive Agreement %) and Notes | Type of Molecular Test | Reference |
|---|---|---|---|
| 74 samples. 53/58 positives | 91.37% positive agreement. The CT value for detection of the SARS-CoV-2 ORF1 gene tendentially lower in saliva than that of NPSs (mean 27.07; 95% CI, 25.62 to 28.52 vs. mean 28.24; 95% CI, 26.62 to 29.85, although nonsignificant statistically (p > 0.05). | RNA extraction + RT-qPCR | [5] |
| 522 samples. 33/39 positives | 84.6% positive agreement (33/39 patients, 95% CI 70.0–93.1%) in opposition to NPSs than only had 39/522 SARS-CoV-2 positive cases (6.3%; 95% CI 4.6–8.5%). The median CT value was lower in NPSs compared with saliva and was correlated with the days from symptom onset in both samples. | RNA extraction + RT-qPCR | [58] |
| 166 samples. 16/16 positives | 93.75% positive agreement using ddPCR (15 out of 16 samples) and 100% using RT-qPCR (16 out of 16 samples). | RNA extraction + RT-qPCR and ddPCR | [52] |
| 459 samples. 31/37 positives | 81.1% positive agreement, and 90.0% in samples with moderate to high viral load. The mean CT values were on higher in saliva compared with NPSs. | RNA extraction + RT-qPCR | [54] |
| 31 samples. 3/31 positives 3/4 critically ill patients | 23.08% positive agreement and 75% positive agreement in critically ill patients | RT-qPCR | [51] |
| 58 samples. 49/58 positives | 84.5% positive agreement. | Xpert Xpress SARS-CoV-2 assay | [59] |
| 185 samples. 6/37 positives | 16.22% positive agreement. The mean viral load was 5677 ± 13,647 compared to 16,224 ± 67,507 at NPSs. Many patients in the recovery period negative for SARS-CoV-2 in NPSs were positive with other specimens, particularly in anal swabs. | RNA extraction + ddPCR | [60] |
| 5 samples. 5/5 positives | 100% positive agreement (first saliva in the morning compared to NPSs). Exception was in children by days 8–10 after the onset of symptoms that were positive for SARS-CoV-2 in NPSs but not in saliva. | RNA extraction + RT-qPCR | [2] |
| 401 samples. 20/35 positives | The virus was detected in 6.5% (n/N = 26/401) cases by swab and 7.0% (n/N = 28/401) by saliva. For saliva the sensitivity was 73.1% (95% CI 52.2–88.4%), the specificity was 97.6% (95% CI 95.5–98.9%), the positive predictive value was 67.9% (95% CI 51.5–80.8%) and the negative predictive value was 98.1% (95% CI 96.5–99.0%). | RNA extraction + RT-qPCR | [6] |
| 28 samples. 19/23 positives | 82.61% positive agreement. In 3 convalescent patients, the virus was detected in saliva in opposition to NPSs, at 4 time points. | RNA extraction + RT-PCR | [56] |
| 36 samples. 20/27positives | 86% positive agreement. The values of PPA and NPA were 85.0% and 28.6%, respectively. Compared with NPSs, OPS, and sputum, saliva had higher detection performance and less false negatives. | RT-dPCR | [61] |
| 432 samples. 46/53 positives | 98.4% positive agreement. | RNA extraction + RT-PCR | [55] |
| NM | Almost equal to or higher viral loads in saliva than in NPSs/OPS (saliva 1.07 ± 0.34–1.65 ± 0.46 log10 copies/mL, versus NPSs/OPS 1.18 ± 0.12–1.34 ± 0.30 log10 copies/mL) | RNA extraction + RT-qPCR | [62] |
| 16 samples. 4/7 positives | Detection of viral RNA in 7 out of 16 saliva specimens (43.8%) and viral antigen in 4 out of 7 positive saliva specimens (47.8%). | Rapid immunochromatographic test | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Silva, M.; Cervantes, R.; Ribeiro, E.; Marques-Ramos, A. Development of an Indexed Score to Identify the Most Suitable Biological Material to Assess SARS-CoV-2. Appl. Sci. 2024, 14, 2761. https://doi.org/10.3390/app14072761
Almeida-Silva M, Cervantes R, Ribeiro E, Marques-Ramos A. Development of an Indexed Score to Identify the Most Suitable Biological Material to Assess SARS-CoV-2. Applied Sciences. 2024; 14(7):2761. https://doi.org/10.3390/app14072761
Chicago/Turabian StyleAlmeida-Silva, Marina, Renata Cervantes, Edna Ribeiro, and Ana Marques-Ramos. 2024. "Development of an Indexed Score to Identify the Most Suitable Biological Material to Assess SARS-CoV-2" Applied Sciences 14, no. 7: 2761. https://doi.org/10.3390/app14072761
APA StyleAlmeida-Silva, M., Cervantes, R., Ribeiro, E., & Marques-Ramos, A. (2024). Development of an Indexed Score to Identify the Most Suitable Biological Material to Assess SARS-CoV-2. Applied Sciences, 14(7), 2761. https://doi.org/10.3390/app14072761







