Investigation of Hydrogen-Blended Natural Gas Pipelines in Utility Tunnel Leakage and Development of an Accident Ventilation Strategy for the Worst Leakage Conditions
Abstract
1. Introduction
2. Physical and Mathematical Model
2.1. Physical Model
2.2. Mathematical Model
2.2.1. Model Simplification
- The gas leakage process at the leakage hole is isentropic, which means that the mass flow rate of HBNG at the leakage hole remains constant.
- The influence of air viscosity is disregarded.
- It is assumed that the HBNG is composed exclusively of methane and hydrogen. Following a leakage, these components become mixed with the surrounding air. These components are also believed to leak, behave as ideal gases, and not undergo any chemical reactions with each other.
- The walls of the natural gas compartment are adiabatic, which signifies the absence of heat transfer between the system and its surroundings.
2.2.2. Leakage Diffusion Modelling
- 1.
- Continuity equations:
- 2.
- Energy equation:
- 3.
- Momentum equation:
- 4.
- Component transport equation:
- 5.
- Turbulence equation:
2.3. Physical Properties of Components
2.4. Boundary Conditions
2.4.1. Boundary Condition Type Setting
2.4.2. Inlet Condition Setting
2.4.3. Leak Hole Condition Setting
2.5. Working Conditions
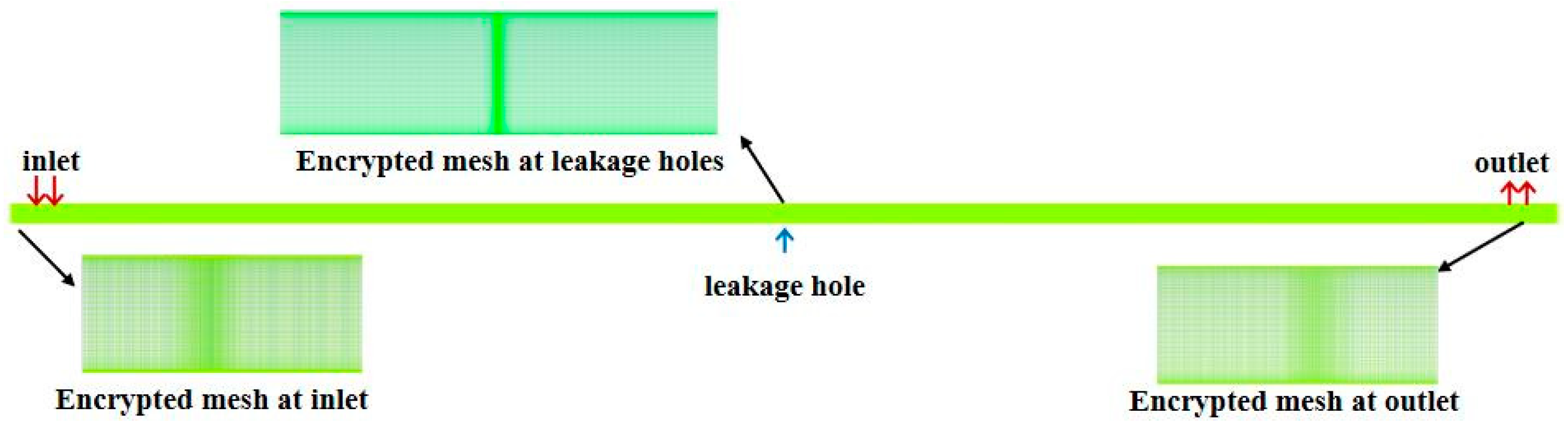
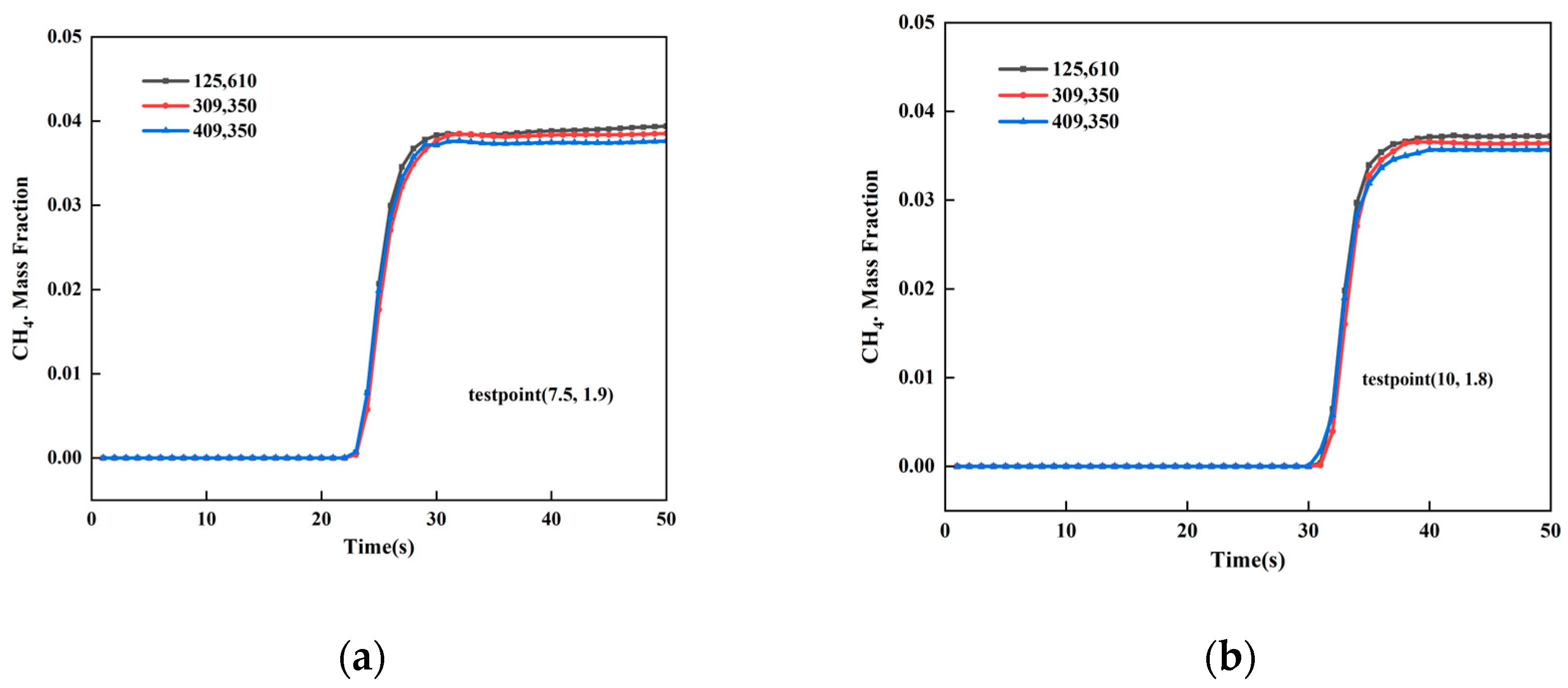
2.6. Verification of Grid-Independence
2.7. Initial Conditions and Solution Methods
- Initial conditions:
- 2.
- Solution methods:
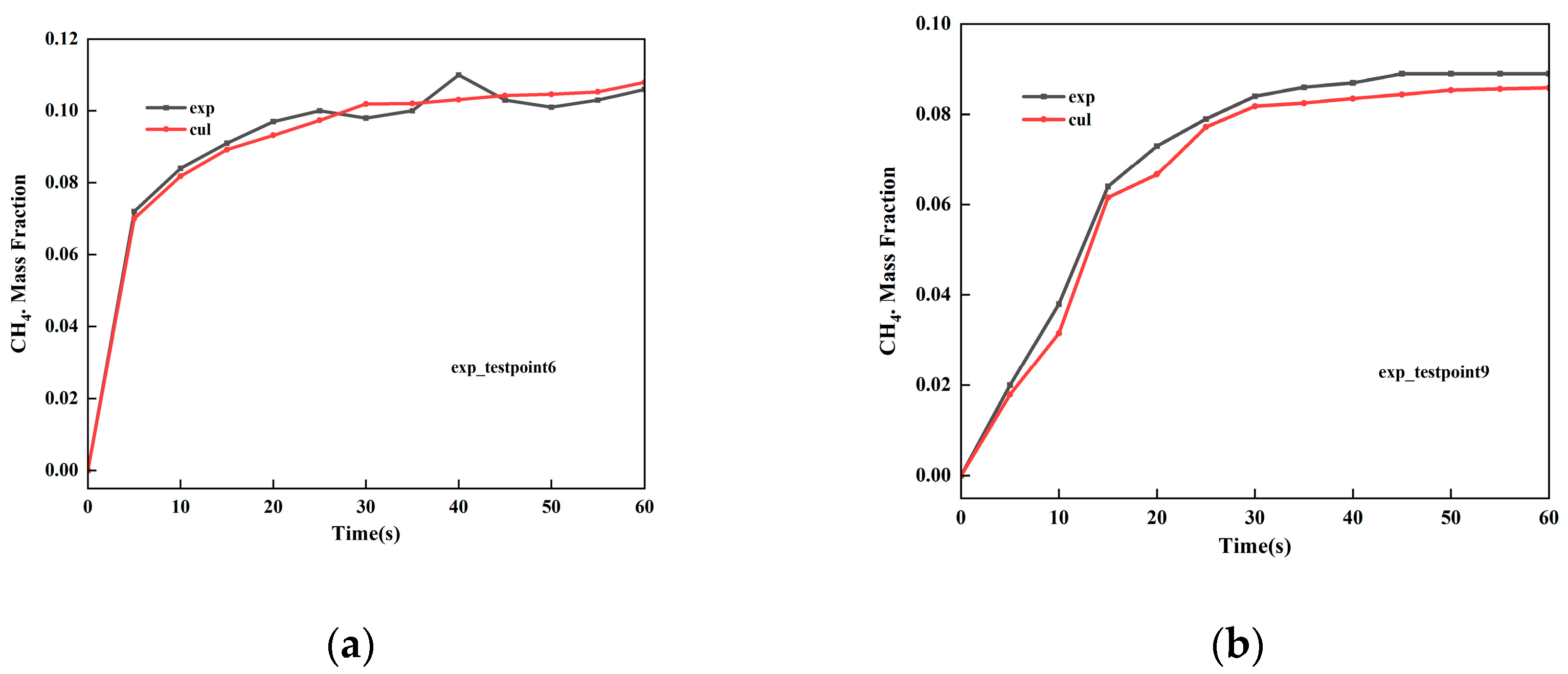
2.8. Model Validation
3. Results and Discussion
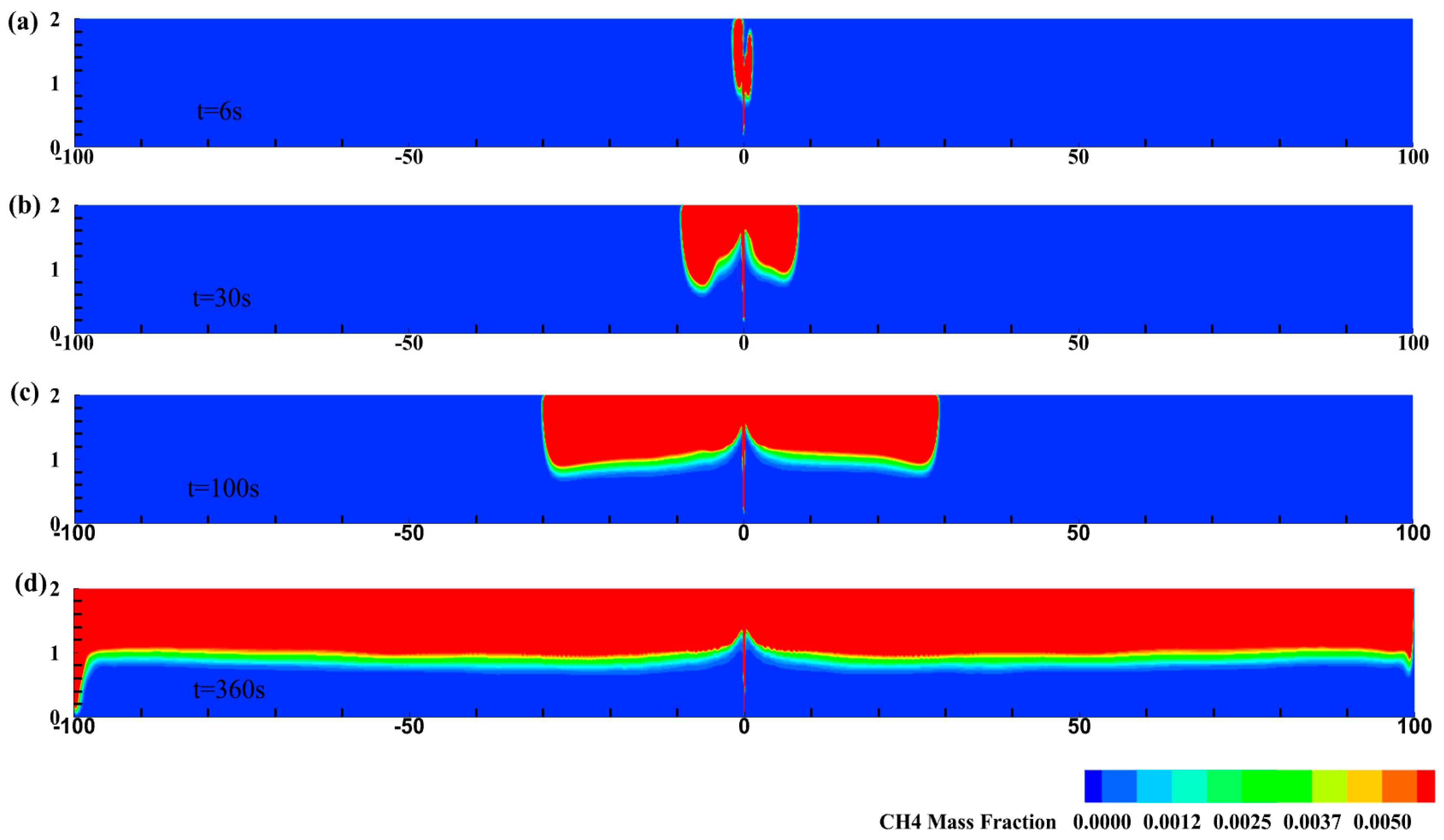
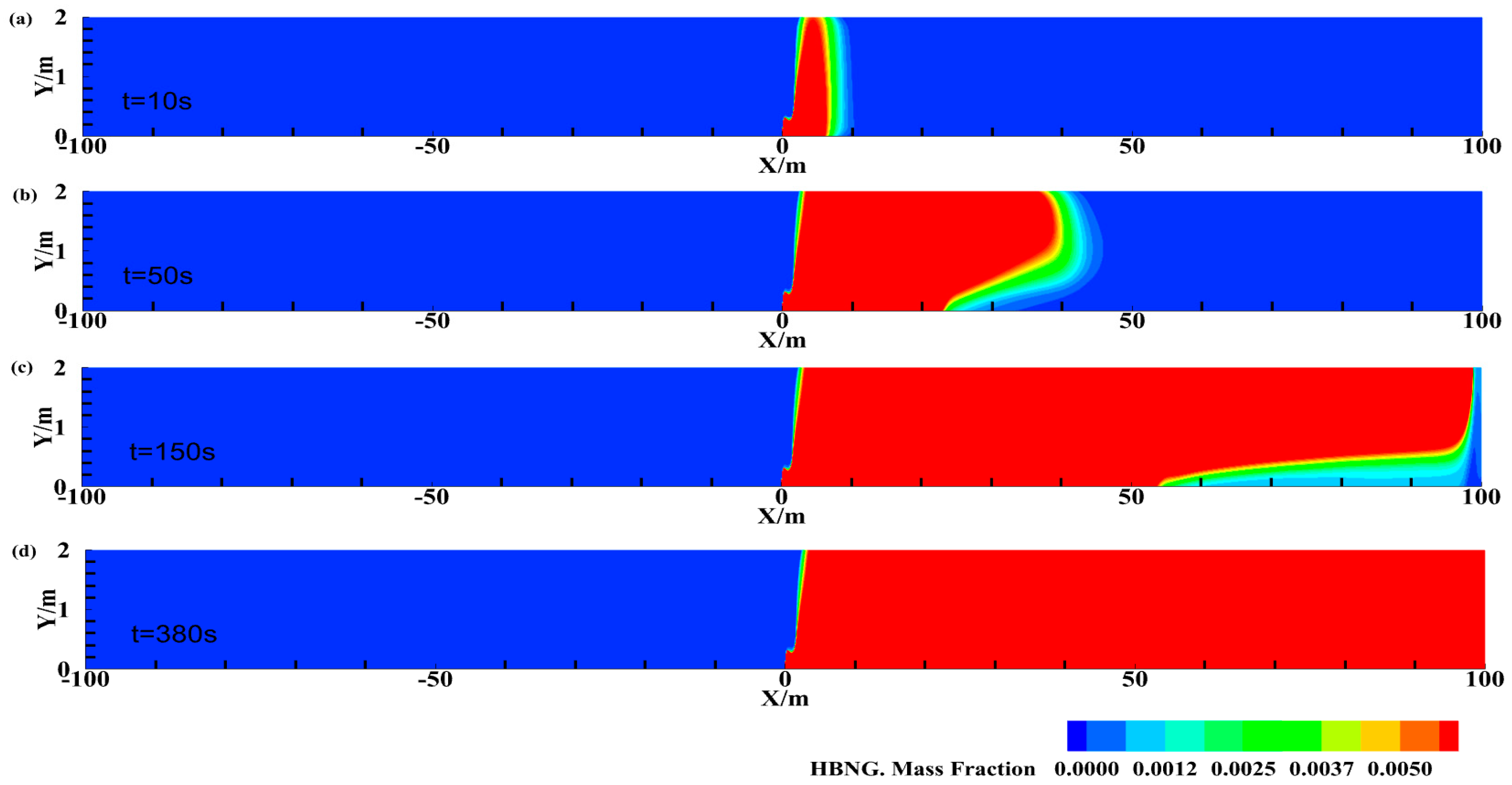
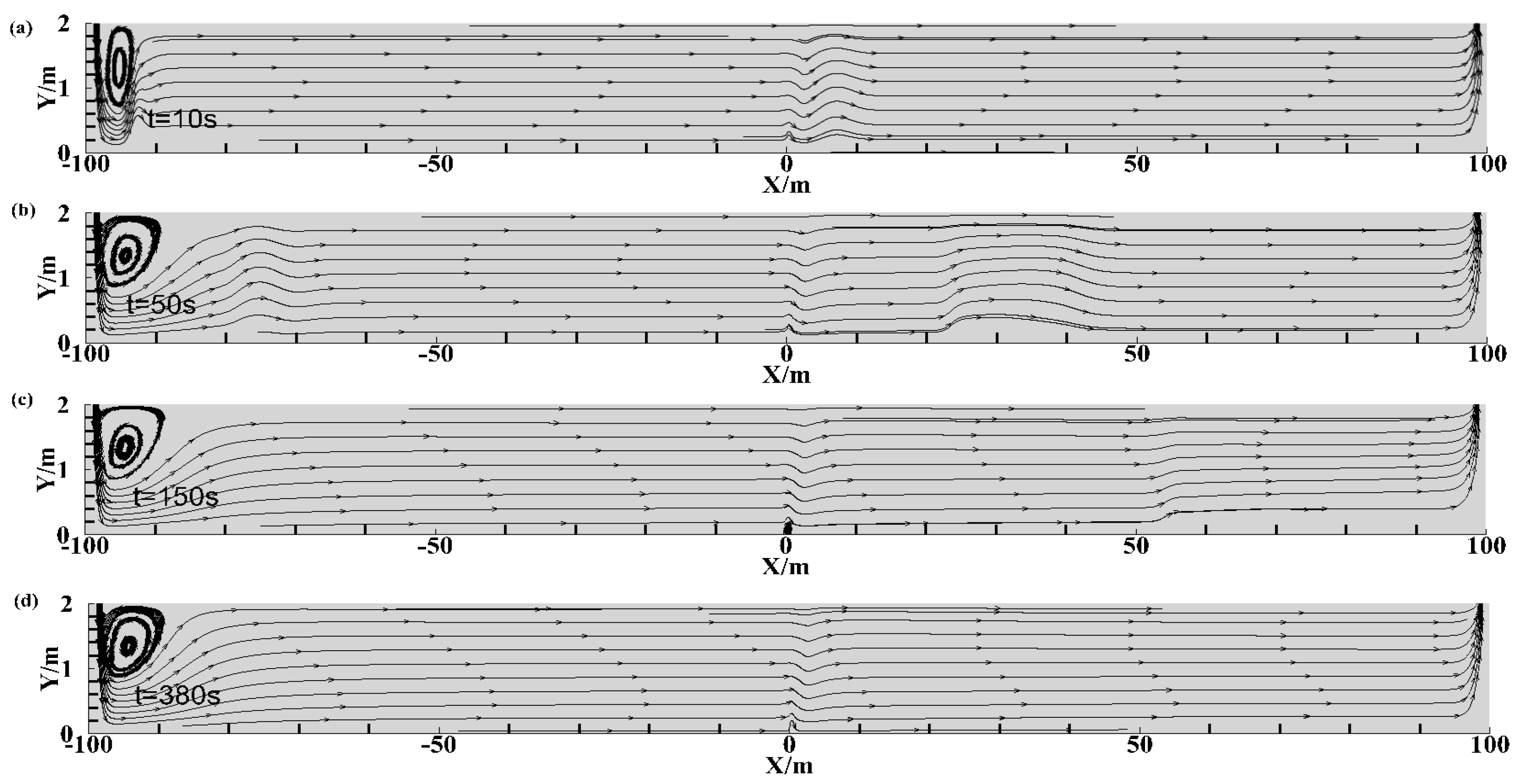
3.1. HBNG Leakage Pattern under Natural Ventilation
3.2. Analysis of Factors Affecting the Spread of HBNG Leakage under Normal Ventilation Conditions
3.2.1. HBNG Leakage Pattern under Normal Ventilation
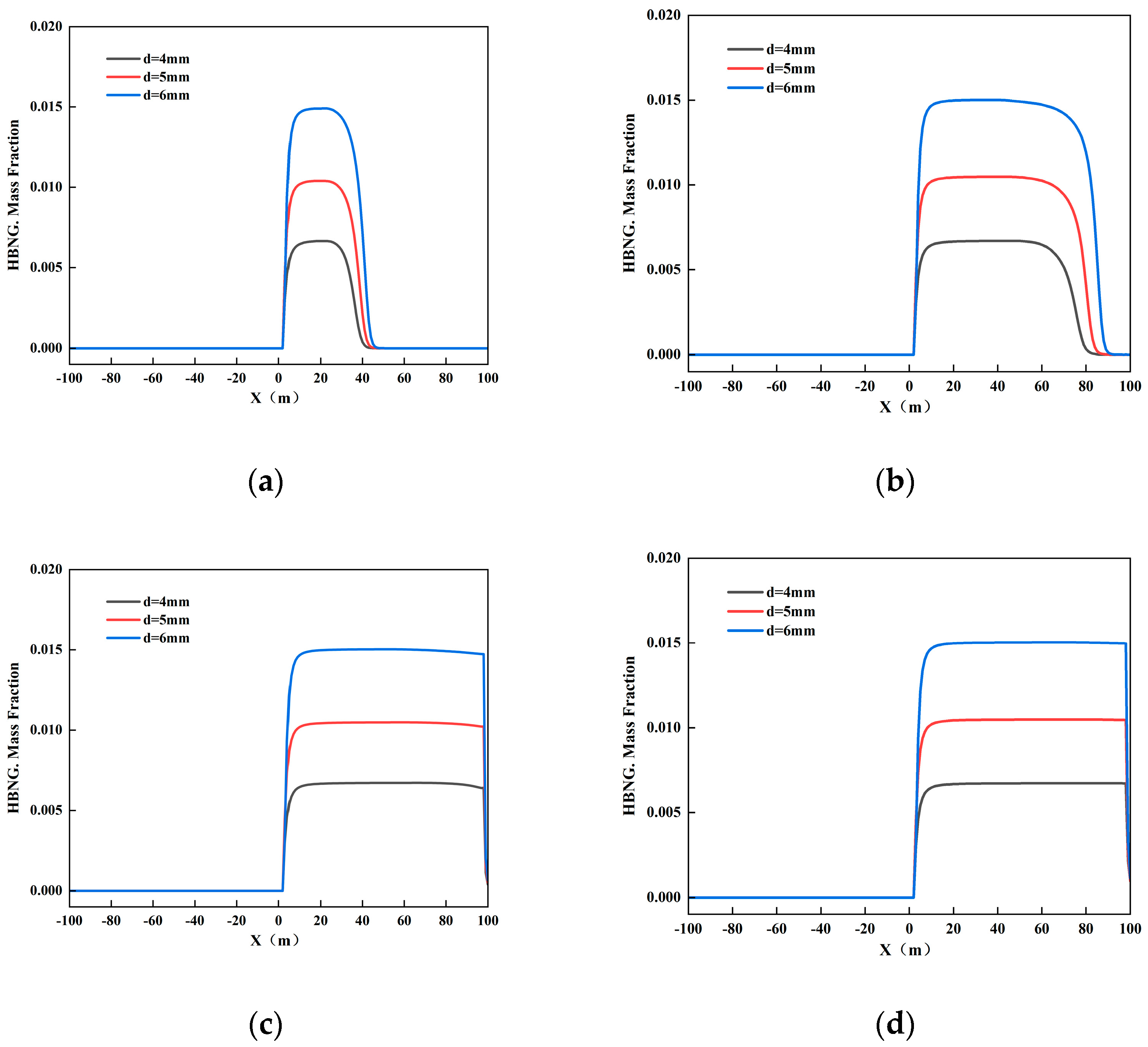
3.2.2. Different Leakage Hole Sizes
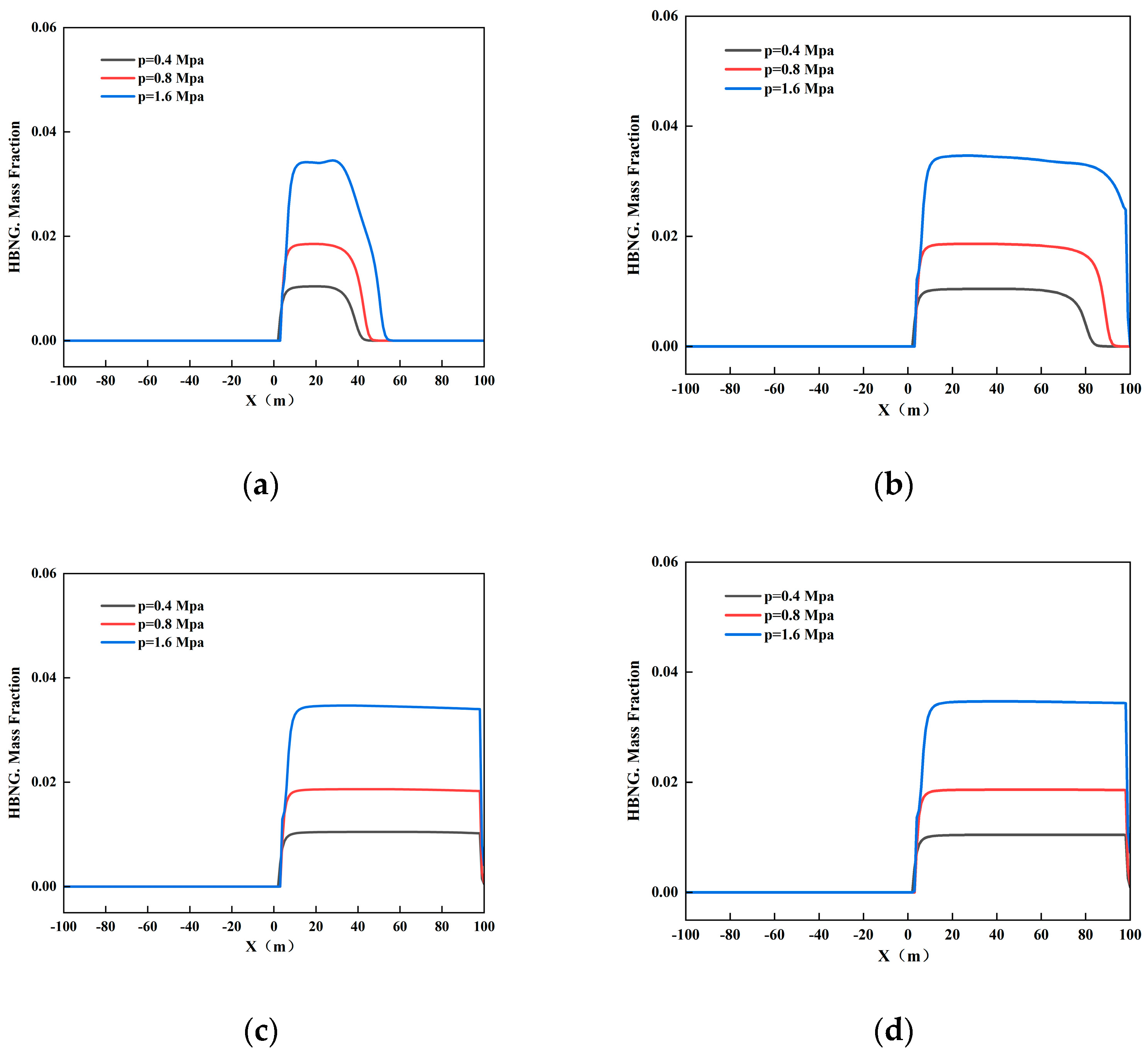
3.2.3. Different Pipeline Pressures
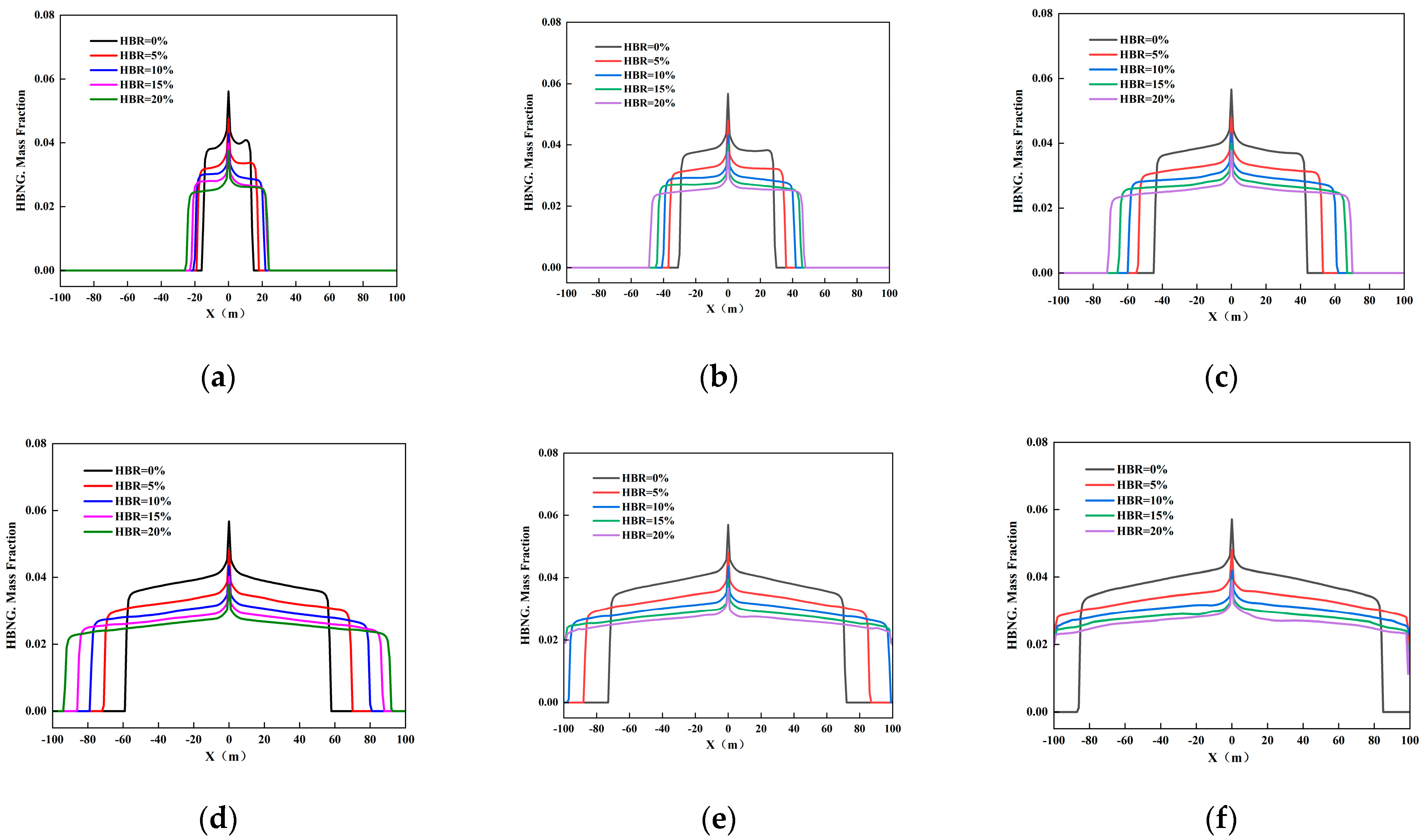
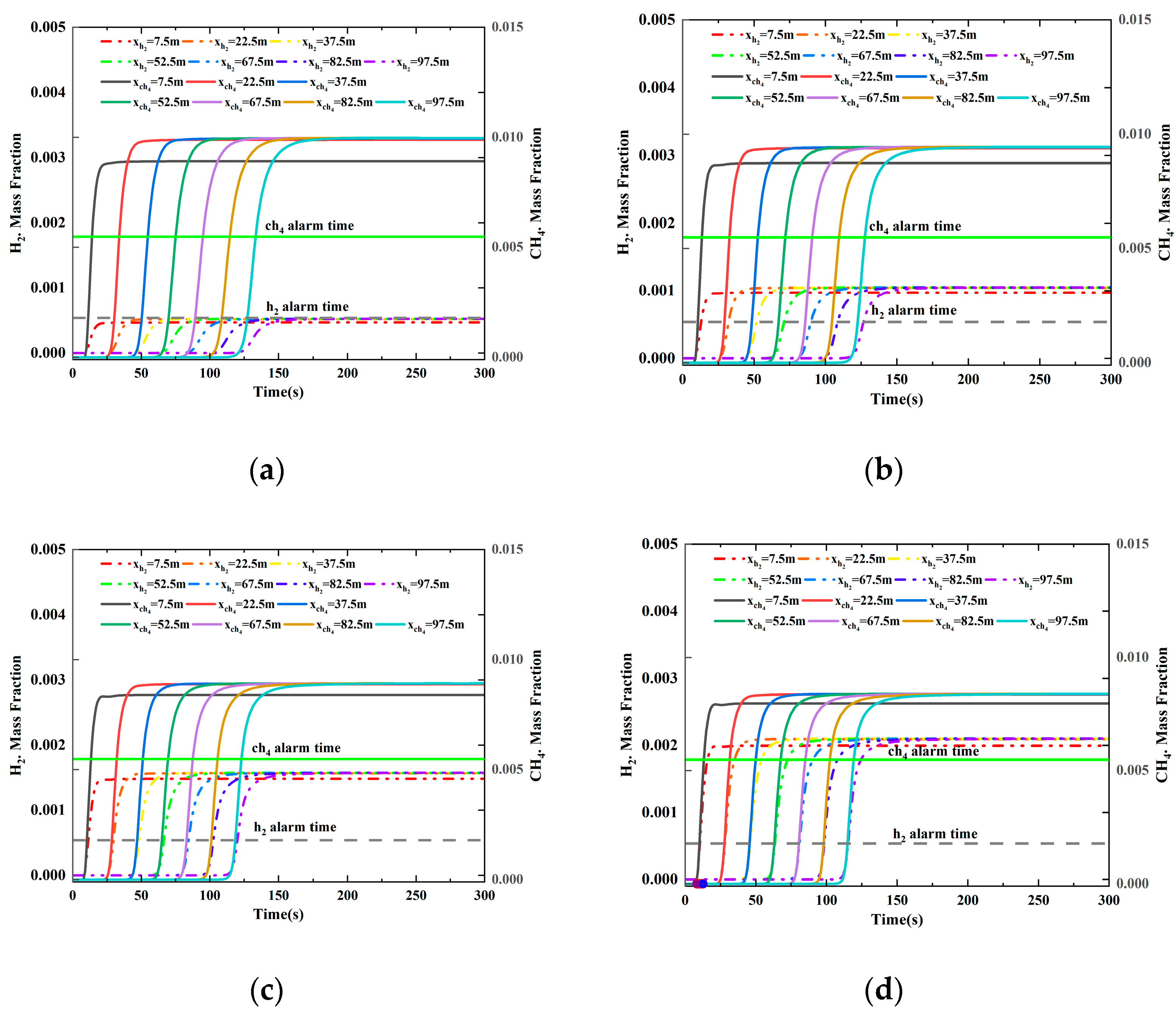
3.2.4. Different HBR
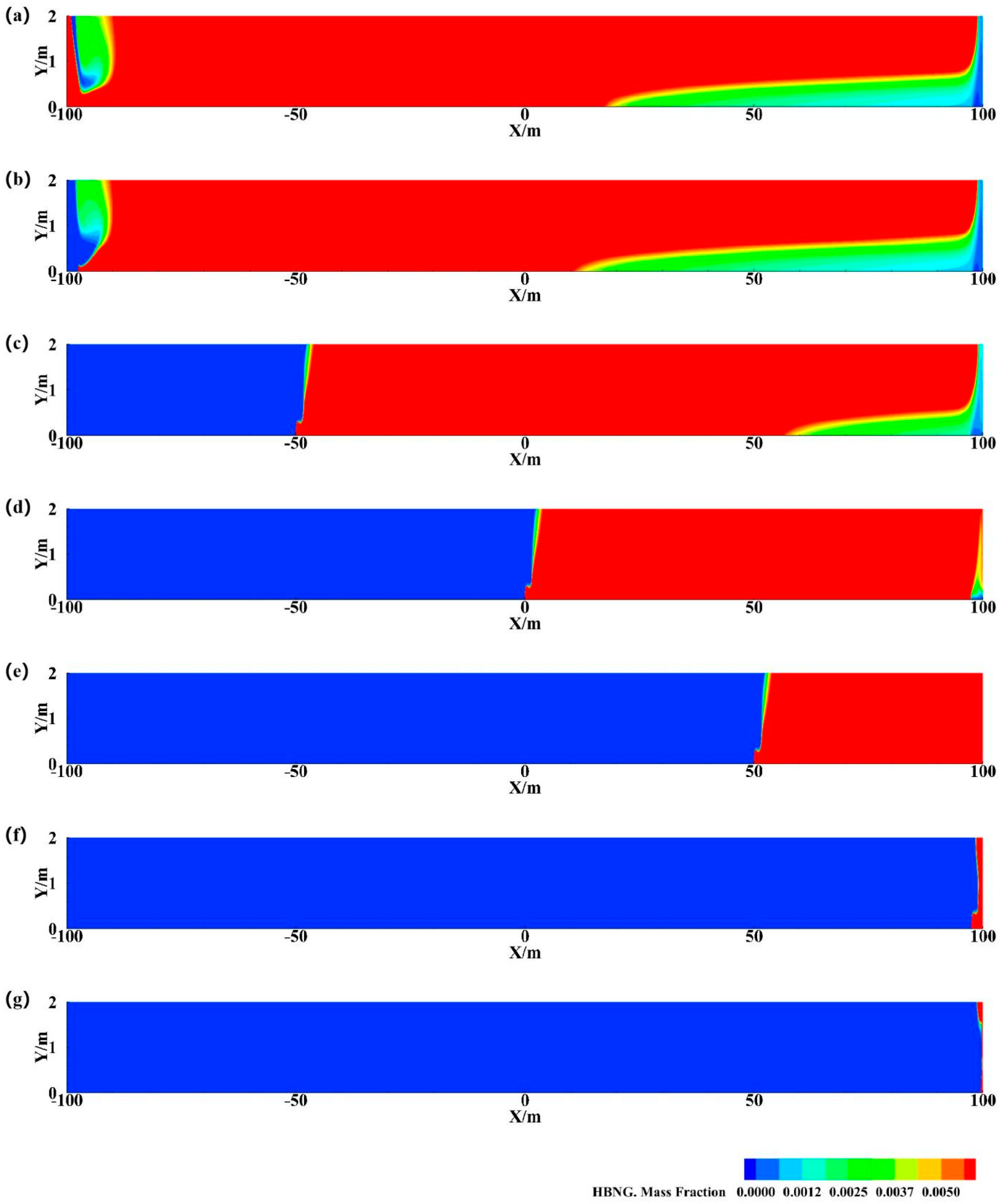
3.2.5. Different Leak Locations
3.3. Ventilation Strategy for HBNG Leakage Accidents Based on the Most Unfavorable Leakage Conditions
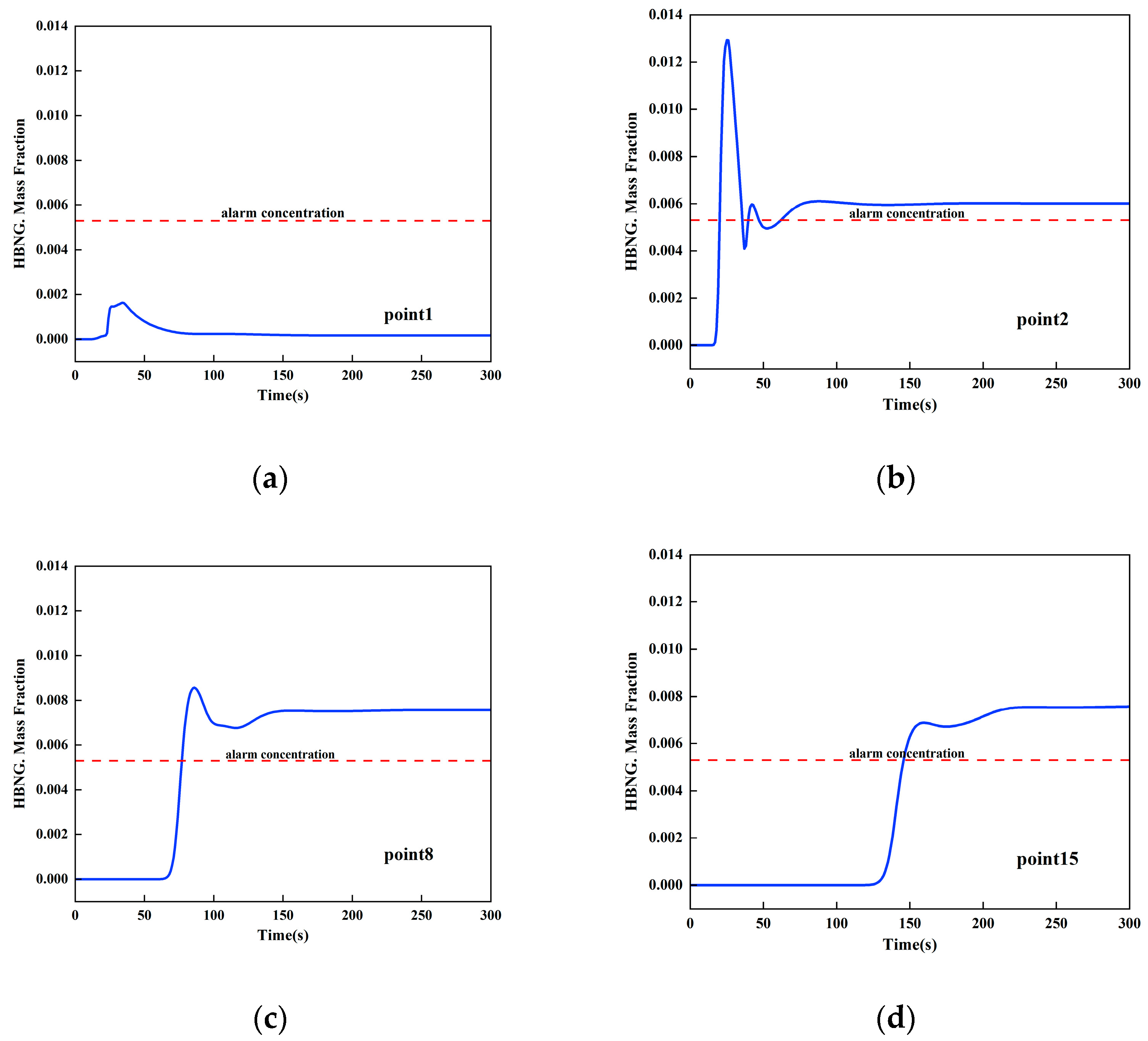
3.3.1. Determination of the Most Unfavorable Leakage Conditions
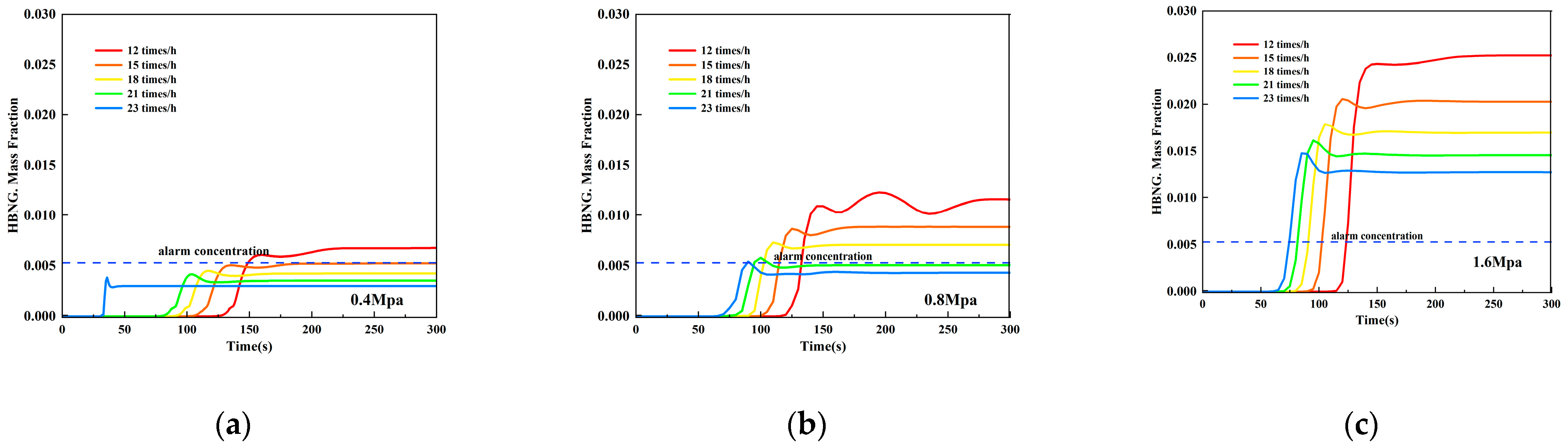
3.3.2. Minimal Accident Ventilation Strategy for Sub-High Pressure HBNG Pipelines under Most Unfavorable Working Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, J.X.; Tang, G.H.; Zhao, R.J.; Hwang, W.S. Carbon Reduction Programs and Key Technologies in Global Steel Industry. J. Iron Steel Res. Int. 2014, 21, 275–281. [Google Scholar] [CrossRef]
- Shen, Y.J.; Su, Z.W.; Huang, G.H.; Khalid, F.; Farooq, M.B.; Akram, R. Firm market value relevance of carbon reduction targets, external carbon assurance and carbon communication. Carbon Manag. 2020, 11, 549–563. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembsky, V.I.; Fateev, V.N. Pure hydrogen production by PEM electrolysis for hydrogen energy. Int. J. Hydrogen Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- Winter, C.J. Into the hydrogen energy economy–milestones. Int. J. Hydrogen Energy 2005, 30, 681–685. [Google Scholar] [CrossRef]
- Noh, H.; Kang, K.; Seo, Y. Environmental and energy efficiency assessments of offshore hydrogen supply chains utilizing compressed gaseous hydrogen, liquefied hydrogen, liquid organic hydrogen carriers and ammonia. Int. J. Hydrogen Energy 2023, 48, 7515–7532. [Google Scholar] [CrossRef]
- Yu, Y.W.; Chen, Y.; Jiang, J.H.; Li, Y.Z. Low-carbon scheduling of integrated hydrogen transport and energy system. Int. J. Hydrogen Energy 2024, 52, 655–666. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Nederlandse Gasunie, N.V. Using the Existing Natural Gas System for Hydrogen; 2009. Available online: https://www.fwg-gross-bieberau.de/fileadmin/user_upload/Erneuerbare_Energie/Naturalhy_Brochure.pdf (accessed on 12 February 2024).
- Kippers, M.J.; De Laat, J.C.; Hermkens, R.J.; Overdiep, J.J.; Van Der Molen, A.; Van Erp, W.C.; Van Der Meer, A. Pilot project on hydrogen injection in natural gas on island of Ameland in the Netherlands. Int. Gas Res. Conf. 2011, 2, 1163–1177. Available online: http://members.igu.org/old/IGU%20Events/igrc/igrc2011/igrc-2011-proceedings-and-presentations/poster%20paper-session%201/P1-34_Mathijs%20Kippers.pdf (accessed on 15 February 2024).
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; National Renewable Energy Laboratory: Golden, CO, USA, 2013.
- Networks, N.G. H21 Leeds City Gate. Available online: https://www.h2knowledgecentre.com/content/project1017 (accessed on 15 February 2024).
- Zhou, H.G. The First Case in China! The First Phase of the HCNG Demonstration Project by the State Power Investment Corporation Limited Was Successfully Completed. Available online: http://news.bjx.com.cn/html/20191015/1013148.shtml (accessed on 15 February 2024).
- Shenzhen State-Owned Assets Supervision and Administration Commission. Shenzhen Built the Country’s First Town Gas Hydrogen Doping Integrated Science and Technology Experimental Platform. Available online: https://www.sz.gov.cn/cn/xxgk/zfxxgj/bmdt/content/post_11103389.html (accessed on 15 February 2024).
- GB 50838-2015; Technical Code for Urban Utility Tunnel Engineering. Ministry of Housing and Urabn-Rural Development of the People’s Republic of China: Beijing, China, 2015.
- GB 50028-2006; Code for Design of City Gas Engineering. North China Municipal Engineering Design & Research Institute: Beijing, China, 2006.
- Wang, X.M.; Tan, Y.F.; Zhang, T.T.; Zhang, J.D.; Yu, K.C. Diffusion process simulation and ventilation strategy for small-hole natural gas leakage in utility tunnels. Tunn. Undergr. Space Technol. 2020, 97, 17. [Google Scholar] [CrossRef]
- Bu, F.X.; Liu, Y.; Wang, Z.X.; Xu, Z.; Chen, S.Q.; Hao, G.W.; Guan, B. Analysis of natural gas leakage diffusion characteristics and prediction of invasion distance in utility tunnels. J. Nat. Gas Sci. Eng. 2021, 96, 19. [Google Scholar] [CrossRef]
- Bai, Y.P.; Wu, J.S.; Yuan, S.Q.; Reniers, G.; Yang, M.; Cai, J.T. Dynamic resilience assessment and emergency strategy optimization of natural gas compartments in utility tunnels. Process Saf. Environ. Protect. 2022, 165, 114–125. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wu, J.S.; Zhou, R.; Cai, J.T.; Bai, Y.P.; Pang, L. Effects of the length and pressure relief conditions on propagation characteristics of natural gas explosion in utility tunnels. J. Loss Prev. Process Ind. 2022, 75, 10. [Google Scholar] [CrossRef]
- Li, M.H.; Chen, S.Q.; Jiang, W.D.; Li, Y.C.; Xu, Z.; Guan, B.; Wang, X.W.; Lin, X.Q.; Liu, T.Q. Numerical Simulation Analysis of the Hydrogen-Blended Natural Gas Leakage and Ventilation Processes in a Domestic House. ACS Omega 2023, 8, 34610–34628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.F.; Su, Y.; Wang, P.; Yu, B. Effects of hydrogen blending on hydraulic and thermal characteristics of natural gas pipeline and pipe network. Oil Gas Sci. Technol. 2021, 76, 15. [Google Scholar] [CrossRef]
- CJJ/T 146-2011; Technical Specification for Urban Gas Alarm Control System. China City Gas Association: Beijing, China, 2011.
- Launder, B.E. Current Capabilities for Modeling Turbulence in Industrial Flows. Appl. Sci. Res. 1991, 48, 247–269. [Google Scholar] [CrossRef]
- Lu, H.F.; Huang, K.; Fu, L.D.; Zhang, Z.H.; Wu, S.J.; Lyu, Y.; Zhang, X.L. Study on leakage and ventilation scheme of gas pipeline in tunnel. J. Nat. Gas Sci. Eng. 2018, 53, 347–358. [Google Scholar] [CrossRef]
- GB/T 50493-2019; Standard for Design of Combustible Gas and Toxic Gas Detection and Alarm for Petrochemical Industry. National Standard of the People’s Republic of China: Beijing, China, 2009.
- Kondo, S.; Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Sekiya, A. A study on flammability limits of fuel mixtures. J. Hazard. Mater. 2008, 155, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X. Experimental and Numerical Research on Gas Leak and Diffusion in the Utility Tunnel. Master’s Thesis, Beijing Architecture University, Beijing, China, 2018. [Google Scholar]
- Hasheminasab, F.; Bagherpour, R.; Aminossadati, S.M. Numerical simulation of methane distribution in development zones of underground coal mines equipped with auxiliary ventilation. Tunn. Undergr. Space Technol. 2019, 89, 68–77. [Google Scholar] [CrossRef]
- Zhang, P. Study on Gas Leakage and Diffusion Law in Underground Utility Tunnel and Risk Assessment Method of Its Ventilation System; Beijing Jiaotong University: Beijing, China, 2021. [Google Scholar]


| Serial Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| X Coordinate | −97.5 | −82.5 | −67.5 | −52.5 | −37.5 | −22.5 | −7.5 | 0 | 7.5 | 22.5 | 37.5 | 52.5 | 67.5 | 82.5 | 97.5 |
| Symbol | Terminology | Unit |
|---|---|---|
| Gas density | ||
| Natural gas enthalpy value | ||
| Surface forces perform work on a micro-unit | ||
| Thermal conductivity | ||
| Thermodynamic temperatures of micro-unit | ||
| Energy dissipation function | ||
| Endothermic terms for micro-unit | ||
| Dynamic viscosity | ||
| , | The force acting on the x y coordinate axes of the micro-unit | |
| Pulsating expansion term for component i in compressible turbulence | ||
| Diffusive flux of component i | ||
| Chemical net source term for a substance i | ||
| Mole fraction of component i | ||
| Diffusion coefficient | ||
| Turbulent kinetic energy | ||
| Turbulent dissipation rate | ||
| Turbulent viscosity | ||
| Turbulent kinetic energy from mean velocity gradient | ||
| Dissipation rate from compressed turbulent dynamic expansion | ||
| , | Custom source items | |
| ,,,, | Model coefficients for turbulence equations | |
| Gas flow time | s | |
| Absolute pressure | Pa | |
| Mass fraction of component | % | |
| The relative molecular mass of air | ||
| The relative molecular mass of methane | ||
| Explosive limits of gas mixtures | % | |
| Volume fraction of hydrogen in the gas mixture | % | |
| Volume fraction of methane in the gas mixture | % | |
| Explosive limit of hydrogen | % | |
| Explosive limit of methane | % | |
| Air velocity | m/s | |
| Gas compartment volume | m3 | |
| Ventilation opening area | m2 |
| Gas Type | ) | LEL (vol%) | UEL (vol%) |
|---|---|---|---|
| CH4 | 0.6679 | 4.9 | 15 |
| H2 | 0.0819 | 4.0 | 75.9 |
| HBR (%) | LEL | UEL |
|---|---|---|
| 0 | 0.0279 | 0.0878 |
| 5 | 0.0275 | 0.0917 |
| 10 | 0.0272 | 0.0960 |
| 15 | 0.0268 | 0.1001 |
| 20 | 0.0265 | 0.1060 |
| Model Boundary | Boundary Type | Setting Parameters |
|---|---|---|
| air inlet | velocity inlet | velocity magnitude, species |
| leakage hole | mass-flow-inlet | mass flow rate, species |
| air outlet | pressure-outlet | |
| wall, firewall, ceiling | wall | |
| fluid domain | interior |
| Ventilation Strategies | 6 Times/h | 12 Times/h | 15 Times/h | 18 Times/h | 21 Times/h | 22 Times/h |
| air velocity | 1.33 m/s | 2.66 m/s | 3.325 m/s | 3.99 m/s | 4.655 m/s | 4.87 m/s |
| Pipeline Pressure (MPa) | Leakage Hole Diameter (mm) | Mass Flow Rate (kg/s) |
|---|---|---|
| 0.4 | 4 | 0.0106 |
| 5 | 0.0166 | |
| 6 | 0.0239 | |
| 0.8 | 5 | 0.0298 |
| 1.6 | 5 | 0.0563 |
| Pipeline Pressure (MPa) | Initial Gauge Pressure (MPa) |
|---|---|
| 0.4 | 0.274 |
| 0.8 | 0.493 |
| 1.6 | 0.932 |
| Case | Pipeline Pressure (MPa) | Leakage Hole Diameter (mm) | Leak Hole Location | HBR (%) |
|---|---|---|---|---|
| Case1 | 0.4 | 5 | X = 0 | 0 |
| Case2 | 0.4 | 5 | X = 0 | 5 |
| Case3 | 0.4 | 5 | X = 0 | 10 |
| Case4 | 0.4 | 5 | X = 0 | 15 |
| Case5 | 0.4 | 5 | X = 0 | 20 |
| Case6 | 0.4 | 4 | X = 0 | 15 |
| Case7 | 0.4 | 6 | X = 0 | 15 |
| Case8 | 0.8 | 5 | X = 0 | 15 |
| Case9 | 1.6 | 5 | X = 0 | 15 |
| Case10 | 0.4 | 5 | X = −50 | 15 |
| Case11 | 0.4 | 5 | X = −97.5 | 15 |
| Case12 | 0.4 | 5 | X = −99.5 | 15 |
| Case13 | 0.4 | 5 | X = 50 | 15 |
| Case14 | 0.4 | 5 | X = 97.5 | 15 |
| Case15 | 0.4 | 5 | X = 99.5 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Guan, B.; Wei, L.; Chen, S.; Li, M.; Jiang, X. Investigation of Hydrogen-Blended Natural Gas Pipelines in Utility Tunnel Leakage and Development of an Accident Ventilation Strategy for the Worst Leakage Conditions. Appl. Sci. 2024, 14, 2667. https://doi.org/10.3390/app14062667
Xu Z, Guan B, Wei L, Chen S, Li M, Jiang X. Investigation of Hydrogen-Blended Natural Gas Pipelines in Utility Tunnel Leakage and Development of an Accident Ventilation Strategy for the Worst Leakage Conditions. Applied Sciences. 2024; 14(6):2667. https://doi.org/10.3390/app14062667
Chicago/Turabian StyleXu, Zhe, Bing Guan, Lixin Wei, Shuangqing Chen, Minghao Li, and Xiaoyu Jiang. 2024. "Investigation of Hydrogen-Blended Natural Gas Pipelines in Utility Tunnel Leakage and Development of an Accident Ventilation Strategy for the Worst Leakage Conditions" Applied Sciences 14, no. 6: 2667. https://doi.org/10.3390/app14062667
APA StyleXu, Z., Guan, B., Wei, L., Chen, S., Li, M., & Jiang, X. (2024). Investigation of Hydrogen-Blended Natural Gas Pipelines in Utility Tunnel Leakage and Development of an Accident Ventilation Strategy for the Worst Leakage Conditions. Applied Sciences, 14(6), 2667. https://doi.org/10.3390/app14062667






