The Limitation of Unproductive Binding of Cellulases to Lignin by Ozone Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Chemicals
2.2. Ozone Pretreatment
2.3. Analysis Procedures
2.3.1. Fourier-Transform Infrared Spectrum (FTIR) Analysis
2.3.2. Phosphorus-31 Nuclear Magnetic Resonance Spectrum (31P-NMR) Analysis
2.3.3. Two-Dimensional Heteronuclear Single Quantum Coherence–Nuclear Magnetic Resonance (HSQC-NMR) Spectral Analysis
2.3.4. Rose Bengal Dye Analysis
2.3.5. Zeta Potential Analysis
2.3.6. Gel Permeation Chromatography (GPC) Analysis
2.4. Lignin and Cellulase Langmuir-Adsorption Analysis
3. Results and Discussion
3.1. FTIR Determination
3.2. 31P-NMR Determination
3.3. HSQC-NMR Determination
3.4. Determination of Lignin Hydrophobicity by Rose Bengal
3.5. Determination of Zeta Potential
3.6. GPC Determination
3.7. Langmuir Isothermal Adsorption Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Chen, L.; Khoo, K.S.; Gupta, V.K.; Sharma, M.; Show, P.L.; Yap, P.S. Exploitation of lignocellulosic-based biomass biorefinery: A critical review of renewable bioresource, sustainability and economic views. Biotechnol. Adv. 2023, 69, 108265. [Google Scholar] [CrossRef] [PubMed]
- Anastasovski, A. Improvement of Energy Efficiency in Ethanol Production Supported with Solar Thermal Energy—A Case Study. Clean. Prod. 2020, 278, 123476. [Google Scholar] [CrossRef]
- Li, Y.; Bhagwat, S.S.; Cortés-Peña, Y.R.; Ki, D.; Rao, C.V.; Jin, Y.; Guest, J.S. Sustainable Lactic Acid Production from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 1341–1351. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Kim, S.W.; Abd-Aziz, S. Advanced bioprocessing strategies for biobutanol production from biomass. Renew. Sustain. Energy Rev. 2018, 91, 1192–1204. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, F.; Wang, J.; Cao, L.; Han, Q. A review on solid acid catalysis for sustainable production of levulinic acid and levulinate esters from biomass derivatives. Bioresour. Technol. 2021, 342, 125977. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Zhang, Y.; Li, J. Effect of adding surfactant for transforming lignocellulose into fermentable sugars during biocatalysing. Biotechnol. Bioprocess Eng. 2011, 16, 930–936. [Google Scholar] [CrossRef]
- Sammond, D.W.; Yarbrough, J.M.; Mansfield, E.; Bomble, Y.J.; Hobdey, S.E.; Decker, S.R.; Taylor, L.E.; Resch, M.G.; Bozell, J.J.; Himmel, M.E. Predicting enzyme adsorption to lignin films by calculating enzyme surface hydrophobicity. Biol. Chem. 2014, 289, 20960–20969. [Google Scholar] [CrossRef]
- Lou, H.; Zhu, J.Y.; Lan, T.Q.; Lai, H.; Qiu, X. pH-Induced lignin surface modification to reduce nonspecific cellulase binding and enhance enzymatic saccharification of lignocelluloses. ChemSusChem 2013, 6, 919–927. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The isolation, characterization, and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef]
- Yu, Z.; Gwak, K.S.; Treasure, T.; Jameel, H.; Chang, H.M.; Park, S. Effect of lignin chemistry on the enzymatic hydrolysis of woody biomass. ChemSusChem 2014, 7, 1942–1950. [Google Scholar] [CrossRef]
- Pan, X. Role of functional groups in lignin inhibition of enzymatic hydrolysis of cellulose to glucose. J. Biobased Mater. Bioenergy 2008, 2, 25–32. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Da Cunha, M.P.; Maciel Filho, R.; Bonomi, A.; Jesus, C.D.F.; Rossell, C.E.V. Simulation of integrated first and second generation bioethanol production from sugarcane: Comparison between different biomass pretreatment methods. J. Ind. Microbiol. Biotechnol. 2011, 38, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Travaini, R.; Martín-Juárez, J.; Lorenzo-Hernando, A.; Bolado-Rodríguez, S. Ozonolysis: An advantageous pretreatment for lignocellulosic biomass revisited. Bioresour. Technol. 2016, 199, 2–12. [Google Scholar] [CrossRef]
- Barrera-Martínez, I.; Guzmán, N.; Peña, E.; Vázquez, T.; Cerón-Camacho, R.; Folch, J.; Honorato Salazar, J.A.; Aburto, J. Ozonolysis of alkaline lignin and sugarcane bagasse: Structural changes and their effect on saccharification. Biomass Bioenergy 2016, 94, 167–172. [Google Scholar] [CrossRef]
- Rosen, Y.; Mamane, H.; Gerchman, Y. Immersed ozonation of agro-wastes as an effective pretreatment method in bioethanol production. Renew. Energy 2021, 174, 382–390. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Ren, J.; He, B. Structural Changes of Alkali Lignin under Ozone Treatment and Effect of Ozone-Oxidized Alkali Lignin on Cellulose Digestibility. Processes 2022, 10, 559. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Klett, A.S.; Thies, M.; Zheng, Y. Inhibitory effects of lignin on enzymatic hydrolysis: The role of lignin chemistry and molecular weight. Renew. Energy 2018, 123, 664–674. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.; Argyropoulos, D. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef]
- Li, G.; Ho, K.K.H.Y.; Zuo, Y.Y. Relative Dye Adsorption Method for Determining the Hydrophobicity of Nanoparticles. J. Phys. Chem. C 2022, 126, 832–837. [Google Scholar] [CrossRef]
- Lange, H.; Rulli, F.; Crestini, C. Gel Permeation Chromatography in Determining Molecular Weights of Lignins: Critical Aspects Revisited for Improved Utility in the Development of Novel Materials. ACS Sustain. Chem. Eng. 2016, 4, 5167–5180. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Yoo, C.G.; Chen, C.; Meng, X.; Dai, J.; Yang, C.; Yu, J.; Ragauskas, A.J.; Chen, X. A mechanistic study of cellulase adsorption onto lignin. Green Chem. 2021, 23, 333–339. [Google Scholar] [CrossRef]
- Yang, H.; Yoo, C.G.; Meng, X.; Pu, Y.; Muchero, W.; Tuskan, G.A.; Tschaplinski, T.J.; Ragauskas, A.J.; Yao, L. Structural changes of lignins in natural Populus variants during different pretreatments. Bioresour. Technol. 2020, 295, 122240. [Google Scholar] [CrossRef] [PubMed]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Sun, W.; Li, X.; Wang, F.; Zhao, J.; Qu, Y. Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism. Biotechnol. Biofuels 2014, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, C.; Tong, S.; Cui, Z.; Liu, P. Enhanced enzymatic hydrolysis and structural features of corn stover by NaOH and ozone combined pretreatment. Molecules 2018, 23, 1300. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E. On the quantification of lignin hydroxyl groups with 31P and 13C NMR spectroscopy. J. Wood Chem. Technol. 2015, 35, 220–237. [Google Scholar] [CrossRef]
- Yao, L.; Xiong, L.; Yoo, C.G.; Dong, C.; Meng, X.; Dai, J.; Ragauskas, A.J.; Yang, C.; Yu, J.; Yang, H. Correlations of the physicochemical properties of organosolv lignins from Broussonetia papyrifera with their antioxidant activities. Sustain. Energy Fuels 2020, 4, 5114–5119. [Google Scholar] [CrossRef]
- More, A.; Elder, T.; Pajer, N.; Argyropoulos, D.S.; Jiang, Z. Novel and Integrated Process for the Valorization of Kraft Lignin to Produce Lignin-Containing Vitrimers. ACS Omega 2023, 8, 1097–1108. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, S.; Wang, W.; Linhardt, R.J.; Ragauskas, A.J. Preparation of Highly Reactive Lignin by Ozone Oxidation: Application as Surfactants with Antioxidant and Anti-UV Properties. ACS Sustain. Chem. Eng. 2020, 8, 22–28. [Google Scholar] [CrossRef]
- Wu, K.; Ying, W.; Shi, Z.; Yang, H.; Zheng, Z.; Zhang, J.; Yang, J. Fenton reaction-oxidized bamboo lignin surface and structural modification to reduce nonproductive cellulase binding and improve enzyme digestion of cellulose. ACS Sustain. Chem. Eng. 2018, 6, 3853–3861. [Google Scholar] [CrossRef]
- Shi, Z.; Xiao, L.; Deng, J.; Xu, F.; Sun, R. Physicochemical characterization of lignin fractions sequentially isolated (Dendrocalamus brandisii) with hot water and alkaline ethanol solution. J. Appl. Polym. Sci. 2012, 125, 3290–3301. [Google Scholar] [CrossRef]
- Wei, X.; Yu, Y.; Shen, Z.; Liu, Y.; Liu, X.; Wang, S.; Zhang, L.; Min, D. Deciphering the linkage type and structural characteristics of the p-hydroxyphenyl unit in Pinus massoniana Lamb compressed wood lignin. Int. J. Biol. Macromol. 2022, 208, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Sánchez, R.; Espinosa, E.; Savy, D.; Mazzei, P.; Piccolo, A.; Rodríguez, A. Isolation and Characterization of Gramineae and Fabaceae Soda Lignins. Int. J. Mol. Sci. 2017, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Figueirêdo, M.B.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Valorization of Pyrolysis Liquids: Ozonation of the Pyrolytic Lignin Fraction and Model Components. ACS Sustain. Chem. Eng. 2019, 7, 4755–4765. [Google Scholar] [CrossRef]
- Figueirêdo, M.B.; Keij, F.W.; Hommes, A.; Deuss, P.J.; Venderbosch, R.H.; Yue, J.; Heeres, H.J. Efficient Depolymerization of Lignin to Biobased Chemicals Using a Two-Step Approach Involving Ozonation in a Continuous Flow Microreactor Followed by Catalytic Hydrotreatment. ACS Sustain. Chem. Eng. 2019, 7, 18384–18394. [Google Scholar] [CrossRef]
- Cheng, K.; Sorek, H.; Zimmermann, H.; Wemmer, D.E.; Pauly, M. Solution-state 2D NMR spectroscopy of plant cellwalls enabled by a dimethylsulfoxide-d 6/1-ethyl-3-methylimidazolium acetate solvent. Anal. Chem. 2013, 85, 3213–3221. [Google Scholar] [CrossRef]
- Wen, J.; Sun, S.; Xue, B.; Sun, R. Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Wang, J.; Hao, X.; Wen, P.; Zhang, T.; Zhang, J. Adsorption and desorption of cellulase on/from lignin pretreated by dilute acid with different severities. Ind. Crops Prod. 2020, 148, 112309. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Lu, Z.; Zhang, J. Adsorption and desorption of cellulase on/from enzymatic residual lignin after alkali pretreatment. Ind. Crops Prod. 2020, 155, 112811. [Google Scholar] [CrossRef]
- Fritz, C.; Ferrer, A.; Salas, C.; Jameel, H.; Rojas, O.J. Interactions between cellulolytic enzymes with native, autohydrolysis, and technical lignins and the effect of a polysorbate amphiphile in reducing nonproductive binding. Biomacromolecules 2015, 16, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, C.; Lai, C.; Huang, C.; Li, X.; Yong, Q. Elucidation of structure-inhibition relationship of monosaccharides derived pseudo-lignin in enzymatic hydrolysis. Ind. Crops Prod. 2018, 113, 368–375. [Google Scholar] [CrossRef]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Yao, L.; Chen, C.; Yoo, C.G.; Meng, X.; Li, M.; Pu, Y.; Ragauskas, A.J.; Dong, C.; Yang, H. Insights of ethanol organosolv pretreatment on lignin properties of Broussonetia papyrifera. ACS Sustain. Chem. Eng. 2018, 6, 14767–14773. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M.; Gilkes, N.; Kadla, J.; Maximenko, V.; Kubo, S.; Saddler, J. Inhibition of cellulase, xylanase and β-glucosidase activities by softwood lignin preparations. J. Biotechnol. 2006, 125, 198–209. [Google Scholar] [CrossRef]
- Li, M.; Yi, L.; Bin, L.; Zhang, Q.; Song, J.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Comparison of nonproductive adsorption of cellulase onto lignin isolated from pretreated lignocellulose. Cellulose 2020, 27, 7911–7927. [Google Scholar] [CrossRef]
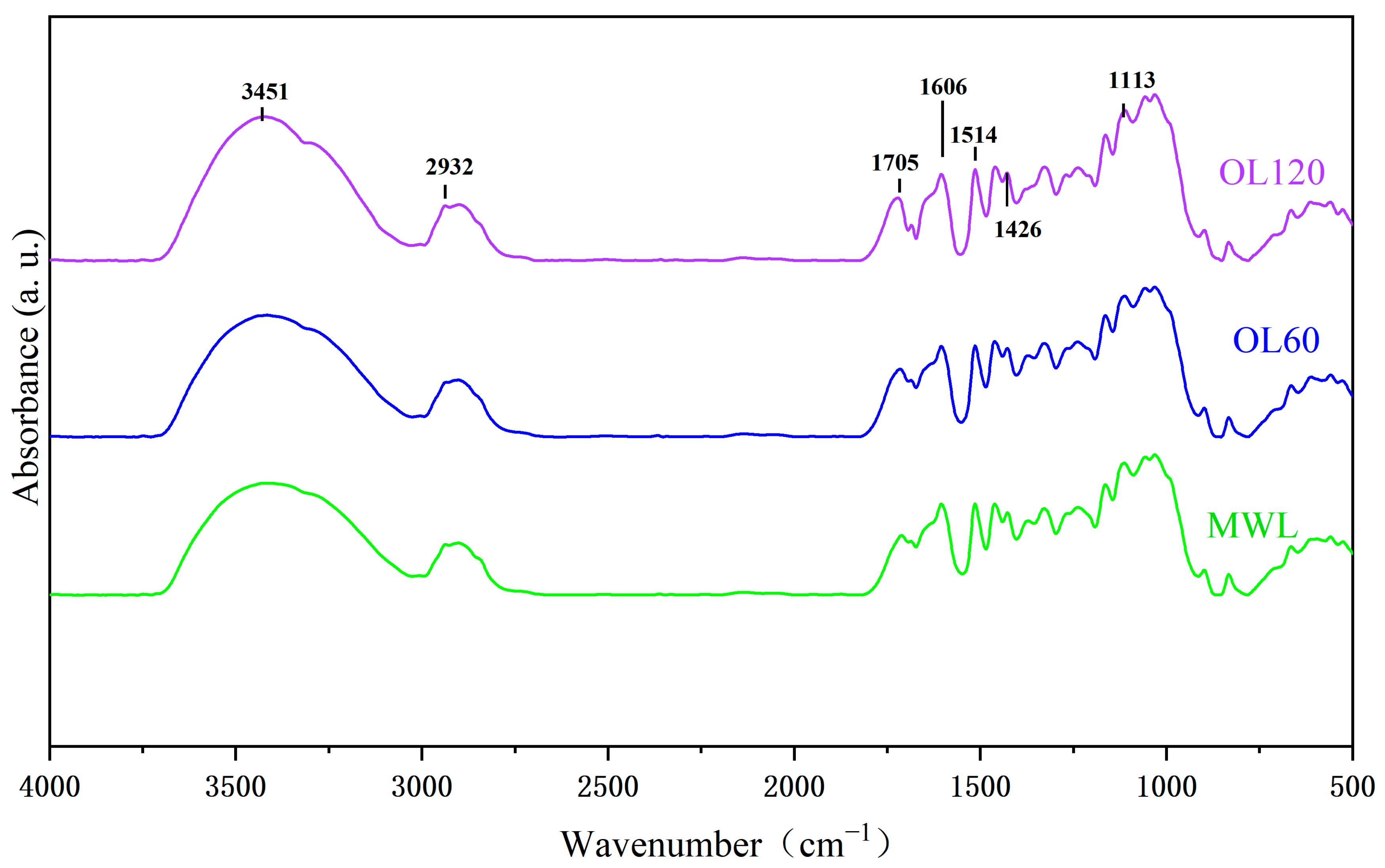

| Assignment | Wavenumber cm−1 | MWL | OL60 | OL120 |
|---|---|---|---|---|
| Hydroxyl group | 3451 | 1.08 | 1.15 | 1.18 |
| C-H stretching | 2932 | 1.03 | 1.01 | 0.98 |
| Carbonyl group | 1705 | 1.06 | 1.11 | 1.12 |
| Aromatic ring | 1606 | 0.97 | 1.02 | 1.05 |
| Aromatic ring | 1514 | 0.98 | 0.97 | 0.98 |
| Aromatic ring | 1426 | 1.00 | 1.00 | 1.00 |
| Aromatic C-H deformation in syringyl | 1113 | 0.81 | 0.77 | 0.76 |
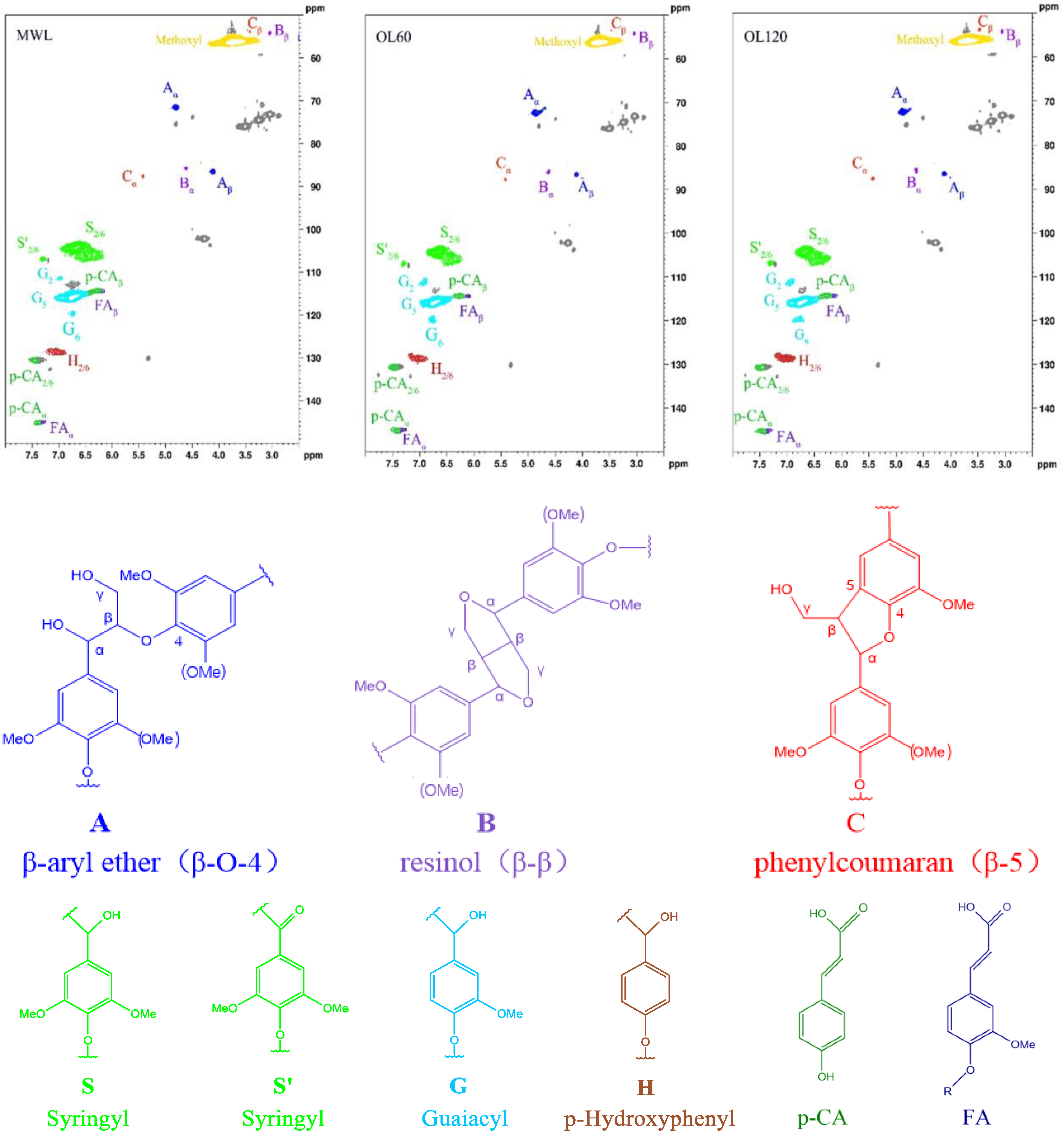
| Lignin Structure | Lignin Samples (mmol/g) | ||
|---|---|---|---|
| MWL | OL60 | OL120 | |
| Aliphatic hydroxyl | 2.13 | 1.88 | 1.75 |
| 5-substituted-OH | 1.08 | 0.88 | 0.79 |
| G-OH | 0.49 | 0.43 | 0.44 |
| H-OH | 0.69 | 0.66 | 0.65 |
| Total phenol hydroxyl | 2.26 | 1.97 | 1.88 |
| COOH | 0.21 | 0.30 | 0.47 |
| Lignin Structure | Lignin Samples (%) | ||
|---|---|---|---|
| MWL | OL60 | OL120 | |
| β-O-4 1 | 23.3 | 24.9 | 26.4 |
| β-β 1 | 5.8 | 6.6 | 8.0 |
| β-5 1 | 4.5 | 5.1 | 6.5 |
| S 2 | 59.9 | 57.8 | 56.1 |
| G 2 | 18.8 | 19.7 | 21.1 |
| H 2 | 20.7 | 21.1 | 22.8 |
| S/G 3 | 3.2 | 3.0 | 2.7 |
| p-CA | 15.0 | 16.2 | 15.9 |
| FA | 1.5 | 1.9 | 1.8 |
| Lignin | Lignin Samples | ||
|---|---|---|---|
| MWL | OL60 | OL120 | |
| Hydrophobicity (L/g) | 0.951 | 0.889 | 0.615 |
| Lignin | Lignin Samples | ||
|---|---|---|---|
| MWL | OL60 | OL120 | |
| zeta (mv) | −10.3 | −11.8 | −15.5 |
| Lignin | Lignin Samples | ||
|---|---|---|---|
| MWL | OL60 | OL120 | |
| Mw (g/mol) | 8879 | 8959 | 9301 |
| Mn (g/mol) | 4125 | 4051 | 4064 |
| PDI (Mw/Mn) | 2.15 | 2.21 | 2.29 |
| Lignin Samples | Γmax (mg/g) | K (mL/mg) | R (mL/g) | R2 | |
|---|---|---|---|---|---|
| Lignin | MWL | 25.77 | 13.86 | 357.14 | 0.980 |
| OL60 | 12.58 | 12.62 | 158.73 | 0.977 | |
| OL120 | 10.09 | 10.11 | 102.04 | 0.958 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhao, L.; Li, W.; Ren, J.; Wang, H.; He, B. The Limitation of Unproductive Binding of Cellulases to Lignin by Ozone Pretreatment. Appl. Sci. 2024, 14, 2318. https://doi.org/10.3390/app14062318
Zhang C, Zhao L, Li W, Ren J, Wang H, He B. The Limitation of Unproductive Binding of Cellulases to Lignin by Ozone Pretreatment. Applied Sciences. 2024; 14(6):2318. https://doi.org/10.3390/app14062318
Chicago/Turabian StyleZhang, Congfei, Lihong Zhao, Weiying Li, Junli Ren, Hongyuan Wang, and Beihai He. 2024. "The Limitation of Unproductive Binding of Cellulases to Lignin by Ozone Pretreatment" Applied Sciences 14, no. 6: 2318. https://doi.org/10.3390/app14062318
APA StyleZhang, C., Zhao, L., Li, W., Ren, J., Wang, H., & He, B. (2024). The Limitation of Unproductive Binding of Cellulases to Lignin by Ozone Pretreatment. Applied Sciences, 14(6), 2318. https://doi.org/10.3390/app14062318







