Abstract
Advanced glycation end-products (AGEs) and their receptor cause diabetic liver disease by increasing oxidative stress and inflammation. We investigate the potential therapeutic benefits of Cirsium japonicum (CJ) in preventing the progression of diabetes, focusing on complications for both liver and kidney health associated with AGEs. Streptozotocin (STZ, 30 mg/kg) was injected into SD rats and CJ (50, 100 mg/kg) was orally administered for 4 weeks. CJ treatment led to a marked reduction in key diabetic markers (glucose, reaction oxygen species, and lactate dehydrogenase), compared with the rats treated only with STZ. Moreover, the hepatic tissues of STZ-treated rats exhibited heightened biomarkers associated with AGE induction and formation, and these were notably attenuated in the CJ-treated rats. This effectively alleviated oxidative stress, inflammation, and AGE accumulation in the liver. Similarly, in the context of diabetic nephropathy, CJ treatment resulted in significant improvements in the rats with STZ-induced diabetes. Biomarkers associated with AGE induction and formation were significantly reduced in CJ-treated rats, demonstrating the ability of CJ to combat renal oxidative stress, inflammation, and AGE-related complications in diabetic nephropathy. CJ thus shows potential as a promising natural remedy that might mitigate the detrimental effects of diabetes on both the liver and kidneys through its anti-oxidation, anti-inflammation, and anti-AGE activities. These findings suggest that CJ is a beneficial agent for preventing and treating diabetic complications.
1. Introduction
Diabetes mellitus is a chronic metabolic disorder often associated with microvascular complications, including nephropathy, that result in significant morbidity and mortality worldwide [1,2]. The most significant challenge for diabetic patients is the complications arising from hyperglycemia. These diabetic complications can lead to disturbances in the eyes, nerves, and kidneys [3]. Although the mechanisms by which hyperglycemia causes these complications have not been clearly elucidated, the most representative pathways include the polyol pathway, the protein kinase C pathway, the hexosamine pathway, and the advanced glycation end-products (AGEs)/receptor of AGEs (RAGE) pathway [4].
AGEs are formed by non-enzymatic reactions between proteins and sugars or amino groups in a hyperglycemic state [5,6]. N(6)-carboxymethyllysine (CML) and N-(6)-(1-carboxyethyl)-L-lysine (CEL) are the most well-characterized glycated amino acids among the AGEs, and others include pentosidine and (2S)-2-amino-5-((3-methyl-2-oxo-1,3-dihydropyrrol-5-yl)amino)pentanoic acid (N5-(5-hydro-5-methyl-4-imidazolon-2-yl)L-ornithine: MG-H1) [6]. Among these, CML has mainly been reported to affect insulin secretion reduction and is associated with kidney and inflammation measurements. AGEs primarily induce oxidative stress, cellular dysfunction, and inflammatory response associated with diabetic complications [7]. Streptozotocin (STZ)-induced diabetic rats express RAGE, and higher levels of AGEs have been reported in the liver, contributing to the onset of inflammatory diseases [8]. Once initiated in the liver, the excessive activity of AGEs/RAGE is considered a central cause of severe diabetic complications, including nephropathy and testicular damage [9,10,11]. Furthermore, the oxidative stress and inflammation induced by these AGEs and RAGE contribute to the sustained activation of inflammation in diabetic conditions [8]. Therefore, the development of substances inhibiting the AGEs/RAGE signaling system would be beneficial for preventing diabetes and its associated complications.
The binding of RAGE and its ligands activates NADPH oxidase, leading to increased formation of reactive oxygen species (ROS) and leading to cell apoptosis [12]. The activation of ROS causes oxidative stress, resulting in the formation of AGEs and triggering all damage mechanisms mediated by AGEs [13]. On a mechanistic level, AGEs activate the mitogen-activated protein kinase (MAPK) pathways, which constitute a family of serine-threonine protein kinases. Specifically, the activation of c-jun N terminal kinase (JNK), a significant subfamily within the MAPK pathways, has been demonstrated to induce cell apoptosis and dysfunction [14]. The activation of the MAPK pathway and JNK subsequently induces the sustained activation of transcription factors such as NF-κB and STAT3, enhancing the expression of various inflammatory cytokines, thereby promoting chronic inflammation in the body [15].
Cirsium japonicum var. maackii (CJ) is a perennial plant native to Korea, Japan, China and other regions, and it has been utilized in traditional folk remedies due to its diverse pharmacological benefits [16,17,18]. CJ has demonstrated diverse physiological activities, including the prevention of Alzheimer’s disease [16] and non-alcoholic fatty liver disease [17], anti-aging effects on the skin [18], the prevention of renal hypertension [19,20], and protection against liver damage [21]. According to Yoon et al. [18], apigenin found in the 70% ethanol extract of CJ flowers was reported to inhibit the formation of AGEs and the oxidative stress induced by AGEs, indicating its role as a primary active ingredient in anti-glycation effects. CJ contains various flavonoids, and it has been reported to be a valuable material for the treatment of various complications associated with diabetes [22]. The water extract of CJ has been confirmed to inhibit α-glucosidase activity, indicating its potential as a therapeutic agent for diabetes [23]. However, the effects of CJ extract on AGEs and the associated mechanisms in STZ-induced diabetic rats has not been investigated. Therefore, we investigated the effects of extracts of CJ on STZ-induced diabetic rats, and found that CJ could prevent hepatic and renal damage triggered by the AGEs/RAGE signaling pathway in diabetic rats.
2. Materials and Methods
2.1. Sample Extraction
Cirsium japonicum var. maackii (CJ) plants were purchased from Imsil Herbal Medicine (Imsil-gun, Jeollabuk-do, Republic of Korea), and underwent a cleaning process and a drying process at 50 °C for 3 days. Dried CJ samples were added with 30% ethanol and the mixture was refluxed at 65 °C for 3 h for extraction, the filtered through 30 μm filter paper. Finally, the CJ extract was frozen and lyophilized.
2.2. Animals and Treatment
Sprague Dawley (SD) male rats (8 weeks old, n = 8/groups) were purchased from Damool Sciences (Daejeon, Republic of Korea). Rats had free access to a standard laboratory pellet diet (Damool Sciences, Daejeon, Republic of Korea) and DW and were housed under conditions with a 12 h light/12 h dark cycle and a constant temperature (22 ± 2 °C) and humidity (50 ± 10%). Rats were treated according to the guidelines prescribed by the Jeonbuk National University Animal Care Committee (Approval Number: NON2023-204).
The rats used in the experiment were subjected to a one-week acclimatization period and were subsequently randomly divided into five groups: (1) Normal, normal control group; (2) Veh, negative control group, diabetic control, STZ 30 mg/kg i.p. only; (3) CJ50, STZ 30 mg/kg i.p. + CJ extract 50 mg/kg/day oral administration group; (4) CJ100, STZ 30 mg/kg i.p. + CJ extract 100 mg/kg/day oral administration group; and (5) AG100, positive control group, aminoguanidine (AG) 100 mg/kg/day administration group.
Diabetes was induced experimentally by administering a single intraperitoneal (i.p.) injection of freshly prepared STZ at a dose of 30 mg/kg (dissolved in a 10 nmol/L of citrate buffer) to SD rats after fasting for 12 h. The Normal group received an equivalent volume of citrate buffer without STZ. After two weeks of injection of STZ, CJ and AG were orally administrated daily in appropriate doses to diabetic rats for a period of 4 weeks. The Normal and Veh groups received an equivalent volume of DW as used for diluting CJ and AG throughout the same 4-week period. The body weights and food and water intakes were determined every day during the experimental period. In addition, the fasting blood glucose levels were measured and recorded every week during the experimental period. The rats were anesthetized using isoflurane (Hana Pharm Co., Ltd., Seoul, Republic of Korea) to facilitate blood collection. The blood was centrifuged at 800× g for 15 min to obtain the serum supernatant. After blood collection, the rats were euthanized upon cessation of breathing and a drop in heart rate, and liver and kidney tissues were harvested for further analysis. The serum, liver, and kidney samples were stored at −80 °C before analysis.
2.3. Serum Biochemical Analysis
After the completion of the experimental period, rat serum was obtained by means of anesthesia using isoflurane and collecting blood from the abdominal vena cava. The blood was then centrifuged at 800× g for 15 min to obtain the final supernatant. The serum blood urea nitrogen (BUN), creatinine, glucose and ROS were measured using a colorimetric assay kit (BIOMAX, Guri-si, Gyeonggi-do, Republic of Korea). The serum and reaction mix were added to a 96-well plate and incubated at room temperature or 37 °C for 30 min. The serum biochemical levels were measured using a microplate reader (Synergy 2, BioTek instrument, Winooski, VT, USA) at 570 nm. The concentration used was according to the manufacturer’s standard sample.
2.4. Hepatic Glucose Levels and Reaction Oxygen Species (ROS) Concentration
To measure hepatic glucose levels and ROS concentration, liver tissue was homogenized with 0.9% NaCl buffer. The supernatant was obtained by means of a centrifuge at 12,000× g for 5 min. Then, the hepatic glucose level and ROS concentration in the supernatant was measured. The hepatic glucose level was measured by using the ASAN glucose kit (ASAN, Seoul, Republic of Korea). The absorbance of each well at 490 nm was measured using a microplate reader. The level of ROS was quantified using 25 mM DCFH-DA (Invitrogen, Carlsbad, CA, USA) added to the homogenates. After incubation at room temperature for 30 min, the change in fluorescence values was determined at an excitation wavelength of 486 nm and an emission wavelength of 530 nm.
2.5. Renal Glucose, Triglyceride, ROS, Lactate Dehydrogenase (LDH) Concentration
To measure renal glucose, triglyceride, ROS and LDH concentrations, kidney tissue was homogenized with 0.9% NaCl buffer. The supernatant was obtained via a centrifuge at 11,000× g for 5 min. Then, the renal glucose, triglyceride, ROS, and LDH concentrations in the supernatant were measured. The renal glucose level was measured by using the ASAN glucose kit (ASAN, Seoul, Republic of Korea), the triglyceride level was quantified by using the Triglyceride Colorimetric Assay kit (Cayman chemical, Ann Arbor, MI, USA), and the LDH level was measured by using the LDH Cytotoxicity Detection Kit (Cayman Chemical, Ann Arbor, MI, USA). The absorbance of each well at 490 nm was measured using a microplate reader. The level of ROS was quantified using 25 mM DCFH-DA (Invitrogen, Carlsbad, CA, USA) added to the homogenates. After incubation at room temperature for 30 min, the change in fluorescence values was determined at an excitation wavelength of 486 nm and an emission wavelength of 530 nm.
2.6. Heaptic and Renal Western Blot Analysis
Liver and kidney protein samples were extracted with RIPA buffer with 1% Duo inhibitor cocktail (GenDEPOT, Katy, TX, USA) and the protein concentration was measured using a BCA assay kit (Thermo Fisher, Middlesex County, MA, USA). Proteins were subjected to 8–15% SDS-polyacrylamide gel and separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked in 5% skim milk in PBS-T at room temperature for 1 h and incubated with a 1:1000 v/v dilution of primary antibodies against NADPH oxidase 4 (NOX-4), neutrophil cytosol factor 1 (p47 phox), receptor for AGE (RAGE), N(epsilon)-(carboxymethyl)lysine (CML), N(epsilon)-(carboxyethyl)lysine (CEL), N(epsilon)-(carboxymethyl)arginine (CMA) and glycolaldehyde-pyridine (GA-pyridine) (Santa Cruz, CA, USA), p38, phospho-p38, extracellular-signal-regulated kinase (ERK), phospho-ERK, Jun N-terminal kinase (JNK), phospho-JNK and β-actin (Cell signaling, Danvers, MA, USA) with 1% skim milk in PBS-T at 4 °C overnight. After the membranes were washed with PBS-T, and the membranes were next incubated with 1:5000 v/v goat-anti rabbit or anti-mouse IgG antibodies (Merck Millipore, Middlesex County, MA, USA) at room temperature for 1 h. Signals were detected via a supersignal west dura extended duration substrate (Thermo Fisher Scientiric Inc., Middlesex County, CA, USA) according to the manufacturer’s instructions. Densitometric analysis was conducted directly from the blotted membrane utilizing a Chemi Imager analyzer system (Alpha Innotech, San Leandro, CA, USA).
2.7. Histological Analysis
To evaluate the effects of CJ on liver and kidney tissues, we performed hematoxylin and eosin (H&E) staining. Each tissue was fixed in 10% formalin with agitation for 24 h and subsequently sectioned and further fixed for an additional 24 h. The fixed tissues were embedded in paraffin wax blocks. Each paraffin block was then cut into 5 μm sections using a microtome. Subsequently, tissue sections were deparaffinized in xylene, rehydrated through a graded series of alcohols, and stained with H&E. Histological changes in the liver and kidney were observed using an optical microscope (Zeiss, Jena, Germany).
2.8. Statistical Analysis
The Normal and Veh groups were compared, and the Veh, CJ, and AG groups were compared separately. Statistical analysis was performed using Prism 5 (GraphPad version 5.0, GraphPad Software, San Diego, CA, USA), Student’s t-test and repeated measures ANOVA followed by a Bonferroni test. Data were expressed as the mean ± SEM. Differences with p values < 0.05 were considered statistically significant.
3. Results
3.1. Effects of CJ on Body and Organ Weight Changes, Food and Water Intakes in STZ-Induced Rats
Table 1 shows the body and liver weights and food and water intakes for 4 weeks. The loss of body weight with STZ injection has been reported in various studies [24,25]. In our results, weight reduction was also observed in the Veh group (Table 1), but the CJ-treated group exhibited a restoration of body weight. The prominent feature of STZ-induced diabetes is weight loss accompanied by an increase in water and food intake [26], and our study confirmed similar results. The administration of CJ appears to counteract these side effects induced by STZ in a dose-dependent manner, suggesting its effectiveness in mitigating weight loss and polyphagia induced by STZ. Additionally, CJ demonstrated the ability to restore liver and kidney weights in the STZ-induced diabetic rat model to a level comparable to the Normal group.

Table 1.
Body and organ weights and food and water intakes in STZ-induced diabetes rats.
3.2. Effects of CJ on Serum Glucose, ROS, BUN and Creatinine in STZ-Induced Rats
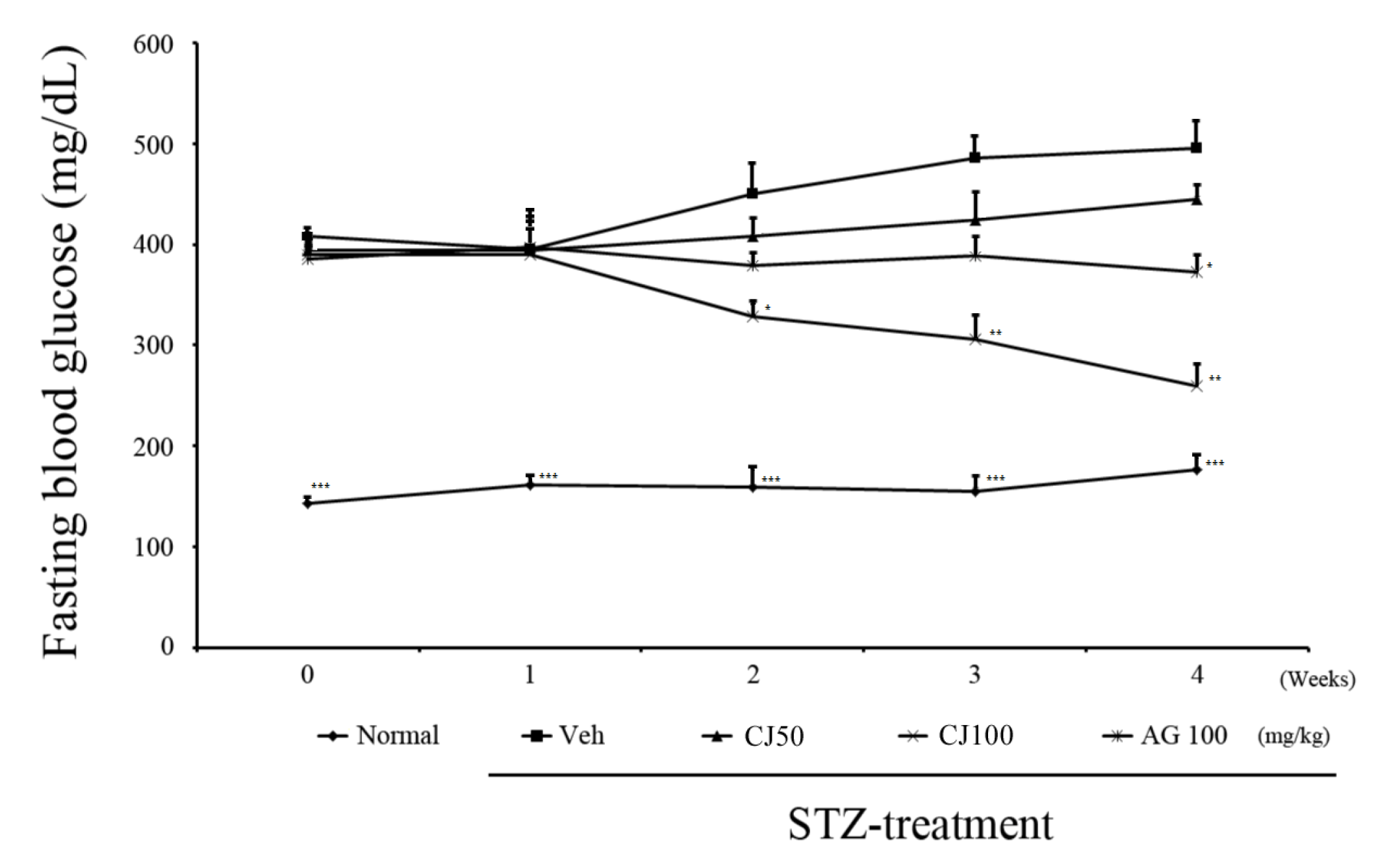
We observed changes in fasting blood glucose levels over a total of 4 weeks following STZ injection (Figure 1). In the first week, there were no significant differences observed among the groups, excluding the Normal group. However, from 2nd week onward, a significant difference was observed between the Veh group and the CJ 100 group. Furthermore, at the end of the 4-week experiment, the average fasting blood glucose level in the Veh group was 489.9 ± 45.8 mg/dL, whereas in the CJ 100 group, a substantial decrease was observed, with a level of 265.71 ± 38.1 mg/dL.
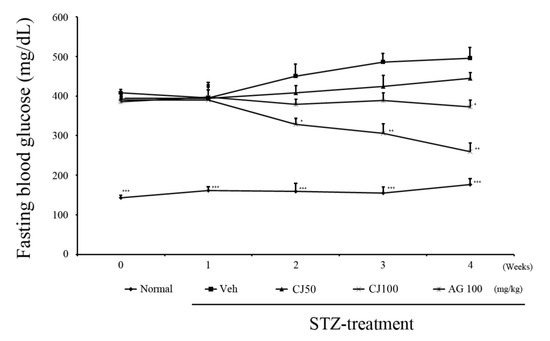
Figure 1.
Effects of CJ on levels of fasting glucose test. * p < 0.05, ** p < 0.01, and *** p < 0.005 compared with the Veh groups. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats.
To assess renal functional variables affected by CJ, we conducted assays for glucose, ROS, BUN, and creatinine in the serum. In comparison to the Normal group, the Veh group exhibited elevated concentrations of serum glucose, ROS, BUN, and creatinine. Notably, these heightened concentrations were significantly reduced in both the CJ and AG groups. Particularly noteworthy is the dose-dependent decrease observed in the CJ groups (Table 2). These findings highlight the potential of CJ in mitigating renal functional abnormalities associated with increased glucose levels and oxidative stress.

Table 2.
Effects of CJ on levels of glucose, ROS, BUN and creatinine in the serum of STZ-induced diabetic rats.
3.3. Effects of CJ on Hepatic ROS, LDH, and Renal Glucose, TC, ROS, and LDH Levels
We evaluated the levels of hepatic ROS and LDH production altered in STZ-induced diabetic rats. In the Veh group, hepatic ROS and LDH levels were significantly increased compared with the Normal group (Figure 2). However, these elevations were observed to decrease with the administration of CJ. The ROS concentration in the CJ 100 group displayed normalization to levels similar to the Normal group. Hence, the administration of CJ is deemed beneficial for hepatic anti-oxidative activity based on these results.
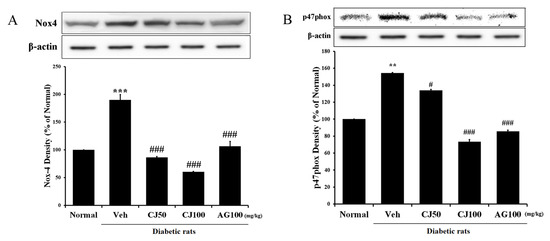
Figure 2.
Effects of CJ on hepatic (A) ROS production and (B) LDH production. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01, *** p < 0.001 compared with Normal. # p < 0.05, and ### p < 0.001 compared with Veh.
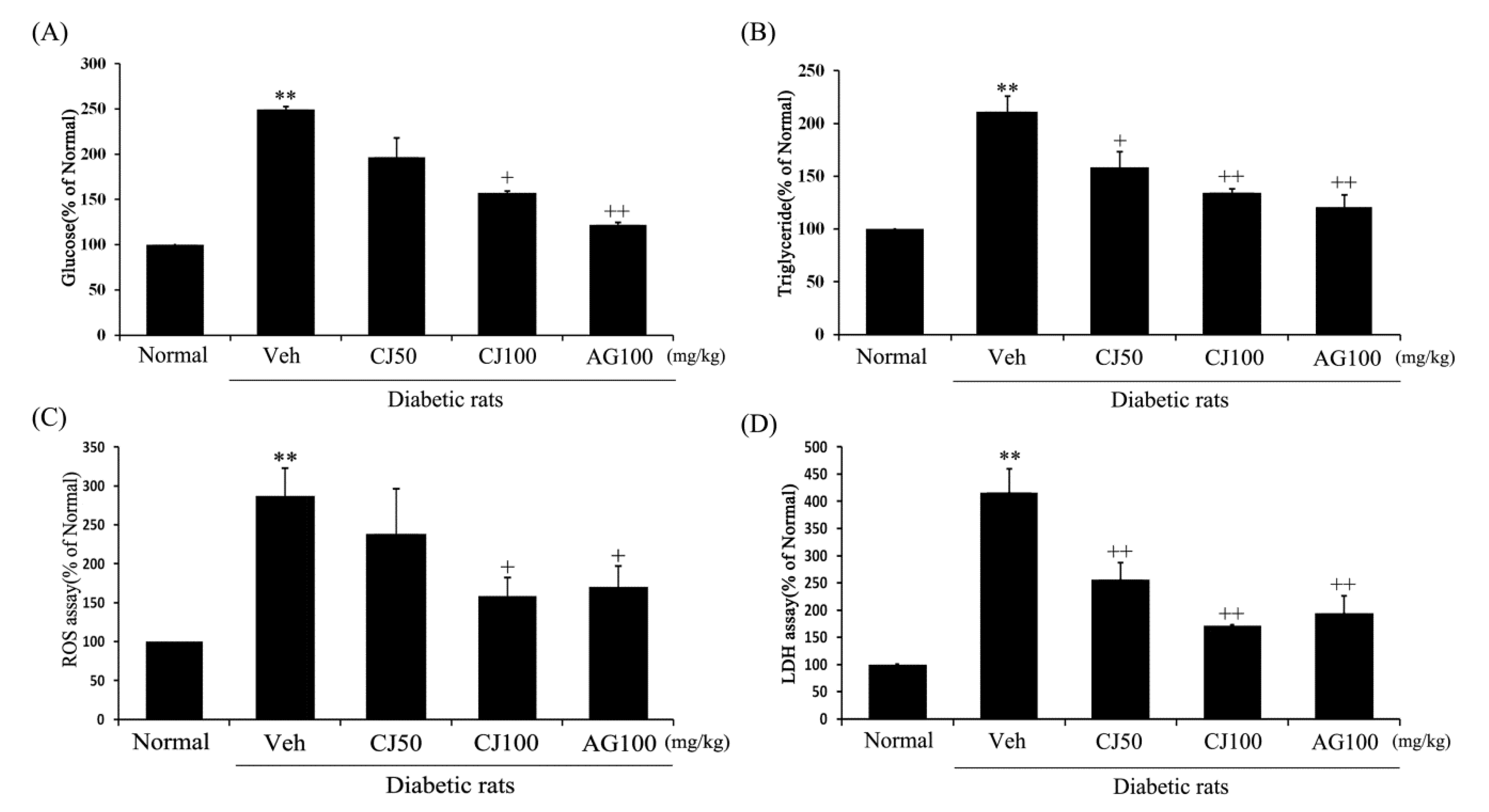
To confirm the renal functional variables affected by CJ, ELISA and ROS assays were conducted on kidney tissue. Renal glucose, triglyceride, ROS and LDH concentrations in the Veh group were increased compared to the Normal group (Figure 3). However, these elevated concentrations were significantly decreased in both the CJ and AG groups. Notably, the CJ groups exhibited a dose-dependent decrease.
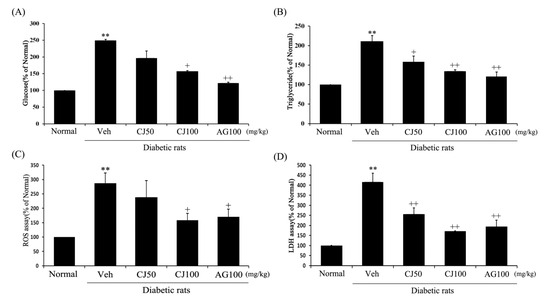
Figure 3.
Effects of CJ on renal (A) glucose concentration, (B) triglyceride production, (C) ROS production, and (D) LDH production in STZ-induced rats. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01 compared with Normal. + p < 0.05 and ++ p < 0.01 compared with Veh.
3.4. Effects of CJ on Hepatic and Renal NADPH Oxidase Expression
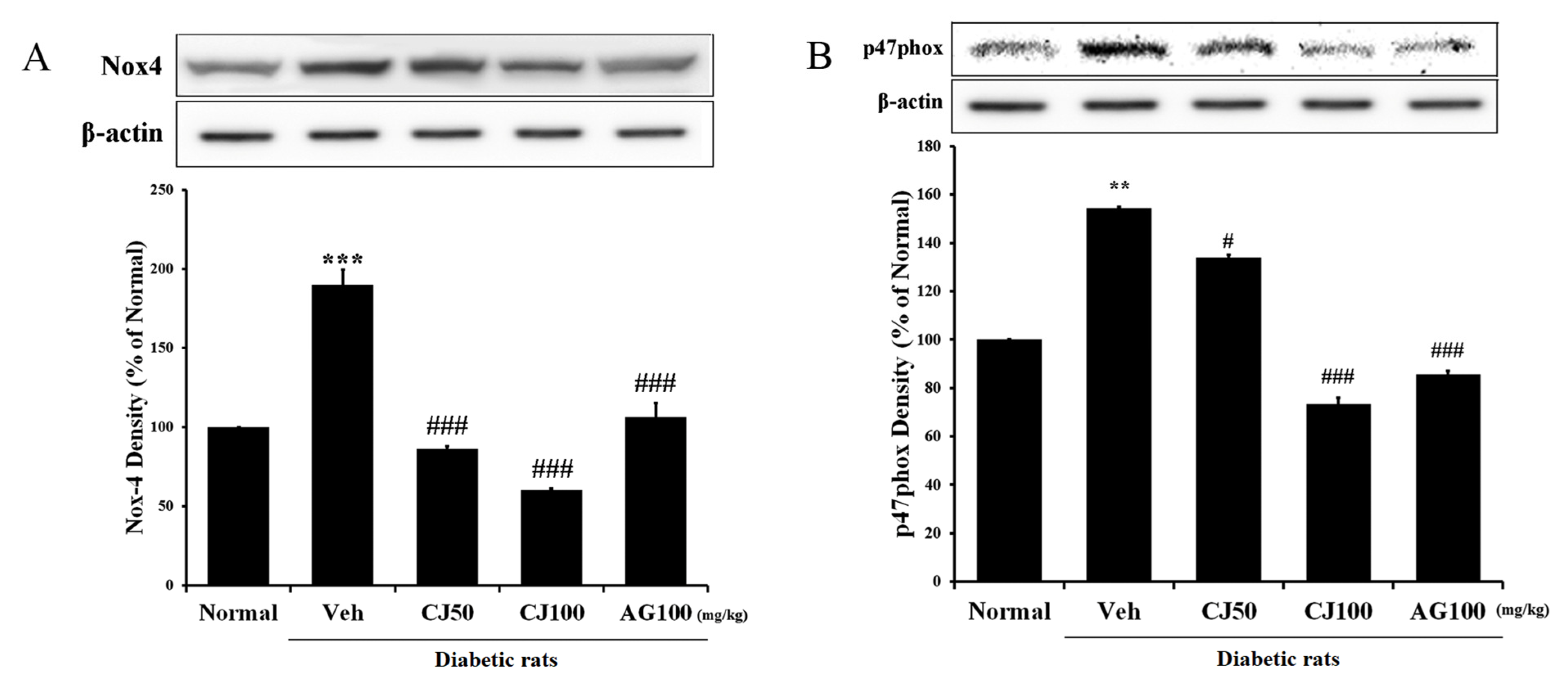
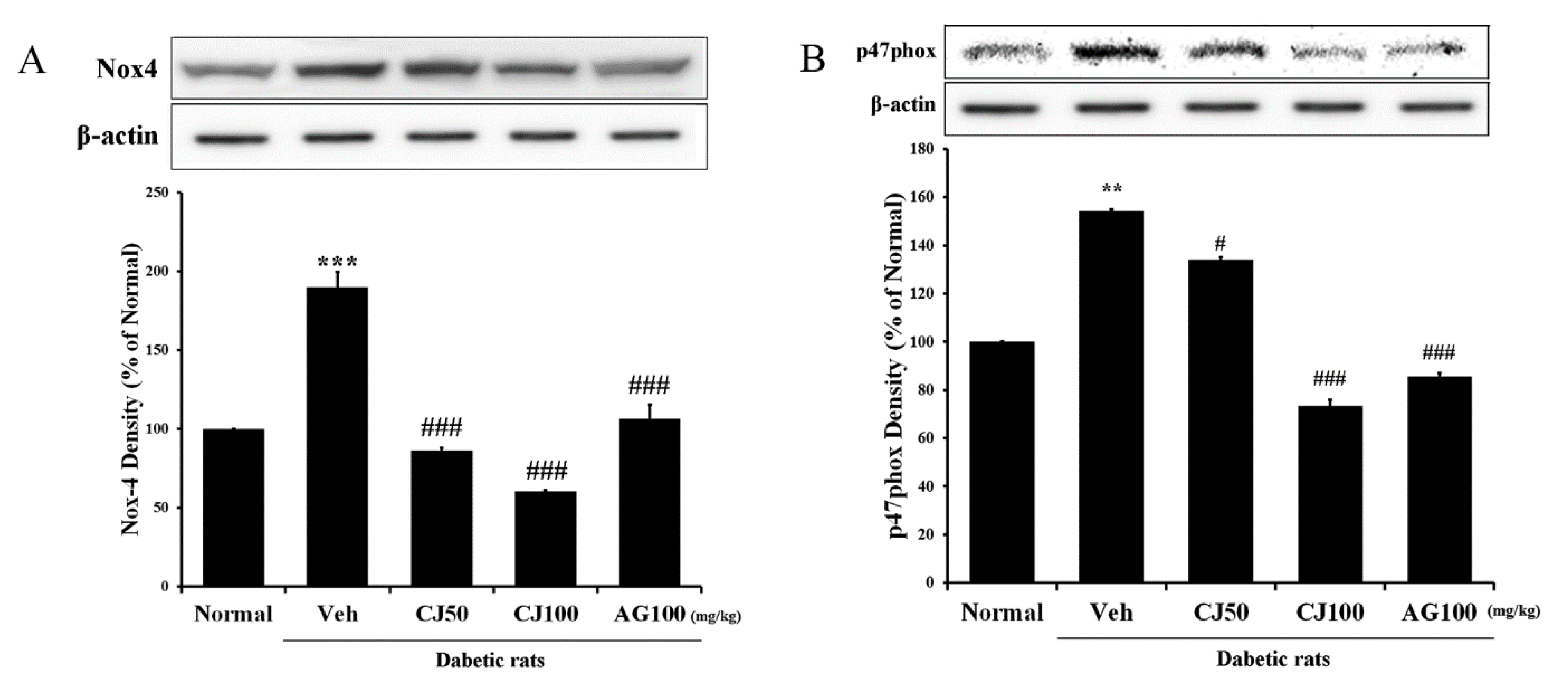
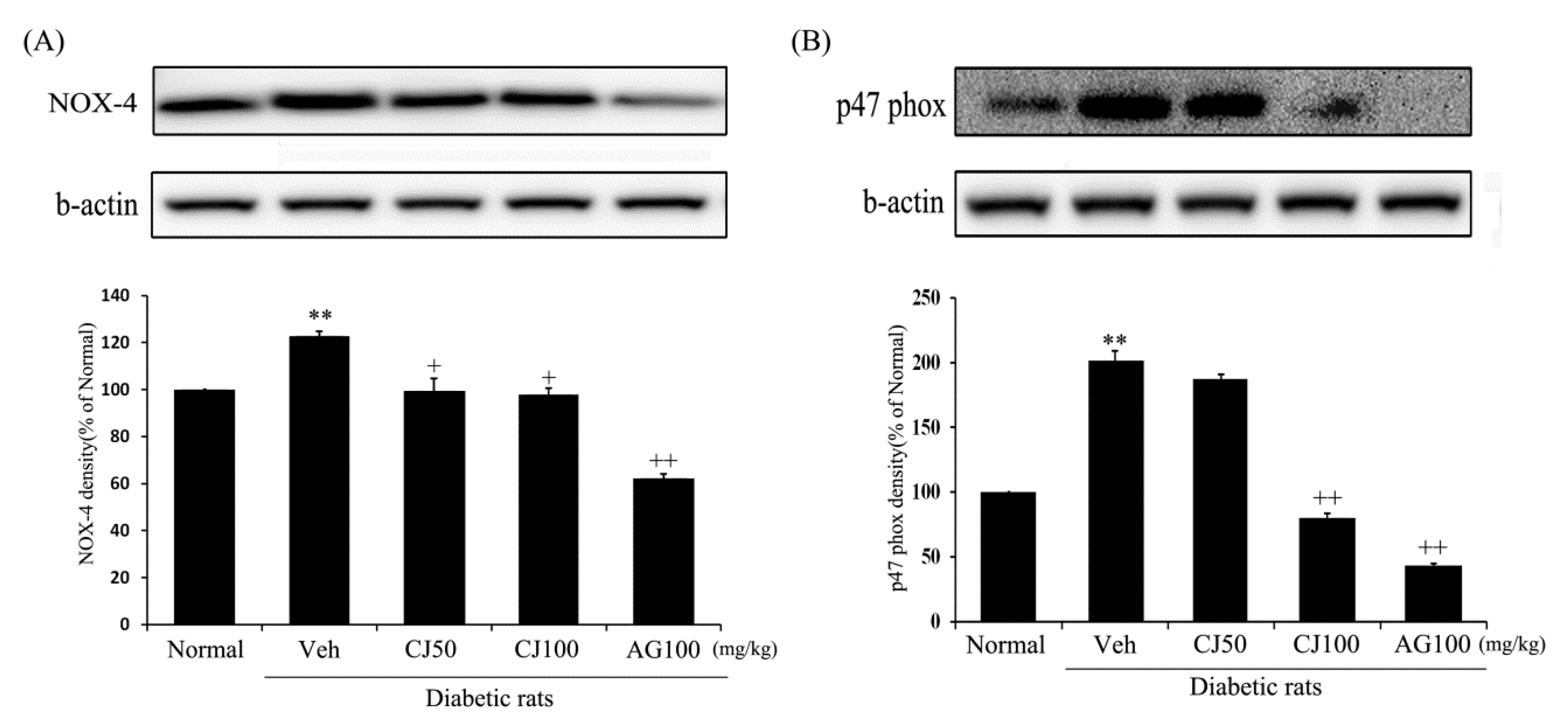
We aimed to measure the NADPH oxidase (NOX) protein, which plays not only a pathophysiological role in diabetic pancreatic β-cells but also a physiological role, responding to various factors as a membrane-associated multi-subunit enzyme [27] in the liver and kidney. The results revealed a significant increase in expression in the Veh group compared with the Normal group (Figure 4), whereas a dose-dependent decrease in expression was observed in the CJ administration group. Additionally, a substantial increase in p47phox expression was observed in the Veh group, while a significant decrease was observed in the CJ 100 group.
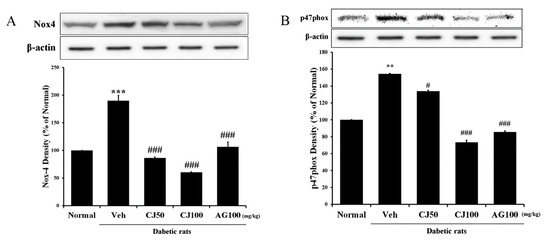
Figure 4.
Hepatic NADPH oxidase subunit related protein expression. (A) NOX-4 expression; (B) p47phox expression. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01, *** p < 0.001 compared with Normal. # p < 0.05, and ### p < 0.001 compared with Veh.
The expression of both of these proteins (NOX-4 and p47 phox) was significantly increased in the Veh group compared with the Normal group. However, the NOX-4 and p47 phox expressions in the CJ and AG groups were significantly decreased compared with the Normal group. In particular, p47 phox expression displayed a dose-dependent decrease in the CJ groups (Figure 5B). These findings suggest a potential inhibitory effect of CJ on hepatic and renal NADPH oxidase activity, as indicated by the reduced expression of key proteins associated with this pathway.
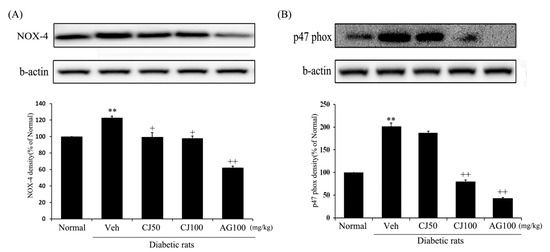
Figure 5.
Effects of CJ on renal (A) NOX-4 and (B) p47 phox protein expression in STZ-induced rats. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01 compared with Normal. + p < 0.05, and ++ p < 0.01 compared with Veh.
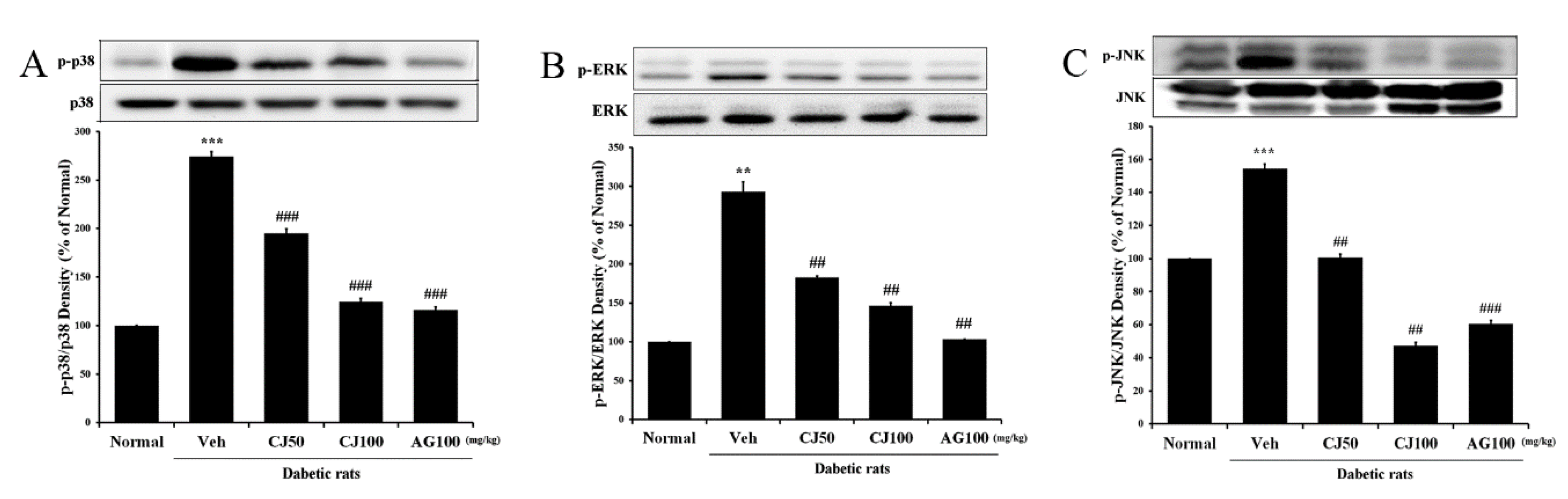
3.5. Effects of CJ on Hepatic and Renal MAPK Pathway Expression
To further investigate whether the effects of CJ in the liver and kidney tissues of STZ-induced diabetic rats involve the MAPK pathway, the expression of markers related to the MAPK pathway was analyzed through Western blot. In the Veh group, the expression of p-p38, p-ERK, and p-JNK was significantly increased compared with the Normal group (Figure 6). However, CJ administration led to a dose-dependent decrease in the overexpression of p38 MAPK and JNK.
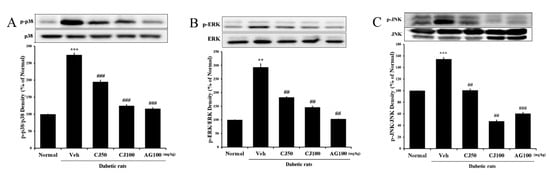
Figure 6.
Effects of CJ on hepatic MAPK-related protein expression in STZ-induced rats. (A) p-p38/p38 expression; (B) p-ERK/ERK expression; (C) p-JNK/JNK expression. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01, *** p < 0.001 compared with Normal. ## p < 0.01, and ### p < 0.001 compared with Veh.
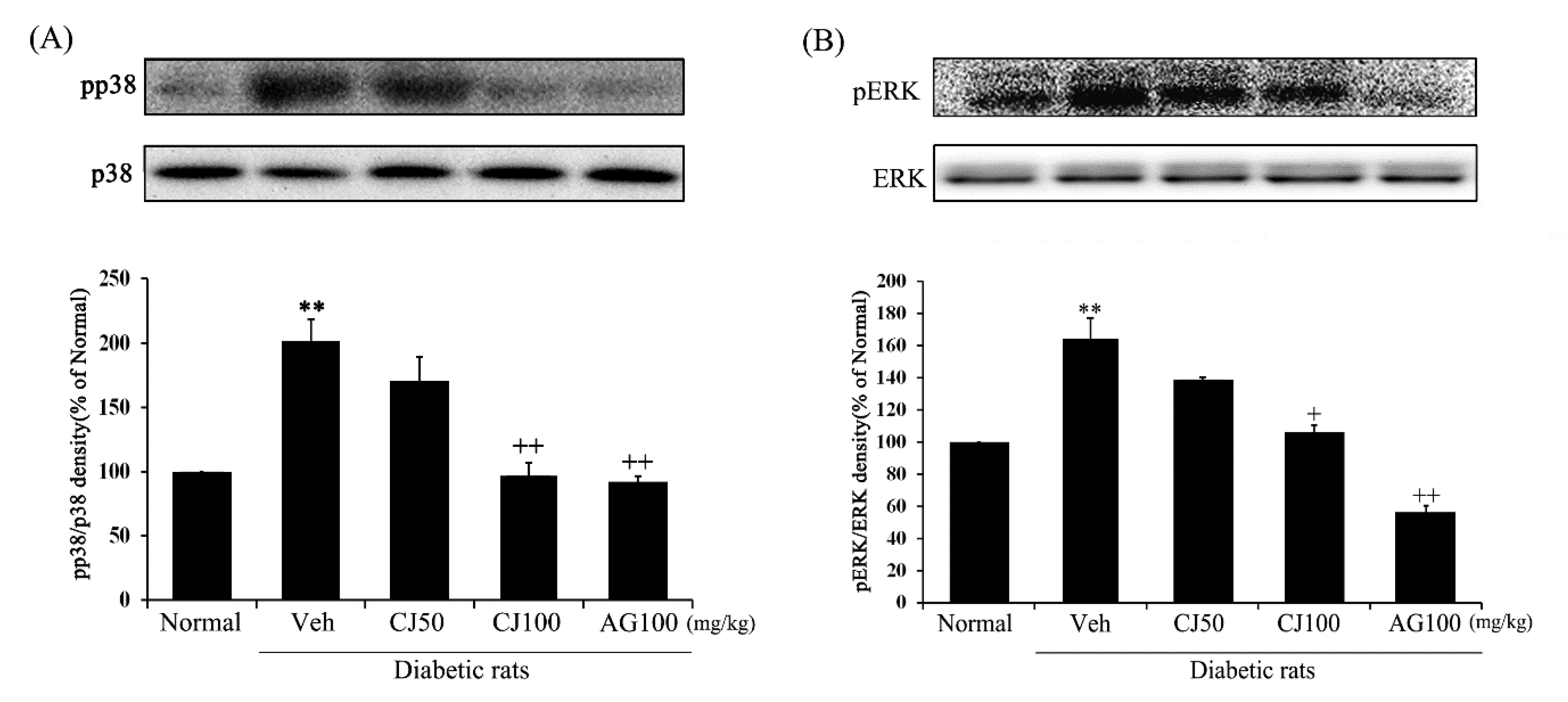
The protein expressions of both p-p38 and p-ERK1/2 were significantly increased in the Veh group compared with the Normal group (Figure 7). However, the protein expressions in the CJ and AG groups were significantly decreased compared to the Veh group. Particularly noteworthy is the dose-dependent decrease observed in the CJ groups. The results of the CJ100 and AG100 groups showed similar outcomes, with levels of p-p38 comparable to the Normal group, while the AG100 group exhibited downregulated levels of both p-p38 and p-ERK1/2 compared to the Normal group. These results indicate that CJ inhibits the activation of the p38 MAPK and JNK pathway in diabetes induced by STZ treatment.
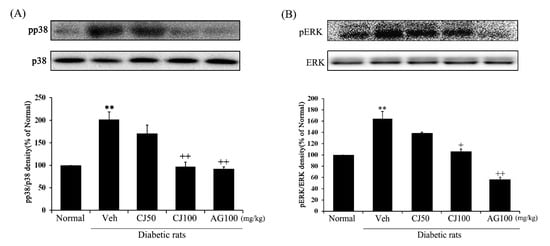
Figure 7.
Effects of CJ on renal MAPKs related protein expression in STZ-induced rats. (A) p-p38/p38; (B) p-ERK/ERK. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01 compared with Normal. + p < 0.05, and ++ p < 0.01 compared with Veh.
3.6. Effects of CJ on Hepatic and Renal AGEs/RAGE Pathway Expression
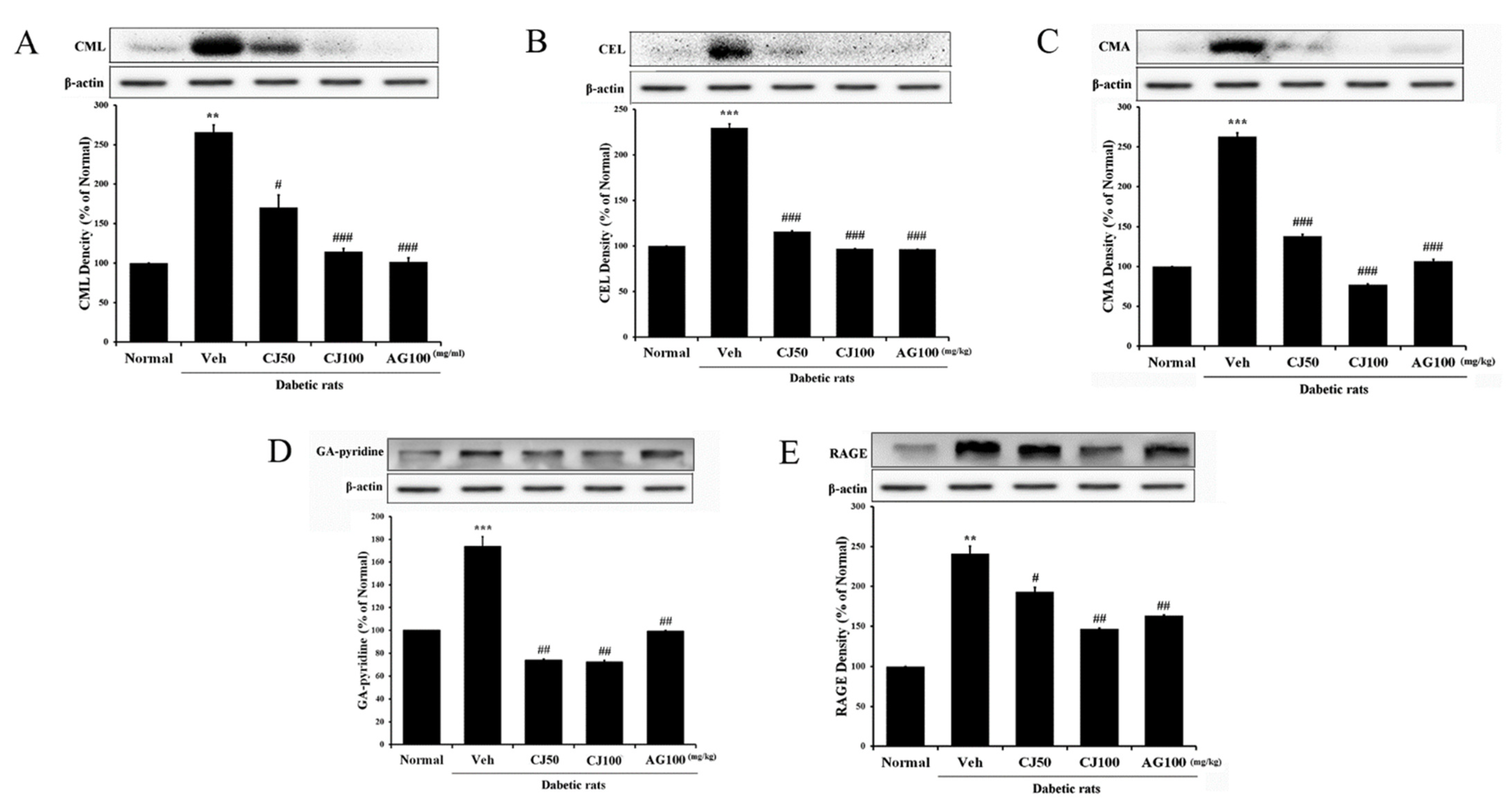
The interaction between AGEs (advanced glycation end products) and RAGE (Receptor for AGEs) is known to induce various complications in diabetes [28]. Inhibiting the effects of the AGEs/RAGE signaling pathway has been recognized as a potential therapeutic approach for new diabetes-related complications. In this study, we confirmed the involvement of the AGEs/RAGE pathway in liver and kidney tissues. In the Veh group, the expression of factors related to the AGEs/RAGE pathway was significantly increased compared with the Normal group (Figure 8). However, in the CJ-treated groups, it was observed that the receptors of these AGEs/RAGE pathway factors were downregulated.
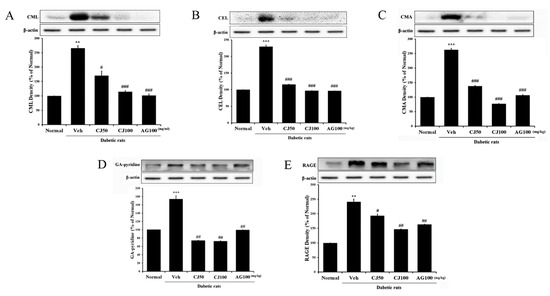
Figure 8.
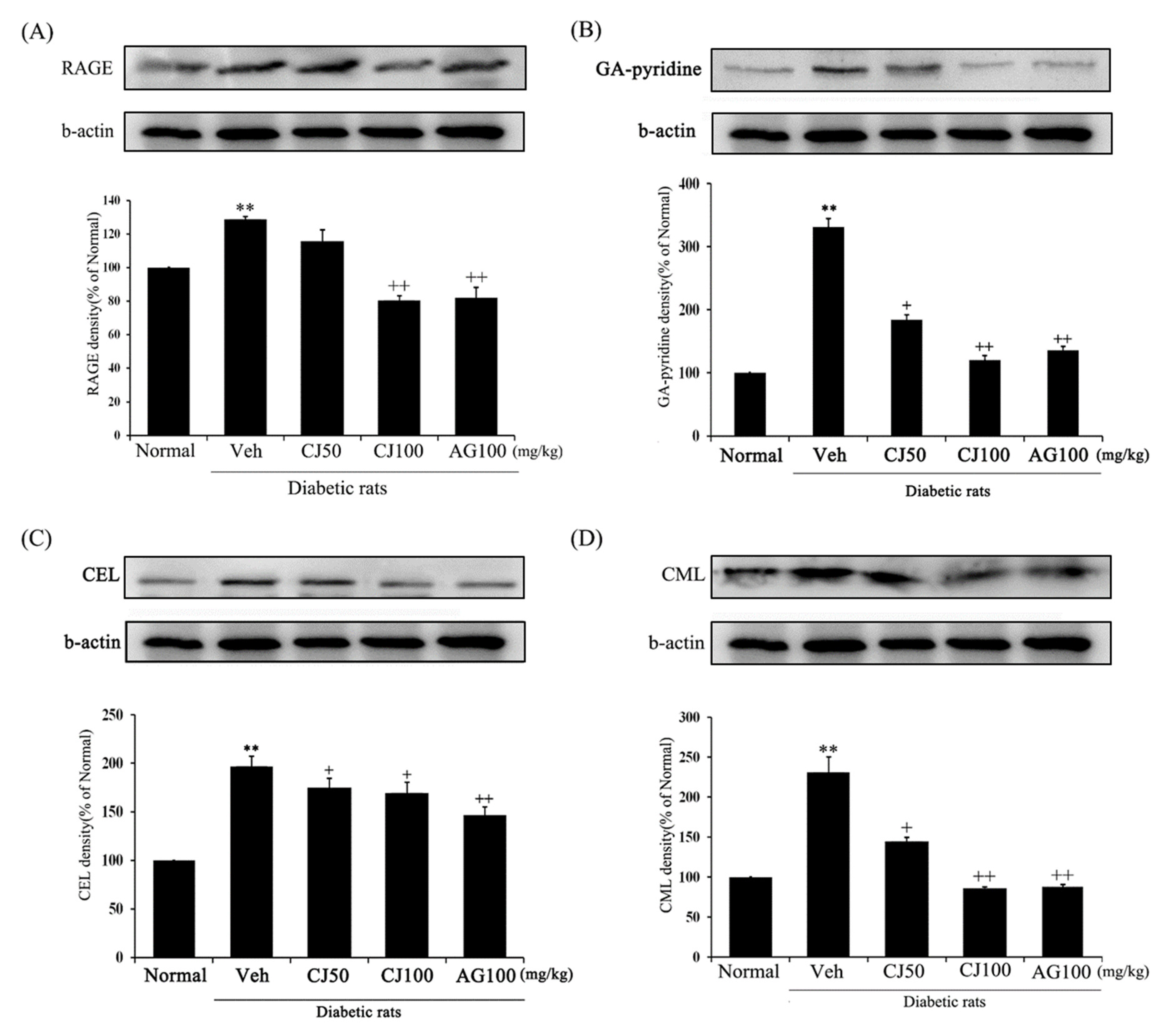
Effects of CJ on hepatic AGEs-related protein expression in STZ-induced rats. (A) CML; (B) CEL; (C) CMA; (D) GA-pyridine; (E) RAGE expression. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01 and *** p < 0.001 compared with Normal. # p < 0.05, ## p < 0.01, and ### p < 0.001 compared with Veh.
To investigate the protein expressions of RAGE, GA-pyridine, anti–CEL, and anti–CML in the kidneys, Western blot analyses were conducted. The expression of AGE-related proteins was significantly increased in the Veh group compared with the Normal group (Figure 9), whereas the CJ and AG groups showed downregulation in the levels of AGE-related protein expression. Notably, the CJ groups exhibited a dose-dependent decrease.
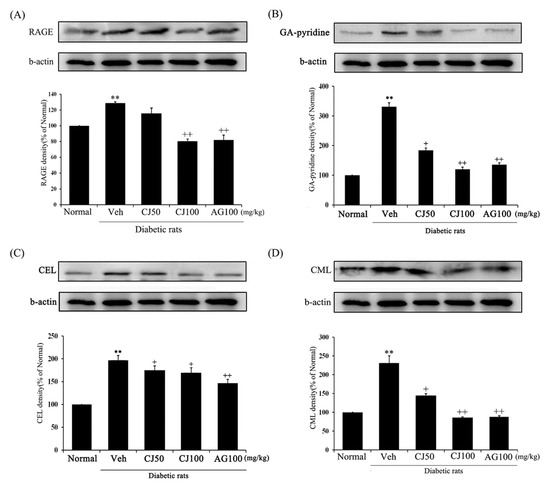
Figure 9.
Effects of CJ on renal AGEs-related protein expression in STZ-induced rats. (A) RAGE; (B) GA-pyridine; (C) CEL; (D) CML expression. Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats. Values are expressed as the mean ± SEM (n = 8). ** p < 0.01 compared with Normal. + p < 0.05, and ++ p < 0.01 compared with Veh.
3.7. Effects of CJ on Hepatic and Renal Histological Analysis
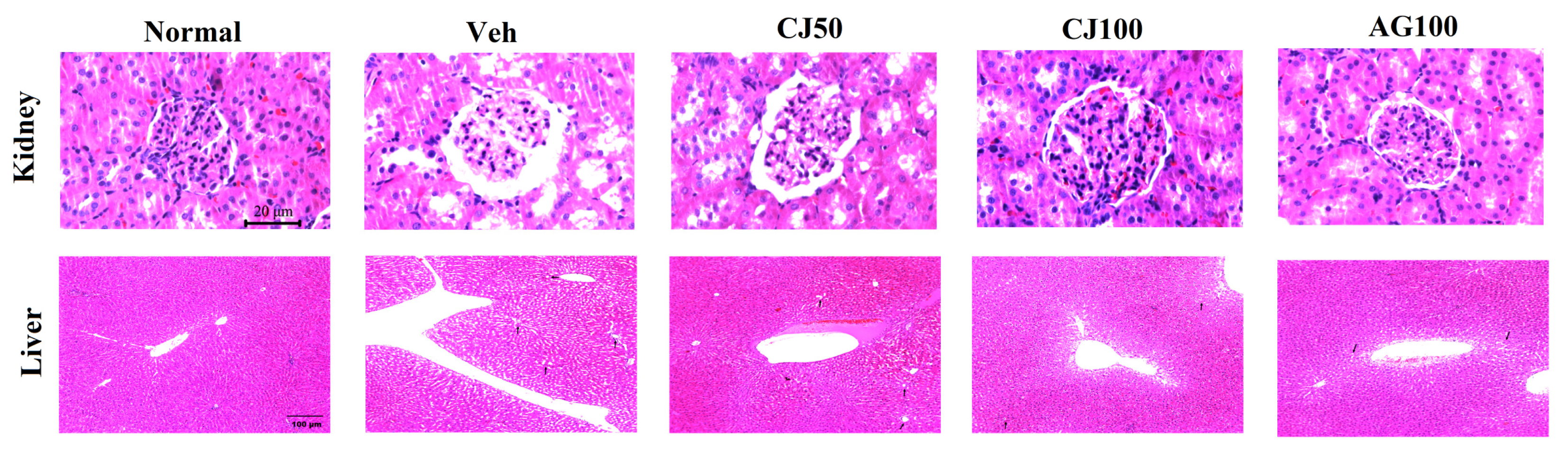
To evaluate the protective effects of CJ on histological changes, hematoxylin and eosin (H&E) staining was performed. Figure 10 showed pathological changes in the liver and kidney of the Veh group compared with the Normal group. The renal dysfunction in diabetic mice is accompanied by structural disturbances in the renal tubular epithelium, glomerular vasculature, and interstitium. Consistent with these findings, our experimental results also showed structural abnormalities in the renal tubular epithelium and interstitium in the Veh group. While these histological changes in the kidney were less pronounced in the CJ50 group, a notable restoration of the morphology of the tubules and glomeruli was observed in the CJ100 group compared to the Veh group.
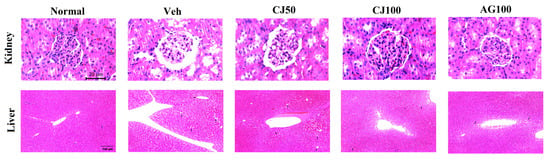
Figure 10.
Effects of CJ on histological changes in renal and hepatic tissues in STZ-induced rats. Rats induced with STZ exhibited centrilobular hepatic vacuolation characterized by circular borders and clear vacuoles indicative of fatty changes (arrows). Normal, non-diabetic rats; Veh, STZ 30 mg/kg-treated rats; CJ 50, STZ 30 mg/kg + CJ 50 mg/kg-treated rats; CJ 100, STZ 30 mg/kg + CJ 100 mg/kg-treated rats; AG 100, STZ 30 mg/kg + aminoguanidine 100 mg/kg-treated rats.
When examining histological images to liver tissues, an increase in necrosis with a fragment nucleus and karyolysis was observed in the Veh group compared with the Normal group. Such observations are known to occur due to STZ injection, and similar patterns were observed in our experimental groups. These patterns were found to decrease in a dose-dependent manner in the CJ-administration groups (Figure 10).
4. Discussion
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder characterized by the immune system’s attack on pancreatic beta cells, resulting in hyperglycemia in the host [29]. Currently, there is no cure for T1DM worldwide, and insulin injection remains the only treatment to manage disease symptoms. STZ, used to induce T1DM in this experiment, cause beta-cell apoptosis in the pancreas via GLUT2 due to its structural similarity to glucose, thereby impairing insulin production [30]. In this study, T1DM was induced in rats via the intraperitoneal administration of 30 mg/kg STZ, resulting in hyperglycemia induction (Figure 1) and histological damage (Figure 10), confirming successful T1DM model induction. The AGEs/RAGE pathway, known to be crucial in the onset and progression of T1DM, induces AGE formation in the body when hyperglycemia persists due to diabetes, serving as a major contributor to diabetic complications [15]. RAGE, a member of the immunoglobulin superfamily capable of binding to AGEs, is known to recognize a three-dimensional structure rather than specific amino acid sequences. Prolonged diabetic conditions lead to an increase in AGE levels and RAGE involvement, inducing signaling events through the interaction of AGEs/RAGE, resulting in various downstream effectors, including MAPK-mediated signal transduction [31]. Activation of the MAPK-mediated signaling pathway induces the generation of ROS and upregulates enzymes such as NADPH oxidase and nitric oxide synthase, thereby playing a significant role in the occurrence and prevention of diabetic complications [15]. Consequently, the AGEs/RAGE signaling pathway serves as a crucial mechanism in the pathogenesis and prevention of diabetes and its complication.
This study explored the potential effects of CJ on the AGEs/RAGE signaling pathway in an STZ-induced diabetic animal model. In several studies on the activity of compounds in CJ, it has been reported that CJ can influence the inhibition of AGE formation [32], providing assistance in the management of diabetic complications and oxidative stress [33]. STZ-induced rats exhibit polyphagia, polydipsia, and polyuria, leading to weight loss associated with intestine dysfunction, muscle catabolism, and other factors [34,35,36]. In our study, the Veh group only lost weight, whereas the CJ-treated groups showed dose-dependent recovery (Table 1). In addition, diabetic rats lose weight and have increased fasting blood glucose levels. These changes contribute to the onset of diabetic complications such as hyperglycemia and lead to the formation of enzymatic immature and mature cross-links and non-enzymatic AGEs.
Oxidative stress plays a crucial role in the onset of diabetic complications; therefore, it is essential to regulate intracellular ROS and manage blood glucose levels for effective control [37]. Under oxidative stress-induced diabetic conditions, endogenous antioxidant enzymes [38] and ROS concentrations are regulated by SOD, CAT, and GSH [33]. We investigated the concentration of ROS in liver and kidney tissue samples. In the CJ 100 group, both showed a significant decrease compared with the Veh group (Figure 2A and Figure 3C). In the intricate landscape of cardiovascular physiology, the assertion that NADPH oxidase (NOX) serves as the primary source of ROS highlights the pivotal role this enzyme plays in the delicate balance of oxidative processes within cardiovascular tissues [39]. Our results showed that the expression of NOX-4 and p47 phox was reduced in the CJ100 group compared with that in the Veh group (Figure 4 and Figure 5). In the context of diabetic nephropathy, the expression of NOX-4 within the kidney is intricately linked to the pathophysiology of the condition [40]. Our findings suggest that CJ, through its regulation of ROS induced by diabetes, may act as a key modulator in various pathological conditions associated with increased NADPH oxidase activity in the kidney and liver. This implies a potential regulatory role for CJ in diverse pathologies related to diabetic complications, hinting at its significance as a major regulator in conditions characterized by heightened oxidative stress in renal and hepatic tissues.
We evaluated serum creatinine and BUN levels (Table 2). Creatinine, a major product of muscle metabolism, is excreted by the kidney and serves as an important indicator of renal function [41]. Elevated creatinine levels are often associated with renal failure. Our results showed that the administration of CJ restored the levels of creatinine and BUN, indicating that CJ may help manage renal failure.
According to various studies, the binding of AGEs and RAGE induces the activation of the p38 MAPK pathway [7]. This activation is implicated in processes such as cell death and is particularly activated in the renal cortex. MAPK is a member of the protein kinase family, composed of extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38, which collectively regulate extracellular signaling [42,43]. In the results of this study, the injection of STZ was found to impact the phosphorylation of MAPK markers in the liver and kidney of rats, confirming the induction of MAPK pathway activation. This indicates that STZ injection induces the activation of MAPK pathway, which in turn can influence the secretion of inflammatory mediators such as proinflammatory cytokines. In our study, the administration of CJ was observed to inhibit the activation of p38, ERK1/2 and JNK in the liver. Furthermore, it was found to be effective in regulating p38 and ERK1/2 levels in the kidney. Notably, the CJ 100 group exhibited a significant decrease compared with the Veh group. These results suggest that CJ can control MAPK activation under STZ-induced diabetic condition.
AGE accumulation can lead to vascular damage and RAGE overexpression in smooth muscle and endothelial cells [44]. In this study, we observed a significant increase in RAGE expression in the liver and kidneys of rats injected with STZ alone compared with the Normal group rats. RAGE expression appeared to be regulated by various factors, with one of the most significant being the accumulation of AGEs. Therefore, the reduced RAGE expression in STZ-induced rats treated with CJ may be attributed, as suggested by other studies, to a probable decrease in AGEs accumulation.
The interaction between AGEs and cell receptor is known to play a crucial role in the development of complications associated with diabetes [45,46]. AGEs accumulate in various diabetic complication-prone areas such as the kidney and retina [46,47]. They act as stimuli, activating intracellular signaling pathway and modifying the function of intracellular proteins. In particular, among various complications induced by diabetes, AGEs in hyperglycemia elevate intracellular oxidative stress, contributing to the initiation of other complications [48]. This ultimately contributes to inflammatory mechanisms such as MAPK and NF-κB activation [7]. The interaction between AGEs and RAGE in macrophage cells induces the activation of the MAPK pathway [49]. This activation subsequently triggers NF-κB, leading to the modulation of the production of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin(IL)-1α, and IL-6 [50]. In addition, it contributes to the enhancement of ROS generation [46]. Therefore, inhibiting the formation of AGEs could contribute to the regulation of various oxidative stress and inflammatory mediators, suggesting a novel approach to diabetes treatment beyond glucose control alone.
In summary, we have shown that CJ extracts in STZ-induced diabetic rats can inhibit AGEs/RAGE levels, followed by oxidative stress and MAPK pathway activation. Taken together, our results suggest that under STZ-induced diabetic conditions, administration of CJ may reduce the expression of AGEs factors and RAGE in liver and kidney tissues, thereby potentially controlling the AGEs/RAGE pathway. Additionally, CJ appears to regulate oxidative stress in the liver and kidney, inhibiting activation of NADPH oxidase and MAPK signaling pathway. This comprehensive approach may propose a new possibility for diabetes treatment by intervening in the AGEs/RAGE pathway.
5. Conclusions
Our results show that STZ treatment causes an increased level of blood glucose and an increased ROS concentration in the serum, and further increases the expression of NADPH oxidase markers, the MAPK signaling pathway, and RAGE in the liver and kidney. In this study, the administration of CJ in an STZ-induced rat model demonstrated the potential for mitigating the pathophysiology of diabetes by regulating the AGEs/RAGE pathway and its downstream sub-pathways, suggesting a possibility for preventing complications associated with diabetes. Our results indicate that CJ, particularly in the content of type 1 diabetes, may offer a novel therapeutic potential by intervening in the AGEs/RAGE pathway.
Author Contributions
Conceptualization, C.-H.P., J.-P.J. and J.K.; methodology, C.-H.P. and I.-B.S.; validation, J.C., I.-B.S., S.L. and H.C.; resources, C.-H.P., D.-H.Y., S.-W.H. and J.-P.J.; writing—original draft preparation, J.C.; writing—review and editing, J.C. and J.K.; visualization, J.C. and I.-B.S.; supervision, C.-H.P., J.-P.J. and J.K. project administration, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Insitutional Ethics Committee of Jeonbuk National University (Protocol code: NON2023-204 and date of approval: 13 December 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments
This research was supported by Hallym University Fund and Industry Co., Ltd. Fund.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balakumar, P.; Arora, M.K.; Ganti, S.S.; Reddy, J.; Singh, M. Recent advances in pharmacotherapy for diabetic nephropathy: Current perspectives and future directions. Pharmacol. Res. 2009, 60, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic complications of diabetes mellitus: A mini review. Curr. Diabetes Res. 2017, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Babel, R.A.; Dandekar, M.P. A review on cellular and molecular mechanisms linked to the development of diabetes complication. Curr. Diabetes Rev. 2021, 17, 457–473. [Google Scholar] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Z.; Yang, S.L.; Wang, J.Y.; Ye, M.; Zhou, X.Y.; Wang, L.T.; Chen, H.; Zhang, H.; Yang, L. Irbesartan attenuates advanced glycation end products-mediated damage in diabetes-associated osteoporosis through the AGEs/RAGE pathway. Life Sci. 2018, 205, 184–192. [Google Scholar] [CrossRef]
- Nomi, Y.; Kudo, H.; Miyamoto, K.; Okura, T.; Yamamoto, K.; Shimohiro, H.; Kitao, S.; Ito, Y.; Egawa, S.; Kawahara, K.; et al. Free advanced glycation end product distribution in blood components and the effect of genetic polymorphisms. Biochimie 2020, 179, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiao, N.; Jiang, M.; Liu, L.; Zhu, Y.; Wu, H.; Chen, J.; Fu, Y.; Du, Q.; Xu, H.; et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J. Cell Mol. Med. 2020, 24, 6083–6095. [Google Scholar] [CrossRef]
- Yue, S.; Zhou, H.M.; Zhu, J.J.; Rao, J.H.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Lu, L.; Zhai, Y. Hyperglycemia and liver ischemia reperfusion injury: A role for the advanced glycation end products and its receptor pathway. Am. J. Transplant. 2015, 15, 2877–2887. [Google Scholar] [CrossRef]
- Penfold, S.A.; Coughlan, M.T.; Patel, S.K.; Srivastava, P.M.; Sourris, K.C.; Steer, D.; Webster, D.E.; Thomas, M.C.; Maclsaac, R.J.; Jerums, G.; et al. Circulating high-molecular-weight RAGE ligand activate pathways implicated in the development of diabetic nephropathy. Kidney Int. 2010, 78, 287–295. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Gan, X.; Liu, K.; Lv, X.; Shen, H.; Dai, G.; Xu, H. Iridoid glycoside from Cornus officinalis ameliorated diabetes mellitus-induced testicular damage in male rats: Involvement of suppression of the AGEs/RAGE/p38 MAPK signaling pathway. J. Ethnopharmacol. 2016, 194, 850–860. [Google Scholar] [CrossRef]
- Jiao, N.; Chen, Y.; Zhu, Y.; Wang, W.; Liu, M.; Ding, W.; Lv, G.; Lu, J.; Yu, B.; Xu, H. Protective effects of catalpol on diabetes mellitus-induced male reproductive damage via suppression of the AGEs/RAGE/Nox 4 signaling pathway. Life Sci. 2020, 256, 116736. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jing, J.; Yu, S.; Song, M.; Tan, H.; Cui, B.; Huang, L. Advanced glycation endproducts induce apoptosis of endothelial progenitor cells by activating receptor RAGE and NADPH oxidase/JNK signaling axis. Am. J. Transl. Res. 2016, 8, 2169–2178. [Google Scholar] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, C.; Wu, S.; Zhang, Y.; Mao, X.; Wang, W. Advanced glycation end products downregulates peroxisome proliferator-activated receptor γ expression in cultured rabbit chondrocyte through MAPK pathway. Eur. J. Pharmacol. 2010, 649, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspective. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Pang, Q.Q.; Kim, J.H.; Choi, J.M.; Song, J.L.; Lee, S.; Cho, E.J. Cirsium japonicum var Maackii improves cognitive impairment under amyloid beta 25-35-induced Alzheimer’s disease model. Biomed. Res. Int. 2022, 2022, 4513998. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Jiang, J.G.; Zhang, X.M.; Zhu, W. Identification of luteolin 7-O-β-D-glucuronide from Cirsium japonicum and its anti-inflammatory mechanism. J. Funct. Foods 2018, 46, 521–528. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, M.; Shin, S.; Woo, J.; Son, D.; Ryu, D.; Yoo, J.; Park, D.; Jung, E. Effect of Cirsium japonicum flower extract on skin aging by glycation. Molecules 2022, 27, 2093. [Google Scholar] [CrossRef]
- Che, D.N.; Shin, J.Y.; Kang, H.J.; Cho, B.O.; Park, J.H.; Wang, F.; Hao, S.; Sim, J.S.; Sim, D.J.; Jang, S.I. Ameliorative effects of Cirsium japonicum extract and main component cirsimaritin in mice model of high-fat diet-induced metabolic dysfunction-associated fatty liver disease. Food Sci. Nutr. 2021, 9, 6060–6068. [Google Scholar] [CrossRef]
- Yang, X.; Shao, H.; Chen, Y.; Ding, N.; Yang, A.; Tian, J.; Jiang, Y.; Li, G.; Jiang, Y. In renal hypertension, Cirsium japonicum strengthens cardiac function via the intermedin/nitric oxide pathway. Biomed. Pharmacother. 2018, 101, 787–791. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.H.; Jiang, J.G. Hepatoprotective effects of flavonoids from Cirsium japonicum DC on hepatotoxicity in comparison with silymarin. Food Funct. 2016, 7, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rodriguez, J.P.; Lee, K.H.; Park, J.Y.; Kang, K.S.; Hahm, D.H.; Huh, C.K.; Lee, S.C.; Lee, S. Determination of flavonoids from Cirsium japonicum var maackii and their inhibitory activities against aldose reductase. Appl. Biol. Chem. 2017, 60, 487–496. [Google Scholar] [CrossRef]
- Yin, J.; Heo, S.I.; Wang, M.H. Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots. Nutr. Res. Pract. 2008, 2, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Jan, B.L.; Alkharfy, K.M.; Ansari, M.A.; Mohsin, K.; Jenoobi, F.A.; Al-Mohizea, A. Momordica charantia polysaccharides mitigate the progression of STZ induced diabetic nephropathy in rats. Int. J. Biol. Macromol. 2016, 91, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Xu, M.; Wang, Y.; Cheng, S.; Liebrecht, A.; Qian, H.; Zhang, H.; Qi, X. Anti-diabetic activity of Vaccinium bracteatum Thunb. Leaves’ polysaccharide in STZ-induced diabetic mice. Int. J. Biol. Macromol. 2013, 61, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, S.; Karunakaran, U.; Moon, J.S.; Won, K.C. NADPH oxidase (NOX) targeting in diabetes: A special emphasis on pancreatic β-cell dysfunction. Cells 2021, 10, 1573. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes and bone: Review of the literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Du, C.; Whiddett, R.O.; Buckle, I.; Chen, C.; Forbes, J.M.; Fotheringham, A.K. Advanced Glycation End Products and Inflammation in Type 1 Diabetes Development. Cells 2022, 11, 3503. [Google Scholar] [CrossRef]
- Marino, F.; Salerno, N.; Scalise, M.; Salerno, L.; Torella, A.; Molinaro, C.; Chiefalo, A.; Filardo, A.; Siracusa, C.; Panuccio, G.; et al. Streptozotocin-Induced Type 1 and 2 Diabetes Mellitus Mouse Models Show Different Functional, Cellular and Molecular Patterns of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 1132. [Google Scholar] [CrossRef]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The role of dietary advanced glycation end products in metabolic dysfunction. Mol. Nutr. Food Res. 2021, 65, e1900934. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, J.J.; Min, B.S.; Jung, H.J.; Islam, M.N.; Choi, J.S. Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium jaackii. Asian Pac. J. Trop. Med. 2015, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Kim, Y.S.; Choi, J.S. Quantitative HPLC analysis of two key flavonoids and inhibitory activities against aldose reductase from different part of the Korean thistle, Cirsium maackii. Food Chem. Toxicol. 2009, 47, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Swanston-Flatt, S.K.; Day, C.; Bailey, C.J.; Flatt, P.R. Traditional plant treatments for diabetes. Studies on normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic effect of Ginko biloba extract in streptozotocin-induced diabetes in rats. Biomed. Res. Int. 2013, 2013, 162724. [Google Scholar] [CrossRef] [PubMed]
- Wang-Fischer, Y.; Garyantes, T. Improving the reliability and utility of streptozotocin-induced rat diabetic model. J. Diabetes Res. 2018, 2018, 8054073. [Google Scholar] [CrossRef] [PubMed]
- Lawal, B.; Sani, S.; Onikanni, A.S.; Ibrahim, Y.O.; Agboola, A.R.; Lukaman, H.Y.; Olawale, F.; Jigam, A.A.; Batiha, G.E.S.; Babalola, S.B.; et al. Preclinical anti-inflammatory and antioxidant effects of Azanza garckeana in STZ-induced glycemic-impaired rats, and pharmacoinformatics of its major phytoconstituents. Biomed. Pharmacother. 2022, 152, 113196. [Google Scholar] [CrossRef]
- Ojo, O.A.; Okesola, M.A.; Ekakitie, L.I.; Ajiboye, B.O.; Oyinloye, B.E.; Agboinghale, P.E.; Onikanni, A.S. Gongronema latifolium Benth. leaf extract attenuates diabetes-induced neuropathy via inhibition of cognitive, oxidative stress and inflammatory response. J. Sci. Food Agric. 2020, 100, 4504–4511. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; Shehatou, G.S.; Abdelsalam, R.A.; Suddek, G.M.; Salem, H.A. Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn Schmiedbergs Arch. Pharmacol. 2019, 392, 243–258. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidase, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Ogunlana, O.O.; Adetuyi, B.O.; Esalomi, C.F.; Rotimi, M.I.; Popoola, J.O.; Ogunlana, O.E.; Adetuyi, O.A. Antidiabetic and antioxidant activities of the twigs of Andrograhis paniculata on streptozocotin-induced diabetic male rats. BioChem 2021, 1, 238–249. [Google Scholar] [CrossRef]
- Suchal, K.; Malik, S.; Gamad, N.; Malhotra, R.K.; Goyal, S.N.; Ojha, S.; Kumari, S.; Bhatia, J.; Arya, D.S. Mangiferin protect myocardial insults through modulation of MAPK/TGF-β pathways. Eur. J. Pharmacol. 2016, 776, 43. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effects of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.H.; Chen, K.H.; Yang, S.H.; Kuo, P.C.; Chen, J.K. Resveratrol ameliorates vasculopathy in STZ-induced diabetic rats: Role of AGE-RAGE signaling. Diabetes Metab. Res. Rev. 2010, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yamamoto, Y.; Munesue, S.; Motoyoshi, S.; Saito, H.; Win, M.T.T.; Watanabe, T.; Tsuneyama, K.; Yamamoto, H. Induction of receptor for advanced glycation end products by insufficient leptin action triggers pancreatic β-cell failure in type 2 diabetes. Genes. Cells 2013, 18, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Res. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef]
- He, S.; Hu, Q.; Xu, X.; Xiu, Y.; Chen, Y.; Lu, Y.; Su, Q.; Qin, L. Advanced glycation end products enhance M1 macrophage polarization by activating the MAPK pathway. Biochem. Biophys. Res. Commun. 2020, 525, 334–340. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Boarescu, P.M.; Bolboacă, S.D.; Balidaru, M.; Feștilă, D.; Dogaru, G.; Nicula, C.A. Comparative effect of curcumin versus liposomal curcumin on systemic pro-inflammatory cytokines profile, MCP-1 and RANTES in experimental diabetes mellitus. Int. J. Nanomed. 2019, 14, 8961–8972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).