Finite Element Model of Canine-Specific Vertebrae Incorporating Biomechanical Tissue Nonlinearity
Abstract
1. Introduction
2. Materials and Methods
2.1. Real-Time Experiment
2.1.1. Specimen Guidelines
2.1.2. Sample Preparation
2.1.3. Equipment Used in the Experiment
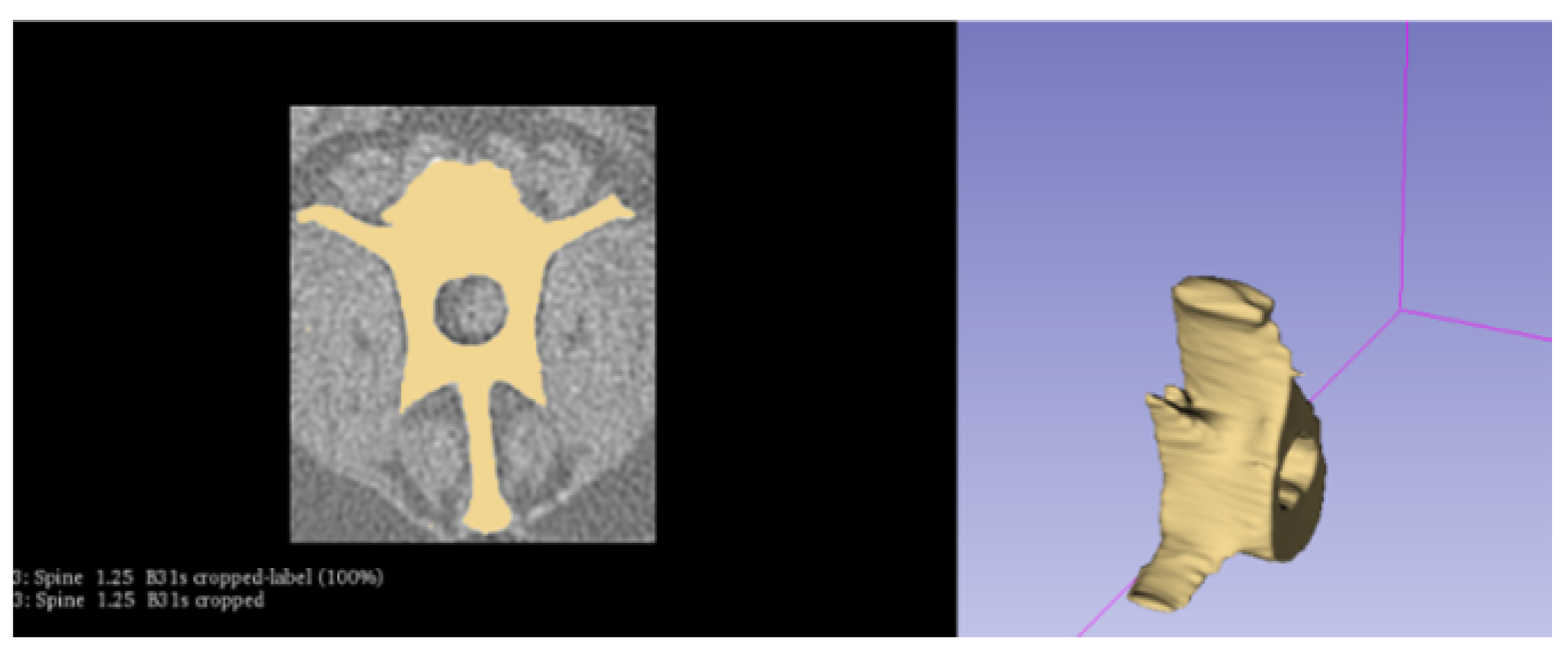
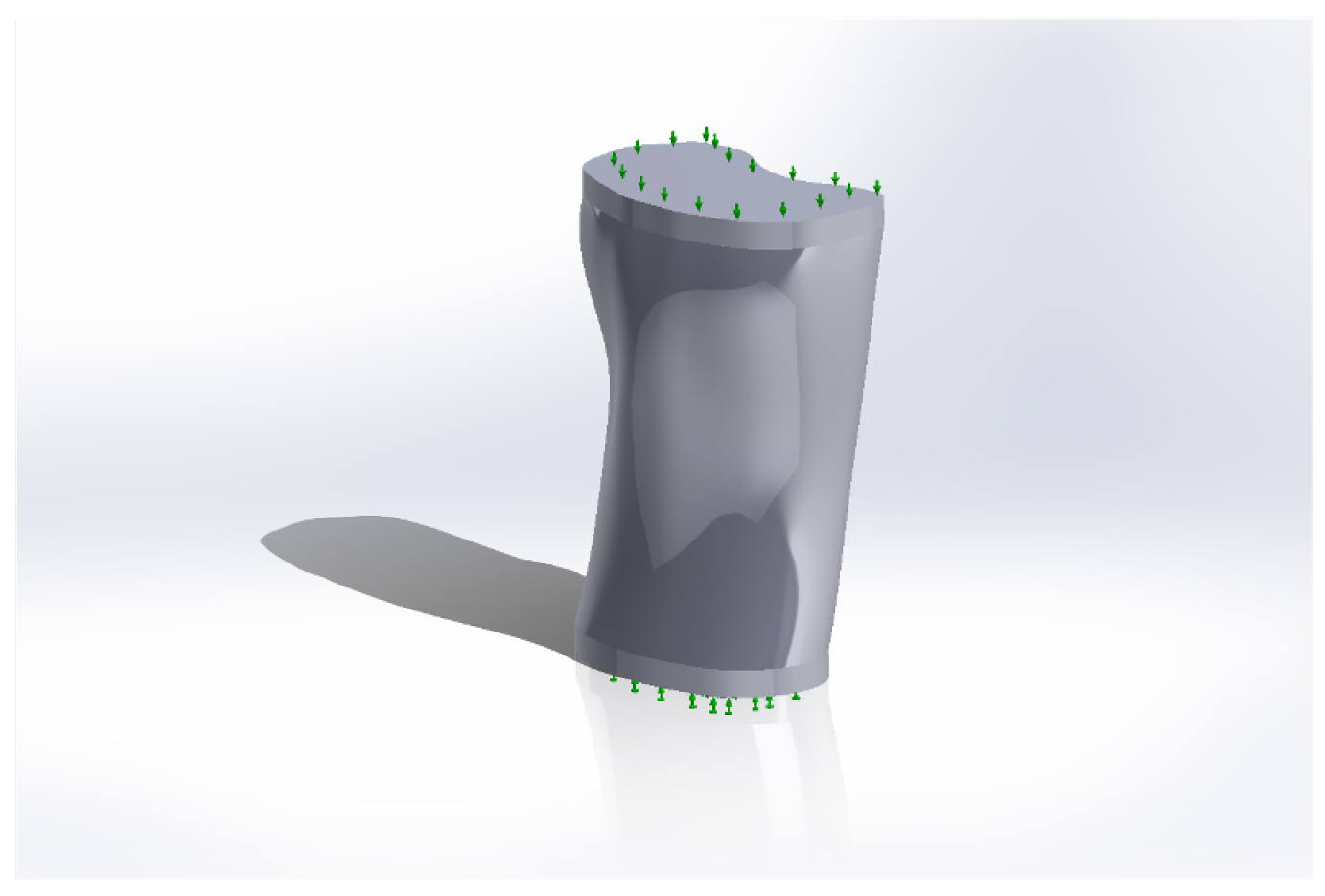
2.2. Creating the Geometry
2.2.1. Simplification of the Model
2.2.2. Mechanical Properties
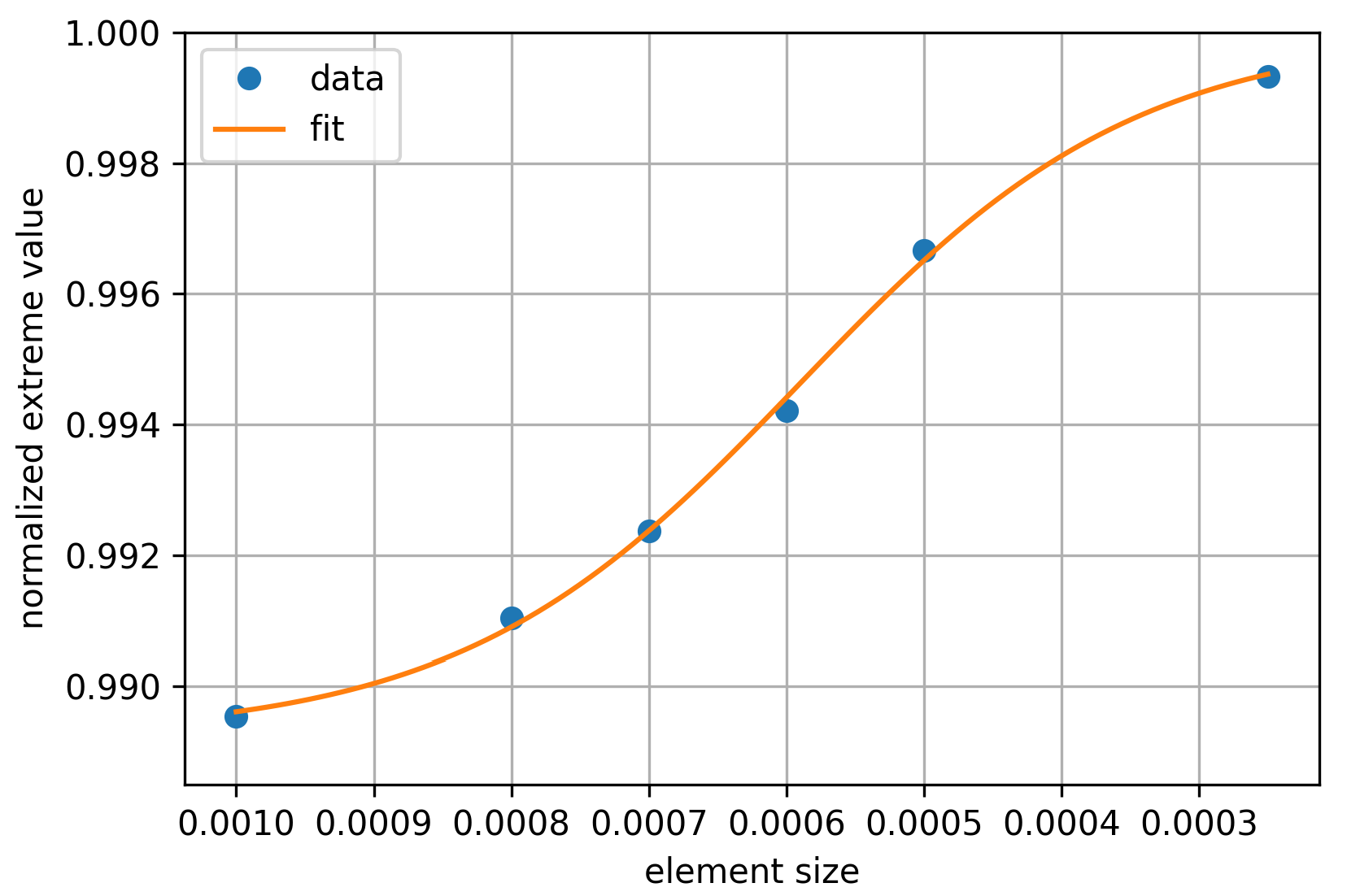
2.2.3. Convergence Analysis
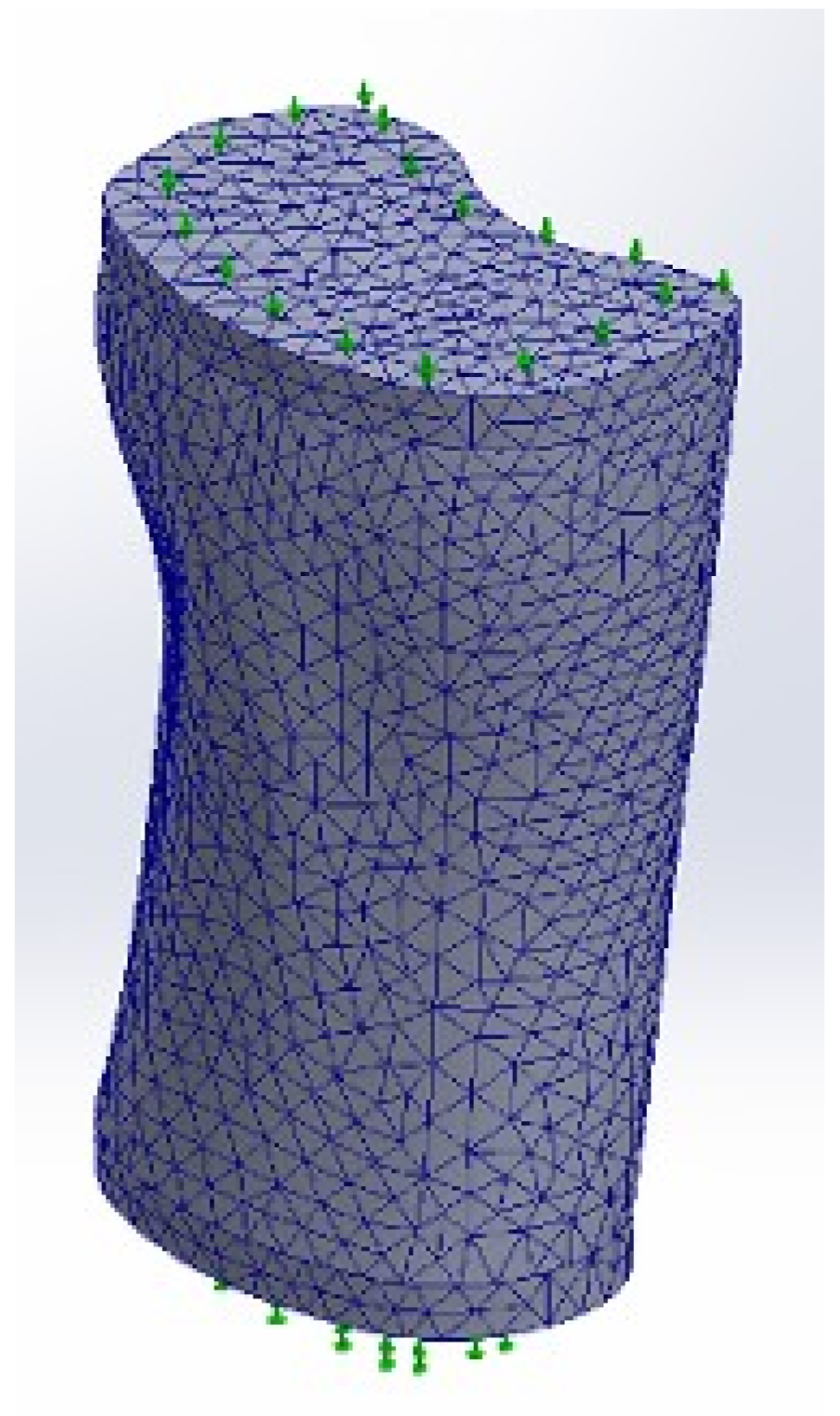
2.2.4. Meshing
2.3. Theoretical Background
2.4. Statistical Approach
3. Results
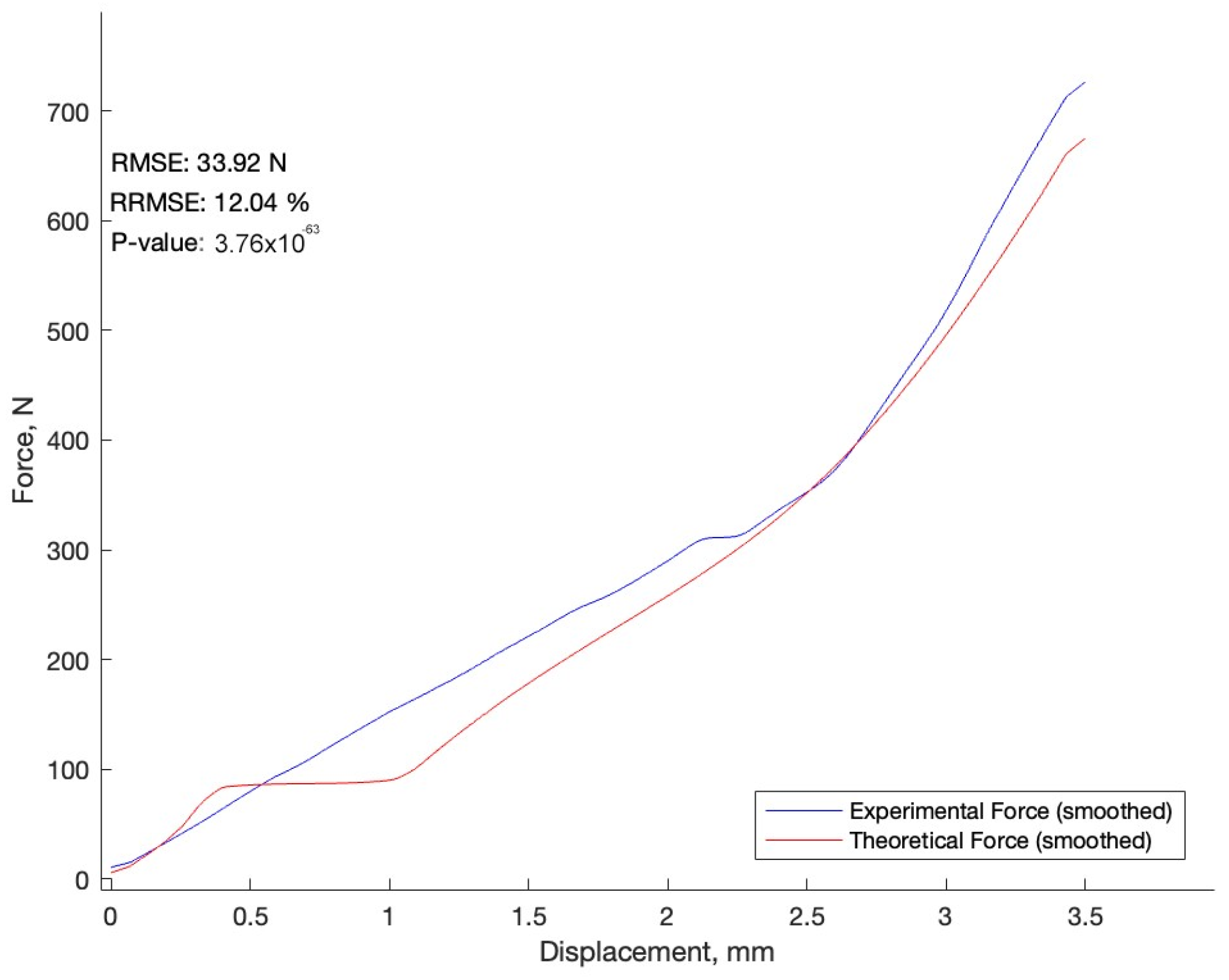
3.1. Comparison of the Experiment and the Numerical Model
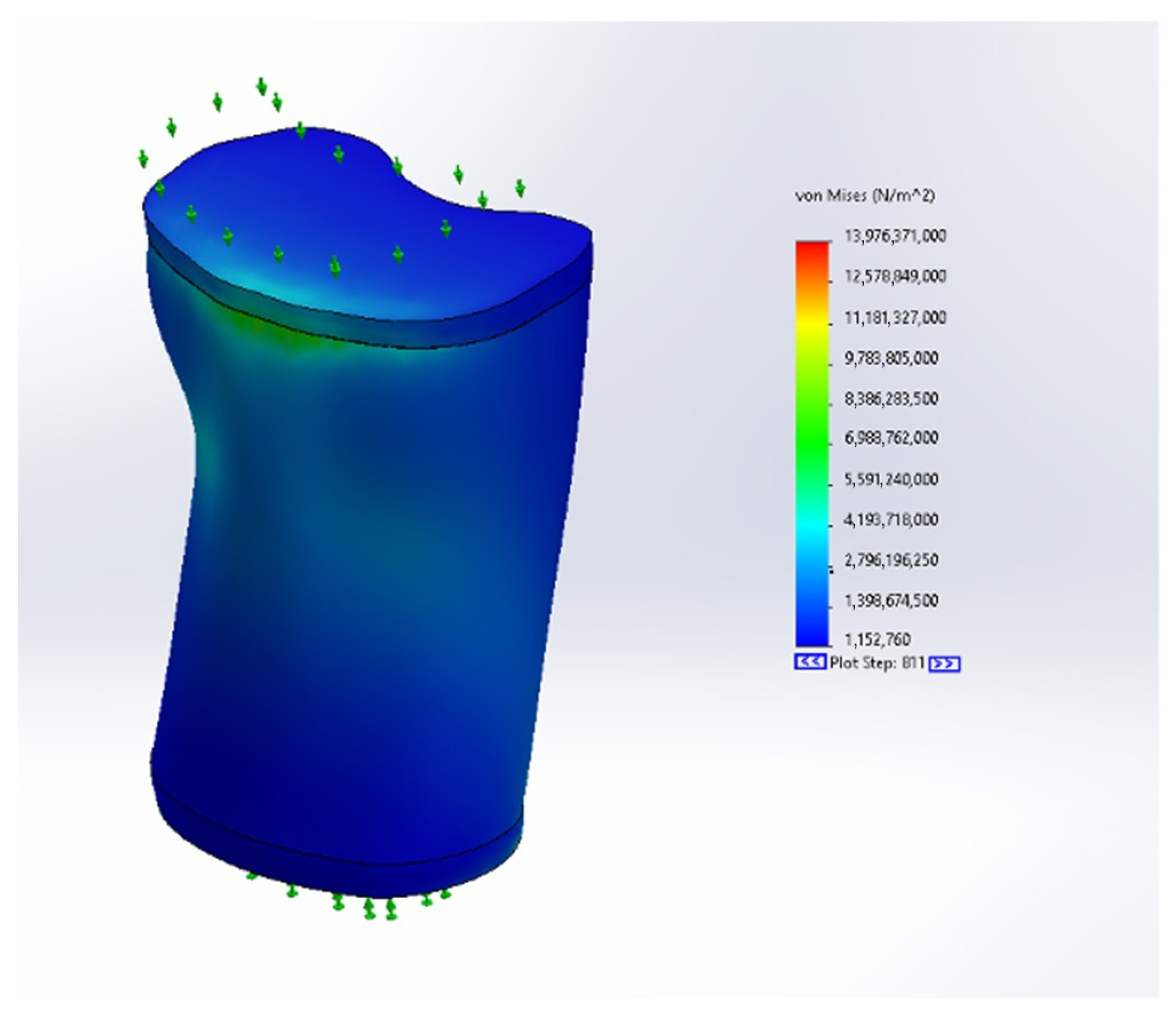
3.2. Stress Results
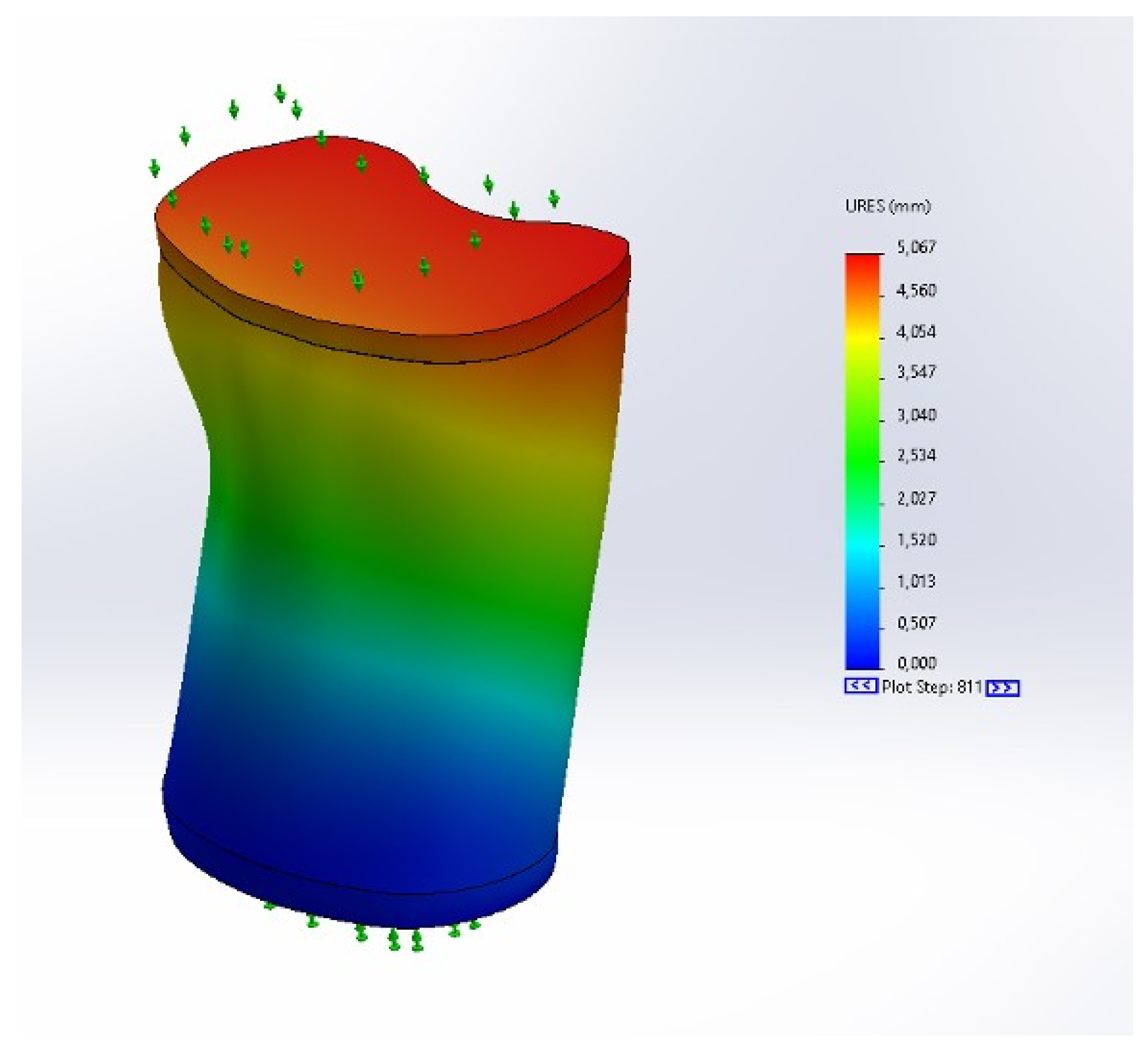
3.3. Displacement Results
4. Discussion
4.1. Role and Significance of the Nonlinear Study
4.2. Bipedal and Quadrupedal Locomotion Mechanical Aspects
4.3. Limitations
4.4. Implications of Breed, Age, and Other Risk Factors’ Variability in Canine Spinal Research
5. Conclusions
- -
- This study’s findings can be applied to research on osteoporosis in dogs to obtain a better understanding of the role that biomechanical factors play in determining fracture risk.
- -
- A displacement of 3.5 mm was observed between the actual experiment and the numerical model. Nevertheless, the maximum force calculated by the numerical model was 690 N, while that of the actual experiment was 740 N.
- -
- The RRMSE of 12.11% indicates that the results are credible, and the investigation into the canine lumbar vertebrae and the numerical model yielded biomechanical insights.
- -
- The study’s findings will enable the development of methods for the prevention and treatment of osteoporosis, reducing the likelihood of fractures, enhancing quality of life, and strengthening numerical models for use in other contexts.
- -
- The analysis of convergence indicates that as the initial size of the finite elements reduces, the error margin of the finite element method (FEM) results is confined within % and approaches the precise values.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BV/TV | Bone Volume per Trabecular Volume |
| FEM | Finite Element Method |
| CT | Computed Tomography |
| C | Cervical vertebra |
| Th | Thoracic vertebra |
| L | Lumbar vertebra |
References
- Bray, E.E.; Gruen, M.E.; Gnanadesikan, G.E.; Horschler, D.J.; Levy, K.M.; Kennedy, B.S.; Hare, B.A.; MacLean, E.L. Dog cognitive development: A longitudinal study across the first 2 years of life. Anim. Cogn. 2021, 24, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Kunkle, B.; Norrdin, R.; Brooks, R.; Thomassen, R. Osteopenia with decreased bone formation in beagles with malabsorption syndrome. Calcif. Tissue Int. 1982, 34, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Lopes, B.; Lanis, A.; De Oliveira, D.; Giannotti, J.; Costa, F. Bone demineralization in the lumbar spine of dogs submitted to prednisone therapy. J. Vet. Pharmacol. Ther. 2010, 33, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, Y.; Choi, W.; Chang, J.; Kang, J.H.; Na, K.J.; Chang, D.W. Quantitative CT assessment of bone mineral density in dogs with hyperadrenocorticism. J. Vet. Sci. 2015, 16, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Popović, T.; Šrbić, R.; Matavulj, M.; Obradović, Z.; Sibinčić, S. Experimental model of osteoporosis on 14 weeks old ovariectomised rats: Biochemical, histological and biomechanical study. Biol. Serbica 2016, 38. [Google Scholar] [CrossRef]
- Böcker, W.; El Khassawna, T.; Bauer, N.; Brodsky, K.; Weisweiler, D.; Govindarajan, P.; Schlewitz, G.; Kampschulte, M.; Dürselen, L.; Thormann, U.; et al. Short-term glucocorticoid treatment causes spinal osteoporosis in ovariectomized rats. Eur. Spine J. 2014, 23, 2437–2448. [Google Scholar] [CrossRef]
- Kharode, Y.P.; Sharp, M.C.; Bodine, P.V. Utility of the ovariectomized rat as a model for human osteoporosis in drug discovery. In Osteoporosis: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2008; pp. 111–124. [Google Scholar]
- Aithal, H.; Singh, G.; Bisht, G. Fractures in dogs: A survey of 402 cases. Indian J. Vet. Surg. 1999, 20, 15–21. [Google Scholar]
- Marshall, W.G.; Gonzalez-Garcia, C.; Trujillo, S.; Alba-Perez, A.; Childs, P.; Shields, D.W.; Tomlinson, A.; Pettitt, R.; Filliquist, B.; Chou, P.Y.; et al. Bioengineering an Osteoinductive Treatment for Bone Healing Disorders: A Small Animal Case Series. VCOT Open 2023, 6, e41–e51. [Google Scholar] [CrossRef]
- Woods, G.; Israeliantz Gunz, N.; Handel, I.; Liuti, T.; Mellanby, R.J.; Schwarz, T. Computed Tomography Osteodensitometry for Assessment of Bone Mineral Density of the Canine Head—Preliminary Results. Animals 2021, 11, 1413. [Google Scholar] [CrossRef]
- Tamburro, R.; Cerasoli, I.; Savino, R.; Terragni, R.; Stehlík, L.; Vignoli, M. Computed tomographic findings in three Pinschers affected by incomplete ossification of the humeral condyle. Acta Vet. Brno 2020, 89, 269–272. [Google Scholar] [CrossRef]
- Tang, S.; Allen, M.R.; Phipps, R.; Burr, D.B.; Vashishth, D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos. Int. 2009, 20, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Watanabe, K. Osteoporosis Research: Animal Models; Springer: London, UK, 2011. [Google Scholar]
- Szotek, S.; Szust, A.; Pezowicz, C.; Majcher, P.; Bedzinski, R. Animal models in biomechanical spine investigations. Bull-Vet. Inst. Pulawy 2004, 48, 163–168. [Google Scholar]
- Kostenko, E.; Stonkus, R.; Šengaut, J.; Maknickas, A. Empirical case report of the mechanical properties of three spayed canine lumbar vertebrae. Open Vet. J. 2022, 12, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Hogan, H.A.; Hobbs, W.A.; Koivuniemi, A.S.; Koivuniemi, M.C.; Burr, D.B. Raloxifene enhances material-level mechanical properties of femoral cortical and trabecular bone. Endocrinology 2007, 148, 3908–3913. [Google Scholar] [CrossRef]
- Mohsin, S.; Kaimala, S.; Sunny, J.J.; Adeghate, E.; Brown, E.M. Type 2 diabetes mellitus increases the risk to hip fracture in postmenopausal osteoporosis by deteriorating the trabecular bone microarchitecture and bone mass. J. Diabetes Res. 2019, 2019, 3876957. [Google Scholar] [CrossRef] [PubMed]
- Nolte, E.; Frisch, F. Changes in Bone Strength and Distal Femur Trabecular Thickness following Sleep-Deprivation in Ovariectomized Rats Treated with Zoledronate. FASEB J. 2020, 34, 1-1. [Google Scholar] [CrossRef]
- Kostenko, E.; Pockevičius, A.; Maknickas, A. Histomorphometry and μCT scan analysis of osteoporosis in spayed female dogs. Open Vet. J. 2023, 13, 1–10. [Google Scholar] [CrossRef]
- Zhao, S.; Arnold, M.; Ma, S.; Abel, R.; Cobb, J.; Hansen, U.; Boughton, O. Standardizing compression testing for measuring the stiffness of human bone. Bone Jt. Res. 2018, 7, 524–538. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Eswaran, S.K.; Bevill, G.; Nagarathnam, P.; Allen, M.R.; Burr, D.B.; Keaveny, T.M. Effects of suppression of bone turnover on cortical and trabecular load sharing in the canine vertebral body. J. Biomech. 2009, 42, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.K.; Xin, H.; Zhang, C.; Wang, C.; Xu, C.; Li, C.; He, Q. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Eng. Part A 2010, 16, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- SolidWorks Corporation. Nonlinear Dynamic Studies. 2021. Available online: https://help.solidworks.com/2021/english/SolidWorks/cworks/c_nonlinear_dynamic_studies.htm (accessed on 10 October 2023).
- Hori, R.; Lewis, J. Mechanical properties of the fibrous tissue found at the bone-cement interface following total joint replacement. J. Biomed. Mater. Res. 1982, 16, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Urban, R.; Lim, T.; Tomlinson, M.; Hall, D.; Renner, S.; An, H. Vertebroplasty using injectable calcium phosphate cement compared to polymethylmethacrylate in a unique canine vertebral body large defect model. In Proceedings of the Trans 49th Annual Meeting of Orthopaedic Research Society, New Orleans, LA, USA, 2–5 February 2003; p. 267. [Google Scholar]
- Moeller, D.; Lioce, L.; Budisalich, K.; Gunter, C.; Maddux, G.; Fogle, I.; Schroer, B. 3D models of compression fracture at lumbar vertebra L1: Analysis of injected bone cement injected by percutaneous kypholoshy. In Proceedings of the EDULEARN21 Proceedings, Online, 5–6 July 2021; pp. 5273–5283. [Google Scholar]
- Chaves, R.O.; Aiello, G.; de Souza, F.W.; Copat, B.; Schimdt, B.; de Oliveira, M.T.; Santalucia, S.; Mazzanti, A.; Brun, M.V. Videolaparoscopia na remoção de pino de Steinmann migrado em canino. Acta Sci. Vet. 2014, 42, 1–5. [Google Scholar]
- Gong, H.; Zhang, M.; Fan, Y. Micro-finite element analysis of trabecular bone yield behavior—effects of tissue nonlinear material properties. J. Mech. Med. Biol. 2011, 11, 563–580. [Google Scholar] [CrossRef]
- Schwiedrzik, J.J.; Wolfram, U.; Zysset, P.K. A generalized anisotropic quadric yield criterion and its application to bone tissue at multiple length scales. Biomech. Model. Mechanobiol. 2013, 12, 1155–1168. [Google Scholar] [CrossRef]
- Goldenblar, I.; Kopnov, A. Strength of Glass Reinforced Plastics in the Complex Stress State. Polym. Mech. 1966, 1, 54–60. [Google Scholar] [CrossRef]
- Von Mises, R. Mechanik der festen Körper im plastisch deformablen Zustand. Göttin. Nachr. Math. Phys. 1913, 1, 582–592. [Google Scholar]
- Hill, R. The Mathematical Theory of Plasticity; Oxford U.P.: Oxford, UK, 1950. [Google Scholar]
- Tsai, S. Strength Theories of Filamentary Structure. Chapter 1, Fundamental Aspects of Fibre Reinforced Plastic Composites; Interscience: New York, NY, USA, 1968. [Google Scholar]
- Korenczuk, C.; Votava, L.; Dhume, R.; Kizilski, S.; Brown, G.; Narain, R.; Barocas, V. Isotropic Failure Criteria Are Not Appropriate for Anisotropic Fibrous Biological Tissues. J. Biomech. Eng. 2017, 139, 071008. [Google Scholar] [CrossRef]

| Animal Species | Dog |
| Sex | Female |
| Weight | 15–25 kg |
| Age | Not specified |
| Reproductive Status | Spayed |
| Breed | Any |
| Nutrition | Poor or average |
| Muscle Development | Not prone to muscle atrophy |
| Joint Damage | Any (but, if present, must be noted) |
| Medical History | The animal should not have suffered from metabolic diseases; thyroid disorders; diseases such as rickets, osteomalacia, osteomyelitis, bone tumors, osteochondritis, dysplasias, spinal hernias, discospondylosis, or discospondylitis; and other osteopathies. Suitable: If there is a recorded bone fracture, except in cases where the required bone samples are damaged. |
| Required Bones | Lumbar vertebrae (L1–L5) |
| Cortical layer thickness, mm [23] | 0.32 |
| Surface area, | 415.0 |
| Intervertebral disc thickness, mm [24] | 2 |
| Total vertebral volume, | 7240.75 |
| Experimental loading rate, mm/min | 7 |
| Type | Rayleigh Damping |
|---|---|
| Alpha Coefficient | 0.01 |
| Beta Coefficient | 0.01 |
| Maximum Element Size | 0.7 mm |
| Minimum Element Size | 0.35 mm |
| Mesh Quality | High |
| Total Nodes | 642,003 |
| Total Elements | 371,706 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostenko, E.; Šengaut, J.; Višniakov, N.; Maknickas, A. Finite Element Model of Canine-Specific Vertebrae Incorporating Biomechanical Tissue Nonlinearity. Appl. Sci. 2024, 14, 2027. https://doi.org/10.3390/app14052027
Kostenko E, Šengaut J, Višniakov N, Maknickas A. Finite Element Model of Canine-Specific Vertebrae Incorporating Biomechanical Tissue Nonlinearity. Applied Sciences. 2024; 14(5):2027. https://doi.org/10.3390/app14052027
Chicago/Turabian StyleKostenko, Ernest, Jakov Šengaut, Nikolaj Višniakov, and Algirdas Maknickas. 2024. "Finite Element Model of Canine-Specific Vertebrae Incorporating Biomechanical Tissue Nonlinearity" Applied Sciences 14, no. 5: 2027. https://doi.org/10.3390/app14052027
APA StyleKostenko, E., Šengaut, J., Višniakov, N., & Maknickas, A. (2024). Finite Element Model of Canine-Specific Vertebrae Incorporating Biomechanical Tissue Nonlinearity. Applied Sciences, 14(5), 2027. https://doi.org/10.3390/app14052027







