Abstract
Amoebiasis, a disease caused by the protozoan Entamoeba histolytica, represents a serious public health problem, mainly in developing countries. The first line of therapy for amoebiasis treatment is metronidazole (MTZ); however, clinical isolates of E. histolytica with resistance to MTZ and varying sensitivity to other antiamoebic drugs threaten the effectiveness of the prevention and treatment of this parasitic infection. Natural products stand out as a promising strategy to develop new, safe and more effective alternatives. In this study, we determined and compared the phytochemical profiles of Agave tequilana, Agave angustifolia, Agave rhodacantha, and Agave maximiliana and described their cytotoxic effect on E. histolytica trophozoites. The results show that the four Agaves kill E. histolytica in a species–time–dose-dependent manner. A morphologic analysis of the treated parasites showed evident morphological alterations suggestive of programmed cell death with nuclear alterations; it also highlighted the presence of rounded cells with protuberances/perforations in the membrane and cells that appeared to have exploded. The overall activity of Agave ethanolic extracts in E. histolytica can help provide new strategies to advance alternative treatments against amoebiasis.
1. Introduction
Human amoebiasis represents a severe public health problem worldwide; millions of people are infected, resulting in over 100,000 deaths yearly. Entamoeba histolytica, the causative agent of amoebiasis, is associated most commonly with intestinal infections but can also present extraintestinal manifestations leading to an amoebic liver abscess; in rare cases, it affects the lungs, heart, and brain [1]. Immunocompromised persons may develop the most severe symptoms and higher case fatality rates due to invasive amoebiasis. In areas with poor sanitation and high-risk hygiene behaviors, its prevalence remains as high as 40% [2]. In Mexico, this parasitosis is classified as one of the main causes of diarrheal disease, with high prevalence and mortality rates; incidences over 70% have been related to geographical, socioeconomic, and environmental factors [3]. There are multiple pharmacological treatments to control amoebiasis which are classified as luminal, systemic, or mixed amoebicides; nitroimidazoles are first-line drugs, in particular, metronidazole (MTZ) is the most widely used standard therapy for invasive amoebiasis. However, the indiscriminate use of amoebicidal compounds has stimulated an increasing presence of strains resistant to these antibiotics and significantly high therapeutic failure rates [4,5,6]. Currently, plants and natural products continue to be important sources of novel bioactive compounds [7,8,9,10]. Several studies have identified, quantified, and classified bioactive compounds, such as flavonoids, saponins, terpenes, and steroids, with potential uses as antioxidant, anti-inflammatory, and antimicrobial compounds [11,12,13,14,15,16]. The genus Agave consists of approximately 200 species; 150 are in Mexico, with 119 endemics [17,18,19]. This plant has been used principally to produce distilled alcoholic beverages, representing an economic benefit of billions of dollars yearly [20,21,22]. However, during production, a considerable amount of waste is generated, including the plant’s spiky leaves [21,22,23]. Due to the presence of secondary metabolites such as phenols, flavonoids, phytosterols, and saponins in Agave crude leaf extracts with well-known biological activities, they have been associated with several benefits for human health, curing and preventing many diseases including infectious diseases and cancer [24,25,26,27,28,29,30,31]. In particular, as antiparasitics, extracts of Agave americana have been reported to exhibit significant leishmanicidal activities; 0.05 mg/mL was enough to eliminate promastigotes and axenic amastigotes after 24 h of treatment [32]. In addition, other studies reported by Botura et al. (2011, 2013) proved that an extract from Agave sisalana has effective activity against the eggs, larvae, and adult worms of gastrointestinal nematodes in in vitro assays [33,34]. Guerra JO et al. (2008) reported cytostatic and growth inhibition activity in steroidal saponins from the plant Agave brittoniana against Trichomonas vaginalis [35]. Other species, such as Agave tequilana and Agave angustifolia, have been highlighted principally for their therapeutic benefits as antibacterial compounds [36,37]. Even though there have been entirely positive results in biological assays, Agave extracts have not been widely evaluated against the most relevant intestinal protozoa. A first report by Quintanilla-Licea et al. (2020) showed 69% growth inhibition against Entamoeba histolytica trophozoites by extracts of Agave lechugilla Torr [38]. In this study, we determined and compared the phytochemical profiles of Agave tequilana, Agave angustifolia, Agave rhodacantha, and Agave maximiliana and their cytotoxic effect on E. histolytica trophozoites. Our investigation supports that Agave crude extract compounds are good candidates for the discovery of the untapped potential of the agro-wastes of the tequila industry in the development of novel amoebicidal alternatives.
2. Materials and Methods
2.1. Harvesting of the Raw Material and Ethanolic Extraction
Leaves of Agave tequilana Weber, Agave angustifolia Haw, Agave rhodacantha Trel, and Agave maximiliana Baker were collected during the period of October to November 2020 at the “Gorupa” property, from 4.5 Km S of Chiquilistlán, and 0.5 km from the “El Agostadero” crossroads, respectively. The collected samples were washed, and once dry, a 60 g sample of dried leaves was cut, placed in flasks containing 600 mL of ethanol, and incubated at 150 rpm for 48 h at room temperature (TA). Subsequently, the extracts were filtered through Whatman paper no.2 to remove fibers. The collected ethanolic extracts were concentrated under vacuum in a rotary evaporator (R-300, BÜCHI Labortechnik AG, Swiss) at 40 °C to yield a 100 mL volume. The samples were frozen for 48 h at −20 °C. The samples were subsequently dried by freeze-drying for 48 h (−36 °C, Scientz-10N Freeze Dryer, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China). The powders collected were stored at 4 °C until use.
2.2. UV–Vis
Absorbance spectra were obtained using a UV–Vis spectrophotometer (Genesys 10UV, Thermo Fisher Scientific, Waltham, MA, USA), scanning wavelengths from 300 to 800 nm with a 1 nm step. For the measurements, 3 mL of each Agave extract was poured into a quartz cell. The acquired data were analyzed using OriginLab software (version OriginPro 2023b).
2.3. Phytochemical Screening
Standard extraction and screening procedures were used to determine the phytochemical components of A. tequilana, A. maximiliana, A. angustifolia, and A. rhodacantha.
2.3.1. Test for Flavonoids
For the identification of flavonoids, the Shinoda test [39,40] was used, in which 1–2 mg of crude extract was dissolved in 1 mL of ethanol in a test tube and a few drops of concentrated hydrochloric acid and two magnesium filings were added; if the solution turned bright red, the test was positive.
2.3.2. Test for Sugars
For the identification of sugars, the Anthrone test [41] was used. This technique consisted of placing 1 mL of the sample in a test tube before dissolving the sample in water. Subsequently, 1–2 mg of Anthrone reagent and about 4 drops of sulfuric acid were added. The test is positive when a blue-green ring appears at the interface.
2.3.3. Test for Sterols and Triterpenes
For the identification of sterols and triterpenes, the Lieberman–Burchard test [39] was used; the reagent was prepared by mixing 1 mL of acetic anhydride, 1 mL of chloroform, and five drops of sulfuric acid. A couple of drops of the reagent were added to l–2 mg of the sample dissolved in chloroform. The appearance of any coloration within an hour indicates a positive test for steroids and triterpenes, particularly when they have a high degree of unsaturation.
2.3.4. Test for Saponins
For the identification of saponins, 2 mg of sample was dissolved with l.5 mL of water and stirred manually for three minutes to observe the formation of foam [42,43].
The overall results were expressed by cross-testing, with (−) negative, (+) weak positive, (++) moderate positive, and (+++) strong positive results.
2.4. Culture and Maintenance of E. histolytica
The trophozoites of E. histolytica (HM1-IMSS strain) were grown axenically in TYI-S-33 medium at a pH of 6.8 supplemented with 15% bovine serum, penicillin, and streptomycin 1% (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C [44]. The culture tubes were monitored via microscopic examination, and subcultures were made twice weekly.
2.5. In Vitro Susceptibility Assay
To evaluate the effect of the ethanolic agave extracts on the growth of E. histolytica, 15,000 trophozoites/mL were grown at 37 °C for 24, 48, and 72 h in the presence of 0, 100, 300, or 600 μg/mL of A. tequilana, A. maximiliana, A. angustifolia, or A. rhodacantha. Untreated cells and the extracts’ diluent, 0.4% dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA), were used as negative controls, and 1.4 μg/mL of MTZ (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control. After the incubation periods, trophozoites were collected by chilling the cell culture tubes on ice for 20 min, and they were counted in a Neubauer chamber. The percentage of parasite growth inhibition was determined in relation to the DMSO control, which was considered to represent 100% parasite growth.
2.6. Cell Viability Assay
To determine the effect of the Agave extracts on the viability of trophozoites, an exclusion staining method with trypan blue was used. From cultures exposed to DMSO, 0, 100, 300, or 600 μg/mL of A. tequilana, A. angustifolia, A. rhodacantha, and A. maximiliana, a volume of 10 μL of culture was mixed with 10 μL of trypan blue (0.4% GibcoBRL, Grant Island, FL, USA) for 24 and 48 h. The total number of parasites, including those in samples which excluded the dye, was counted in a Neubauer chamber, and cell viability was expressed as the percentage of viable cells: [100 × (living cells)/(dead cells + living cells)].
2.7. Bright-Field Microscopy
For a microscopy analysis, Agave- or DMSO-treated trophozoites were washed and adhered to poly-L-lysine pretreated coverslips. Then, the adhered parasites were fixed with 4% paraformaldehyde for 30 min. Finally, the coverslips were washed in phosphate-buffered saline (PBS) and mounted on glass slides using ProLong Gold mounting medium containing DAPI (ProLong Gold, Thermo Fisher Scientific, USA). The cells were analyzed under an Eclipse Ts2 microscope (Nikon Instruments Inc., Melville, NY, USA)
2.8. Statistical Analysis
All the results obtained were the average of three independent experiments, each in triplicate. All data are expressed as mean ± standard deviation values. Data were statistically analyzed using a one-way ANOVA (GraphPad Prism version 6.01 for Windows, GraphPad Software, La Jolla, CA, USA), and p values ≤ 0.05 were considered significantly different.
3. Results and Discussion
3.1. Ethanolic Extracts of Agave tequilana, Agave angustifolia, Agave rhodacantha, and Agave maximiliana UV–Vis Analysis and Phytochemical Screening
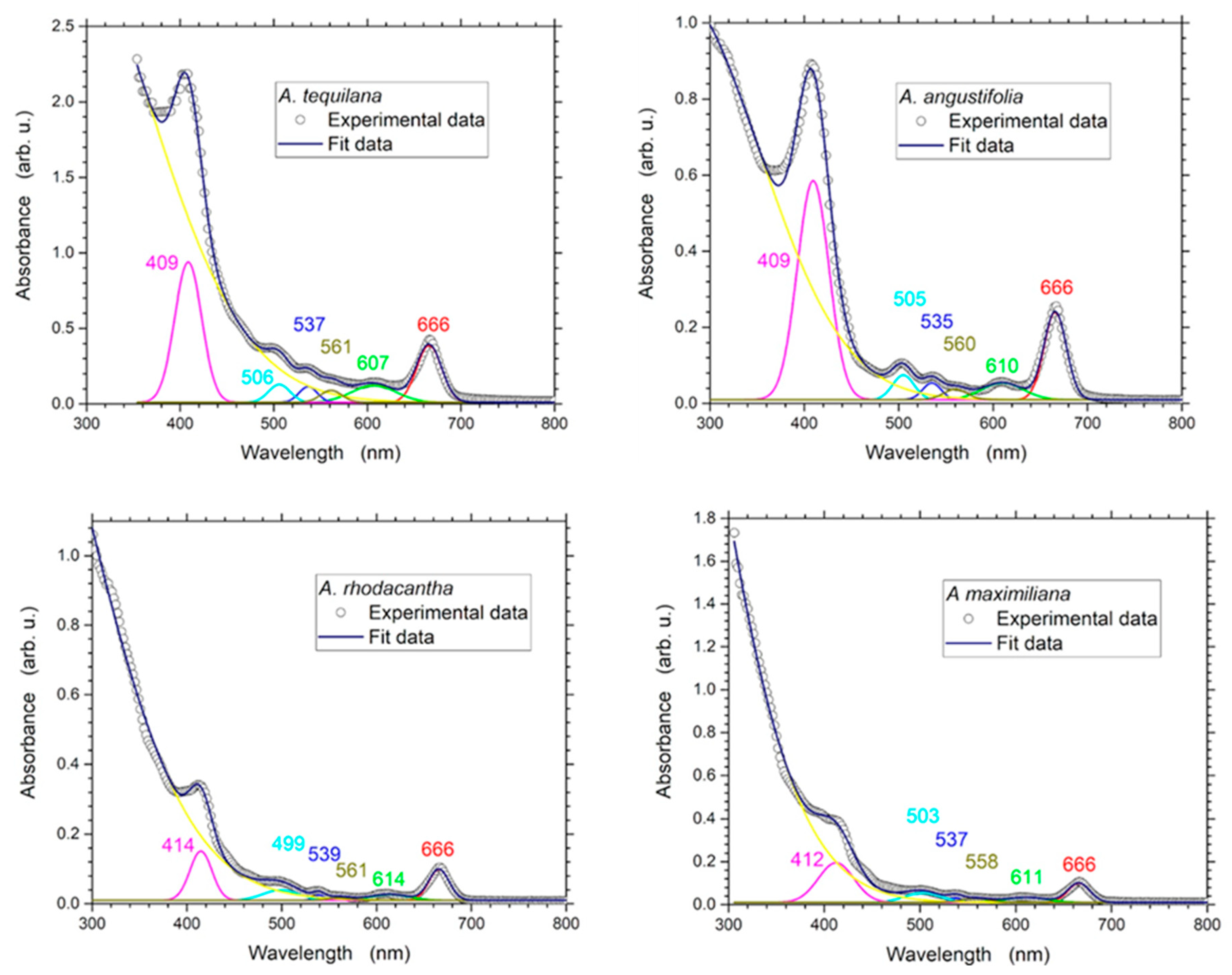
Several authors have proved that during phytochemical extraction, solvents have a significant impact on the level of polyphenols extracted [45]. In general, tannins, polyphenols, polyacetylenes, flavonols, terpenoids, sterols, and alkaloids can be readily extracted with ethanol [46,47,48]. In this study, a UV–Vis spectroscopy analysis revealed six weak and strong, intense bands ranging in wavelength from 400 to 700 nm (Figure 1); as reported by other authors, these bands reveal the presence of alkaloids, flavonoids, terpenes, steroids, saponins, tannins, and coumarins [45,48,49,50,51,52]. The profile showed the peaks at 409–414 nm, 499–506 nm, 535–539 nm, 558–561 nm, 607–614 nm, and 666 nm. The higher absorbance values were obtained from A. tequilana, followed by A. angustifolia (Table 1). The fact that the Agave species had absorbance peaks of unequal intensity is not surprising to us since the phytochemical constituents of plants vary among species; also, the concentration depends on the metabolite type, the characteristics of the plant, the tissue, the stage of development, and climatic factors [53,54,55,56,57]. The phytochemical screening of the Agave extracts confirmed the presence of flavonoids, terpenes, steroids, saponins, tannins, and coumarins, the contents of which varied considerably among the Agave species. The concentration of terpenes was high (+++) in the four species analyzed, while tannins were moderately (++) present. Only A. angustifolia and A. rhodacanta presented high concentrations of flavonoids (+++). A. maximiliana was found to be the species with the lowest concentrations of flavonoids, steroids, and coumarins (Table 2).
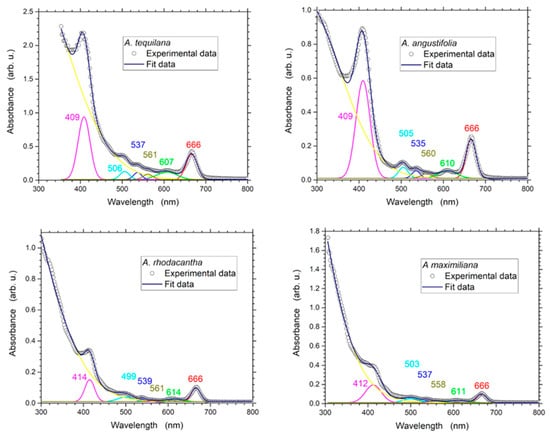
Figure 1.
UV–Vis absorbance spectra analysis of the ethanolic extracts of A. angustifolia, A. maximiliana, A. rhodacantha, and A. tequilana from 300 to 800 nm.

Table 1.
UV–Vis absorbance peak intensity values of the ethanolic extracts of A. angustifolia, A. maximiliana, A. rhodacantha, and A. tequilana..

Table 2.
Screening of ethanolic Agave extracts.
3.2. Ethanolic Extracts of Agave Leaves Inhibit the Growth of E. histolytica Trophozoites
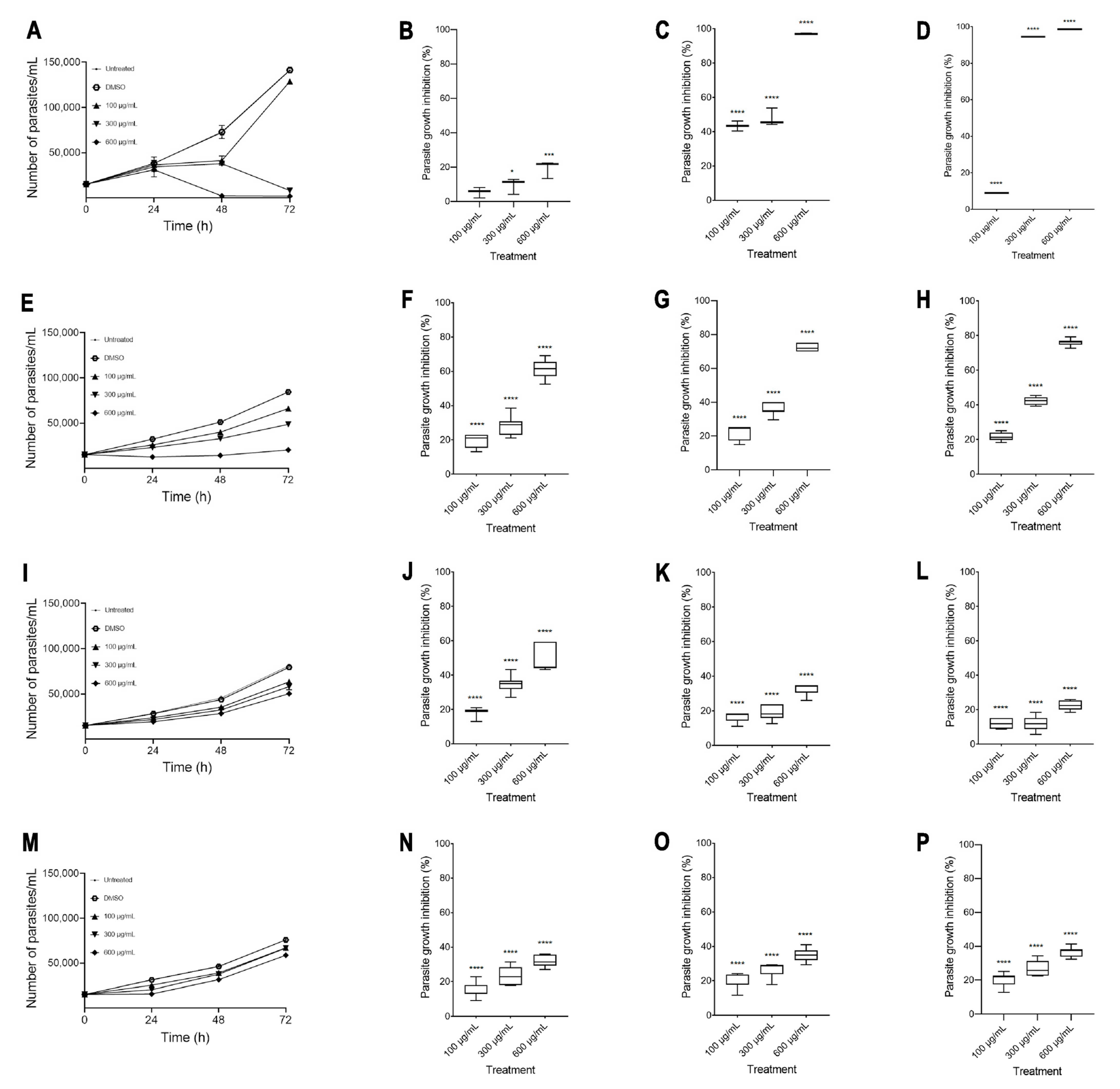
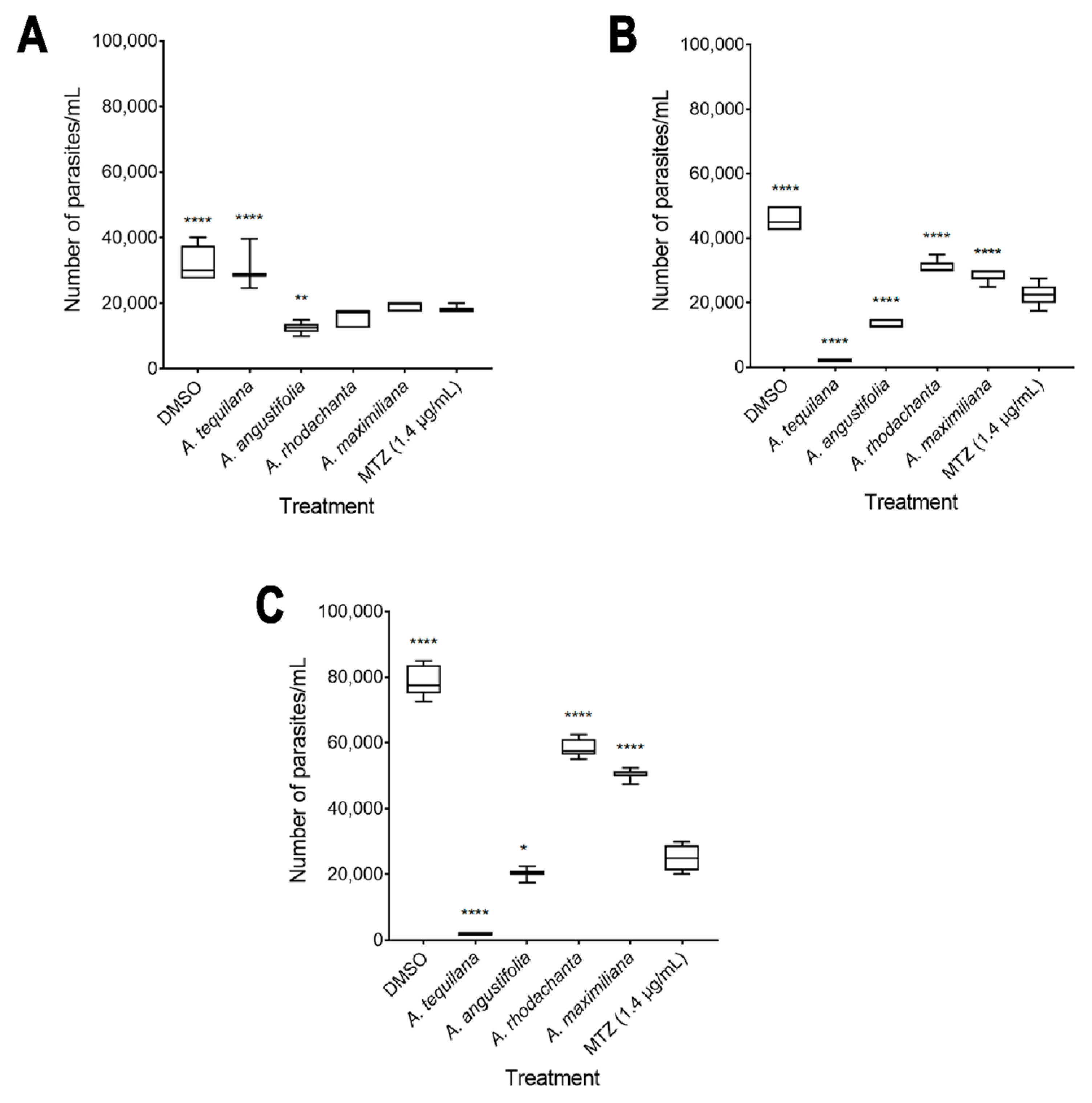
The in vitro amoebicidal activity of ethanolic extracts of the species Agave tequilana, Agave angustifolia, Agave rhodacantha, and Agave maximiliana was evaluated. Our results showed that all the Agave species studied in the present investigation showed promising amoebicidal activity (Figure 2). The negative control (DMSO 0.4%) did not exhibit any significant differences compared with untreated cells. Remarkably, the highest amoebicidal activity was observed with A. tequilana; at 72 h, with concentrations of 300 and 600 µg/mL, a more than 90% decrease in the trophozoite count was observed compared to the negative controls (Figure 2D), with IC50 = 193 µg/mL (Table 3). With A. angustifolia, a growth inhibitory effect of more than 60% was seen only at 600 µg/mL during the entire exposure period (Figure 2F–H), with IC50 = 364 µg/mL (Table 3). The extracts with moderate activity were A. rodhacantha and A. maximiliana (Figure 2I–P); large and undefined IC50 values were set at 1220 and 1824 µg/mL, respectively, for purposes of illustration (Table 3). Our results correspond with those reported by Quintanilla-Licea et al. (2014), who showed the amoebicidal activity of A. lechuguilla [38]; however, our results showed that for E. histolytica, higher doses are necessary to achieve a reduction of more than 90%. In addition, we confirmed that other species of the Agave genus present varying degrees of amoebicidal activity. The phytochemical analysis (Table 2), showing differences in the presence and relative abundance of some metabolites, could explain the different efficacies of the Agave extracts when killing E. histolytica. In addition, in this research study, there was a positive correlation between the higher absorbance values of A. tequilana and A. angustifolia (Figure 1 and Table 1) and the higher amoebicidal properties observed for these species. Chromatographic separation, chromatography–mass spectrometry, and a UV–Vis analysis confirmed the abundant presence of quercetin, kaempferol, gallic acid, β-sitosterols, and steroidal saponins in A. tequilana and A. angustifolia [36,58,59,60,61]. This variety of compounds has been associated with numerous medicinal uses and biological activities. In E. histolytica, studies by Moises Martines-Castillo et al. (2018) demonstrated the amoebicidal activity of epicatechin, kaempferol, and quercetin [62]. Similar amoebicidal activity has been reported by Arrieta et al. (2001) for β-sitosterol and β-sitosterol glucoside phenolic [63]. In the latter work, the authors described that pure compounds were less active than complete plant extracts, suggesting an important synergist activity with the presence of several metabolites. On the other hand, MTZ is considered the drug of choice for treating amoebiasis; in this study, at 24 h, 600 µg/mL of A. angustifolia showed greater efficacy than MTZ (Figure 3A). After 48 h, A. tequilana and A. angustifolia were demonstrated to be more effective than MTZ (Figure 3B,C). Even though Agave extracts showed higher amoebicidal activity in comparison to MTZ, the plants use natural toxins as a defense mechanism. Additional studies are necessary to establish the toxicity or adverse health effects of Agave extracts for their use as amoebicidal alternatives.
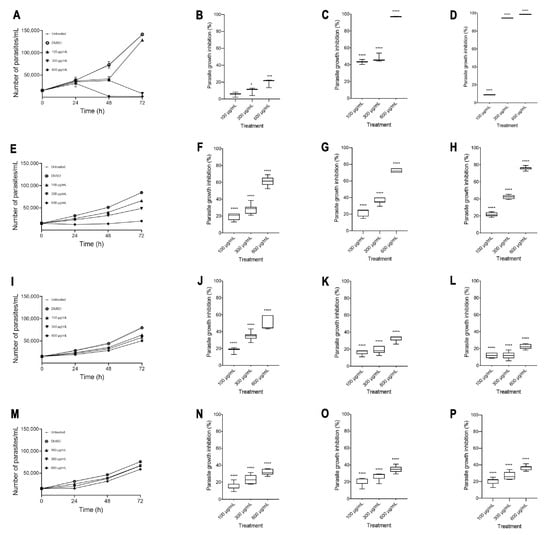
Figure 2.
Extracts of Agave inhibit E. histolytica growth. Growth inhibition curves of A. tequilana (A), A. angustifolia (E), A. rhodacantha (I), and A. maximiliana (M). Percentages of inhibition at 24 h of A. tequilana (B), A. angustifolia (F), A. rhodacantha (J), and A. maximiliana (N). Percentages of inhibition at 48 h of A. tequilana (C), A. angustifolia (G), A. rhodacantha (K), and A. maximiliana (O). Percentages of inhibition at 72 h of A. tequilana (D), A. angustifolia (H), A. rhodacantha (L), and A. maximiliana (P). The boxes represent the 25–75th percentiles, and the medians are indicated. The whiskers show the range. Data are representative of at least three independent experiments and represented as mean ± SEM values. * p ≤ 0.05, *** p ≤ 0.0005, and **** p ≤ 0.0001 compared to the control group (DMSO-treated cells).

Table 3.
IC50 value of Agave extracts on E. histolytica growth after 72 h of incubation.
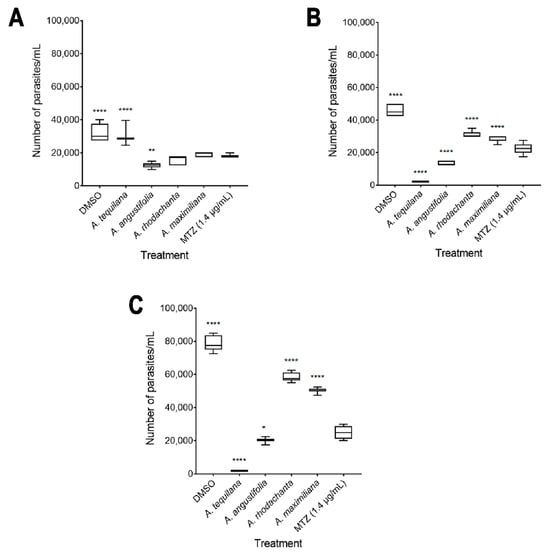
Figure 3.
Comparison of growth inhibition rates of Agave extracts (600 µg/mL) and MTZ (1.4 µg/mL) at 24 h (A), 48 h (B), and 72 h (C). The boxes represent the 25–75th percentiles, and the medians are indicated. The whiskers show the range. Error bars represent the standard deviation (n = 3 experiments performed in triplicate; * p ≤ 0.05, ** p ≤ 0.005, and **** p ≤ 0.0001).
3.3. Agave Extracts Reduced the Cell Viability of E. histolytica Trophozoites
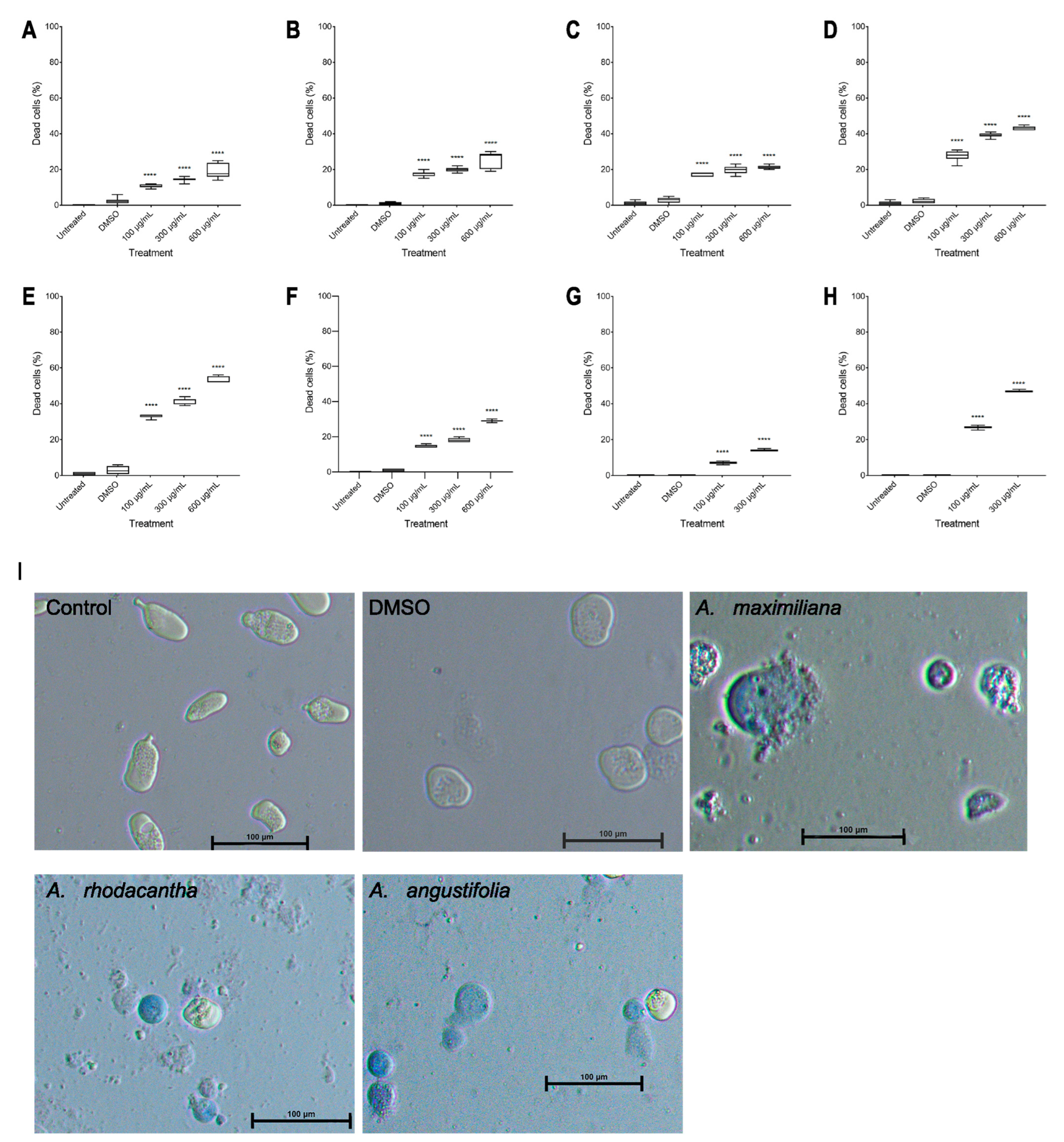
The percentage of viable parasites was measured after 48 h of incubation with Agave extracts using the trypan blue exclusion test. Interestingly, the extracts that showed less amoebicidal activity turned out to be from the species with the highest presence of blue-stained cells (nonviable cells) (Figure 4I). With A. maximiliana, at 24 h, only 73%, 61%, and 52% were viable at 100, 300, and 600 µg/mL, respectively (Figure 4E). A. maximiliana was the species with the highest concentration of saponins (Table 2). Saponins have properties that facilitate the formation of insoluble complexes with components of the cell membrane (steroids, proteins, and phospholipids, especially cholesterol), which contributes to the permeability of the cell membrane, leading to the formation of pores, leading to a massive release of cytoplasmic content and the formation of apoptotic bodies, which eventually induces the death of the parasite [64,65]. In other Agave extracts, it has been reported that saponins from A. lophantha and A. brittoniana at concentrations of 500, 100, and 10 µg/mL decreased the viability of T. vaginalis, G. lamblia, and E. histolytica [35,66]. The DMSO-treated cells did not exhibit any significant difference compared with the untreated cells.
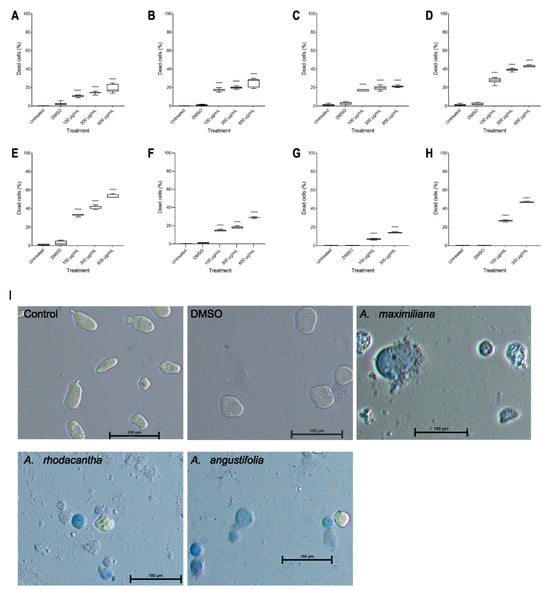
Figure 4.
Percentage inhibition of viability due to the effect of Agave ethanolic extracts in E. histolytica. A. angustifolia at 24 h (A) and 48 h (B), A. rhodacantha at 24 h (C) and 48 h (D), A. maximiliana at 24 h (E) and 48 h (F), and A. Tequilana at 24 h (G) and 48 h (H). Inverted optical microscope (Nikon Eclipse Ts2) pictures of trypan-blue-stained trophozoites after DMSO or Agave extract treatment (600 µg/mL, 48 h) (I). The boxes represent the 25–75th percentiles, and the medians are indicated. The whiskers show the range. Results were obtained from three experiments independently using the triplicate SD. **** p ≤ 0.0001. Bar = 100 µm.
3.4. Agave Extracts Induce Morphological Alterations in E. histolytica Trophozoites
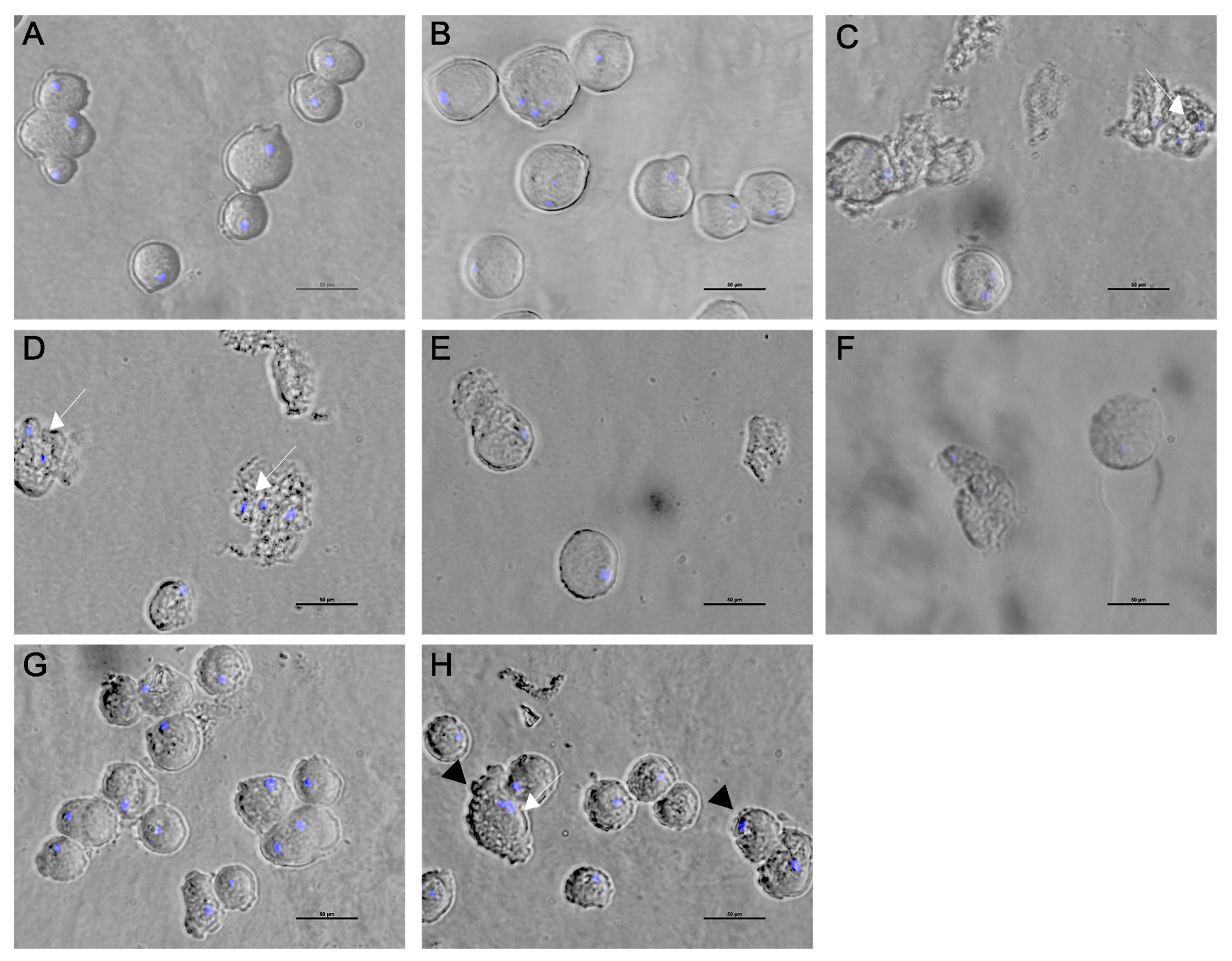
The effect of the Agave extracts on trophozoite morphology was evaluated using bright-field microscopy. All concentrations of the Agave extracts stimulated a wide variety of morphological alterations compared to the control trophozoites. No differences were found in the morphology of untreated and DMSO-treated trophozoites; the cells showed a distinctive pleomorphic form and the presence of pseudopods (Figure 5A,B). After Agave exposure, there was a prevalence of detached trophozoites with a rounded form; there were parasites with apparent protuberances in the membrane, and cell lysis and DAPI staining revealed apparent changes in the diameter of nuclei (Figure 5). With A. tequilana and A. angustifolia, more than 60% of cells showed damage; ruptured trophozoites and cell debris were observed (Figure 5C,D). With A. maximilana, 80% of the parasite population presented a granular surface, and an accumulation of filopodial protrusions prevailed (Figure 4G,H). Phytochemical screening indicated a greater presence of flavonoids and steroids in A. tequilana, A. angustifolia, and A. rhodacantha (Table 2), which could be responsible for the drastic morphological alterations observed due to these extracts. Several studies support the amoebicidal activity of flavonoids, and (−)-epicatechin and kaempferol have been related to growth inhibition, decreased cell viability, and nuclear and morphological alterations in E. histolytica trophozoites. Damage to cytoskeletal structures with changes in essential mechanisms such as adhesion, cytolysis, and nuclear alterations followed by programmed cell death are the principal findings [67,68]. In addition, it has been demonstrated that the motility of E. histolytica depends on the sustained instability of the intracellular hydrostatic pressure that drives the cyclic generation and healing of membrane blebs [69]. Furthermore, actin filaments constitute the physical backbone of membrane protrusions [70]. The prevalence of the filopodial protrusions that A. maximiliana caused in trophozoites could be related to alterations in intracellular hydrostatic pressure and the actin cytoskeleton. Our results suggest that the trophozoites’ deaths occurred principally due to a loss of membrane integrity, changes within the nucleus, and cytoskeletal alterations; biochemical studies are necessary to obtain evidence of programmed cell death in Entamoeba histolytica due to Agave extracts.
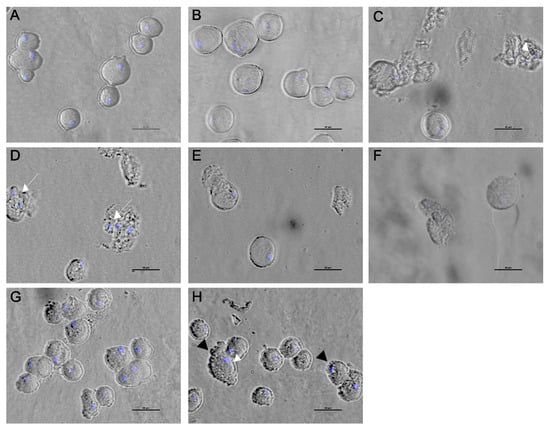
Figure 5.
Inverted optical microscope (Nikon Eclipse Ts2) pictures of trophozoites treated with ethanolic extracts of Agave. Untreated trophozoites (A) and those treated with 0.4% DMSO (B). A. Tequilana (C), A. angustifolia (D,E), A. rhodachanta (F), and A. maximiliana (G,H). White arrows indicate alterations in nuclei. Black arrows indicate filopodial protrusions. Bar = 50 µm.
4. Conclusions
The results obtained in this study demonstrate that the ethanolic leaf extracts of Agave tequilana, Agave angustifolia, Agave rhodacantha, and Agave maximiliana present different efficacies of amoebicidal activity. This is the first study focused on identifying structural damage in E. histolytica due to Agave extracts. The result of this study supports the traditional claim that the plants are antiparasitic alternatives, and efforts are currently under way to identify the bioactive component(s) of the Agave species and their cytotoxicity and selectivity.
Author Contributions
Conceptualization, R.R.-O. and A.C.-R.; data curation, A.L.R.-Z., L.B.-R. and M.N.-V.; formal analysis, A.C.-R.; funding acquisition, A.C.-R.; investigation, A.L.R.-Z., J.I.M.-F., M.A.B.-E., A.P.-C. and A.C.-R.; methodology, A.L.R.-Z., J.I.M.-F., M.A.B.-E., A.P.-C., L.B.-R. and A.C.-R.; project administration, A.C.-R.; resources, L.B.-R. and A.C.-R.; software, A.L.R.-Z. and A.P.-C.; supervision, A.C.-R.; validation, A.P.-C., L.B.-R., R.R.-O., M.N.-V. and A.C.-R.; visualization, A.L.R.-Z.; writing—original draft, A.L.R.-Z., L.B.-R. and A.C.-R.; writing—review and editing, A.P.-C., L.B.-R., R.R.-O., M.N.-V. and A.C.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Consejo Estatal de Ciencia y Tecnología de Jalisco (Coecytjal, Fodecijal 2019-8129).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Ana Laura Rodríguez-Zapata thanks CONACYT for the undergraduate scholarship (761449) received. Miguel Angel Briano-Elias thanks COECYTJAL for the scholarship received.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carrero, J.C.; Reyes-Lopez, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; Leon-Sicairos, N.; de la Garza, M. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef]
- Shirley, D.T.; Farr, L.; Watanabe, K.; Moonah, S. A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect. Dis. 2018, 5, ofy161. [Google Scholar] [CrossRef]
- Boletin Epidemiológico. Salud Pública Mex. Volume 41. Available online: https://www.gob.mx/cms/uploads/attachment/file/893627/sem06.pdf (accessed on 22 February 2024).
- Penuliar, G.M.; Nakada-Tsukui, K.; Nozaki, T. Phenotypic and transcriptional profiling in Entamoeba histolytica reveal costs to fitness and adaptive responses associated with metronidazole resistance. Front. Microbiol. 2015, 6, 354. [Google Scholar] [CrossRef]
- Powell, R. Inhibition of PI 3-Kinase Signaling Contributes to Metronidazole Resistance in the Protozoan Parasite, Entamoeba histolytica. 2009. Available online: https://tigerprints.clemson.edu/all_theses/684/ (accessed on 22 February 2021).
- Dhand, A.; Snydman, D.R. Mechanism of Resistance in Metronidazole. In Antimicrobial Drug Resistance; Springer: Berlin/Heidelberg, Germany, 2009; pp. 223–227. [Google Scholar] [CrossRef]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018, 17, 420–451. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Wang, C.; Wu, J.; Li, Y.; Zhang, X.; Li, H.; Zhang, H.; Zhou, Y.; Lu, A.; et al. Natural products for infectious microbes and diseases: An overview of sources, compounds, and chemical diversities. Sci. China Life Sci. 2022, 65, 1123–1145. [Google Scholar] [CrossRef] [PubMed]
- Khater, H.; Govindarajan, M.; Benelli, G. Natural Remedies in the Fight Against Parasites; IntechOpen: London, UK, 2017; Chapter 3. EBOOK (PDF); ISBN 978-953-51-4740-4. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Cantun, D.; Ramos-Cassellis, M.E.; Marin-Castro, M.A.; Castelan-Vega, R.D.C. Secondary Metabolites and Antioxidant Activity of the Solid-State Fermentation in Apple (Pirus malus L.) and Agave Mezcalero (Agave angustifolia H.) Bagasse. J. Fungi 2020, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Sharma, R.; Cruz-Martins, N.; Valko, M.; Upadhyay, N.K.; Kuca, K.; Bhardwaj, P. Studies of Phytochemicals, Antioxidant, and Antibacterial Activities of Pinus gerardiana and Pinus roxburghii Seed Extracts. BioMed Res. Int. 2022, 2022, 5938610. [Google Scholar] [CrossRef]
- Yangui, I.; Younsi, F.; Ghali, W.; Boussaid, M.; Messaoud, C. Phytochemicals, antioxidant and anti-proliferative activities of Myrtus communis L. genotypes from Tunisia. S. Afr. J. Bot. 2021, 137, 35–45. [Google Scholar] [CrossRef]
- Rosmalena, R.; Widyastuti, P.A.; Yazid, F.; Ambarwati, N.S.S.; Ahmad, I. Phytochemicals and Antioxidant Activities Evaluation of Origanum vulgare (L.) Stem Bark Extracts. Pharmacogn. J. 2021, 13, 965–970. [Google Scholar] [CrossRef]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253. [Google Scholar] [CrossRef] [PubMed]
- Alducin-Martinez, C.; Ruiz Mondragon, K.Y.; Jimenez-Barron, O.; Aguirre-Planter, E.; Gasca-Pineda, J.; Eguiarte, L.E.; Medellin, R.A. Uses, Knowledge and Extinction Risk Faced by Agave Species in Mexico. Plants 2022, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lemus, A.; Casas, A.; Tellez, O. Distribution, abundance and traditional management of Agave potatorum in the Tehuacan Valley, Mexico: Bases for sustainable use of non-timber forest products. J. Ethnobiol. Ethnomed. 2014, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- García Mendoza, A. Distribution of Agave (Agavaceae) in Mexico. Cactus Suculent J. 2002, 74, 177–187. Available online: https://www.researchgate.net/publication/303263665_Distribution_of_Agave_Agavaceae_in_Mexico (accessed on 22 February 2024).
- Martínez, S.; Nuñez-Guerrero, M.; Gurrola-Reyes, J.N.; Rutiaga-Quiñones, O.M.; Paredes-Ortíz, A.; Soto, O.N.; Flores-Gallegos, A.C.; Rodriguez-Herrera, R. Mescal an Alcoholic Beverage from Agave spp. with Great Commercial Potential. In Alcoholic Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–140. [Google Scholar] [CrossRef]
- Arellano-Plaza, M.; Paez-Lerma, J.B.; Soto-Cruz, N.O.; Kirchmayr, M.R.; Gschaedler Mathis, A. Mezcal Production in Mexico: Between Tradition and Commercial Exploitation. Front. Sustain. Food Syst. 2022, 6, 832532. [Google Scholar] [CrossRef]
- Fonseca Varela, M.; Chalita Tovar, L.E. Financial evaluation of Agave and mezcal production: Case study Caltepec, Puebla. Rev. Mex. Cienc. Agríc. 2021, 12, 263–273. [Google Scholar] [CrossRef]
- Tetreault, D.; McCulligh, C.; Lucio, C. Distilling agro-extractivism: Agave and tequila production in Mexico. J. Agrar. Chang. 2021, 21, 219–241. [Google Scholar] [CrossRef]
- Avila-Gaxiola, E.; Avila-Gaxiola, J.; Velarde-Escobar, O.; Ramos-Brito, F.; Atondo-Rubio, G.; Yee-Rendon, C. Effect of Drying Temperature on Agave tequilana Leaves: A Pretreatment for Releasing Reducing Sugars for Biofuel Production. J. Food Process Eng. 2016, 40, e12455. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; El-Kammar, H.A.; Farag, M.A.; Saleh, D.O.; El Dine, R.S. Metabolomic profiling of five Agave leaf taxa via UHPLC/PDA/ESI-MS inrelation to their anti-inflammatory, immunomodulatory and ulceroprotective activities. Steroids 2020, 160, 108648. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Valle, E.; Herrera-Ruiz, M.; Salgado, G.R.; Zamilpa, A.; Ocampo, M.L.; Aparicio, A.J.; Tortoriello, J.; Jimenez-Ferrer, E. Anti-inflammatory effect of 3-O-[(6′-O-palmitoyl)-beta-D-glucopyranosyl sitosterol] from Agave angustifolia on ear edema in mice. Molecules 2014, 19, 15624–15637. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Nava, Z.J.; Jimenez-Aparicio, A.R.; Herrera-Ruiz, M.L.; Jimenez-Ferrer, E. Immunomodulatory Effect of Agave tequilana Evaluated on an Autoimmunity Like-SLE Model Induced in Balb/c Mice with Pristane. Molecules 2017, 22, 848. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; Jimenez-Ferrer, E.; Tortoriello, J.; Zamilpa, A.; Alegria-Herrera, E.; Jimenez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Martinez-Duncker, I.; Monterrosas-Brisson, N. Anti-neuroinflammatory effect of Agaves and cantalasaponin-1 in a model of LPS-induced damage. Nat. Prod. Res. 2021, 35, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Santos Cerqueira, G.; dos Santos e Silva, G.; Rios Vasconcelos, E.; Fragoso de Freitas, A.P.; Arcanjo Moura, B.; Silveira Macedo, D.; Lopes Souto, A.; Barbosa Filho, J.M.; de Almeida Leal, L.K.; de Castro Brito, G.A.; et al. Effects of hecogenin and its possible mechanism of action on experimental models of gastric ulcer in mice. Eur. J. Pharmacol. 2012, 683, 260–269. [Google Scholar] [CrossRef]
- Araldi, R.P.; Dos Santos, M.O.; Barbon, F.F.; Manjerona, B.A.; Meirelles, B.R.; de Oliva Neto, P.; da Silva, P.I.J.; Dos Santos, L.; Camargo, I.C.C.; de Souza, E.B. Analysis of antioxidant, cytotoxic and mutagenic potential of Agave sisalana Perrine extracts using Vero cells, human lymphocytes and mice polychromatic erythrocytes. Biomed. Pharmacother. 2018, 98, 873–885. [Google Scholar] [CrossRef]
- Singh, S.K.; Bimal, S.; Narayan, S.; Jee, C.; Bimal, D.; Das, P.; Bimal, R. Leishmania donovani: Assessment of leishmanicidal effects of herbal extracts obtained from plants in the visceral leishmaniasis endemic area of Bihar, India. Exp. Parasitol. 2011, 127, 552–558. [Google Scholar] [CrossRef]
- Botura, M.B.; Silva, G.D.; Lima, H.G.; Oliveira, J.V.; Souza, T.S.; Santos, J.D.; Branco, A.; Moreira, E.L.; Almeida, M.A.; Batatinha, M.J. In vivo anthelmintic activity of an aqueous extract from sisal waste (Agave sisalana Perr.) against gastrointestinal nematodes in goats. Vet. Parasitol. 2011, 177, 104–110. [Google Scholar] [CrossRef]
- Botura, M.B.; dos Santos, J.D.; da Silva, G.D.; de Lima, H.G.; de Oliveira, J.V.; de Almeida, M.A.; Batatinha, M.J.; Branco, A. In vitro ovicidal and larvicidal activity of Agave sisalana Perr. (sisal) on gastrointestinal nematodes of goats. Vet. Parasitol. 2013, 192, 211–217. [Google Scholar] [CrossRef]
- Guerra, J.O.; Meneses, A.; Simonet, A.M.; Macias, F.A.; Nogueiras, C.; Gomez, A.; Escario, J.A. Steroidal saponins from the plant Agave brittoniana with activity against the parasite Trichomona vaginalis. Rev. Biol. Trop. 2008, 56, 1645–1652. [Google Scholar]
- Lopez-Romero, J.C.; Ayala-Zavala, J.F.; Pena-Ramos, E.A.; Hernandez, J.; Gonzalez-Rios, H. Antioxidant and antimicrobial activity of Agave angustifolia extract on overall quality and shelf life of pork patties stored under refrigeration. J. Food Sci. Technol. 2018, 55, 4413–4423. [Google Scholar] [CrossRef]
- Monterrosas-Brisson, N.; Ocampo, M.L.; Jimenez-Ferrer, E.; Jimenez-Aparicio, A.R.; Zamilpa, A.; Gonzalez-Cortazar, M.; Tortoriello, J.; Herrera-Ruiz, M. Anti-inflammatory activity of different Agave plants and the compound cantalasaponin-1. Molecules 2013, 18, 8136–8146. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Mata-Cardenas, B.D.; Vargas-Villarreal, J.; Bazaldua-Rodriguez, A.F.; Kavimngeles-Hernandez, I.; Garza-Gonzalez, J.N.; Hernandez-Garcia, M.E. Antiprotozoal activity against Entamoeba histolytica of plants used in northeast Mexican traditional medicine. Bioactive compounds from Lippia graveolens and Ruta chalepensis. Molecules 2014, 19, 21044–21065. [Google Scholar] [CrossRef] [PubMed]
- Anairis Pujol Garcia, B.T.; Salas, E.; Calzadilla, C.; Acevedo, R.; Sierra, G. Tamizaje fitoquímico de extractos obtenidos de la planta Sapindus saponaria L que crece en Cuba. Bionatura 2020, 5. [Google Scholar] [CrossRef]
- Pant, D.R.; Pant, N.D.; Saru, D.B.; Yadav, U.N.; Khanal, D.P. Phytochemical screening and study of antioxidant, antimicrobial, antidiabetic, anti-inflammatory and analgesic activities of extracts from stem wood of Pterocarpus marsupium Roxburgh. J. Intercult. Ethnopharmacol. 2017, 6, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.F.; Garms, R. The detection of plant sugars in Simulium damnosum s.l. by means of the cold Anthrone test. Trans. R. Soc. Trop. Med. Hyg. 1980, 74, 811–813. [Google Scholar] [CrossRef]
- Leandro Machado, R.; Nikolai, S. Fundamentos de Tecnología de Fitofármacos y Productos Naturales; Editorial Secretaría Ejecutiva del Convenio Andrés Bello, Impreso, Idioma Español: Madrid, Spain, 1999; ISBN 13978-958-698-001-2. [Google Scholar]
- Domínguez, S.; Xorge, A. Métodos de Investigación Fitoquímica. Editor México: Centro Regional de Ayuda Técnica, 1973. 281p. Materia Química Vegetal. Disponible, Ciencias Agronómicas y Forestales Colección General; 547 D671. Impreso, idioma Español. Available online: https://bibliotecadigital.uchile.cl/permalink/56UDC_INST/1rhgcaj/alma991002648959703936 (accessed on 22 February 2024).
- Diamond, L.S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica like amebae. J. Parasitol. 1968, 54, 1047–1056. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV-vis and FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef]
- Mukadam, M.; Khan, N.A.M.; Khan, S.; Kauchali, A. Uv-Vis Spectroscopy in Analysis of Phytochemicals. Int. J. Pharm. Res. Appl. 2021, 6, 482–499. [Google Scholar]
- Thouri, A.; Chahdoura, H.; El Arem, A.; Omri Hichri, A.; Ben Hassin, R.; Achour, L. Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti). BMC Complement. Altern. Med. 2017, 17, 248. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Mabasa, X.E.; Mathomu, L.M.; Madala, N.E.; Musie, E.M.; Sigidi, M.T. Molecular Spectroscopic (FTIR and UV-Vis) and Hyphenated Chromatographic (UHPLC-qTOF-MS) Analysis and In Vitro Bioactivities of the Momordica balsamina Leaf Extract. Biochem. Res. Int. 2021, 2021, 2854217. [Google Scholar] [CrossRef]
- Hernandez, V.; Botella, M.A.; Hellin, P.; Cava, J.; Fenoll, J.; Mestre, T.; Martinez, V.; Flores, P. Phenolic and Carotenoid Profile of Lamb’s Lettuce and Improvement of the Bioactive Content by Preharvest Conditions. Foods 2021, 10, 188. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Effect of Impregnation of Biodegradable Polyesters with Polyphenols from Cistus linnaeus and Juglans regia Linnaeus Walnut Green Husk. Polymers 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hao, Z.; Li, M. Isolation and Structure Identification of Flavonoids. In Flavonoids—From Biosynthesis to Human Health; Chapter 2; Justino, G.C., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Quinones-Munoz, T.A.; Villanueva-Rodriguez, S.J.; Torruco-Uco, J.G. Nutraceutical Properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L.: A Review of Metabolites and Mechanisms. Metabolites 2022, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Biondi, F.; Balducci, F.; Capocasa, F.; Visciglio, M.; Mei, E.; Vagnoni, M.; Mezzetti, B.; Mazzoni, L. Environmental Conditions and Agronomical Factors Influencing the Levels of Phytochemicals in Brassica Vegetables Responsible for Nutritional and Sensorial Properties. Appl. Sci. 2021, 11, 1927. [Google Scholar] [CrossRef]
- Bjorkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Radácsi, P.; Gosztola, B.; Németh, É.Z. Effects of temperature and light intensity on morphological and phytochemical characters and antioxidant potential of wormwood (Artemisia absinthium L.). Biochem. Syst. Ecol. 2018, 79, 1–7. [Google Scholar] [CrossRef]
- Ahumada-Santos, Y.P.; Montes-Avila, J.; Uribe-Beltrán, M.d.J.; Díaz-Camacho, S.P.; López-Angulo, G.; Vega-Aviña, R.; López-Valenzuela, J.Á.; Heredia, J.B.; Delgado-Vargas, F. Chemical characterization, antioxidant and antibacterial activities of six Agave species from Sinaloa, Mexico. Ind. Crops Prod. 2013, 49, 143–149. [Google Scholar] [CrossRef]
- Almazán-Morales, A.; Moreno-Godínez, M.E.; Hernández-Castro, E.; Vázquez-Villamar, M.; Mora-Aguilera, J.A.; Cabrera-Huerta, E.; Alvarez-Fitz, P. Phytochemical profile and in vitro activity of Agave angustifolia and A. cupreata extracts against phytopathogenic fungi. Rev. Mex. De Fitopatol. Mex. J. Phytopathol. 2022, 40, 169–187. [Google Scholar] [CrossRef]
- Pereira, G.M.; Ribeiro, M.G.; da Silva, B.P.; Parente, J.P. Structural characterization of a new steroidal saponin from Agave angustifolia var. Marginata and a preliminary investigation of its in vivo antiulcerogenic activity and in vitro membrane permeability property. Bioorg. Med. Chem. Lett. 2017, 27, 4345–4349. [Google Scholar] [CrossRef]
- Bermudez-Bazan, M.; Castillo-Herrera, G.A.; Urias-Silvas, J.E.; Escobedo-Reyes, A.; Estarron-Espinosa, M. Hunting Bioactive Molecules from the Agave Genus: An Update on Extraction and Biological Potential. Molecules 2021, 26, 6789. [Google Scholar] [CrossRef]
- Martinez-Castillo, M.; Pacheco-Yepez, J.; Flores-Huerta, N.; Guzman-Tellez, P.; Jarillo-Luna, R.A.; Cardenas-Jaramillo, L.M.; Campos-Rodriguez, R.; Shibayama, M. Flavonoids as a Natural Treatment against Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2018, 8, 209. [Google Scholar] [CrossRef]
- Arrieta, J.; Reyes, B.; Calzada, F.; Cedillo-Rivera, R.; Navarrete, A. Amoebicidal and giardicidal compounds from the leaves of Zanthoxylum liebmannianun. Fitoterapia 2001, 72, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.V.; Santos, F.O.; Lima, H.G.; Silva, G.D.D.; Uzeda, R.S.; Dias, E.R.; Branco, A.; Cardoso, K.V.; David, J.M.; Botura, M.B.; et al. In Vitro ovicidal and larvicidal activities of some saponins and flavonoids against parasitic nematodes of goats. Parasitology 2018, 145, 1884–1889. [Google Scholar] [CrossRef]
- Fleck, J.D.; Betti, A.H.; da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. Molecules 2019, 24, 171. [Google Scholar] [CrossRef]
- Oranday, C.R.M.; Morales, V.; Mata, C.; Gutiérrez, G.J.J. Determinación de la Concentración Inhibitoria Media (CI50) del Extracto Etanólico Obtenido del Agave Lophantha Sobre el Crecimiento in vitro de Entamoeba Histolytica, Trichomonas Vaginalis y Giardia Lamblia; Repositorio Universidad Autónoma de Nuevo León: San Nicolás de los Garza, México, 2002; TLRC119.G8. 1080124437. [Google Scholar]
- Soto, J.; Gomez, C.; Calzada, F.; Ramirez, M.E. Ultrastructural changes on Entamoeba histolytica HM1-IMSS caused by the flavan-3-ol, (-)-epicatechin. Planta Med. 2010, 76, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, V.; Diaz-Martinez, A.; Soto, J.; Marchat, L.A.; Sanchez-Monroy, V.; Ramirez-Moreno, E. Kaempferol inhibits Entamoeba histolytica growth by altering cytoskeletal functions. Mol. Biochem. Parasitol. 2015, 204, 16–25. [Google Scholar] [CrossRef]
- Maugis, B.; Brugues, J.; Nassoy, P.; Guillen, N.; Sens, P.; Amblard, F. Dynamic instability of the intracellular pressure drives bleb-based motility. J. Cell Sci. 2010, 123, 3884–3892. [Google Scholar] [CrossRef]
- Sierra-Lopez, F.; Baylon-Pacheco, L.; Espiritu-Gordillo, P.; Lagunes-Guillen, A.; Chavez-Munguia, B.; Rosales-Encina, J.L. Influence of Micropatterned Grill Lines on Entamoeba histolytica Trophozoites Morphology and Migration. Front. Cell. Infect. Microbiol. 2018, 8, 295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).