Exploring the Potential of Nanoparticles in the Treatment of Breast Cancer: Current Applications and Future Directions
Abstract
Featured Application
Abstract
1. Introduction
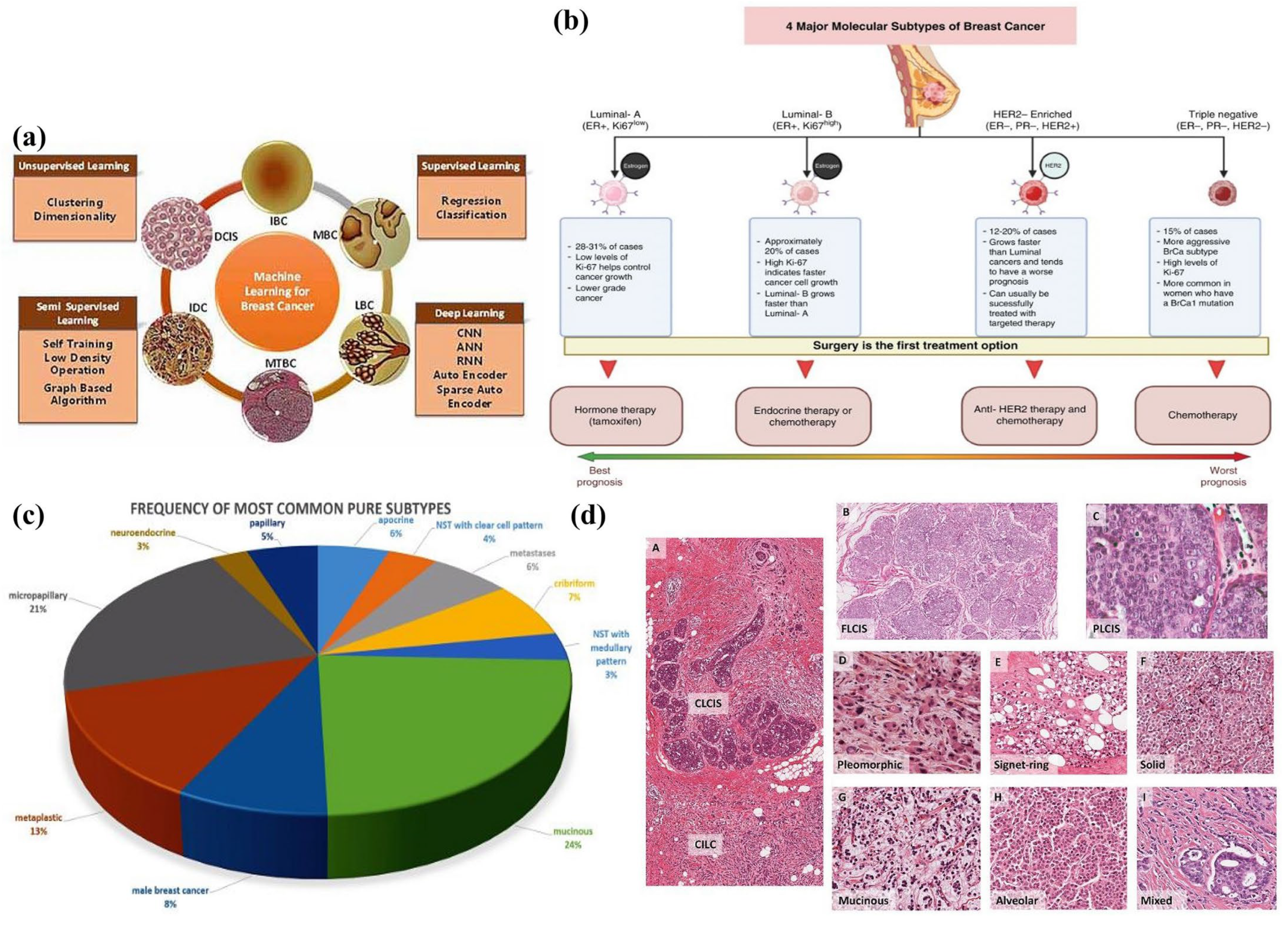
2. Classification of Breast Cancer by Histological, Molecular, and Morphological Characteristics
2.1. Histopathological Classification of Breast Cancer
- Medullary carcinoma is characterized by its slow growth, featuring soft and fleshy tumors that bear a resemblance to the medulla of the brain. This subtype accounts for less than 1% of all breast cancers [18];
- Tubular carcinoma is a rare histological subtype of invasive ductal carcinoma, constituting 1% to 5% of all invasive breast cancers. These tumors exhibit slow growth and are characterized by cancer cells with tube-like structures [19]. TC is recognized as a well-differentiated invasive carcinoma and is further classified into two categories: pure TC and mixed TC. Pure TC refers to tumors with a tubular content of more than 90% and a low nuclear grade, with few-to-no mitoses [20]. On the other hand, mixed TC has a tubular composition of less than 75% [20];
- Mucinous carcinoma is characterized by low-grade tumors composed of cancer cells that are situated within the mucin substance found in mucus. This subtype accounts for fewer than 2% of all breast cancers [21];
- Papillary carcinomas are tiny cancerous cells that have finger-like appendages. Less than 1% of all breast cancers are papillary carcinomas, making them extremely uncommon [22];
- Cribriform carcinoma is characterized as an unusual subtype with a Swiss cheese-like pattern of holes. This type of cancer accounts for fewer than 1% of breast cancer cases [23];
- Adenoid cystic carcinoma: In contrast to typical ductal cancer cells, adenoid cystic carcinoma resembles cancerous cells found in the salivary gland. This subtype constitutes less than 1% of all breast cancers [24];
- Metaplastic carcinoma: This takes place when ductal cells transform into new cell types. Less than 1% of all breast cancers are metaplastic carcinomas, which are typically more aggressive cancers [25].
2.2. Molecular Classification of Breast Cancer
3. Traditional Methods for Breast Cancer Treatment
4. Nanotechnology and Cancer
4.1. Nanoparticles in Cancer Therapy
4.2. Targeting Strategies
4.3. Targeted Drug Delivery
5. Therapeutic Properties of Nanoparticles in Breast Cancer Treatment
5.1. pH-Responsive NPs
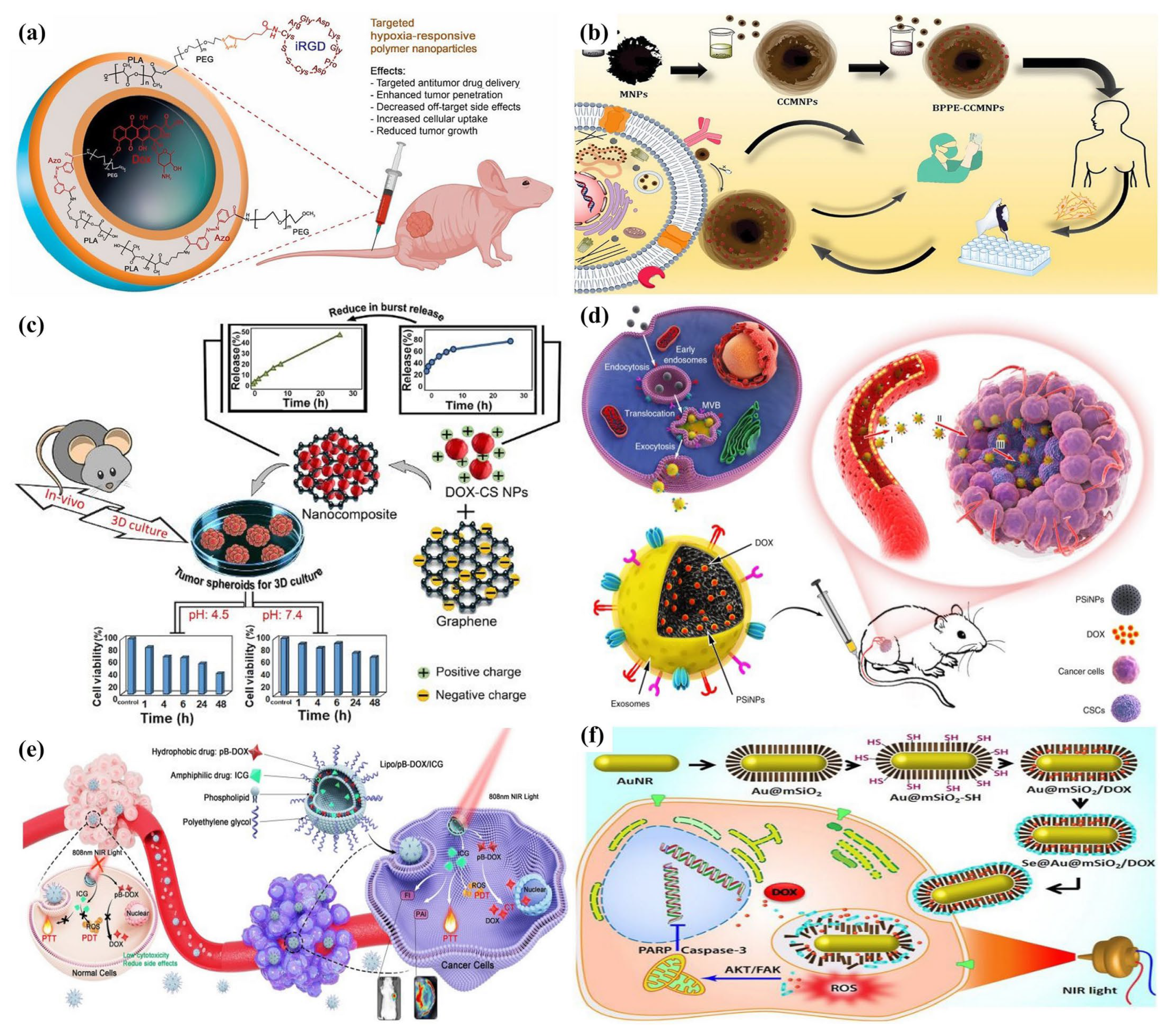
5.2. Temperature-Sensitive Nanoparticles
5.3. Enzyme-Responsive Nanoparticles
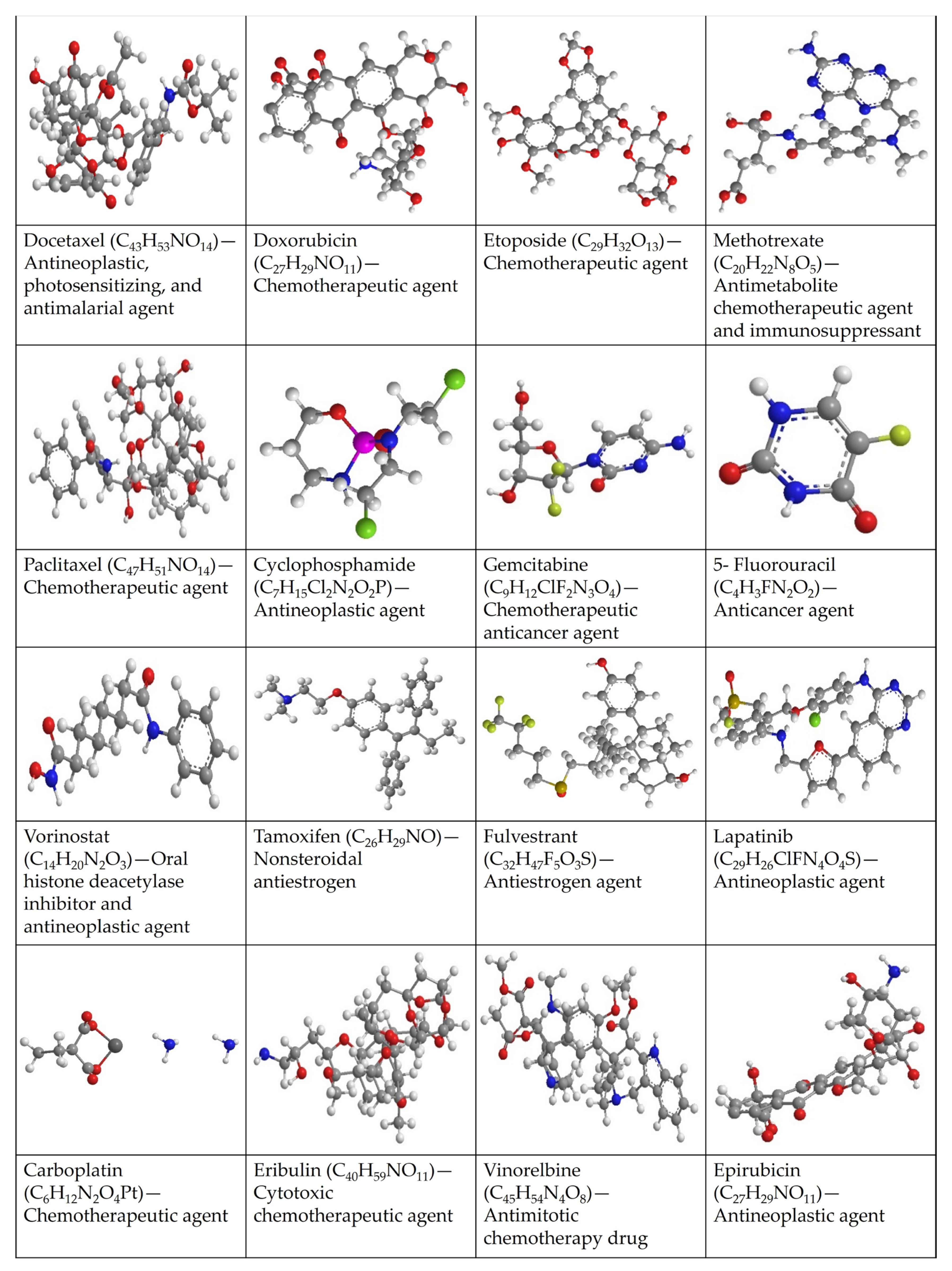
6. Properties of Breast Cancer Drugs
7. Nanomedicine to Enhance the Therapeutic Effectiveness of BC
7.1. Nanoparticle-Mediated Drug Delivery
7.1.1. Liposomal Nanocarriers
7.1.2. Solid Lipid Nanoparticles
7.1.3. Other Polymeric Nanoparticles
7.1.4. Carbon-Based Nanoparticles
7.1.5. Other Novel Nanoparticles
7.2. Nanotechnology to Enhance Immunotherapy for BC Treatment
7.3. Nanotechnology-Augmented Gene Therapy for BC Treatment
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA—A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA—A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Ben-Dror, J.; Shalamov, M.; Sonnenblick, A. The History of Early Breast Cancer Treatment. Genes 2022, 13, 960. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. Breast Cancer Treatment (PDQ®). 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65969/ (accessed on 6 December 2023).
- Dissanayake, R.; Towner, R.; Ahmed, M. Metastatic Breast Cancer: Review of Emerging Nanotherapeutics. Cancers 2023, 15, 2906. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Michaeli, D.T.; Michaeli, T. Overall survival, progression-free survival, and tumor response benefit supporting initial US food and drug administration approval and indication extension of new cancer drugs, 2003–2021. J. Clin. Oncol. 2022, 40, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- Goransson, S.; Chen, S.; Olofsson, H.; Larsson, O.; Stromblad, S. An extracellular matrix stiffness-induced breast cancer cell transcriptome resembles the transition from ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC). Biochem. Biophys. Res. Commun. 2023, 654, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, N.; Fehm, T.; Banys-Paluchowski, M. DCIS in Male and Aged Women with Comorbidities. Chirurgia 2021, 116, S120–S127. [Google Scholar] [CrossRef] [PubMed]
- Rodin, D.; Sutradhar, R.; Nofech-Mozes, S.; Gu, S.M.; Faught, N.; Hahn, E.; Fong, C.; Trebinjac, S.; Paszat, L.; Rakovitch, E. Long-term outcomes of women with large DCIS lesions treated with breast-conserving therapy. Breast Cancer Res. Treat. 2022, 192, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Liu, L.; Hong, S.; Ahmed, H. Prediction of Breast Cancer, Comparative Review of Machine Learning Techniques, and Their Analysis. IEEE Access 2020, 8, 150360–150376. [Google Scholar] [CrossRef]
- Girithar, H.N.; Pires, A.S.; Ahn, S.B.; Guillemin, G.J.; Gluch, L.; Heng, B. Involvement of the kynurenine pathway in breast cancer: Updates on clinical research and trials. Br. J. Cancer 2023, 129, 185–203. [Google Scholar] [CrossRef]
- Rechsteiner, A.; Dietrich, D.; Varga, Z. Prognostic relevance of mixed histological subtypes in invasive breast carcinoma: A retrospective analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 4967–4978. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.M.; Kalinowski, L.; Simpson, P.T.; Lakhani, S.R. Invasive lobular carcinoma of the breast: The increasing importance of this special subtype. Breast Cancer Res. 2021, 23, 6. [Google Scholar] [CrossRef]
- Takada, K.; Kashiwagi, S.; Asano, Y.; Goto, W.; Morisaki, T.; Takahashi, K.; Fujita, H.; Takashima, T.; Tomita, S.; Hirakawa, K.; et al. Factors predictive of invasive ductal carcinoma in cases preoperatively diagnosed as ductal carcinoma in situ. BMC Cancer 2020, 20, 513. [Google Scholar] [CrossRef]
- Aihara, T.; Kumamaru, H.; Ishitobi, M.; Miyashita, M.; Miyata, H.; Tamura, K.; Yoshida, M.; Ogo, E.; Nagahashi, M.; Asaga, S.; et al. Prognosis and effectiveness of chemotherapy for medullary breast carcinoma. Breast Cancer Res. Treat. 2022, 196, 635–645. [Google Scholar] [CrossRef]
- Nakagawa, T.; Oda, G.; Mori, H.; Uemura, N.; Onishi, I.; Sagawa, N.; Fujioka, T.; Mori, M.; Kubota, K.; Ishikawa, T.; et al. Prognosis of Subcutaneous Mastectomy for Special Types of Breast Cancer. Med.-Lith. 2022, 58, 112. [Google Scholar] [CrossRef]
- Min, Y.; Bae, S.Y.; Lee, H.C.; Lee, J.H.; Kim, M.; Kim, J.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Lee, J.E.; et al. Tubular Carcinoma of the Breast: Clinicopathologic Features and Survival Outcome Compared with Ductal Carcinoma In Situ. J. Breast Cancer 2013, 16, 404–409. [Google Scholar] [CrossRef][Green Version]
- Tsoukalas, N.; Kiakou, M.; Tolia, M.; Kostakis, I.D.; Galanopoulos, M.; Nakos, G.; Tryfonopoulos, D.; Kyrgias, G.; Koumakis, G. Mucinous breast carcinoma with tall columnar cells. Ann. R. Coll. Surg. Engl. 2018, 100, E132–E135. [Google Scholar] [CrossRef]
- Arif, F.Z.; Breese, R.O.; Friend, K. Tall Cell Variant of Invasive Papillary Breast Carcinoma. Am. Surg. 2023, 89, 3875–3876. [Google Scholar] [CrossRef]
- Xia, L.Q.; Chen, X. Invasive cribriform carcinoma of the breast: A case report. Asian J. Surg. 2023, 46, 1130–1131. [Google Scholar] [CrossRef]
- Samar, M.R.; Khan, W.; Mooghal, M.; Anjum, S.; Mohammad, A.T.V.; Vohra, L.M. Breast adenoid cystic carcinoma: An uncommon neoplasm- Case report. Int. J. Surg. Case Rep. 2023, 107, 108333. [Google Scholar] [CrossRef]
- Tower, A.; Hughes, J.; Moore, L.; Srivastava, K. Mixed metaplastic carcinoma of the breast: A case report. J. Surg. Case Rep. 2023, 2023, rjad144. [Google Scholar] [CrossRef]
- Goetz, M.P.; Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D. NCCN guidelines insights: Breast cancer, Version 3.2018: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bulut, N.; Ulas, A.; Altundag, K.; Altundag, K.; Bulut, N.; Ulas, A.; Bulut, N. Histopathological Characteristics, Clinical Course, and 10-year Follow-up Results of Breast Cancer Subtypes with Regard to ER (+)(−)/PR (+)(−) Receptor Status. Available online: https://www.researchgate.net/profile/Kadri-Altundag/publication/311949386_Histopathological_characteristics_clinical_course_and_10-_year_follow-up_results_of_breast_cancer_subtypes_with_regard_to_ER_-PR_-_receptor_status_Short_title_Clinic_course_in_subtypes_of_breast_cance/links/5864253408aebf17d3974378/Histopathological-characteristics-clinical-course-and-10-year-follow-up-results-of-breast-cancer-subtypes-with-regard-to-ER-PR-receptor-status-Short-title-Clinic-course-in-subtypes-of-breast.pdf (accessed on 30 December 2016).
- Subramaniam, S.; Bhoo-Pathy, N.; Taib, N.; Tan, G.; See, M.; Jamaris, S.; Ho, G.; Looi, L.; Yip, C. Breast cancer outcomes as defined by the estrogen receptor, progesterone receptor, and human growth factor receptor-2 in a multi-ethnic asian country. World J. Surg. 2015, 39, 2450–2458. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Hernando, C.; Ortega-Morillo, B.; Tapia, M.; Moragon, S.; Martinez, M.T.; Eroles, P.; Garrido-Cano, I.; Adam-Artigues, A.; Lluch, A.; Bermejo, B.; et al. Oral Selective Estrogen Receptor Degraders (SERDs) as a Novel Breast Cancer Therapy: Present and Future from a Clinical Perspective. Int. J. Mol. Sci. 2021, 22, 7812. [Google Scholar] [CrossRef]
- Yu, N.Y.; Iftimi, A.; Yau, C.; Tobin, N.P.; van ‘t Veer, L.; Hoadley, K.A.; Benz, C.C.; Nordenskjold, B.; Fornander, T.; Stal, O.; et al. Assessment of Long-term Distant Recurrence-Free Survival Associated With Tamoxifen Therapy in Postmenopausal Patients With Luminal A or Luminal B Breast Cancer. JAMA Oncol. 2019, 5, 1304–1309. [Google Scholar] [CrossRef]
- Goncalves, A.; Finetti, P.; Birnbaum, D.; Bertucci, F. The CINSARC signature predicts the clinical outcome in patients with Luminal B breast cancer. NPJ Breast Cancer 2021, 7, 48. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, Y.X.; Huang, Y.; Chen, Z.J.; Zhang, H.Z.; Yu, Y.; Wang, X.; Cao, X.C. The regrouping of Luminal B (HER2 negative), a better discriminator of outcome and recurrence score. Cancer Med. 2022, 12, 2493–2504. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef]
- Shen, M.Y.; Pan, H.W.; Chen, Y.X.; Xu, Y.H.; Yang, W.X.; Wu, Z.J. A review of current progress in triple-negative breast cancer therapy. Open Med. 2020, 15, 1143–1149. [Google Scholar] [CrossRef]
- Lukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanislawek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Sakach, E.; O’Regan, R.; Meisel, J.; Li, X.X. Molecular Classification of Triple Negative Breast Cancer and the Emergence of Targeted Therapies. Clin. Breast Cancer 2021, 21, 509–520. [Google Scholar] [CrossRef]
- Chu, C.R.K.; Bedrosian, I. Prophylactic Mastectomy and Breast Reconstruction in Patients at High Risk for Breast Cancer. Curr. Breast Cancer Rep. 2020, 12, 13–20. [Google Scholar] [CrossRef]
- Pandit, S.; Sapkota, S.; Suwal, S.; Adhikari, A.; Karki, P.; Jha, A.K. Interstitial brachytherapy for internal mammary node in breast cancer: A case report. J. Contemp. Brachyther. 2023, 15, 229–233. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Patel, A.P. Passive targeting of nanoparticles to cancer. Surf. Modif. Nanopart. Target. Drug Deliv. 2019, 2019, 125–143. [Google Scholar]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- Felice, B.; Prabhakaran, M.P.; Rodríguez, A.P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C 2014, 41, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer nanotechnology: A new revolution for cancer diagnosis and therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Vaez, A.; Abbasi, M.; Shabani, L.; Azizipour, E.; Shafiee, M.; Zare, M.A.; Rahbar, O.; Azari, A.; Amani, A.M.; Golchin, A. A Bright Horizon of Intelligent Targeted-cancer Therapy: Nanoparticles Against Breast Cancer Stem Cells. Curr. Stem Cell Res. Ther. 2023, 18, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, M. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. 2018, 38, 25–40. [Google Scholar] [CrossRef]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Ganesan, K.; Wang, Y.; Gao, F.; Liu, Q.; Zhang, C.; Li, P.; Zhang, J.; Chen, J. Targeting engineered nanoparticles for breast cancer therapy. Pharmaceutics 2021, 13, 1829. [Google Scholar] [CrossRef]
- Ulldemolins, A.; Seras-Franzoso, J.; Andrade, F.; Rafael, D.; Abasolo, I.; Gener, P.; Schwartz, S., Jr. Perspectives of nano-carrier drug delivery systems to overcome cancer drug resistance in the clinics. Cancer Drug Resist. 2021, 4, 44. [Google Scholar] [CrossRef]
- De Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Grumezescu, A.; Holban, A.M. Materials for Biomedical Engineering: Organic Micro and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef] [PubMed]
- Hirsjarvi, S.; Passirani, C.; Benoit, J.-P. Passive and active tumour targeting with nanocarriers. Curr. Drug Discov. Technol. 2011, 8, 188–196. [Google Scholar] [CrossRef]
- Ejigah, V.; Owoseni, O.; Bataille-Backer, P.; Ogundipe, O.D.; Fisusi, F.A.; Adesina, S.K. Approaches to improve macromolecule and nanoparticle accumulation in the tumor microenvironment by the enhanced permeability and retention effect. Polymers 2022, 14, 2601. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Mirza, Z.; Karim, S. Nanoparticles-Based Drug Delivery and Gene Therapy for Breast Cancer: Recent Advancements and Future Challenges. Semin. Cancer Biol. 2021, 69, 226–237. [Google Scholar]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Yadav, P.; Jain, J.; Sherje, A.P. Recent advances in nanocarriers-based drug delivery for cancer therapeutics: A review. React. Funct. Polym. 2021, 165, 104970. [Google Scholar] [CrossRef]
- Vordos, N.; Gkika, D.A.; Pradakis, N.; Mitropoulos, A.C.; Kyzas, G.Z. Therapeutic and Diagnostic Potential of Nanomaterials for Enhanced Biomedical Applications. In Advanced and Innovative Approaches of Environmental Biotechnology in Industrial Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 277–300. [Google Scholar]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Akhoundi Kharanaghi, E.; Mohammadnejad Arough, J.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and Innate Immunity: New Perspectives on Host Defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar]
- Parks, S.K.; Mueller-Klieser, W.; Pouysségur, J. Lactate and acidity in the cancer microenvironment. Annu. Rev. Cancer Biol. 2020, 4, 141–158. [Google Scholar] [CrossRef]
- Li, J.P.; Wang, Y.S.; Xu, C.Q.; Yu, Q.W.; Wang, X.H.; Xie, H.B.; Tian, L.F.; Qiu, Y.; Guo, R.; Lu, Z.Z.; et al. Rapid pH-responsive self-disintegrating nanoassemblies balance tumor accumulation and penetration for enhanced anti-breast cancer therapy. Acta Biomater. 2021, 134, 546–558. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, X.Y.; Zhang, G.Y.; Li, J.N.; Liu, Y.; Yan, X. Novel targeted pH-responsive drug delivery systems based on PEGMA-modified bimetallic Prussian blue analogs for breast cancer chemotherapy. RSC Adv. 2023, 13, 1684–1700. [Google Scholar] [CrossRef]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Qiao, L.N.; Zhang, S.P.; Wan, G.Y.; Chen, B.W.; Zhou, P.; Zhang, N.; Wang, Y.S. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Ghosh, N.; Kundu, M.; Ghosh, S.; Das, A.K.; De, S.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of chrysin via folic acid-functionalized mesoporous silica nanocarrier for breast cancer therapy. Int. J. Pharm. 2023, 631, 122555. [Google Scholar] [CrossRef]
- Mamnoon, B.; Loganathan, J.; Confeld, M.I.; De Fonseka, N.; Feng, L.; Froberg, J.; Choi, Y.; Tuvin, D.M.; Sathish, V.; Mallik, S. Targeted Polymeric Nanoparticles for Drug Delivery to Hypoxic, Triple-Negative Breast Tumors. Acs Appl. Bio Mater. 2021, 4, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Taherian, A.; Esfandiari, N.; Rouhani, S. Breast cancer drug delivery by novel drug-loaded chitosan-coated magnetic nanoparticles. Cancer Nanotechnol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Gooneh-Farahani, S.; Naghib, S.M.; Naimi-Jamal, M.R.; Seyfoori, A. A pH-sensitive nanocarrier based on BSA-stabilized graphene-chitosan nanocomposite for sustained and prolonged release of anticancer agents. Sci. Rep. 2021, 11, 17404. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.Y.; Zhang, X.Q.; Bie, N.N.; Zhang, H.B.; Zhang, X.T.; Li, F.Y.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.X.; Lu, W.X.; Liu, F.; Zhang, G.Q.; Xie, F.F.; Liu, W.J.; Wang, L.; Zhou, W.H.; Cheng, Z.N. ROS-responsive liposomes with NIR light-triggered doxorubicin release for combinatorial therapy of breast cancer. J. Nanobiotechnol. 2021, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Ruttala, H.B.; Sundaramoorthy, P.; Poudel, B.K.; Youn, Y.S.; Ku, S.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Multimodal selenium nanoshell-capped Au@mSiO(2) nanoplatform for NIR-responsive chemo-photothermal therapy against metastatic breast cancer. NPG Asia Mater. 2018, 10, 197–216. [Google Scholar] [CrossRef]
- Cen, J.Q.; Huang, Y.Q.; Liu, J.; Liu, Y.A. Thermo-responsive palladium-ruthenium nanozyme synergistic photodynamic therapy for metastatic breast cancer management. J. Mater. Chem. B 2022, 10, 10027–10041. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, Y.; Li, C.; Yang, C.; Xu, H.; Liang, Q.; Zhou, Y.; Zhang, L.; He, Y.; Tong, H.; et al. Gold-promoting-satellite to boost photothermal conversion efficiency of Cu2-xSe for triple-negative breast cancer targeting therapy. Mater. Today Nano 2022, 18, 100211. [Google Scholar] [CrossRef]
- Dorjsuren, B.; Chaurasiya, B.; Ye, Z.X.; Liu, Y.Y.; Li, W.; Wang, C.Y.; Shi, D.; Evans, C.E.; Webster, T.J.; Shen, Y. Cetuximab-Coated Thermo-Sensitive Liposomes Loaded with Magnetic Nanoparticles and Doxorubicin for Targeted EGFR-Expressing Breast Cancer Combined Therapy. Int. J. Nanomed. 2020, 15, 8201–8215. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Li, J.J.; Hu, Y.C.; Gao, F.; Leung, G.P.H.; Geng, F.N.; Fu, C.M.; Zhang, J.M. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on “two strikes” effects. Acta Pharm. Sin. B 2020, 10, 2227–2245. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; De Giorgi, M.L.; Leporatti, S.; Rinaldi, R. Encapsulation of Thermo-Sensitive Lauric Acid in Silica Shell: A Green Derivate for Chemo-Thermal Therapy in Breast Cancer Cell. Molecules 2019, 24, 2034. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Khizar, S.; Zine, N.; Errachid, A.; Elaissari, A. Introduction to Stimuli-Responsive Materials and Their Biomedical Applications. In Stimuli-Responsive Materials for Biomedical Applications; ACS Publications: Washington, DC, USA, 2023; pp. 1–30. [Google Scholar]
- Moll, U.M.; Youngleib, G.L.; Rosinski, K.B.; Quigley, J.P. Tumor promoter-stimulated M r 92,000 gelatinase secreted by normal and malignant human cells: Isolation and characterization of the enzyme from HT1080 tumor cells. Cancer Res. 1990, 50, 6162–6170. [Google Scholar]
- Aslzad, S.; Heydari, P.; Abdolahinia, E.D.; Amiryaghoubi, N.; Safary, A.; Fathi, M.; Erfan-Niya, H. Chitosan/gelatin hybrid nanogel containing doxorubicin as enzyme-responsive drug delivery system for breast cancer treatment. Colloid Polym. Sci. 2023, 301, 273–281. [Google Scholar] [CrossRef]
- Xiao, P.F.; Tao, X.G.; Wang, H.X.; Liu, H.B.; Feng, Y.P.; Zhu, Y.Q.; Jiang, Z.Z.; Yin, T.; Zhang, Y.; He, H.B.; et al. Enzyme/pH dual stimuli-responsive nanoplatform co-deliver disulfiram and doxorubicin for effective treatment of breast cancer lung metastasis. Expert Opin. Drug Deliv. 2023, 20, 1015–1031. [Google Scholar] [CrossRef]
- Sun, Y.A.; Lyu, B.; Yang, C.; He, B.; Zhang, H.; Wang, X.Q.; Zhang, Q.; Dai, W.B. An enzyme-responsive and transformable PD-L1 blocking peptide-photosensitizer conjugate enables efficient photothermal immunotherapy for breast cancer. Bioact. Mater. 2023, 22, 47–59. [Google Scholar] [CrossRef]
- Fernando, I.R.; Ferris, D.P.; Frasconi, M.; Malin, D.; Strekalova, E.; Yilmaz, M.D.; Ambrogio, M.W.; Algaradah, M.M.; Hong, M.P.; Chen, X.Q.; et al. Esterase- and pH-responsive poly(beta-amino ester)-capped mesoporous silica nanoparticles for drug delivery. Nanoscale 2015, 7, 7178–7183. [Google Scholar] [CrossRef]
- Gong, D.; Sun, L.; Li, X.; Zhang, W.; Zhang, D.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based on Microorganisms: Recent Advances and Perspectives. Small Struct. 2023, 4, 2200356. [Google Scholar] [CrossRef]
- Gong, D.; Celi, N.; Zhang, D.; Cai, J. Magnetic biohybrid microrobot multimers based on chlorella cells for enhanced targeted drug delivery. ACS Appl. Mater. Interfaces 2022, 14, 6320–6330. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Jia, H.R.; Jiang, Y.W.; Guo, Y.; Duan, Q.Y.; Xu, K.F.; Shan, B.H.; Liu, X.; Chen, X.; Wu, F.G. A Red Blood Cell-Derived Bionic Microrobot Capable of Hierarchically Adapting to Five Critical Stages in Systemic Drug Delivery; Exploration, 2023; Wiley Online Library: Hoboken, NJ, USA, 2023; p. 20230105. [Google Scholar]
- Zhong, L.; Li, Y.S.; Xiong, L.; Wang, W.J.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.Y.; Miao, Z.; Wang, T.Q.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Duan, C.Y.; Yu, M.J.; Xu, J.Y.; Li, B.Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-drug Resistance (MDR): Reasons, mechanisms, nanotherapeutic solutions, and challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef]
- Omidi, Y.; Mobasher, M.; Castejon, A.M.; Mahmoudi, M. Recent advances in nanoscale targeted therapy of HER2-positive breast cancer. J. Drug Target. 2022, 30, 687–708. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.Y.; Chen, X.Z. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Tagde, P.; Najda, A.; Nagpal, K.; Kulkarni, G.T.; Shah, M.D.S.; Ullah, O.; Balant, S.; Rahman, M.H. Nanomedicine-Based Delivery Strategies for Breast Cancer Treatment and Management. Int. J. Mol. Sci. 2022, 23, 2856. [Google Scholar] [CrossRef]
- Behl, A.; Chhillar, A.K. Nano-Based Drug Delivery of Anticancer Chemotherapeutic Drugs Targeting Breast Cancer. Recent Pat. Anti-Cancer Drug Discov. 2023, 18, 325–342. [Google Scholar] [CrossRef]
- Bonizzi, A.; Truffi, M.; Sevieri, M.; Allevi, R.; Sitia, L.; Ottria, R.; Sorrentino, L.; Sottani, C.; Negri, S.; Grignani, E.; et al. Everolimus Nanoformulation in Biological Nanoparticles Increases Drug Responsiveness in Resistant and Low-Responsive Breast Cancer Cell Lines. Pharmaceutics 2019, 11, 384. [Google Scholar] [CrossRef]
- Hu, C.; Song, Y.J.; Zhang, Y.W.; He, S.Q.; Liu, X.Y.; Yang, X.T.; Gong, T.; Huang, Y.; Gao, H.L. Sequential delivery of PD-1/PD-L1 blockade peptide and IDO inhibitor for immunosuppressive microenvironment remodeling via an MMP-2 responsive dual-targeting liposome. Acta Pharm. Sin. B 2023, 13, 2176–2187. [Google Scholar] [CrossRef]
- Luo, Z.M.; Wu, S.Y.; Zhou, J.F.; Xu, W.X.; Xu, Q.Z.; Lu, L.W.; Xie, C.; Liu, Y.; Lu, W.Y. All-stage targeted therapy for the brain metastasis from triple-negative breast cancer. Acta Pharm. Sin. B 2023, 13, 359–371. [Google Scholar] [CrossRef]
- Singh, M.K.; Pindiprolu, S.; Sanapalli, B.K.R.; Yele, V.; Ganesh, G.N.K. Tumor homing peptide modified liposomes of capecitabine for improved apoptotic activity and HER2 targeted therapy in breast cancer: In vitro studies. RSC Adv. 2019, 9, 24987–24994. [Google Scholar] [CrossRef]
- Surve, C.; Banerjee, A.; Anupriya, S.; Chakraborty, R.; Kumar, D.; Butti, R.; Gorain, M.; Parida, S.; Kundu, G.C.; Shidhaye, S.; et al. Antiproliferative and apoptotic potential of methotrexate lipid nanoparticle in murine breast cancer model. Nanomedicine 2022, 17, 753–764. [Google Scholar] [CrossRef]
- Abd-Ellatef, G.E.F.; Gazzano, E.; Chirio, D.; Hamed, A.R.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Marie, M.A.S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Liu, F.J.; Li, L.H.; Lan, M.; Zou, T.T.; Kong, Z.D.; Cai, T.G.; Wu, X.Y.; Cai, Y. Psoralen-loaded polymeric lipid nanoparticles combined with paclitaxel for the treatment of triple-negative breast cancer. Nanomedicine 2021, 16, 2411–2430. [Google Scholar] [CrossRef]
- Kefayat, A.; Vaezifar, S. Biodegradable PLGA implants containing doxorubicin-loaded chitosan nanoparticles for treatment of breast tumor-bearing mice. Int. J. Biol. Macromol. 2019, 136, 48–56. [Google Scholar] [CrossRef]
- Verma, R.; Rani, V.; Kumar, M. In-vivo anticancer efficacy of self-targeted methotrexate-loaded polymeric nanoparticles in solid tumor-bearing rat. Int. Immunopharmacol. 2023, 119, 110147. [Google Scholar] [CrossRef]
- Chang, J.; Mo, L.F.; Song, J.F.; Wang, X.C.; Liu, H.H.; Meng, C.C.; Wu, Y.J. A pH-responsive mesoporous silica nanoparticle-based drug delivery system for targeted breast cancer therapy. J. Mater. Chem. B 2022, 10, 3375–3385. [Google Scholar] [CrossRef]
- Chaudhari, D.; Kuche, K.; Yadav, V.; Ghadi, R.; Date, T.; Bhargavi, N.; Jain, S. Exploring paclitaxel-loaded adenosine-conjugated PEGylated PLGA nanoparticles for targeting triple-negative breast cancer. Drug Deliv. Transl. Res. 2023, 13, 1074–1087. [Google Scholar] [CrossRef]
- Wang, M.C.; Chen, J.; Li, W.J.; Zang, F.; Liu, X.X.; Qin, S. Paclitaxel-nanoparticles-loaded double network hydrogel for local treatment of breast cancer after surgical resection. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 114, 111046. [Google Scholar] [CrossRef]
- Moammeri, A.; Abbaspour, K.; Zafarian, A.; Jamshidifar, E.; Motasadizadeh, H.; Moghaddam, F.D.; Salehi, Z.; Makvandi, P.; Dinarvand, R. pH-Responsive, Adorned Nanoniosomes for Codelivery of Cisplatin and Epirubicin: Synergistic Treatment of Breast Cancer. ACS Appl. Bio Mater. 2022, 5, 675–690. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Tewari, C.; Pandey, N.; Rana, A.; Rana, S.; Pal, M.; Sahoo, N.G. Dual Drug Loaded Potassium-contained Graphene Oxide as a Nanocarrier in Cocktailed Drug Delivery for the Treatment of Human Breast Cancer. Curr. Drug Deliv. 2023, 20, 943–950. [Google Scholar] [CrossRef]
- Ramadan, I.; Nassar, M.Y.; Gomaa, A. In-vitro Investigation of the Anticancer Efficacy of Carboplatin-Loaded Chitosan Nanocomposites Against Breast and Liver Cancer Cell Lines. J. Polym. Environ. 2023, 31, 1102–1115. [Google Scholar] [CrossRef]
- Sharma, S.; Naskar, S.; Kuotsu, K. Metronomic chemotherapy of carboplatin-loaded PEGylated MWCNTs: Synthesis, characterization and in vitro toxicity in human breast cancer. Carbon Lett. 2020, 30, 435–447. [Google Scholar] [CrossRef]
- Sahrayi, H.; Hosseini, E.; Karimifard, S.; Khayam, N.; Meybodi, S.M.; Amiri, S.; Bourbour, M.; Far, B.F.; Akbarzadeh, I.; Bhia, M.; et al. Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression. Pharmaceuticals 2022, 15, 6. [Google Scholar] [CrossRef]
- Tohidi, S.; Aghaie-Khafri, M. Cyclophosphamide Loading and Controlled Release in MIL-100(Fe) as an Anti-breast Cancer Carrier: In vivo In vitro Study. Curr. Drug Deliv. 2023, 21, 283–294. [Google Scholar] [CrossRef]
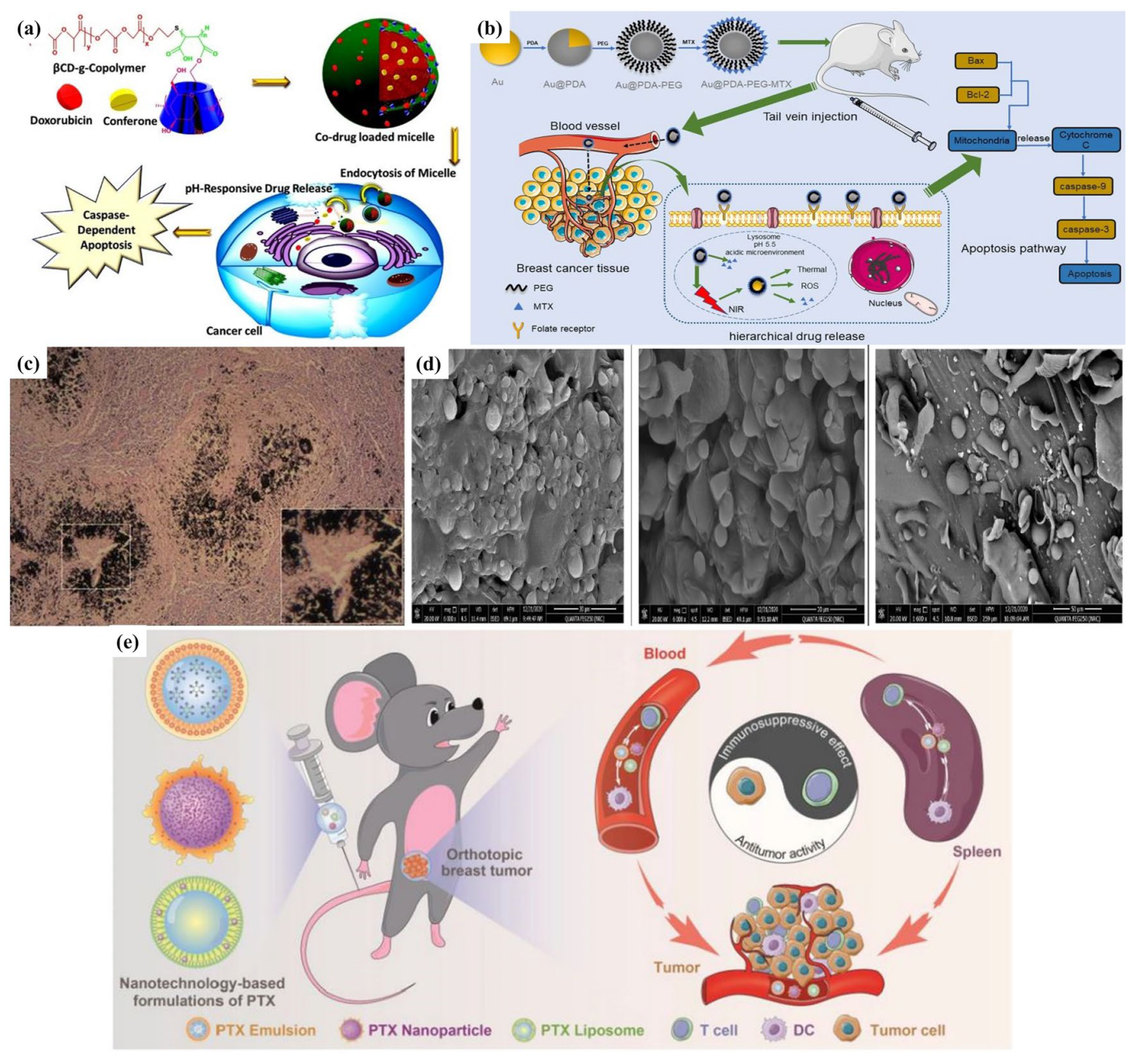
- Rahmani, A.; Rahimi, F.; Iranshahi, M.; Kahroba, H.; Zarebkohan, A.; Talebi, M.; Salehi, R.; Mousavi, H.Z. Co-delivery of doxorubicin and conferone by novel pH-responsive beta-cyclodextrin grafted micelles triggers apoptosis of metastatic human breast cancer cells. Sci. Rep. 2021, 11, 21425. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.W.; Yu, L.C.Y.; Huang, Q.C.; Zhu, D.J.; Lu, C.; Lu, A.P.; Liu, Y.Y. Hierarchical drug release designed Au @PDA-PEG-MTX NPs for targeted delivery to breast cancer with combined photothermal-chemotherapy. J. Nanobiotechnol. 2021, 19, 143. [Google Scholar] [CrossRef]
- Du, J.Z.; Zhang, Y.S.; Ming, J.; Liu, J.; Zhong, L.; Liang, Q.K.; Fan, L.J.; Jiang, J. Evaluation of the tracing effect of carbon nanoparticle and carbon nanoparticle-epirubicin suspension in axillary lymph node dissection for breast cancer treatment. World J. Surg. Oncol. 2016, 14, 164. [Google Scholar] [CrossRef]
- Samy, M.; Abdallah, H.M.; Awad, H.M.; Ayoub, M.M.H. Preparation, Characterization and In vitro Biological activity of 5-Fluorouracil Loaded onto poly (D, L-lactic-co-glycolic acid) Nanoparticles. Polym. Bull. 2023, 80, 6197–6219. [Google Scholar] [CrossRef]
- Ye, J.; Li, R.J.; Yang, Y.F.; Dong, W.J.; Wang, Y.J.; Wang, H.L.; Sun, T.; Li, L.; Shen, Q.Q.; Qin, C.Y.; et al. Comparative colloidal stability, antitumor efficacy, and immunosuppressive effect of commercial paclitaxel nanoformulations. J. Nanobiotechnol. 2021, 19, 199. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Cimino-Mathews, A.; Disis, M.L.; Gatti-Mays, M.E.; Ho, A.Y.; Kalinsky, K.; McArthur, H.L.; Mittendorf, E.A.; Nanda, R. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of breast cancer. J. Immunother. Cancer 2021, 9, e002597. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Xu, J.; Qiu, N.; Zhai, G. Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: The horizons in cancer treatment. ACS Nano 2021, 15, 12567–12603. [Google Scholar] [CrossRef]
- Kakwere, H.; Zhang, H.; Ingham, E.S.; Nura-Raie, M.; Tumbale, S.K.; Allen, R.; Tam, S.M.; Wu, B.; Liu, C.; Kheirolomoom, A.; et al. Systemic Immunotherapy with Micellar Resiquimod-Polymer Conjugates Triggers a Robust Antitumor Response in a Breast Cancer Model. Adv. Healthc. Mater. 2021, 10, 2100008. [Google Scholar] [CrossRef]
- Mobarez, A.M.; Soleimani, N.; Esmaeili, S.A.; Farhangi, B. Nanoparticle-based immunotherapy of breast cancer using recombinant Helicobacter pylori proteins. Eur. J. Pharm. Biopharm. 2020, 155, 69–76. [Google Scholar] [CrossRef]
- Bai, X.W.; Zhou, Y.M.; Yokota, Y.; Matsumoto, Y.; Zhai, B.; Maarouf, N.; Hayashi, H.; Carlson, R.; Zhang, S.H.; Sousa, A.; et al. Adaptive antitumor immune response stimulated by bio-nanoparticle based vaccine and checkpoint blockade. J. Exp. Clin. Cancer Res. 2022, 41, 132. [Google Scholar] [CrossRef]
- Bahreyni, A.; Mohamud, Y.; Zhang, J.C.; Luo, H.L. Engineering a facile and versatile nanoplatform to facilitate the delivery of multiple agents for targeted breast cancer chemo-immunotherapy. Biomed. Pharmacother. 2023, 163, 114789. [Google Scholar] [CrossRef]
- Tunc, C.U.; Aydin, O. Co-delivery of Bcl-2 siRNA and doxorubicin through gold nanoparticle-based delivery system for a combined cancer therapy approach. J. Drug Deliv. Sci. Technol. 2022, 74, 103603. [Google Scholar] [CrossRef]
- Chaudhari, R.; Nasra, S.; Meghani, N.; Kumar, A. MiR-206 conjugated gold nanoparticle based targeted therapy in breast cancer cells. Sci. Rep. 2022, 12, 4713. [Google Scholar] [CrossRef]
- Han, R.; Zhao, J.; Lu, L.G. MicroRNA-34a expression affects breast cancer invasion in vitro and patient survival via downregulation of E2F1 and E2F3 expression. Oncol. Rep. 2020, 43, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kong, C.; Wu, Y.L. Long intergenic non-protein coding RNA 1094 (LINC01094) promotes the progression of breast cancer (BC) by regulating the microRNA-340-5p (miR-340-5p)/E2F transcription factor 3 (E2F3) axis. Bioengineered 2021, 12, 9046–9057. [Google Scholar] [CrossRef] [PubMed]

| Nanomaterials/Nanoformulations | Nature of Nanomaterial/Delivery Mechanism | Results | References |
|---|---|---|---|
| Bimetallic Prussian blue analogs and cobalt–iron loaded with Dox | pH-dependent mechanism | Good biocompatibility of PBA-DDSs | [66] |
| Curcumin-loaded ZnO nanoparticles conjugated with phenylboronic acid (PBA) | pH-dependent | Successful reduction in tumor growth in mice with Ehrlich ascites carcinoma (EAC) tumors | [66] |
| HA-Dox/PHIS/R848 nanoparticles: R848, was encapsulated with poly(L-histidine) (PHIS) to form PHIS/R848 nanocores, and doxorubicin (Dox) was conjugated to hyaluronic acid (HA) | Dual pH-dependent multifunctional nanoparticle combining chemotherapy and immunotherapy | Impressive tumor-targeting ability and effective inhibition tumor growth by regulating immunity and directly eliminating cancer cells | [66] |
| Folic acid-conjugated polyacrylic acid-coated mesoporous silica nanoparticles loaded with chrysin | pH-sensitive | In vivo regression of tumors, restoration of normal tissue structure, and the preservation of a healthy body weight | [66] |
| Phenylboronic acid (PBA)-conjugated zinc oxide nanoparticles (PBA-ZnO), loaded with quercetin | pH-sensitive | Reduction in tumor growth | [77] |
| BSA-stabilized graphene/chitosan nanocomposites conjugated with breast cancer drugs | pH-sensitive | Reduced the burst release observed compared to that for pure chitosan nanoparticles | [72] |
| Palladium–ruthenium nanohybrid with polypyridyl complex (PdRu-RCE@PCM NPs) | Temperature-sensitive (photothermal targeting therapy) | Inhibition of primary tumor growth and tumor metastasis | [77] |
| Programmed death-1 (PD-1)-modified gold-promoting satellite copper selenide nanocrystals | Photothermal targeting therapy | Apoptosis of cancer cells | [77] |
| Cetuximab- and doxorubicin-conjugated liposome-mediated magnetic nanoparticles | Combined treatment of photothermal therapy and targeted chemotherapy in thermo-sensitive nanocarriers | Reduction in viability of breast cancer cells | [77] |
| (Triptolide) TPL@nanogel | Injectable thermo-responsive hydrogel | Reduced systemic toxicity and increased antitumor efficacy | [77] |
| Lauric acid encapsulated in a biocompatible silica shell | Combined effect of temperature and lauric acid activity | Dual activity in anticancer treatments due to the two combined mechanisms | [77] |
| Hollow gold nanostars (HGNSs) and gold nanocages (GNCs) with doxorubicin (Dox) attached | Temperature/pH-dependent mechanism | A high cell mortality and apoptotic effects were observed | [80] |
| Chitosan/gelatin hybrid nanogel incorporating gold nanoparticles (CS/AuNPs@Gel-Dox nanogel) | Enzyme responsive | Successful absorption by cells. Cell cytotoxicity revealed that the drug carrier was efficient | [84] |
| Disulfiram and doxorubicin conjugated with polymeric nanoparticles | Enzyme/pH dual responsive | Increased cytotoxicity against 4T1 cells | [84] |
| Peptide conjugate crafted to combined mild photothermal therapy with immunotherapy in a unified nanosystem | pH and enzyme responsive | Effective inhibition of tumor growth while preventing the formation of lung metastases | [86] |
| (POL-MSN) Dox-loaded MSNs with poly (β-amino ester) and boronate esters | pH and enzyme responsive | Reduced cancer cell viability | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.; Vedarethinam, V.; Korsah, M.A.; Danquah, M.K.; Jeevanandam, J. Exploring the Potential of Nanoparticles in the Treatment of Breast Cancer: Current Applications and Future Directions. Appl. Sci. 2024, 14, 1809. https://doi.org/10.3390/app14051809
Patel P, Vedarethinam V, Korsah MA, Danquah MK, Jeevanandam J. Exploring the Potential of Nanoparticles in the Treatment of Breast Cancer: Current Applications and Future Directions. Applied Sciences. 2024; 14(5):1809. https://doi.org/10.3390/app14051809
Chicago/Turabian StylePatel, Puja, Vadanasundari Vedarethinam, Maame A. Korsah, Michael K. Danquah, and Jaison Jeevanandam. 2024. "Exploring the Potential of Nanoparticles in the Treatment of Breast Cancer: Current Applications and Future Directions" Applied Sciences 14, no. 5: 1809. https://doi.org/10.3390/app14051809
APA StylePatel, P., Vedarethinam, V., Korsah, M. A., Danquah, M. K., & Jeevanandam, J. (2024). Exploring the Potential of Nanoparticles in the Treatment of Breast Cancer: Current Applications and Future Directions. Applied Sciences, 14(5), 1809. https://doi.org/10.3390/app14051809









