Abstract
Diabetic keratopathy (DK) is a common ocular complication of diabetes, characterized by alteration of the normal wound-healing mechanism, reduction of epithelial hemidesmosomes, disruption of the basement membrane, impaired barrier function, reduced corneal sensitivity, corneal ulcers, and corneal edema. The limited number of clinical studies do not allow a full characterization of the pathophysiology of DK and, until now, effective therapeutic approaches have not been available. However, in recent years, neuropeptides gained great attention for their biochemical characteristics and therapeutic potential. This review focuses on the role of neuropeptides vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) in the eye and, in particular, in the cornea, in physiological conditions, or during DK, by providing an overview of this diabetes mellitus complication.
1. Introduction
Diabetes mellitus (DM) is an endemic disease occurring all over the world, which is characterized by chronic hyperglycemia. It is caused by total or relative absence in insulin secretion and/or insulin action by the pancreatic β cells [1]. According to the International Diabetes Federation, 537 million adults (20–79 years) are living with diabetes, and this number is predicted to rise to 643 million by 2030 and 783 million by 2045, resulting in a huge health burden on society.
Chronic hyperglycemia gradually induces several complications affecting almost every organ system, including the ocular tissues [2]. Diabetic retinopathy (DR) is the most common ophthalmic complication of DM. However, corneal abnormalities (diabetic keratopathy) are also common in patients with DM and determine the increased morbidity of these patients [3]. Although the relationship between DR and DK is not fully characterized, a decrease in corneal sensitivity is known to affect both insulin-dependent and non-insulin-dependent diabetic patients [4]. Moreover, corneal sensitivity is lower in diabetic as compared to non-diabetic eyes, and it is lower in patients with DR as compared to no DR. Moreover, corneal sensitivity is more altered with the progression of DR [5] and particularly impaired in eyes with proliferative DR as compared to non-proliferative DR [4]. Different instrumental tests can be used to visualize the severity of DK and DR, as well as choroidal damage. Among them, digital retinal fundus image analysis can detect early DR, although it has been shown to have a low negative predictive value [6,7]. Optical Coherence Tomography (OCT) is a non-invasive test to acquire bidimensional images of the different retinal layers, and optical coherence tomography angiography (OCTA) is a novel diagnostic tool to observe the microvasculature of the retina and choroid without the need for dye injection [8]. Since changes in choroidal thickness, retinal thickness, vessel density of the superficial capillary plexus, and deep capillary plexus can be signs of endothelial damage and dysfunction, OCTA could also be a valid modality to detect diabetic-induced abnormalities [9,10,11,12]. Another useful test is in vivo corneal confocal microscopy (IVCCM), a non-invasive and reproducible technique that allows for the study of the living human cornea, including the cellular structure, as well as sub-basal nerve plexus [13,14].
DK affects 47–64% of patients with DM; therefore, it has a profound social and economic impact. In particular, according to the Italian National Health Service, the mean annual treatment cost of neurotrophic keratopathy per patient is around EUR 5167 in the case of persistent epithelial defect, and EUR 10,885 in the case of corneal ulcer without perforation [15].
The human cornea (Figure 1), forming with the sclera the outermost part of the eye, is mechanically strong and transparent since it exerts barrier and refractive functions. The cornea comprises five different layers: the epithelium, Bowman’s layer, Stroma, Descemet’s membrane, and endothelium. The epithelium, the outermost layer of the cornea, acts as a barrier by protecting the eye against the external insult. It is formed by four to six layers of nonkeratinized stratified squamous epithelial cells. These cells show different morphology comprising the basal columnar, wing, and superficial squamous cells. The corneal epithelium has high regenerative capacity due to the presence of limbal epithelial stem cells (LESCs), which reside in an annular transition zone known as the limbus, laying at the junction area between the cornea and the sclera. Below the epithelium is the Bowman’s membrane (BM), composed of collagen fibrils, which are involved in the cornea’s shape [16]. The major part of the cornea thickness is represented by the stroma, whose transparency, avascularity, and strength depend on its accurate composition. In fact, it is formed by extracellular matrix (ECM) molecules, water, and a communicating network of neural crest-derived keratocytes, synthesizing the stromal extracellular matrix [17,18]. Between the posterior stroma and the corneal endothelial layer, there is Descemet’s membrane which is an acellular extracellular matrix composed of hexagonal collagen VIII networks, as well as associated collagens IV and XII [18]. The inner corneal layer is represented by the endothelium, which is formed by a single layer of flat hexagonal cells. Corneal endothelium plays a dual essential role as a barrier and active pump, by regulating the movement of water from the anterior chamber to stroma, thus maintaining its hydration and transparency. Unlike corneal epithelial cells, endothelial cells are not able to regenerate in vivo since they are blocked to the G1 phase of the cell cycle due to cell–cell contact inhibition and a lack of growth factors [19].
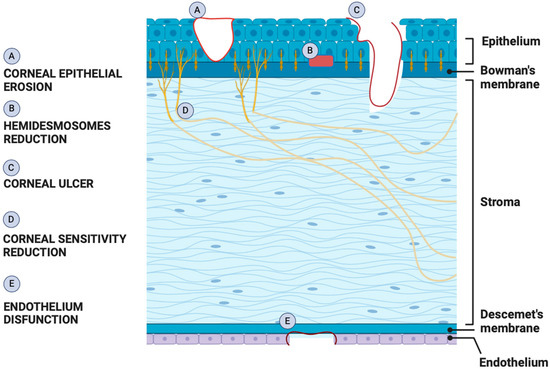
Figure 1.
Schematic showing pathogenesis of DK. Effects triggered by hyperglycemia in different parts of the cornea result in three main types of dysfunctions characterizing DK: epitheliopathy, neuropathy, and endotheliopathy.
Several neuropeptides and relative receptors are largely expressed in the cornea. The present review provides an overview of the pathophysiology of DK and summarizes recent research findings on the role of vasoactive intestinal peptide (VIP) family members including VIP, pituitary adenylate cyclase-activating polypeptide (PACAP), and activity-dependent neuroprotective protein (ADNP) in this diabetic ocular complication. They are involved in corneal wound healing by promoting the proliferation and migration of epithelial cells, keratocytes, and endothelial cells [20]. Moreover, both VIP and PACAP are involved in corneal sensory nerve regeneration and modulation of corneal immune cells [21].
2. Overview of Diabetic Keratopathy
Chronic exposure to hyperglycemia triggers pathophysiological changes in cells, tissues, and organ systems, due to the promotion of oxidative stress, the activation of polyol pathway and protein kinase C (PKC), the formation of advanced glycation end-products (AGEs), and alteration of gene expressions [22]. The cornea is an avascular structure containing no blood vessels, receiving glucose via trans-corneal transport from the aqueous humor. Glucose is also present in tears, but its levels are lower than in the aqueous humor and serum [23]. Given that the cornea receives glucose from the aqueous and not the adjacent tear film, it is not surprising that in patients with diabetes, the cornea is exposed to high levels of oxidative stress and inflammation representing distinct features of diabetes in all other tissues [24,25]. Corneal complications range from mild to severe manifestations and comprise epithelial defects, corneal thickness, erosions, and corneal nerve abnormalities [26] (Figure 1).
Corneal epithelial alterations observed in patients with DM include epithelial fragility, non-healing corneal ulcers, and superficial punctate keratitis (SPK). The latter is characterized by scattered areas of punctate corneal epithelial loss causing photophobia, foreign body sensation, tearing, redness, irritation, and reduced visual acuity [27]. The reduction of corneal epithelial density and thickness is due to the imbalance between cell proliferation, differentiation, migration, and death. Moreover, the accumulation of AGEs counteracts the effective migration of epithelial cells essential for wound healing, leading to recurrent erosions [28]. The impairment of corneal epithelium is closely linked to the increase in glycosylated hemoglobin levels [29], and corneal epithelium barrier alterations expose patients to a higher risk of developing ocular infections than healthy people [30]. Moreover, cataract and laser-assisted in situ keratomileusis (LASIK) surgeries are some of the high-risk interventions for patients with DM, since corneal damage after or during surgery may lead to slow healing and thus frequent corneal erosion damage [31,32]. DK is also characterized by a loss of corneal sensitivity, which could be used by clinicians for the early diagnosis of diabetic peripheral neuropathy and/or DK [33]. In fact, corneal epithelium represents the most innervated and sensible epithelial surface of the human body. In particular, it is innervated by free nerve endings of the ophthalmic division of the trigeminal nerve (cranial nerve V) [34], and corneal nerves are responsible for the sensations of pain from mechanical, thermal, and chemical stimulation [35]. Furthermore, corneal nerves regulate tear secretion and via the regulation of neurotrophic factors maintain ocular surface homeostasis, corneal sensitivity, epithelial health, and wound healing [36,37]. Recent studies showed that, in patients with DM, the density of corneal nerve fiber and branch and the corneal nerve fiber length are significantly reduced. Furthermore, 17% of these patients undergo the loss of 6% or more of corneal nerve fibers per year [38,39,40]. The stroma also shows structural alterations in patients with diabetes, due to the accumulation of AGEs, which provokes non-enzymatic cross-linking between collagen molecules and proteoglycans, thus causing the cornea to stiffen and thicken [41]. Changes in diabetic corneas were also found in corneal endothelial cells, whose density was decreased in patients with diabetes as compared to healthy subjects [32,42]. Accordingly, a recent study involving 120 patients with diabetes and 120 healthy patients demonstrated that hyperglycemia altered corneal endothelium (counts, morphology, and structure) as well as corneal thickness.
Available treatment options for patients affected by DK comprise the use of topical lubricants and antibiotic ointments to increase corneal surface lubrication and prevent infections. However, many potential therapeutic agents such as neuropeptides, growth factors, or cytokines could be used to promote the normalization and regeneration of the impaired human corneal epithelium [27,43].
3. Expression of Neuropeptides in the Cornea
Neuropeptides are signaling molecules of 3 to 100 amino acids that exert key roles in different physiological processes, such as reproduction, body weight regulation, pain, memory, sleep/wake cycles, long-lasting modulation of synaptic transmission, inflammation, tissue repair, and glucose metabolism [44,45]. They exert their functions through the activation of G protein-coupled receptors (GPCRs), which are integral membrane glycoproteins containing seven transmembrane domains [46]. GPCRs are coupled with intracellular heterotrimeric G proteins, which consist of three subunits, the α, β, and γ subunits. Upon receptor activation, the G protein is activated and the α subunit separates from the βγ dimer, and then both α and βγ can modulate the activity of target effectors.
G proteins are classified according to the activity of the Gα subunit as either Gs, Gi/o, or Gq/11 [45]:
- Gs signaling is involved in adenylyl cyclase (AC) activity, which regulates intracellular adenosine 3′,5′-cyclic monophosphate (cAMP). cAMP is an intracellular signal transmitter that, in turn, acts as a second messenger and activator of cAMP-dependent protein kinase A (PKA).
- Gi/o signaling is involved in the inhibition of AC activity, resulting in decreased intracellular cAMP production.
- Gq/11 signaling activates phospholipase Cβ (PLCβ), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2), releasing diacylglycerol (DAG) and 1,4,5-inositol trisphosphate (IP3). DAG activates protein kinase C (PKC), whereas IP3 diffuses to the endoplasmic reticulum (ER) and binds to IP3 receptors on ligand-gated calcium channels on the surface of ER leading to the release of calcium ions.
Neuropeptides are largely synthesized and secreted in the central and peripheral nervous system, as well as in other organs and tissue, including the cornea. Corneal nerves produce various neuropeptides, displayed in Table 1, that play neuromodulatory functions in the healthy and diseased cornea.

Table 1.
Neuropeptides expressed in the cornea.
3.1. Vasoactive Intestinal Peptide (VIP)
VIP is a 28-amino-acid peptide first isolated from the duodenums of pigs by Said and Mutt in 1970 [65]. It belongs to the secretin/glucagon family of peptide hormones, sharing 70% sequence identity with the neuropeptide PACAP. VIP is secreted by neurons, endocrine, and immune cells, and it is largely distributed throughout the central nervous system (CNS), peripheral nervous system (PNS), and peripheral organs and tissues, including heart, eye, lung, thyroid, kidney, urinary and gastrointestinal tracts, genital organs, and the immune system [66] (Figure 2).
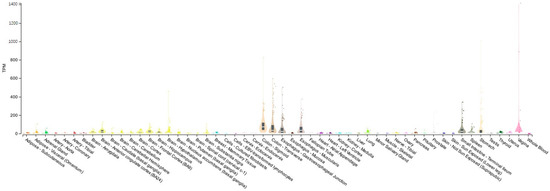
Figure 2.
Tissue distribution of VIP. Boxplot of transcripts per million (TPM) showing the bulk tissue gene expression for VIP. The Genotype-tissue Expression (GTEx) Portal on 25 October 2023.
The mechanism of action of VIP implicates the activation of two subtypes of receptors, VPAC1 and VPAC2, which bind with the same affinity as the other neuropeptide PACAP. VPAC1 and VPAC2 receptors are mainly coupled to the G-protein Gs, stimulating cellular AC activity [67,68]. The two specific receptors for VIP are largely expressed throughout the human body, including the pancreatic islets, where the VPAC1 receptor regulates glucagon secretion and hepatic glucose production, whereas the VPAC2 receptor improves glucose tolerance through the stimulation of insulin secretion [69,70,71,72]. These data suggest that VPAC1 and VPAC2 receptors can potentially be targeted for the treatment of type 2 diabetes.
VIP exerts different biological functions, as a neuromodulator, neurotransmitter, and vasodilator [73,74,75]. It regulates smooth muscle activity, epithelial cell secretion, and blood flow in the gastrointestinal tract [65,76]. VIP is also involved in neurological diseases [77] and in different types of cancers, where it can have a stimulatory or inhibitory role in the growth of neoplastic cells [78,79,80,81]. VIP is also involved in immunomodulation and inflammation by regulating the immunogenic/mature conventional dendritic cells phenotype [82,83].
In the ocular system, VIP and its receptors are distributed in the retina [84,85], showing a protective role in ischemic retinal degeneration [86,87] and in diabetic retinopathy [88,89,90,91]. VIP is also produced at the ocular surface and contributes to the immunosuppressive activity of normal aqueous humor [92]. The peptide is normally present in the tears of healthy subjects, and its levels increased after conjunctival allergen challenge in allergic patients. These data suggest that the local release of VIP can act directly on the epithelia, blood vessels, and immune cells, exerting protective and inflammatory responses [93]. VPAC1 and VPAC2 receptor expression was found at high-intensity levels in the epithelium and stroma of the rabbit cornea [8]. Moreover, in the cornea after P. aeruginosa infection, the treatment with VIP significantly increased ECM molecules linked to the healing/homeostasis process, confirming the protective role played by VIP to promote the integrity of the corneal stroma [94]. VIP enhanced also the integrity of corneal endothelial cells in precut human donor corneas [95]. VIP treatment, by increasing the expression of the VPAC1 receptor on inflammatory cells, exerted a modulatory role on cytokine release leading to less stromal destruction and prevention of corneal perforation [96]. Furthermore, Jiang et al. [97] demonstrated both in vitro and in vivo models that the treatment with VIP reduced and increased the pro- and anti-inflammatory Toll-like receptors, respectively, by improving the outcome of the infected cornea by P. aeruginosa.
3.2. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)
PACAP, encoded by the gene Adcyap1, exists in 27 and 38 amino acid forms. Both forms are bioactive; however, PACAP38 is more than 100-fold more abundant than PACAP27 in neuronal tissues [98,99]. PACAP cytoprotective effects are mediated by the activation of specific receptors, which share with VIP. However, the type I PACAP receptor (PAC1R) has a high affinity for PACAP as compared to VIP [100]. Neurons and endocrine cells express different PAC1R splice variants, which differ at the N terminus or in the third intracellular loop (IC3) of this GPCR [101]. Therefore, the multimodal effects played by PACAP depend on its concentration as well as on the receptor splice variants expressed in the specific tissue and cell, leading to adenylate cyclase (AC) or phospholipase C (PLC) pathways activation [102,103]. The effects of PACAP are also mediated by the stimulation of an intracellular astrocyte-derived neurotrophic factor known as activity-dependent protein (ADNP) [104,105,106,107]. Furthermore, through the PKA signaling pathway, the activation of PAC1R also transactivates EGFR, resulting in the activation of the ERK signaling pathway as detected in different pathological conditions, such as cancers, amyotrophic lateral sclerosis, diabetic retinopathy, and corneal alteration [108,109,110,111,112,113].
PACAP is largely distributed in the body (Figure 3) where it plays different roles at behavioral and cognitive levels [114,115,116,117]. The peptide is involved in the regulation of cardiovascular, gastrointestinal, urinary, and endocrine functions [118,119,120,121,122,123,124]. Moreover, it exerts an important role in reproduction, pregnancy, early development, and aging [125,126,127,128,129,130,131].
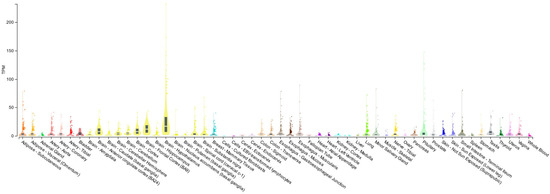
Figure 3.
Tissue distribution of PACAP. Boxplot of transcripts per million (TPM) showing the bulk tissue gene expression for PACAP. The Genotype-tissue Expression (GTEx) Portal on 25 October 2023.
In the eye, PACAP and its high-affinity receptor PAC1 are expressed in the retinal layers [132,133,134,135,136,137], in the ciliary body, in the optic nerve, in the conjunctiva, and in the iris [50,133,138]. Moreover, the expression of PACAP and its receptors has been largely demonstrated in the cornea. In fact, PACAP was detected in nerve terminals running in the stroma and sending off some branches into the epithelium [50]. PACAP and PAC1R immunoreactivity was detected in corneal epithelium, endothelium, as well as in stroma [50,139]. The literature largely described the protective role played by PACAP in the cornea. The treatment with the peptide enhanced cell proliferation and ensured the integrity of the corneal endothelium barrier subjected to growth factor deprivation [139]. Moreover, PACAP shown to protect the human corneal endothelial cells (HCECs) derived from donors’ cornea against the ultraviolet B radiation insult, leading to cell death and the alteration of barrier integrity [140].
The exogenous peptide accelerated corneal epithelial wound healing after laser-assisted in situ keratomileuses (LASIK) surgery, increasing up to 75% of the corneal sensitivity eight weeks after the operation. In fact, the peptide significantly increased the neurite outgrowth of trigeminal ganglion cells [141], confirming the positive role played by PACAP in corneal nerve regeneration and corneal sensitivity. PACAP alone or connected to the N-terminal agrin domain (NtA) with a genetic engineering method promoted corneal epithelial cell proliferation and differentiation, preventing the apoptosis of injured nerves and promoting the growth of nervous processes [142]. Moreover, a new recombinant PACAP-derived peptide (named MPAPO) was shown to promote corneal epithelial cell proliferation and trigeminal ganglion cell axon regeneration. PACAP exerts also a key role in tear secretion. In fact, PACAP knockout mice developed dry eye-like symptoms, which comprised corneal keratinization and reduced tear production [143]. Interestingly, PACAP eyedrops promoted tear secretion through the activation of the AC/cAMP/PKA pathway, leading to the translocation of aquaporin 5 from the cytosol to the membrane of lacrimal acinar cells [144]. The protective role of PACAP was also investigated in ocular inflammation. In fact, high levels of the peptide were found in the rabbit aqueous humor in response to electroconvulsive treatment or other noxious stimuli [145,146]. These data are in line with other studies showing the active role played by PACAP in neurogenic inflammation response [147,148].
3.3. Activity-Dependent Neuroprotective Protein (ADNP)
In 1999, Bassan et al. [104] identified the human ADNP gene, localized on the q13.13 band of chromosome 20, and contains 5 exons and 4 introns with alternative splicing of an untranslated second exon. This gene interacts with nuclear chromatin, regulating more than 400 genes involved in brain formation and plasticity, organ development, autophagy, and axonal transport [149,150,151]. ADNP de novo mutations are responsible for ADNP syndrome, also known as Helsmoortel-Van der Aa syndrome (HVDAS) [105,152]. This syndrome, belonging to autism spectrum disorders (ASDs), is characterized by intellectual development delay, motor abnormalities, facial dysmorphisms, hypotonia, and also ocular problems, such as convergent strabismus, astigmatism, hyperopia, unilateral iris coloboma, bilateral optic nerve coloboma, and retinal dysfunctions [153,154,155]. The neuroprotective functions of ADNP are attributed to the octapeptide NAP sequence (NAPVSIPQ = Asn-Ala-Pro-Val-Ser-Ile-Pro-Gln), which exert several beneficial effects in different neurodegenerative disorders at femtomolar concentration [104,156,157]. NAP induces neuroplasticity, since through the SIP motif, interacts with the end-binding proteins 1 and 3 (EB1 and EB3), promoting the microtubule intervention [158,159]. It is well known that EB3 exerts a key role in dendritic spine formation, and the effect of NAP is EB3-dependent. Moreover, NAP strongly increases EB proteins–Tau interaction protecting microtubule [160].
ADNP is expressed in different tissues and organs of the body with the highest expression in the human brain, gastrointestinal system, respiratory, and reproductive system (Figure 4) [161]. The expression of ADNP was also detected in the eye, particularly in the retina and cornea [51,162,163].
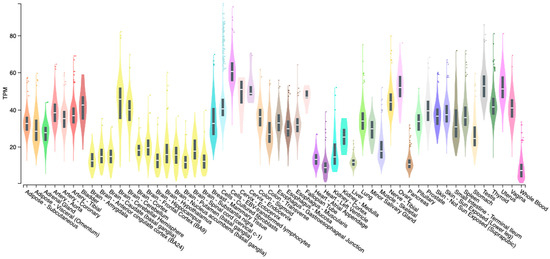
Figure 4.
Tissue distribution of ADNP. Boxplot of transcripts per million (TPM) showing the bulk tissue gene expression for PACAP. The Genotype-tissue Expression (GTEx) on 10 January 2024.
In the retina, the administration of NAP was shown to exert protective effects against different types of insults, including neurotrophic factor deprivation, retinal ischemia, optic nerve crush, and laser-induced retinal damage [164,165,166]. Moreover, targeted NT4-NAP fusion gene transfection of rat retinal Müller cells (RMC), protected from hypoxic–ischemic injury not only RMCs, but also retinal neurons, by promoting their viability and neurite growth [167]. The role of NAP in diabetic retinopathy (DR) was also largely demonstrated [107]. In particular, the treatment with the ADNP active fragment protected the integrity of the outer blood–retinal barrier (BRB), modulating the expression of hypoxic inducible factors (HIFs), and counteracting human retinal pigmental epithelium apoptosis induced by hyperglycemic/hypoxic insult [168,169,170]. Furthermore, NAP treatment modulated the inflammatory event characterizing the early phase of DR, downregulating interleukin (IL)-1β and its related receptors and upregulating IL-1Ra expression [168].
4. Discussion
VIP family members have shown great promise as agents in the treatment of different ocular diseases including DK. In particular, VIP/VPAC receptor signaling exerted multiple positive active roles [83]. VIP, in combination with thymosin beta-4 (Tβ4), was shown to protect corneal epithelial cells against hyperglycemia-induced damage by promoting tissue wound healing and barrier integrity [171]. Furthermore, the exogenous treatment with the peptide restored corneal epithelial wound closure in locally denervated corneas by inhibiting the overexpressed proinflammatory factors. This aspect is perfectly coherent with the large benefits exerted by VIP in inflammatory diseases [172]. Moreover, several animal and human studies showed that VIP regulates the balance of pro- and anti-inflammatory cytokines by blocking the release of pro-inflammatory cytokines and chemokines and promoting the expression of anti-inflammatory cytokines [173]. Second, VIP/VPAC receptor signaling impairment contributed to the alteration in corneal epithelial wound closure and delayed sensory nerve regeneration in diabetic corneas. Accordingly, VIP increased the release of nerve growth factor (NGF) and ciliary neurotrophic factor (CNTF) during DK through the activation of Sonic Hedgehog (Shh), which is required for proper wound healing in the corneas [83]. In fact, the mouse eye organ culture demonstrated that adding Shh into the culture medium promotes the proliferation of corneal epithelial cells by inducing the nuclear accumulation of cyclin D1 [174]. Furthermore, it was described that sympathetic overactivation, induced by hyperglycemia, activates the norepinephrine-β2-adrenoceptor pathway, which, in turn, inhibits Shh activity, leading to a reduction of corneal epithelial wound healing in diabetic mice [175].
PACAP has also been shown to have protective effects in DK. A recent study showed that the expression of PACAP and PAC1R is drastically reduced in the whole cornea of diabetic rats, suggesting that PACAP/PAC1R signaling alteration can concur with the corneal epithelium impairment induced by the hyperglycemic state. The exogenous administration of PACAP, in an in vitro model of DK, characterized by human corneal epithelial cells exposed to sustained levels of high glucose, promoted cell viability, and corneal epithelial wound healing through EGFR/ERK1/2 signaling pathway activation [176]. This mechanism, played by PACAP, confirms the key role of EGFR in corneal epithelial homeostasis [177]. In accord, the delay in corneal epithelial healing seems to be also associated with alterations in cell signaling of EGFR due to the hyperglycemic environment [178]. In fact, diabetes compromises EGFR signaling, acting at the level of the receptor, and the addition of ligand (EGF) is not sufficient to boost downstream signaling pathways [25]. Of note, hyperglycemia perturbs not only EGFR phosphorylation but also its downstream effectors, such as ERK1/2. The reduction of ERK1/2 signaling is correlated to the increase in apoptosis and decrease in cell proliferation concurring with the impairment of corneal epithelial wound healing in diabetic corneas [179]. Therefore, PACAP by stimulating EGFR/ERK1/2 signaling activity could promote the physiological state of corneal epithelium damaged by the hyperglycemic environment.
In the cornea, NAP treatment was shown to reduce the generation of reactive oxygen species (ROS) by decreasing UV-B-irradiation-induced apoptotic cell death and c-Jun NH2-terminal kinase (JNK) signaling pathway activation [51]. It is well known that the pathological mechanism in diabetes is sustained by hyperglycemia, which in turn is responsible for excess oxidative stress, due to the increase in ROS and altered antioxidant capabilities [180]. Otherwise, the reduction of ROS has been shown to reduce diabetes complications, including complications in the cornea [181]. Therefore, NAP’s ability to counteract the generation of ROS sets the stage for investigating its effect against hyperglycemia-induced ROS. Furthermore, NAP mitigated the UV-B-induced inflammatory event and the subsequent epithelial corneal barrier damage by reducing the expression of IL-1β in corneal epithelial cells, which affects NF-κB activation and protects the integrity of the corneal epithelial barrier [182]. It is important to underline that, in addition to epithelial cells, keratocytes, and endothelial cells being present in the cornea, so, too, are antigen-presenting cells (APCs) comprising dendritic cells and macrophages [183]. In the cornea of patients with DM, APCs acquire an immunostimulatory role, inducing immune cell activation with an increase in graft failure risk [184]. Therefore, it will be important to analyze the role of NAP on APCs, since these could represent a key target in the management of hyperglycemia-induced corneal epithelial inflammation. In light of the protective effects performed by NAP in the cornea, and given that treatment with this peptide exerted beneficial effects in in vitro and in vivo models of diabetes, it is desirable to test NAP or its analogue as a treatment for DK [163,185].
5. Conclusions and Future Directions
Diabetes mellitus is a global epidemic and its ocular complications are very common. Long-term poor glycemic control in patients with diabetes may be associated with morphological and functional corneal alterations, particularly affecting nerve terminations, endothelial, and epithelial cells, as well as microvascular changes including capillary remodeling, regression, and decreased density [186,187,188]. Recent studies showed a potential protective role in DK exerted by VIP family members. After all, it is well known that VIP and PACAP have therapeutic potential in diabetes by stimulating insulin secretion in a glucose-dependent manner and promoting glucagon secretion [69]. Moreover, both peptides induced the transactivation of EGFR [113,189], whose signaling is drastically compromised by the hyperglycemia microenvironment [178]. Alongside VIP and PACAP, ADNP has also been shown to have a protective role against diabetic complications. Its active fragment NAP was used as a preventative treatment for CNS complications in a diabetes rat model and it was shown to protect the retina against early diabetic injury [97,170]. To date, the limits characterizing the clinical use of these peptides, including short half-time due to rapid enzymatic degradation and poor in vivo stability, can be easily overcome. A possible strategy is the development of innovative nanoformulation platforms for topical VIP family members delivery, or the use of appropriate peptides-enriched scaffolds able to promote corneal tissue regeneration and restore normal tissue function in vivo [139,140]. Also, not to be underestimated are the differences in the expression of neuropeptide receptor isoforms and the cell-type specific differences in downstream effectors. Therefore, the synthesis of molecules able to activate a specific receptor isoform remains a great promise for developing new therapies considering the positive effects played by VIP, PACAP, and ADNP could be beneficially valuable as a treatment for DK.
Author Contributions
Conceptualization, G.M. and V.D.; writing—original draft preparation, G.M.; writing—review and editing, G.M., A.G.D., B.M. and V.D.; visualization, A.G.D. and B.M.; supervision, V.D.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Education, University and Research (MUR) PRIN PNRR 2022, grant number P2022JRBMB, SmarthyVision.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Purushothaman, I.; Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Ocular surface complications in diabetes: The interrelationship between insulin and enkephalin. Biochem. Pharmacol. 2021, 192, 114712. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef]
- Singer, M.; O’Brien, P.; Mein, L.; Olvera, A. Corneal Sensitivity Is Inversely Correlated with Severity of Diabetic Retinopathy in a Predominantly Underrepresented Population. Am. J. Ophthalmol. 2023, 259, 53–61. [Google Scholar] [CrossRef]
- Salami, M.O.; Aribaba, O.T.; Musa, K.O.; Rotimi-Samuel, A.; Onakoya, A.O. Relationship between corneal sensitivity and diabetic retinopathy among diabetics attending a Nigerian Teaching Hospital. Int. Ophthalmol. 2020, 40, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Tey, K.Y.; Teo, K.; Tan, A.C.S.; Devarajan, K.; Tan, B.; Tan, J.; Schmetterer, L.; Ang, M. Optical coherence tomography angiography in diabetic retinopathy: A review of current applications. Eye Vis. 2019, 6, 37. [Google Scholar] [CrossRef]
- D’Aloisio, R.; Giglio, R.; Di Nicola, M.; De Giacinto, C.; Pastore, M.R.; Tognetto, D.; Peto, T. Diagnostic Accuracy of Digital Retinal Fundus Image Analysis in Detecting Diabetic Maculopathy in Type 2 Diabetes Mellitus. Ophthalmic Res. 2019, 61, 100–106. [Google Scholar] [CrossRef]
- Ang, M.; Tan, A.C.S.; Cheung, C.M.G.; Keane, P.A.; Dolz-Marco, R.; Sng, C.C.A.; Schmetterer, L. Optical coherence tomography angiography: A review of current and future clinical applications. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 237–245. [Google Scholar] [CrossRef]
- Ferrara, M.; Loda, A.; Coco, G.; Grassi, P.; Cestaro, S.; Rezzola, S.; Romano, V.; Semeraro, F. Diabetic Retinopathy: Soluble and Imaging Ocular Biomarkers. J. Clin. Med. 2023, 12, 912. [Google Scholar] [CrossRef]
- Matulevičiūtė, I.; Sidaraitė, A.; Tatarūnas, V.; Veikutienė, A.; Dobilienė, O.; Žaliūnienė, D. Retinal and Choroidal Thinning-A Predictor of Coronary Artery Occlusion? Diagnostics 2022, 12, 2016. [Google Scholar] [CrossRef]
- Ong, C.J.T.; Wong, M.Y.Z.; Cheong, K.X.; Zhao, J.; Teo, K.Y.C.; Tan, T.E. Optical Coherence Tomography Angiography in Retinal Vascular Disorders. Diagnostics 2023, 13, 1620. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Camacho, P. The role of Optical Coherence Tomography Angiography to detect early microvascular changes in Diabetic Retinopathy: A systematic review. J. Diabetes Metab. Disord. 2021, 20, 1957–1974. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.L.; Craig, J.P.; Patel, D.V.; McGhee, C.N.; Pradhan, M.; Ellyett, K.; Kilfoyle, D.; Braatvedt, G.D. In Vivo Confocal Microscopy of Corneal Nerves: An Ocular Biomarker for Peripheral and Cardiac Autonomic Neuropathy in Type 1 Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5060–5065. [Google Scholar] [CrossRef] [PubMed]
- Cosmo, E.; Midena, G.; Frizziero, L.; Bruno, M.; Cecere, M.; Midena, E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J. Clin. Med. 2022, 11, 5130. [Google Scholar] [CrossRef]
- Mansoor, H.; Tan, H.C.; Lin, M.T.; Mehta, J.S.; Liu, Y.C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef]
- Dewitt, E.N. The Histopathology of Bowman’s Membrane. Trans. Am. Ophthalmol. Soc. 1931, 29, 461–485. [Google Scholar]
- Bron, A.J. The architecture of the corneal stroma. Br. J. Ophthalmol. 2001, 85, 379–381. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Kenyon, B.M.; Hamrah, P. Immunomodulatory Role of Neuropeptides in the Cornea. Biomedicines 2022, 10, 1985. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Lane, J.D.; Krumholz, D.M.; Sack, R.A.; Morris, C. Tear glucose dynamics in diabetes mellitus. Curr. Eye Res. 2006, 31, 895–901. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Sohn, E.; Jeong, I.H.; Kim, H.; Kim, J.S. Involvement of advanced glycation end products, oxidative stress and nuclear factor-kappaB in the development of diabetic keratopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 529–536. [Google Scholar] [CrossRef]
- Zhu, L.; Titone, R.; Robertson, D.M. The impact of hyperglycemia on the corneal epithelium: Molecular mechanisms and insight. Ocul. Surf. 2019, 17, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A. Effects of diabetes on the eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, Orsf81–Orsf87. [Google Scholar] [CrossRef]
- Yeung, A.; Dwarakanathan, S. Diabetic keratopathy. Dis. Mon. 2021, 67, 101135. [Google Scholar] [CrossRef]
- Shi, L.; Chen, H.; Yu, X.; Wu, X. Advanced glycation end products delay corneal epithelial wound healing through reactive oxygen species generation. Mol. Cell. Biochem. 2013, 383, 253–259. [Google Scholar] [CrossRef]
- Gekka, M.; Miyata, K.; Nagai, Y.; Nemoto, S.; Sameshima, T.; Tanabe, T.; Maruoka, S.; Nakahara, M.; Kato, S.; Amano, S. Corneal epithelial barrier function in diabetic patients. Cornea 2004, 23, 35–37. [Google Scholar] [CrossRef]
- Dan, J.; Zhou, Q.; Zhai, H.; Cheng, J.; Wan, L.; Ge, C.; Xie, L. Clinical analysis of fungal keratitis in patients with and without diabetes. PLoS ONE 2018, 13, e0196741. [Google Scholar] [CrossRef]
- Simpson, R.G.; Moshirfar, M.; Edmonds, J.N.; Christiansen, S.M. Laser in-situ keratomileusis in patients with diabetes mellitus: A review of the literature. Clin. Ophthalmol. 2012, 6, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Javadi, M.A.; Zarei-Ghanavati, S. Cataracts in diabetic patients: A review article. J. Ophthalmic. Vis. Res. 2008, 3, 52–65. [Google Scholar] [PubMed]
- Sitompul, R. Corneal Sensitivity as a Potential Marker of Diabetic Neuropathy. Acta Med. Indones 2017, 49, 166–172. [Google Scholar] [PubMed]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benítez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Zhou, T.; Lee, A.; Lo, A.C.Y.; Kwok, J. Diabetic Corneal Neuropathy: Pathogenic Mechanisms and Therapeutic Strategies. Front. Pharmacol. 2022, 13, 816062. [Google Scholar] [CrossRef]
- Bu, Y.; Shih, K.C.; Tong, L. The ocular surface and diabetes, the other 21st Century epidemic. Exp. Eye Res. 2022, 220, 109099. [Google Scholar] [CrossRef]
- Lewis, E.J.H.; Lovblom, L.E.; Ferdousi, M.; Halpern, E.M.; Jeziorska, M.; Pacaud, D.; Pritchard, N.; Dehghani, C.; Edwards, K.; Srinivasan, S.; et al. Rapid Corneal Nerve Fiber Loss: A Marker of Diabetic Neuropathy Onset and Progression. Diabetes Care 2020, 43, 1829–1835. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Wang, Q.; Li, Y.; Wei, C.; Xie, L. Mechanistic investigations of diabetic ocular surface diseases. Front. Endocrinol. 2022, 13, 1079541. [Google Scholar] [CrossRef]
- Zou, C.; Wang, S.; Huang, F.; Zhang, Y.A. Advanced glycation end products and ultrastructural changes in corneas of long-term streptozotocin-induced diabetic monkeys. Cornea 2012, 31, 1455–1459. [Google Scholar] [CrossRef]
- Inoue, K.; Kato, S.; Inoue, Y.; Amano, S.; Oshika, T. The corneal endothelium and thickness in type II diabetes mellitus. Jpn. J. Ophthalmol. 2002, 46, 65–69. [Google Scholar] [CrossRef]
- Abdelkader, H.; Patel, D.V.; McGhee, C.; Alany, R.G. New therapeutic approaches in the treatment of diabetic keratopathy: A review. Clin. Exp. Ophthalmol. 2011, 39, 259–270. [Google Scholar] [CrossRef]
- Strand, F.L. Neuropeptides: General characteristics and neuropharmaceutical potential in treating CNS disorders. Prog. Drug Res. 2003, 61, 1–37. [Google Scholar] [CrossRef]
- Amram, N.; Hacohen-Kleiman, G.; Sragovich, S.; Malishkevich, A.; Katz, J.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.C.; Giladi, E.; Yeheskel, A.; et al. Sexual divergence in microtubule function: The novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol. Psychiatry 2016, 21, 1467–1476. [Google Scholar] [CrossRef]
- Li, C.; Kim, K. Neuropeptides. WormBook 2008, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.W.; Waschek, J.A. Corneal endothelial cell survival in organ cultures under acute oxidative stress: Effect of VIP. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4085–4092. [Google Scholar]
- Sacchetti, M.; Lambiase, A. Neurotrophic factors and corneal nerve regeneration. Neural Regen. Res. 2017, 12, 1220–1224. [Google Scholar] [CrossRef]
- Koh, S.W. Corneal endothelial autocrine trophic factor VIP in a mechanism-based strategy to enhance human donor cornea preservation for transplantation. Exp. Eye Res. 2012, 95, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Alm, P.; Håkanson, R. Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience 1995, 69, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Giunta, S.; Giallongo, C.; Tibullo, D.; Bucolo, C.; Saccone, S.; Federico, C.; Scollo, D.; Longo, A.; et al. Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) Counteracts UV-B Radiation-Induced ROS Formation in Corneal Epithelium. Antioxidants 2022, 11, 128. [Google Scholar] [CrossRef]
- Beckers, H.J.; Klooster, J.; Vrensen, G.F.; Lamers, W.P. Substance P in rat corneal and iridal nerves: An ultrastructural immunohistochemical study. Ophthalmic Res. 1993, 25, 192–200. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakayasu, K.; Iwatsu, M.; Kanai, A. Endogenous substance P in corneal epithelial cells and keratocytes. Jpn. J. Ophthalmol. 2002, 46, 616–620. [Google Scholar] [CrossRef]
- Lambiase, A.; Micera, A.; Sacchetti, M.; Cortes, M.; Mantelli, F.; Bonini, S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol. 2011, 129, 981–986. [Google Scholar] [CrossRef]
- Jones, M.A.; Marfurt, C.F. Peptidergic innervation of the rat cornea. Exp. Eye Res. 1998, 66, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Kangawa, K.; Eto, T. Adrenomedullin and PAMP: Discovery, structures, and cardiovascular functions. Microsc. Res. Tech. 2002, 57, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Słoniecka, M.; Le Roux, S.; Boman, P.; Byström, B.; Zhou, Q.; Danielson, P. Expression Profiles of Neuropeptides, Neurotransmitters, and Their Receptors in Human Keratocytes In Vitro and In Situ. PLoS ONE 2015, 10, e0134157. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C. Molecular pharmacology of somatostatin receptor subtypes. J. Endocrinol. Investig. 1997, 20, 348–367. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, P.W.; Fridland, G.H.; Killmar, J.T.; Desiderio, D.M. Purification, characterization, and localization of neuropeptides in the cornea. Peptides 1988, 9, 1373–1379. [Google Scholar] [CrossRef]
- Schrödl, F.; Kaser-Eichberger, A.; Trost, A.; Strohmaier, C.; Bogner, B.; Runge, C.; Bruckner, D.; Motloch, K.; Holub, B.; Kofler, B.; et al. Distribution of galanin receptors in the human eye. Exp. Eye Res. 2015, 138, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Rondeau, N.; Paquet, S.; Forgez, P.; Lombet, A.; Pouzaud, F.; Rostène, W.; Borderie, V.; Laroche, L. Expression of neurotensin receptors in human corneal keratocytes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1765–1771. [Google Scholar]
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069. [Google Scholar]
- Zagon, I.S.; Verderame, M.F.; McLaughlin, P.J. The biology of the opioid growth factor receptor (OGFr). Brain Res. Brain Res. Rev. 2002, 38, 351–376. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Sassani, J.W.; Allison, G.; McLaughlin, P.J. Conserved expression of the opioid growth factor, [Met5]enkephalin, and the zeta (zeta) opioid receptor in vertebrate cornea. Brain Res. 1995, 671, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Said, S.I.; Mutt, V. Polypeptide with broad biological activity: Isolation from small intestine. Science 1970, 169, 1217–1218. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Sawmiller, D.R. Vasoactive intestinal peptide: Cardiovascular effects. Cardiovasc. Res. 2001, 49, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Couvineau, A.; Laburthe, M. VPAC receptors: Structure, molecular pharmacology and interaction with accessory proteins. Br. J. Pharmacol. 2012, 166, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: Structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. Role of VIP and PACAP in islet function. Peptides 2007, 28, 1805–1813. [Google Scholar] [CrossRef]
- Fabricius, D.; Karacay, B.; Shutt, D.; Leverich, W.; Schafer, B.; Takle, E.; Thedens, D.; Khanna, G.; Raikwar, S.; Yang, B.; et al. Characterization of intestinal and pancreatic dysfunction in VPAC1-null mutant mouse. Pancreas 2011, 40, 861–871. [Google Scholar] [CrossRef]
- Bertrand, G.; Puech, R.; Maisonnasse, Y.; Bockaert, J.; Loubatières-Mariani, M.M. Comparative effects of PACAP and VIP on pancreatic endocrine secretions and vascular resistance in rat. Br. J. Pharmacol. 1996, 117, 764–770. [Google Scholar] [CrossRef]
- Hou, X.; Yang, D.; Yang, G.; Li, M.; Zhang, J.; Zhang, J.; Zhang, Y.; Liu, Y. Therapeutic potential of vasoactive intestinal peptide and its receptor VPAC2 in type 2 diabetes. Front. Endocrinol. 2022, 13, 984198. [Google Scholar] [CrossRef]
- Vu, J.P.; Larauche, M.; Flores, M.; Luong, L.; Norris, J.; Oh, S.; Liang, L.J.; Waschek, J.; Pisegna, J.R.; Germano, P.M. Regulation of Appetite, Body Composition, and Metabolic Hormones by Vasoactive Intestinal Polypeptide (VIP). J. Mol. Neurosci. 2015, 56, 377–387. [Google Scholar] [CrossRef]
- Gozes, I. VIP, from gene to behavior and back: Summarizing my 25 years of research. J. Mol. Neurosci. 2008, 36, 115–124. [Google Scholar] [CrossRef]
- Hill, J.M.; Hauser, J.M.; Sheppard, L.M.; Abebe, D.; Spivak-Pohis, I.; Kushnir, M.; Deitch, I.; Gozes, I. Blockage of VIP during mouse embryogenesis modifies adult behavior and results in permanent changes in brain chemistry. J. Mol. Neurosci. 2007, 31, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Said, S.I.; Mutt, V. Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature 1970, 225, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; Souza-Moreira, L.; González-Rey, E. VIP in neurological diseases: More than a neuropeptide. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Nuche-Berenguer, B.; Jensen, R.T. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Gozes, I. Vasoactive intestinal peptide receptors: A molecular target in breast and lung cancer. Curr. Pharm. Des. 2007, 13, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. PACAP and VIP regulate hypoxia-inducible factors in neuroblastoma cells exposed to hypoxia. Neuropeptides 2018, 69, 84–91. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.M.; Reitano, R.; Saccone, S.; Federico, C.; Magro, G.; D’Agata, V. Modulatory role of PACAP and VIP on HIFs expression in lung adenocarcinoma. Peptides 2021, 146, 170672. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Chorny, A.; Fernandez-Martin, A.; Ganea, D.; Delgado, M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood 2006, 107, 3632–3638. [Google Scholar] [CrossRef]
- Delgado, M.; Gonzalez-Rey, E.; Ganea, D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J. Immunol. 2005, 175, 7311–7324. [Google Scholar] [CrossRef] [PubMed]
- Dragich, J.M.; Loh, D.H.; Wang, L.M.; Vosko, A.M.; Kudo, T.; Nakamura, T.J.; Odom, I.H.; Tateyama, S.; Hagopian, A.; Waschek, J.A.; et al. The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur. J. Neurosci. 2010, 31, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Akrouh, A.; Kerschensteiner, D. Morphology and function of three VIP-expressing amacrine cell types in the mouse retina. J. Neurophysiol. 2015, 114, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Tunçel, N.; Başmak, H.; Uzuner, K.; Tunçel, M.; Altiokka, G.; Zaimoğlu, V.; Ozer, A.; Gürer, F. Protection of rat retina from ischemia-reperfusion injury by vasoactive intestinal peptide (VIP): The effect of VIP on lipid peroxidation and antioxidant enzyme activity of retina and choroid. Ann. N. Y. Acad. Sci. 1996, 805, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Danyadi, B.; Kiss, P.; Tamas, A.; Fabian, E.; Gabriel, R.; Reglodi, D. Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J. Mol. Neurosci. 2012, 48, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Carion, T.W.; Jiang, Y.; Steinle, J.J.; Berger, E.A. VIP protects human retinal microvascular endothelial cells against high glucose-induced increases in TNF-α and enhances RvD1. Prostaglandins Other Lipid Mediat. 2016, 123, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. PACAP and VIP Inhibit HIF-1α-Mediated VEGF Expression in a Model of Diabetic Macular Edema. J. Cell. Physiol. 2017, 232, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Gagliano, C.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. VIP Family Members Prevent Outer Blood Retinal Barrier Damage in a Model of Diabetic Macular Edema. J. Cell. Physiol. 2017, 232, 1079–1085. [Google Scholar] [CrossRef]
- Troger, J.; Neyer, S.; Heufler, C.; Huemer, H.; Schmid, E.; Griesser, U.; Kralinger, M.; Kremser, B.; Baldissera, I.; Kieselbach, G. Substance P and vasoactive intestinal polypeptide in the streptozotocin-induced diabetic rat retina. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1045–1050. [Google Scholar]
- Taylor, A.W.; Streilein, J.W.; Cousins, S.W. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J. Immunol. 1994, 153, 1080–1086. [Google Scholar] [CrossRef]
- Sacchetti, M.; Micera, A.; Lambiase, A.; Speranza, S.; Mantelli, F.; Petrachi, G.; Bonini, S.; Bonini, S. Tear levels of neuropeptides increase after specific allergen challenge in allergic conjunctivitis. Mol. Vis. 2011, 17, 47–52. [Google Scholar]
- Berger, E.A.; Vistisen, K.S.; Barrett, R.P.; Hazlett, L.D. Effects of VIP on corneal reconstitution and homeostasis following Pseudomonas aeruginosa induced keratitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7432–7439. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.M.; Coll, T.; Gloria, D.; Sprehe, N. Corneal Endothelial Cell Integrity in Precut Human Donor Corneas Enhanced by Autocrine Vasoactive Intestinal Peptide. Cornea 2017, 36, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Szliter, E.A.; Lighvani, S.; Barrett, R.P.; Hazlett, L.D. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J. Immunol. 2007, 178, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; McClellan, S.A.; Barrett, R.P.; Zhang, Y.; Hazlett, L.D. Vasoactive intestinal peptide downregulates proinflammatory TLRs while upregulating anti-inflammatory TLRs in the infected cornea. J. Immunol. 2012, 189, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A.; Somogyvári-Vigh, A.; Miyata, A.; Mizuno, K.; Coy, D.H.; Kitada, C. Tissue distribution of PACAP as determined by RIA: Highly abundant in the rat brain and testes. Endocrinology 1991, 129, 2787–2789. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A.; Shioda, S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: Neuroendocrine and endocrine interaction. Front. Neuroendocrinol. 1995, 16, 53–88. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, T.; Grimaldi, M.; Eiden, L.E. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J. Biol. Chem. 2007, 282, 8079–8091. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.; Finlayson, K. VPAC and PAC receptors: From ligands to function. Pharmacol. Ther. 2009, 121, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Reglodi, D.; Mester, L.; Szabo, A.; Szabadfi, K.; Tamas, A.; Toth, G.; Kovacs, K. Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J. Mol. Neurosci. 2012, 48, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bassan, M.; Zamostiano, R.; Davidson, A.; Pinhasov, A.; Giladi, E.; Perl, O.; Bassan, H.; Blat, C.; Gibney, G.; Glazner, G.; et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999, 72, 1283–1293. [Google Scholar] [CrossRef]
- Zamostiano, R.; Pinhasov, A.; Gelber, E.; Steingart, R.A.; Seroussi, E.; Giladi, E.; Bassan, M.; Wollman, Y.; Eyre, H.J.; Mulley, J.C.; et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001, 276, 708–714. [Google Scholar] [CrossRef]
- Magrì, B.; D’Amico, A.G.; Maugeri, G.; Morello, G.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Neuroprotective effect of the PACAP-ADNP axis on SOD1G93A mutant motor neuron death induced by trophic factors deprivation. Neuropeptides 2023, 102, 102386. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Musumeci, G.; Reglodi, D.; D’Agata, V. PACAP and NAP: Effect of Two Functionally Related Peptides in Diabetic Retinopathy. J. Mol. Neurosci. 2021, 71, 1525–1535. [Google Scholar] [CrossRef]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Bombesin, endothelin, neurotensin and pituitary adenylate cyclase activating polypeptide cause tyrosine phosphorylation of receptor tyrosine kinases. Peptides 2021, 137, 170480. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Reitano, R.; Magro, G.; Cavallaro, S.; Salomone, S.; D’Agata, V. PACAP and VIP Inhibit the Invasiveness of Glioblastoma Cells Exposed to Hypoxia through the Regulation of HIFs and EGFR Expression. Front. Pharmacol. 2016, 7, 139. [Google Scholar] [CrossRef]
- Moody, T.W.; Lee, L.; Jensen, R.T. The G Protein-Coupled Receptor PAC1 Regulates Transactivation of the Receptor Tyrosine Kinase HER3. J. Mol. Neurosci. 2021, 71, 1589–1597. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Bucolo, C.; D’Agata, V. Protective effect of PACAP-38 on retinal pigmented epithelium in an in vitro and in vivo model of diabetic retinopathy through EGFR-dependent mechanism. Peptides 2019, 119, 170108. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Federico, C.; Saccone, S.; Morello, G.; La Cognata, V.; Cavallaro, S.; D’Agata, V. Molecular mechanisms involved in the protective effect of pituitary adenylate cyclase-activating polypeptide in an in vitro model of amyotrophic lateral sclerosis. J. Cell. Physiol. 2019, 234, 5203–5214. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Castrogiovanni, P.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. PACAP through EGFR transactivation preserves human corneal endothelial integrity. J. Cell. Biochem. 2019, 120, 10097–10105. [Google Scholar] [CrossRef]
- Farkas, J.; Kovács, L.; Gáspár, L.; Nafz, A.; Gaszner, T.; Ujvári, B.; Kormos, V.; Csernus, V.; Hashimoto, H.; Reglődi, D.; et al. Construct and face validity of a new model for the three-hit theory of depression using PACAP mutant mice on CD1 background. Neuroscience 2017, 354, 11–29. [Google Scholar] [CrossRef]
- Gupta, A.; Gargiulo, A.T.; Curtis, G.R.; Badve, P.S.; Pandey, S.; Barson, J.R. Pituitary Adenylate Cyclase-Activating Polypeptide-27 (PACAP-27) in the Thalamic Paraventricular Nucleus Is Stimulated by Ethanol Drinking. Alcohol. Clin. Exp. Res. 2018, 42, 1650–1660. [Google Scholar] [CrossRef]
- Han, P.; Tang, Z.; Yin, J.; Maalouf, M.; Beach, T.G.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol. Aging 2014, 35, 2064–2071. [Google Scholar] [CrossRef]
- King, S.B.; Lezak, K.R.; O’Reilly, M.; Toufexis, D.J.; Falls, W.A.; Braas, K.; May, V.; Hammack, S.E. The Effects of Prior Stress on Anxiety-Like Responding to Intra-BNST Pituitary Adenylate Cyclase Activating Polypeptide in Male and Female Rats. Neuropsychopharmacology 2017, 42, 1679–1687. [Google Scholar] [CrossRef][Green Version]
- Heppner, T.J.; Hennig, G.W.; Nelson, M.T.; May, V.; Vizzard, M.A. PACAP38-Mediated Bladder Afferent Nerve Activity Hyperexcitability and Ca(2+) Activity in Urothelial Cells from Mice. J. Mol. Neurosci. 2019, 68, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.L.; May, V. PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability. J. Mol. Neurosci. 2019, 68, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Illes, A.; Opper, B.; Schafer, E.; Tamas, A.; Horvath, G. Presence and Effects of Pituitary Adenylate Cyclase Activating Polypeptide Under Physiological and Pathological Conditions in the Stomach. Front. Endocrinol. 2018, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Bardosi, S.; Bardosi, A.; Nagy, Z.; Reglodi, D. Expression of PACAP and PAC1 Receptor in Normal Human Thyroid Gland and in Thyroid Papillary Carcinoma. J. Mol. Neurosci. 2016, 60, 171–178. [Google Scholar] [CrossRef]
- Egri, P.; Fekete, C.; Dénes, Á.; Reglődi, D.; Hashimoto, H.; Fülöp, B.D.; Gereben, B. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Regulates the Hypothalamo-Pituitary-Thyroid (HPT) Axis via Type 2 Deiodinase in Male Mice. Endocrinology 2016, 157, 2356–2366. [Google Scholar] [CrossRef]
- Prevost, G.; Arabo, A.; Jian, L.; Quelennec, E.; Cartier, D.; Hassan, S.; Falluel-Morel, A.; Tanguy, Y.; Gargani, S.; Lihrmann, I.; et al. The PACAP-regulated gene selenoprotein T is abundantly expressed in mouse and human β-cells and its targeted inactivation impairs glucose tolerance. Endocrinology 2013, 154, 3796–3806. [Google Scholar] [CrossRef]
- Sasaki, S.; Watanabe, J.; Ohtaki, H.; Matsumoto, M.; Murai, N.; Nakamachi, T.; Hannibal, J.; Fahrenkrug, J.; Hashimoto, H.; Watanabe, H.; et al. Pituitary adenylate cyclase-activating polypeptide promotes eccrine gland sweat secretion. Br. J. Dermatol. 2017, 176, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Lajko, A.; Meggyes, M.; Fulop, B.D.; Gede, N.; Reglodi, D.; Szereday, L. Comparative analysis of decidual and peripheral immune cells and immune-checkpoint molecules during pregnancy in wild-type and PACAP-deficient mice. Am. J. Reprod. Immunol. 2018, 80, e13035. [Google Scholar] [CrossRef]
- Reglodi, D.; Tamas, A.; Koppan, M.; Szogyi, D.; Welke, L. Role of PACAP in Female Fertility and Reproduction at Gonadal Level-Recent Advances. Front. Endocrinol. 2012, 3, 155. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A.; Leon, S.; Madara, J.C.; Schafer, D.; Fergani, C.; Maguire, C.A.; Verstegen, A.M.; Brengle, E.; Kong, D.; Herbison, A.E.; et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. Elife 2018, 7, e35960. [Google Scholar] [CrossRef]
- Fulop, B.D.; Sandor, B.; Szentleleky, E.; Karanyicz, E.; Reglodi, D.; Gaszner, B.; Zakany, R.; Hashimoto, H.; Juhasz, T.; Tamas, A. Altered Notch Signaling in Developing Molar Teeth of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)-Deficient Mice. J. Mol. Neurosci. 2019, 68, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Jungling, A.; Longuespée, R.; Kriegsmann, J.; Casadonte, R.; Kriegsmann, M.; Juhasz, T.; Bardosi, S.; Tamas, A.; Fulop, B.D.; et al. Accelerated pre-senile systemic amyloidosis in PACAP knockout mice—A protective role of PACAP in age-related degenerative processes. J. Pathol. 2018, 245, 478–490. [Google Scholar] [CrossRef]
- Watanabe, J.; Nakamachi, T.; Matsuno, R.; Hayashi, D.; Nakamura, M.; Kikuyama, S.; Nakajo, S.; Shioda, S. Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain development. Peptides 2007, 28, 1713–1719. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of Pacap on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef]
- D’Agata, V.; Cavallaro, S. Functional and molecular expression of PACAP/VIP receptors in the rat retina. Brain Res. Mol. Brain Res. 1998, 54, 161–164. [Google Scholar] [CrossRef]
- Patko, E.; Szabo, E.; Toth, D.; Tornoczky, T.; Bosnyak, I.; Vaczy, A.; Atlasz, T.; Reglodi, D. Distribution of PACAP and PAC1 Receptor in the Human Eye. J. Mol. Neurosci. 2022, 72, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Shioda, S.; Ogino, D.; Nakai, Y.; Arimura, A.; Koide, R. Distribution and ultrastructural localization of a receptor for pituitary adenylate cyclase activating polypeptide and its mRNA in the rat retina. Neurosci. Lett. 1997, 238, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Izumi, S.; Shioda, S.; Zhou, C.J.; Arimura, A.; Koide, R. Gene expression for PACAP receptor mRNA in the rat retina by in situ hybridization and in situ RT-PCR. Ann. N. Y. Acad. Sci. 2000, 921, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Matkovits, A.; Seki, T.; Shioda, S. Distribution and protective function of pituitary adenylate cyclase-activating polypeptide in the retina. Front. Endocrinol. 2012, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.G.; Maugeri, G.; Magrì, B.; Lombardo, C.; Saccone, S.; Federico, C.; Cavallaro, P.; Giunta, S.; Bucolo, C.; D’Agata, V. Pacap-adnp axis prevents outer retinal barrier breakdown and choroidal neovascularization by interfering with vegf secreted from retinal pigmented epitelium cells. Peptides 2023, 168, 171065. [Google Scholar] [CrossRef] [PubMed]
- Elsås, T.; Uddman, R.; Sundler, F. Pituitary adenylate cyclase-activating peptide-immunoreactive nerve fibers in the cat eye. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 573–580. [Google Scholar] [CrossRef]
- Maugeri, G.; Longo, A.; D’Amico, A.G.; Rasà, D.M.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; D’Agata, V. Trophic effect of PACAP on human corneal endothelium. Peptides 2018, 99, 20–26. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Amenta, A.; Saccone, S.; Federico, C.; Reibaldi, M.; Russo, A.; Bonfiglio, V.; Avitabile, T.; Longo, A.; et al. Protective effect of PACAP against ultraviolet B radiation-induced human corneal endothelial cell injury. Neuropeptides 2020, 79, 101978. [Google Scholar] [CrossRef]
- Fukiage, C.; Nakajima, T.; Takayama, Y.; Minagawa, Y.; Shearer, T.R.; Azuma, M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am. J. Ophthalmol. 2007, 143, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, J.; Chen, X.; Hong, A. Expression, identification and biological effects of the novel recombination protein, PACAP38-NtA, with high bioactivity. Int. J. Mol. Med. 2015, 35, 376–382. [Google Scholar] [CrossRef]
- Shioda, S.; Takenoya, F.; Hirabayashi, T.; Wada, N.; Seki, T.; Nonaka, N.; Nakamachi, T. Effects of PACAP on Dry Eye Symptoms, and Possible Use for Therapeutic Application. J. Mol. Neurosci. 2019, 68, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Ohtaki, H.; Seki, T.; Yofu, S.; Kagami, N.; Hashimoto, H.; Shintani, N.; Baba, A.; Mark, L.; Lanekoff, I.; et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat. Commun. 2016, 7, 12034. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Waldeck, K.; Grundemar, L.; Håkanson, R. Ocular inflammation induced by electroconvulsive treatment: Contribution of nitric oxide and neuropeptides mobilized from C-fibres. Br. J. Pharmacol. 1997, 120, 1491–1496. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Alm, P.; Håkanson, R. PACAP occurs in sensory nerve fibers and participates in ocular inflammation in the rabbit. Ann. N. Y. Acad. Sci. 1996, 805, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Danielsen, N.; Sundler, F.; Mulder, H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport 1998, 9, 2833–2836. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shintani, N.; Baba, A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann. N. Y. Acad. Sci. 2006, 1070, 75–89. [Google Scholar] [CrossRef]
- Mandel, S.; Gozes, I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J. Biol. Chem. 2007, 282, 34448–34456. [Google Scholar] [CrossRef]
- Gozes, I. The ADNP Syndrome and CP201 (NAP) Potential and Hope. Front. Neurol. 2020, 11, 608444. [Google Scholar] [CrossRef]
- Mandel, S.; Rechavi, G.; Gozes, I. Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev. Biol. 2007, 303, 814–824. [Google Scholar] [CrossRef]
- Helsmoortel, C.; Vulto-van Silfhout, A.T.; Coe, B.P.; Vandeweyer, G.; Rooms, L.; van den Ende, J.; Schuurs-Hoeijmakers, J.H.; Marcelis, C.L.; Willemsen, M.H.; Vissers, L.E.; et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat. Genet. 2014, 46, 380–384. [Google Scholar] [CrossRef]
- Pascolini, G.; Agolini, E.; Majore, S.; Novelli, A.; Grammatico, P.; Digilio, M.C. Helsmoortel-Van der Aa Syndrome as emerging clinical diagnosis in intellectually disabled children with autistic traits and ocular involvement. Eur. J. Paediatr. Neurol. 2018, 22, 552–557. [Google Scholar] [CrossRef]
- Levine, J.; Cohen, D.; Herman, C.; Verloes, A.; Guinchat, V.; Diaz, L.; Cravero, C.; Mandel, A.; Gozes, I. Developmental Phenotype of the Rare Case of DJ Caused by a Unique ADNP Gene De Novo Mutation. J. Mol. Neurosci. 2019, 68, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.J.; Titus, H.E.; Harman, G.A.; Alabduljalil, T.; Dennis, A.; Wilson, J.L.; Koeller, D.M.; Finanger, E.; Blasco, P.A.; Chiang, P.W.; et al. Longitudinal ophthalmic findings in a child with Helsmoortel-Van der Aa Syndrome. Am. J. Ophthalmol. Case Rep. 2018, 10, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Gozes, I.; Ivashko-Pachima, Y. ADNP: In search for molecular mechanisms and innovative therapeutic strategies for frontotemporal degeneration. Front. Aging Neurosci. 2015, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Magen, I.; Gozes, I. Davunetide: Peptide therapeutic in neurological disorders. Curr. Med. Chem. 2014, 21, 2591–2598. [Google Scholar] [CrossRef]
- Oz, S.; Kapitansky, O.; Ivashco-Pachima, Y.; Malishkevich, A.; Giladi, E.; Skalka, N.; Rosin-Arbesfeld, R.; Mittelman, L.; Segev, O.; Hirsch, J.A.; et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol. Psychiatry 2014, 19, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Tortosa, E.; Bollati, F.; Ramírez-Ríos, S.; Arnal, I.; Avila, J. Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells. J. Neurochem. 2015, 133, 653–667. [Google Scholar] [CrossRef]
- Ivashko-Pachima, Y.; Sayas, C.L.; Malishkevich, A.; Gozes, I. ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: A novel avenue for protection against tauopathy. Mol. Psychiatry 2017, 22, 1335–1344. [Google Scholar] [CrossRef]
- D’Incal, C.P.; Van Rossem, K.E.; De Man, K.; Konings, A.; Van Dijck, A.; Rizzuti, L.; Vitriolo, A.; Testa, G.; Gozes, I.; Vanden Berghe, W.; et al. Chromatin remodeler Activity-Dependent Neuroprotective Protein (ADNP) contributes to syndromic autism. Clin. Epigenetics 2023, 15, 45. [Google Scholar] [CrossRef]
- Teuchner, B.; Dimmer, A.; Humpel, C.; Amberger, A.; Fischer-Colbrie, R.; Nemeth, J.; Waschek, J.A.; Kieselbach, G.; Kralinger, M.; Schmid, E.; et al. VIP, PACAP-38, BDNF and ADNP in NMDA-induced excitotoxicity in the rat retina. Acta Ophthalmol. 2011, 89, 670–675. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Magrì, B.; Musumeci, G.; D’Agata, V. Activity-Dependent Neuroprotective Protein (ADNP): An Overview of Its Role in the Eye. Int. J. Mol. Sci. 2022, 23, 3654. [Google Scholar] [CrossRef] [PubMed]
- Lagrèze, W.A.; Pielen, A.; Steingart, R.; Schlunck, G.; Hofmann, H.D.; Gozes, I.; Kirsch, M. The peptides ADNF-9 and NAP increase survival and neurite outgrowth of rat retinal ganglion cells in vitro. Investig. Ophthalmol. Vis. Sci. 2005, 46, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Jehle, T.; Dimitriu, C.; Auer, S.; Knoth, R.; Vidal-Sanz, M.; Gozes, I.; Lagrèze, W.A. The neuropeptide NAP provides neuroprotection against retinal ganglion cell damage after retinal ischemia and optic nerve crush. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1255–1263. [Google Scholar] [CrossRef]
- Belokopytov, M.; Shulman, S.; Dubinsky, G.; Gozes, I.; Belkin, M.; Rosner, M. Ameliorative effect of NAP on laser-induced retinal damage. Acta Ophthalmol. 2011, 89, e126–e131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zeng, H.; She, H.; Liu, H.; Sun, N. Expression of peptide NAP in rat retinal Müller cells prevents hypoxia-induced retinal injuries and promotes retinal neurons growth. Biomed. Pharmacother. 2010, 64, 417–423. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.; Federico, C.; Saccone, S.; Lazzara, F.; Fidilio, A.; Drago, F.; Bucolo, C.; D’Agata, V. NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell. Physiol. 2019, 234, 5230–5240. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.M.; La Cognata, V.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. NAP counteracts hyperglycemia/hypoxia induced retinal pigment epithelial barrier breakdown through modulation of HIFs and VEGF expression. J. Cell. Physiol. 2018, 233, 1120–1128. [Google Scholar] [CrossRef]
- D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Saccone, S.; Federico, C.; Cavallaro, S.; D’Agata, V. Nap Interferes with Hypoxia-Inducible Factors and VEGF Expression in Retina of Diabetic Rats. J. Mol. Neurosci. 2017, 61, 256–266. [Google Scholar] [CrossRef]
- Ebrahim, A.S.; Carion, T.W.; Ebrahim, T.; Win, J.; Kani, H.; Wang, Y.; Stambersky, A.; Ibrahim, A.S.; Sosne, G.; Berger, E.A. A Novel Combination Therapy Tβ4/VIP Protects against Hyperglycemia-Induced Changes in Human Corneal Epithelial Cells. Biosensors 2023, 13, 974. [Google Scholar] [CrossRef]
- Martínez, C.; Juarranz, Y.; Gutiérrez-Cañas, I.; Carrión, M.; Pérez-García, S.; Villanueva-Romero, R.; Castro, D.; Lamana, A.; Mellado, M.; González-Álvaro, I.; et al. A Clinical Approach for the Use of VIP Axis in Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2019, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Gomariz, R.P.; Martinez, C.; Abad, C.; Leceta, J.; Delgado, M. Immunology of VIP: A review and therapeutical perspectives. Curr. Pharm. Des. 2001, 7, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Muragaki, Y.; Okada, Y.; Miyamoto, T.; Ohnishi, Y.; Ooshima, A.; Kao, W.W. Sonic hedgehog expression and role in healing corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, L.; Li, Y.; Sun, D.; Chen, R.; Dou, S.; Liu, T.; Zhang, S.; Zhou, Q.; Xie, L. Interference of sympathetic overactivation restores limbal stem/progenitor cells function and accelerates corneal epithelial wound healing in diabetic mice. Biomed. Pharmacother. 2023, 161, 114523. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Magrì, B.; Giunta, S.; Saccone, S.; Federico, C.; Bucolo, C.; Musumeci, G.; D’Agata, V. Protective effect of pituitary adenylate cyclase activating polypeptide in diabetic keratopathy. Peptides 2023, 170, 171107. [Google Scholar] [CrossRef] [PubMed]
- Sibilia, M.; Kroismayr, R.; Lichtenberger, B.M.; Natarajan, A.; Hecking, M.; Holcmann, M. The epidermal growth factor receptor: From development to tumorigenesis. Differentiation 2007, 75, 770–787. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.P.; Li, Y.; Ljubimov, A.V.; Yu, F.S. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 2009, 58, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yu, F.S. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3301–3308. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Kaili, D.; Freeman, J.; Lei, C.Y.; Geng, B.C.; Tan, T.; He, J.F.; Shi, Z.; Ma, J.J.; Luo, Y.H.; et al. Diabetes inhibits corneal epithelial cell migration and tight junction formation in mice and human via increasing ROS and impairing Akt signaling. Acta Pharmacol. Sin. 2019, 40, 1205–1211. [Google Scholar] [CrossRef]
- Ljubimov, A.V. Diabetic complications in the cornea. Vision Res. 2017, 139, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Magrì, B.; Giunta, S.; Musumeci, G.; Saccone, S.; Federico, C.; Scollo, D.; Longo, A.; Avitabile, T.; et al. Regulation of UV-B-Induced Inflammatory Mediators by Activity-Dependent Neuroprotective Protein (ADNP)-Derived Peptide (NAP) in Corneal Epithelium. Int. J. Mol. Sci. 2023, 24, 6895. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Blanco, T.; Musayeva, A.; Singh, R.B.; Nakagawa, H.; Lee, S.; Alemi, H.; Gonzalez-Nolasco, B.; Ortiz, G.; Wang, S.; Kahale, F.; et al. The impact of donor diabetes on corneal transplant immunity. Am. J. Transplant. 2023, 23, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Idan-Feldman, A.; Schirer, Y.; Polyzoidou, E.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.C.; Gozes, I. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol. Dis. 2011, 44, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Verma, A.; Alagorie, A.R. Association of severity of diabetic retinopathy with corneal endothelial and thickness changes in patients with diabetes mellitus. Eye 2022, 36, 1202–1208. [Google Scholar] [CrossRef]
- Saif, P.S.; Salman, A.E.G.; Omran, N.A.H.; Farweez, Y.A.T. Assessment of Diabetic Retinopathy Vascular Density Maps. Clin. Ophthalmol. 2020, 14, 3941–3953. [Google Scholar] [CrossRef]
- Buonfiglio, F.; Wasielica-Poslednik, J.; Pfeiffer, N.; Gericke, A. Diabetic Keratopathy: Redox Signaling Pathways and Therapeutic Prospects. Antioxidants 2024, 13, 120. [Google Scholar] [CrossRef]
- Valdehita, A.; Bajo, A.M.; Schally, A.V.; Varga, J.L.; Carmena, M.J.; Prieto, J.C. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol. Cell. Endocrinol. 2009, 302, 41–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).