Quantification of Heavy Metal Content in Anadara tuberculosa from the Gulf of Guayaquil Using ICP-OES: Assessing Marine Contamination
Abstract
1. Introduction
2. Materials and Methods
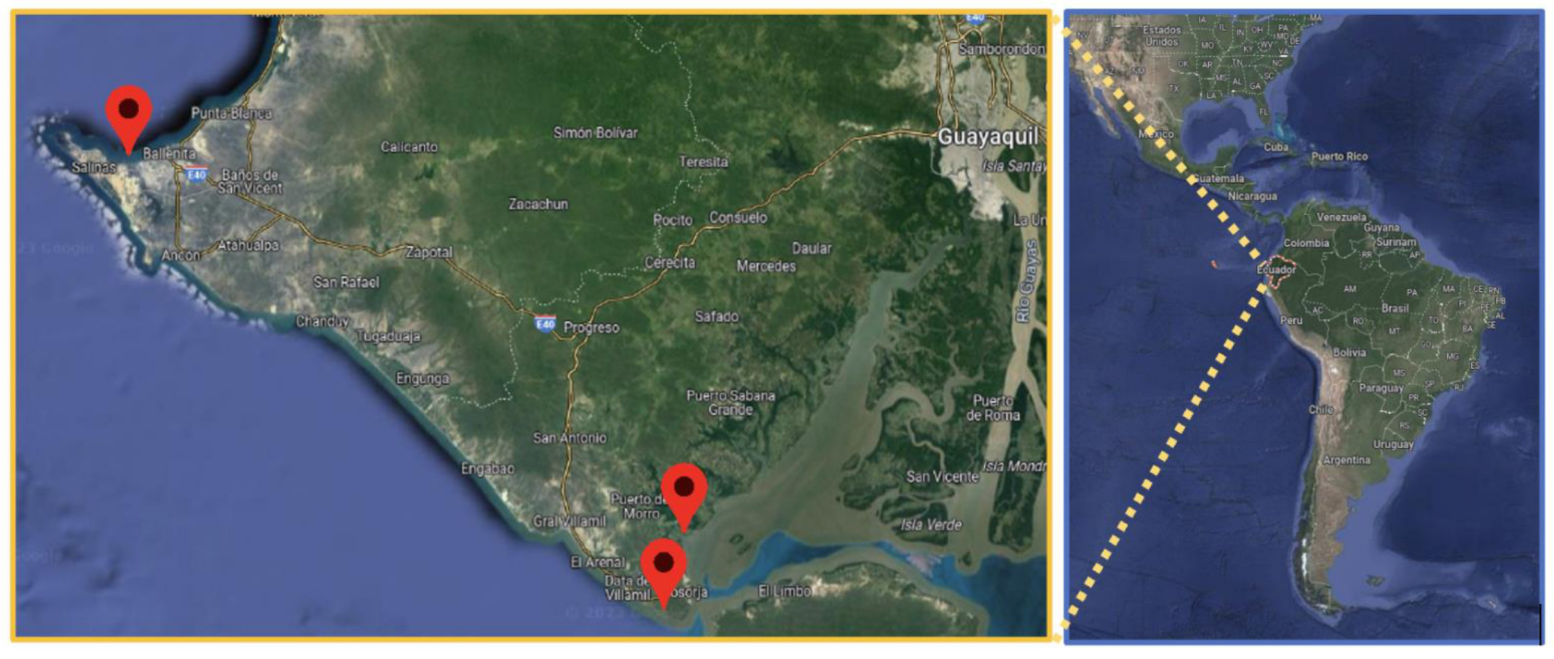
2.1. Study Area
2.2. Sampling
2.3. Sample Preparation and Analysis
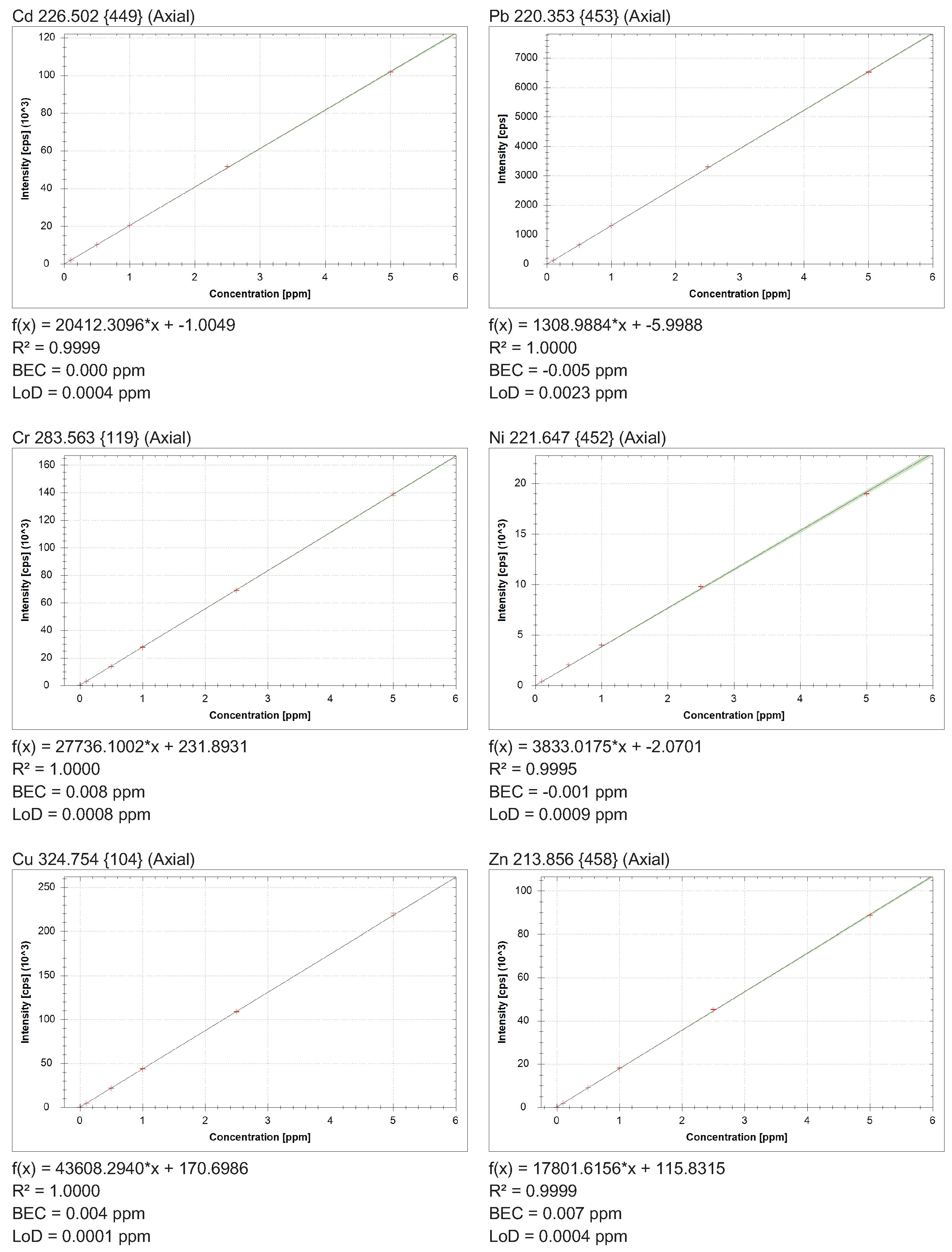
2.4. Quality Assurance and Quality Control (QA/QC)
2.5. Statiscal Analysis
3. Results and Discussion
3.1. Concentrations of Cd, Pb, Cr, Ni, Cu, and Zn
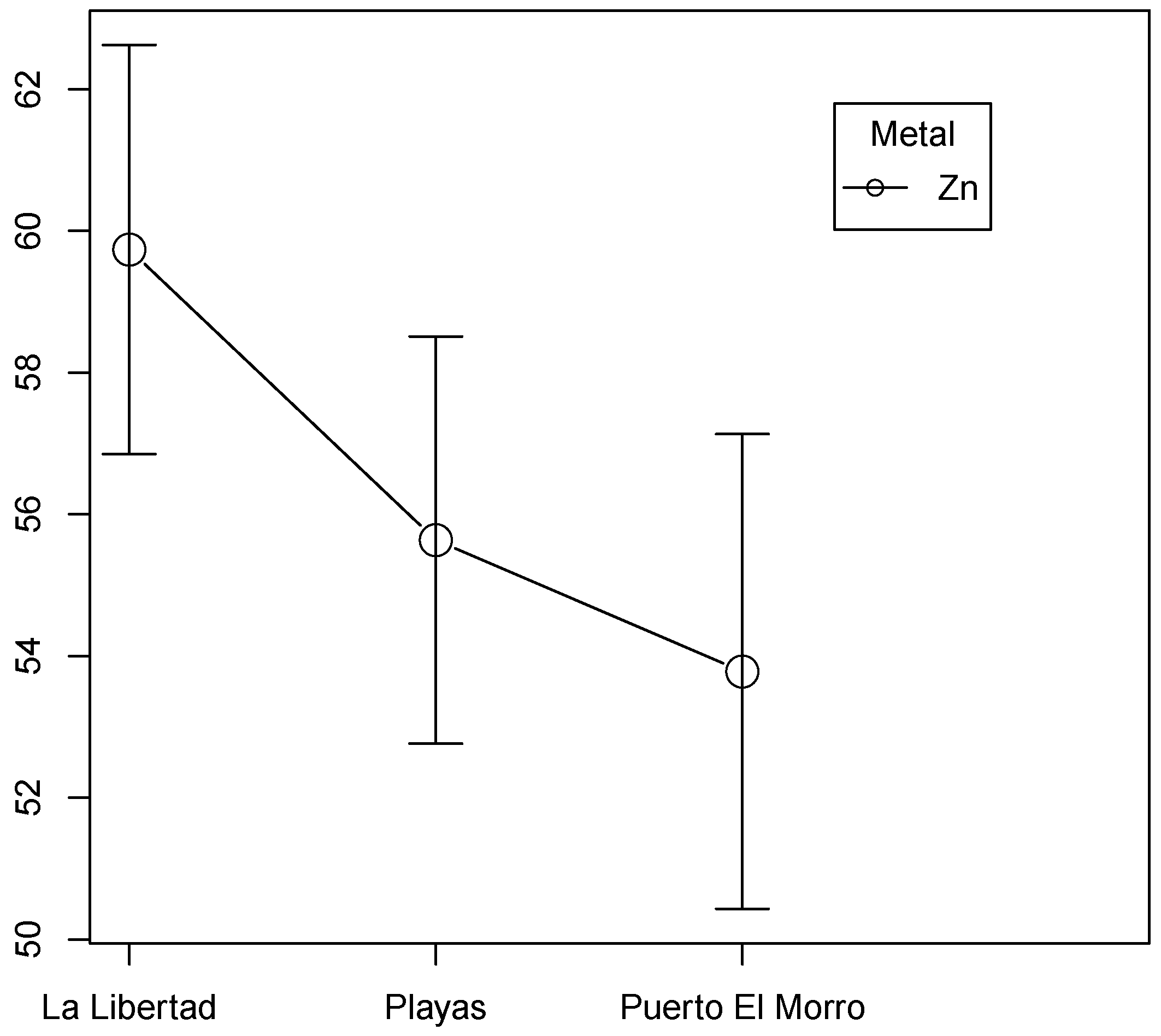
3.2. Tukey’s Test
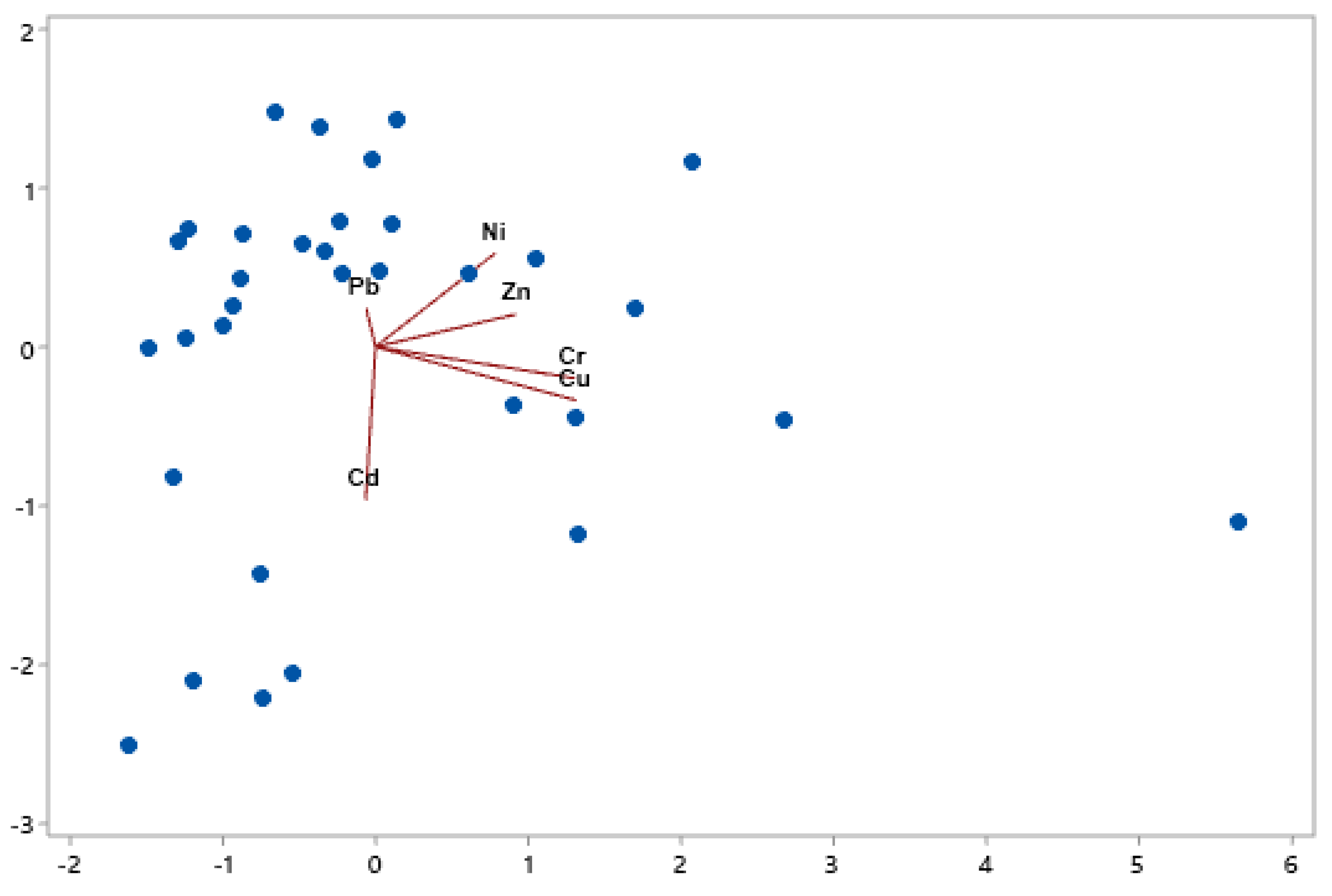
3.3. Principal Component Analysis (PCA)
3.4. Cadmium
3.5. Lead
3.6. Chromium
3.7. Nickel
3.8. Copper
3.9. Zinc
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanhan, P.; Lansubsakul, N.; Phaochoosak, N.; Sirinupong, P.; Yeesin, P.; Imsilp, K. Human Health Risk Assessment of Heavy Metal Concentration in Seafood Collected from Pattani Bay, Thailand. Toxics 2023, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, X.; Wang, L.; Han, J.; Katuwal, H.B.; Jiao, S.; Qiu, G. Human Dietary Exposure to Heavy Metals via Rice in Nepal. Int. J. Environ. Res. Public Health 2023, 20, 4134. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, T.; Kargın, D.; Çoğun, H.Y.; Temiz, Ö.; Varkal, H.S.; Kargın, F. Accumulation and health risk assessment of heavy metals in tissues of the shrimp and fish species from the Yumurtalik coast of Iskenderun Gulf, Turkey. Heliyon 2019, 5, e02131. [Google Scholar] [CrossRef]
- Kang, Y.; Zheng, S.; Wan, T.; Wang, L.; Yang, Q.; Zhang, J. Nematode as a biomonitoring model for evaluating ecological risks of heavy metals in sediments from an urban river. Ecol. Indic. 2023, 147, 110013. [Google Scholar] [CrossRef]
- Márquez, A.; Senior, W.; Martínez, G.; Castañeda, J.; González, Á. Concentraciones de metales en sedimentos y tejidos musculares de algunos peces de la Laguna de Castillero, Venezuela. Rev. Cient. 2008, 18, 121–133. [Google Scholar]
- Ahmed, M.I.; Ambali, A.; Karshima, S.N.; Mohammed, K.M. Heavy metal concentrations, water quality and health risk assessment of freshwater fish from the Lake Chad basin. Limnologica 2023, 103, 126135. [Google Scholar] [CrossRef]
- Anagha, B.; Athira, P.S.; Anisha, P.; Charles, P.E.; Anandkumar, A.; Rajaram, R. Biomonitoring of heavy metals accumulation in molluscs and echinoderms collected from southern coastal India. Mar. Pollut. Bull. 2022, 184, 114169. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jeon, H.; Shin, H.S. Risk Assessment and Determination of Arsenic and Heavy Metals in Fishery Products in Korea. Foods 2023, 12, 3750. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, C.; Sun, Q.; Wang, Y.; Gong, Z.; Zhang, K.; Da, J.; Zou, H.; Zhu, J.; Zhao, H.; et al. Cadmium exposure exacerbates kidney damage by inhibiting autophagy in diabetic rats. Ecotoxicol. Environ. Saf. 2023, 267, 115674. [Google Scholar] [CrossRef]
- Larsen, B.; Sánchez-Triana, E. Global health burden and cost of lead exposure in children and adults: A health impact and economic modelling analysis. Lancet Planet. Health 2023, 7, e831–e840. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.C. Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes schlegelii under the different levels of dietary chromium (Cr6+) exposure. Ecotoxicol. Environ. Saf. 2016, 125, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Nibe-Shirakihara, Y.; Tamura, A.; Aonuma, E.; Arakawa, S.; Otsubo, K.; Nemoto, Y.; Nagaishi, T.; Tsuchiya, K.; Shimizu, S.; et al. Nickel particles are present in Crohn’s disease tissue and exacerbate intestinal inflammation in IBD susceptible mice. Biochem. Biophys. Res. Commun. 2022, 592, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C. Aluminum. Encycl. Toxicol. 2024, 1, 329–334. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Spatial Variation of Metallic Contamination and Its Ecological Risk in Sediment and Freshwater Mollusk: Melanoides tuberculata (Müller, 1774) (Gastropoda: Thiaridae). Water 2020, 12, 206. [Google Scholar] [CrossRef]
- Prado-Carpio, E.C.; Martínez-Soto, M.E.; Rodríguez-Monroy, C.; Quiñonez-Cabeza, M.; Olivo-Garrido, M.L. Biología, productividad y atributos comerciales del molusco bivalvo «concha prieta» (Anadara tuberculosa) Biology, productivity and commercial attributes of the «black ark Shell» bivalve mollusk (Anadara tuberculosa). Rev. Espac. 2021, 42, 2. [Google Scholar] [CrossRef]
- Navarrete-Forero, G.; Morales Baren, L.; Dominguez-Granda, L.; Pontón Cevallos, J.; Marín Jarrín, J.R. Contaminación por Metales Pesados en el Golfo de Guayaquil: Incluso Datos Limitados Reflejan Impactos Ambientales de las Actividades Antrópicas. Rev. Int. Contam. Ambient. 2019, 35, 731–755. [Google Scholar] [CrossRef]
- Pontón-Cevallos, J.; Marín Jarrín, J.R.; Rosado-Moncayo, A.M.; Bonifaz, M.J.; Quiroga, M.d.M.; Espinoza, M.E.; Borbor-Córdova, M.J.; Pozo-Cajas, M.; Goethals, P.L.; Domínguez-Granda, L.E. Spatio-temporal variability of Brachyura larval assemblages in mangroves of the Gulf of Guayaquil’s inner estuary. Reg. Stud. Mar. Sci. 2021, 41, 101601. [Google Scholar] [CrossRef]
- Wojtkowski, K.; Wojtkowska, M.; Długosz-Lisiecka, M.; Walczak, A. Assessment of the Radiation Situation and the Presence of Heavy Metals in the Soil in the Poleski National Park. Appl. Sci. 2023, 13, 11699. [Google Scholar] [CrossRef]
- Rajasekar, A.; Murava, R.T.; Norgbey, E.; Zhu, X. Spatial Distribution, Risk Index, and Correlation of Heavy Metals in the Chuhe River (Yangtze Tributary): Preliminary Research Analysis of Surface Water and Sediment Contamination. Appl. Sci. 2024, 14, 904. [Google Scholar] [CrossRef]
- Pinto, E.B.; Slowey, N.C. Stable isotope evidence for the origins of waters in the Guayas estuary and Gulf of Guayaquil. Estuarine Coast. Shelf Sci. 2021, 250, 107151. [Google Scholar] [CrossRef]
- Calle, P.; Monserrate, L.; Medina, F.; Calle Delgado, M.; Tirapé, A.; Montiel, M.; Ruiz Barzola, O.; Cadena, O.A.; Dominguez, G.A.; Alava, J.J. Mercury assessment, macrobenthos diversity and environmental quality conditions in the Salado Estuary (Gulf of Guayaquil, Ecuador) impacted by anthropogenic influences. Mar. Pollut. Bull. 2018, 136, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Félix, F.; Fernández, J.E.; Paladines, A.; Centeno, R.; Romero, J.; Burneo, S.F. Habitat use of the common bottlenose dolphin (Tursiops truncatus) in the Gulf of Guayaquil, Ecuador: Management needs for a threatened population. Ocean. Coast. Manag. 2022, 223, 106174. [Google Scholar] [CrossRef]
- Zambrano-Monserrate, M.A.; Silva-Zambrano, C.A.; Ruano, M.A. The economic value of natural protected areas in Ecuador: A case of Villamil Beach National Recreation Area. Ocean. Coast. Manag. 2018, 157, 193–202. [Google Scholar] [CrossRef]
- US EPA. EPA Method 6010C (SW-846): Inductively Coupled Plasma—Atomic Emission Spectrometry; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- CE. Reglamento (CE) N∘ 1881/2006 De La Comisión; Technical report; European Comission: Brussels, Belgium, 2006. [Google Scholar]
- Lucero Rincón, C.H.; Peña Salamanca, E.J.; Cantera Kintz, J.R.; Lizcano, O.V.; Cruz-Quintana, Y.; Neira, R. Assessment of mercury and lead contamination using the bivalve Anadara tuberculosa (Arcidae) in an estuary of the Colombian Pacific. Mar. Pollut. Bull. 2023, 187. [Google Scholar] [CrossRef]
- Aldás, A.S.; Grimón, R.R.; Moreno, J.; Chollet-Villalpando, J.G. Análisis de la forma de la concha de Anadara tuberculosa como indicador de contaminación en manglares.: Forma de concha de A. tuberculosa y contaminación en manglares. CICIMAR Oceán. 2024, 38, 7–18. [Google Scholar] [CrossRef]
- Nasevilla, M.; Fernández, L.; Yánez-Jácome, G.S.; Pozo, P.; Dominguez-Granda, L.; Romero, H.; Espinoza-Montero, P. Total mercury determination in bivalves Anadara tuberculosa sold in open markets from Quito, Ecuador. Heliyon 2022, 8, e12451. [Google Scholar] [CrossRef] [PubMed]
- Tuñón-Pineda, O.; Chang, J.C.; Del Cid, A.; Goti, I.; Gómez, J.A. ConcentraciÓn de Metales Pesados (Cu y Cd), en Tejido Gonadal de A. tuberculosa en el Estero FarfÁn, Golfo de Montijo. Tecnociencia 2020, 22, 227–243. [Google Scholar] [CrossRef]
- Collaguazo, N.Y.; Armijos, H.A.; Loja, G.M. Cuantificación de metales pesados en Anadara tuberculosa(Mollusca:bivalvia) del estero Huaylá de Puerto Bolívar, por espectrofotometría de absorción atómica. // Quantification of heavy metals in Anadara tuberculosa, (Mollusca: Bivalvia) from the Huaylá e estuary of Puerto Bolívar, by atomic absorption spectrophotometry. Cienc. UNEMI 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Flores, E.; Pozo, W.; Pernía, B.; Sánchez, W. Niveles de cadmio en atún fresco y enlatado para consumo humano en Ecuador. Maskana 2018, 9, 35–40. [Google Scholar] [CrossRef]
- National Institutes of Health. Cobre—Datos en Español; National Institutes of Health: Madrid, Spain, 2022. [Google Scholar]
- US EPA. Chromium(III), Insoluble Salts (CASRN 16065-83-1); IRIS, US EPA: Washington, DC, USA, 2021. [Google Scholar]
- AESAN. Risk Assessment. Gob.es. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/gestion_riesgos/FICHA_NIQUEL.pdf (accessed on 10 October 2023).
- Heavy Metals. Ministry for Ecological Transition and Demographic Challenge. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/atmosfera-y-calidad-del-aire/emisiones/prob-amb/metales_pesados.html (accessed on 10 October 2023).
- FAO; WHO. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the Gsctff; FAO: Rome, Italy, 2011. [Google Scholar]
- Kadmium, T.; Zink Di Dalam Kerang, D.; Anadara; Selangor, K.; Sabarina, M.; Yunus, M.; Hamzah, Z.; Azlin, N.; Ariffin, N.; Muslim, M.B. Cadmium, Chromium, Copper, Lead, Ferum and Zinc LEVELS in the Cockles (Anadara granosa) from Kuala Selangor, Malaysia. Malays. J. Anal. Sci. 2014, 18, 514–521. [Google Scholar]


| Sample Area | Mean ± SD (mg/kg) (Minimum–Maximum) | |||||
|---|---|---|---|---|---|---|
| Cd | Pb | Cr | Ni | Cu | Zn | |
| Puerto El Morro | 3.75 ± 2.56 | 0.39 ± 0.34 | 0.19 ± 0.17 | 0.80 ± 0.51 | 6.70 ± 1.64 | 53.78 ± 11.10 |
| (1.10−8.20) | (0.0−1.20) | (0.0−0.60) | (0.20−1.70) | (4.30−10.00) | (81.60−40.00) | |
| General Villamil Playas | 2.14 ± 2.00 | 0.68 ± 0.75 | 0.30 ± 0.34 | 0.57 ± 0.21 | 5.3 ± 1.02 | 55.63 ± 9.52 |
| (0.50−8.00) | (0.10−2.8) | (0.0−1.1) | (0.30−1.1) | (4.1−7.50) | (73.80−43.90) | |
| La Libertad | 2.90 ± 1.16 | 0.56 ± 0.33 | 1.34 ± 1.27 | 0.93 ± 0.24 | 8.56 ± 4.27 | 59.73−9.57 |
| (1.60−5.20) | (0.20−1.50) | (0.10−4.50) | (0.60−1.20) | (4.20−18.20) | (75.30−45.70) | |
| Accumulation | Location | Sampling Year | Reference |
|---|---|---|---|
| Pb 0.87 ± 0.68 mg/kg | Colombia, Valle del Cauca | 2023 | Lucero Rincón et al., 2023 [26] |
| Hg 0.57 ± 0.74 mg/kg | |||
| Cd 0.55 mg/kg | Ecuador, Esmeraldas | 2023 | Aldás et al., 2024 [27] |
| Pb 0.03 mg/kg | |||
| Cd 0.76 mg/kg | Ecuador, Guayas | ||
| Pb 0.06 mg/kg | |||
| Cd 0.64 mg/kg | Ecuador, El Oro | ||
| Pb 0.02 mg/kg | |||
| Hg 0.055 mg/kg | Ecuador, Quito | 2022 | Nasevilla et al., 2022 [28] |
| Cd 0.096 ± 0.06 µg/g | Golfo de Montijo, Panamá | 2020 | Tuñon et al., 2020 [29] |
| Cu 1.59 ± 1.10 µg/g | |||
| Pb 7.5 ± 0.45 mg/kg | Ecuador, Puerto Bolívar. | 2017 | Collaguazo et al., 2017 [30] |
| As 12.43 ± 0.08 mg/kg | |||
| Hg 364.67 ± 91.37 mg/kg | |||
| Cd 1.67 ± 0.27 mg/kg | |||
| Cr 4.40 ± 2.15 mg/kg | |||
| Co 2.71 ± 0.34 mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinces, K.C.; Pacheco Flores de Valgaz, A.; Ballesteros, J. Quantification of Heavy Metal Content in Anadara tuberculosa from the Gulf of Guayaquil Using ICP-OES: Assessing Marine Contamination. Appl. Sci. 2024, 14, 1704. https://doi.org/10.3390/app14051704
Vinces KC, Pacheco Flores de Valgaz A, Ballesteros J. Quantification of Heavy Metal Content in Anadara tuberculosa from the Gulf of Guayaquil Using ICP-OES: Assessing Marine Contamination. Applied Sciences. 2024; 14(5):1704. https://doi.org/10.3390/app14051704
Chicago/Turabian StyleVinces, Kevin Cedeño, Angela Pacheco Flores de Valgaz, and Jose Ballesteros. 2024. "Quantification of Heavy Metal Content in Anadara tuberculosa from the Gulf of Guayaquil Using ICP-OES: Assessing Marine Contamination" Applied Sciences 14, no. 5: 1704. https://doi.org/10.3390/app14051704
APA StyleVinces, K. C., Pacheco Flores de Valgaz, A., & Ballesteros, J. (2024). Quantification of Heavy Metal Content in Anadara tuberculosa from the Gulf of Guayaquil Using ICP-OES: Assessing Marine Contamination. Applied Sciences, 14(5), 1704. https://doi.org/10.3390/app14051704







