Abstract
As an oxygen carrier and a strong oxidising agent, ammonium nitrate can create an explosive mixture when mixed with organic material. A typical example is the mixture of ammonium nitrate and fuel oil (ANFO), which is the most used explosive for civilian applications. In this work, we studied the detonability and detonation properties of mixtures of ammonium nitrate with recycled rubber and hay. The main goal of this study was to determine the optimal volume ratio of ammonium nitrate and organic materials in terms of achieving the best performance (working capacity). Using small experiments, it was determined that the maximum burst velocity for the ammonium nitrate/hay mixture is achieved at 8% hay by volume, while the maximum burst velocity for the ammonium nitrate/rubber mixture is achieved at 15% rubber by volume. A thermochemical calculation has shown that the maximum detonation heat is achieved at the zero oxygen balance at the volume ratios of 89.2/10.8 for AN/Rubber and 72.85/27.15 for AN/Hay.
1. Introduction
Ammonium nitrate (AN, H4N2O3) plays an important role in agriculture as a high-nitrogen fertiliser, but also in the production of commercial explosives. It is present in the form of colourless or white-to-grey crystals or odourless globules, with a molecular weight of 80.06 and a specific weight of 1.725 g/cm3. It has a melting point of 169.51 °C and boils at 210 °C, forming nitrous oxide [1].
As an oxygen carrier and strong oxidising agent, ammonium nitrate readily forms explosive mixtures with other explosives or with fuels (such is fuel oil, various organic materials, aluminium powder, etc.). The typical examples of commercial AN-based explosives are ANFO (mixture of 94% AN and 6% fuel oil) and emulsion explosives (an aqueous solution of AN separated by thin films of fuel/emulsifier) [1]. By changing the type and content of organic material in AN-based explosives, the detonation properties can be adjusted so to obtain the best blasting effect. If recycled organic materials (e.g., recycled rubber, hay, etc.) are used as ingredients, the additional benefit of the disposal or exploitation of such materials may be obtained.
The first research on the development of AN-based explosives with the addition of organic materials started in the 1960s, with the addition of various low-density materials to commercial ANFO explosives. The main goal of this research was to develop simple-to-use and cost-effective explosives with appropriate detonation properties [2,3]. Similar research was conducted in Norway, where a blend of ANFO and sawdust in a 50:50 volume ratio was used [4]. The first commercialized explosive with organic material was Isanol, an ANFO explosive with the addition of expanded polystyrene (EPS) [4,5,6]. The modification of ANFO detonation properties by decreasing its density by adding polystyrene [7], expanded polystyrene [2,8,9,10], sawdust [11,12], sawdust and used mineral oil [12], organic waste from sugar manufacturing [3,12], perlite [13,14,15], rubber [16], corn, coal, and fly ash [17], wheat husk [18], and rice husks [6] has been also investigated. The effects of different parameters on the detonation performance of ANFO have been well researched and published. The main parameters include [19] the properties of AN prills [20,21,22] and the density of the mixture and charge diameter [22]. The effects of other parameters, such as the initiation energy [23], charge temperature [24], presence of additives [25], and presence of confinement [26,27], have also been reported.
During the planning, monitoring, and execution of blasting operations, there is a need to develop an explosive suitable for blasting in the vicinity of inhabited areas or surrounding buildings. Such an explosive should be made of products with sufficient heat and volume properties to carry out the mechanical work of the fragmentation of rock. In addition, it should have a favourable ratio between the density of the explosive and the detonation velocity and pressure. The detonation pressure level and the duration of the resulting stress impulse, i.e., the duration of the expansion of detonation products due to the ambient pressure, ensure sufficient mechanical work is performed on the rock, which leads to the formation of new cracks, the expansion of existing cracks, the formation of fragments, and the displacement of blasted rocks.
A part of the blasting energy that is not used for crushing spreads as elastic waves in the surrounding area and leads to undesirable seismic effects. By reducing the density of the explosive mixture by adding some organic waste material, the detonation velocity and detonation pressure change, which can reduce the unwanted seismic effect of the explosion.
In this study, we investigated explosive mixtures based on AN and recycled rubber or hay as organic additives. The main goal of the study was to determine, experimentally and by employing thermochemical calculations, the optimal composition of AN/Rubber and AN/Hay mixtures in terms of detonation properties (detonation velocity and pressure, detonation heat and temperature, detonation energy, gas volume, etc.). In addition, we intended to better understand the effect of the content of organic material on the detonation characteristics of the mixtures through the analysis of the composition and concentration of detonation products.
2. Materials and Methods
2.1. Ammonium Nitrate (NH4NO3)
Ammonium nitrate is available in two forms: as a commercial fertiliser (high-density prills) and for the manufacture of explosives (low-density prills). Low-density prills are more porous and are suitable for the production of AN-based commercial explosives. In this study, we used AN prills with a density of 0.804 g/cm3 and a porosity of 6%. The prills were ground using a mortar and pestle to obtain powdered AN with a density of 0.942 g/cm3 [28]. The appearance of the AN produced in this way is shown in Figure 1.

Figure 1.
Appearance of ground ammonium nitrate.
Ground AN was mixed in a specific volume ratio with ground organic material (rubber or hay) to obtain the explosive mixtures used in this work. The mixing was performed in a plastic bucket with a laboratory mixer.
AN/Hay mixtures were prepared in volume ratios from 99%/1% to 85%/15%, with the hay content being increased by 1%. AN/Rubber mixtures were prepared in volume ratios from 99%/5% up to 50%/50%, with an increase of 5%.
2.2. Recycled Rubber
Rubber is a mechanically stiff and extremely elastic polymer material obtained by the vulcanization of raw rubber [29]. The ground rubber used in this study came from recycled old car tires and was purchased as such. The density of the rubber was experimentally determined to be 0.466 g/cm3, and the particle size was between 0.15 mm and 0.5 mm (Figure 2).

Figure 2.
Appearance of ground recycled rubber.
2.3. Hay
Hay is sun-dried grass with a low moisture content of 12–15%. The volume content of moisture is reduced by drying from about 3/4 of the volume of green grass to 1/5 of the volume of dried hay. Hay is an organic “waste” that is a lignocellulosic raw material and consists of cellulose (40–50%), hemicellulose (25–35%), and lignin (15–20%) [30].
The hay used in this study was ground in a Retsch SM 2000 mill. A hay fraction from 0.25 mm to 0.5 mm with a density of 0.391 g/cm3 was used. Its appearance is shown in Figure 3.

Figure 3.
Dried hay.
2.4. Burst Velocity Measurement
Since, in the small-scale experiments we performed, we are not sure whether the explosive charge detonated, deflagrated, or burnt fast, we will use the term “burst velocity”, or just “velocity”, to describe the propagation mechanism of the reactions in the test charge. This issue is discussed in more detail in Section 3.
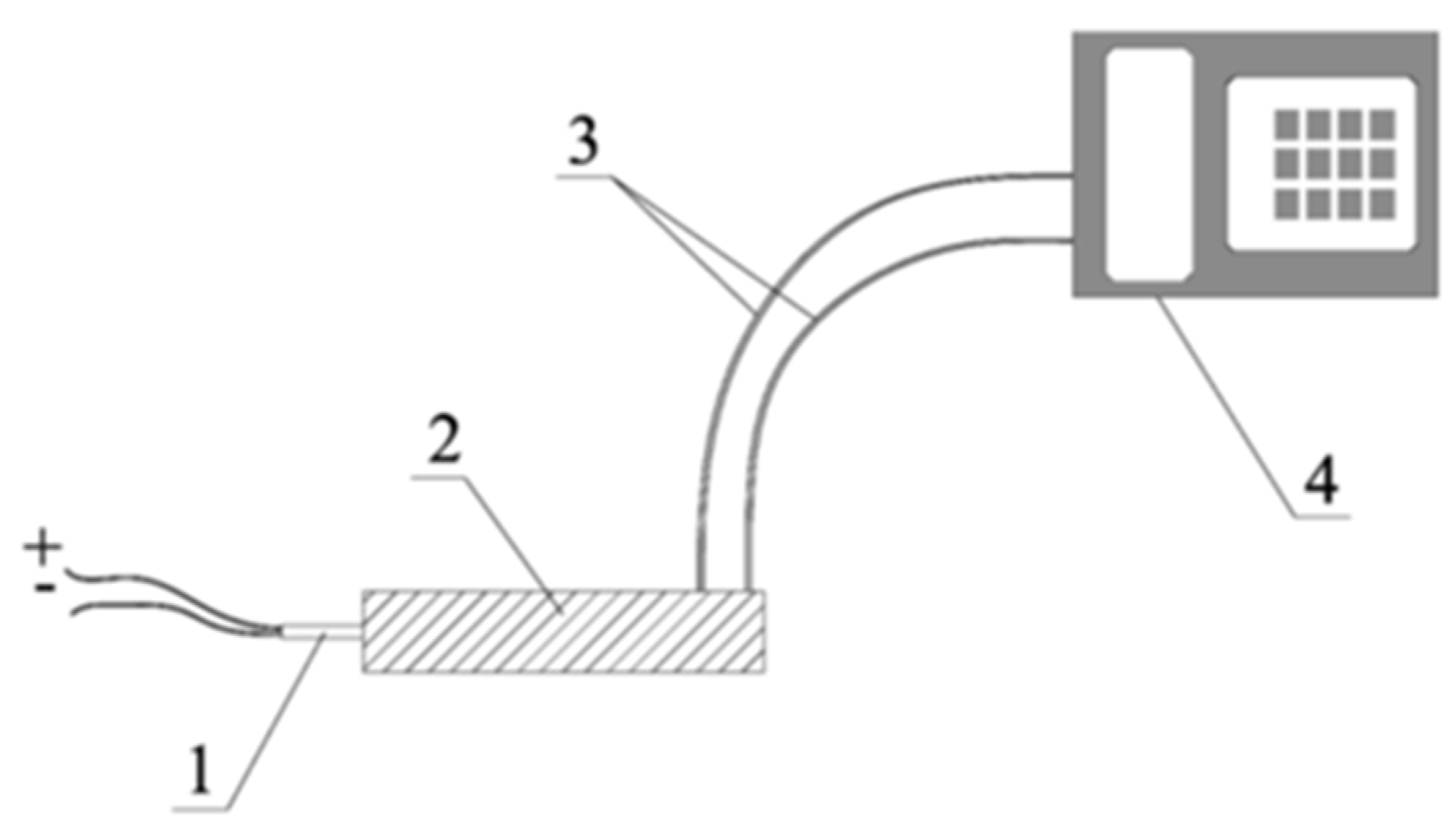
The burst velocity measurements were performed on samples filled in steel tubes, with an inner diameter of 21.5 mm, a wall thickness of 2.7 mm, and a length of 150 mm. The velocity was measured using an electro-optical method, with a distance of 50 mm between sensors. The explosive charges were detonated in a detonation chamber (1 m3 in volume) using electric detonators, whose total energy of the initial impulse corresponds to the energy of reference detonator no. 4, according to EN 13763-15: 2004 [31].
The velocity was measured for 75 samples, of which 45 samples were mixtures of AN/Rubber, and 30 samples were made of AN/Hay. Three shots were fired for each composition. Figure 4 shows the measurement setup.
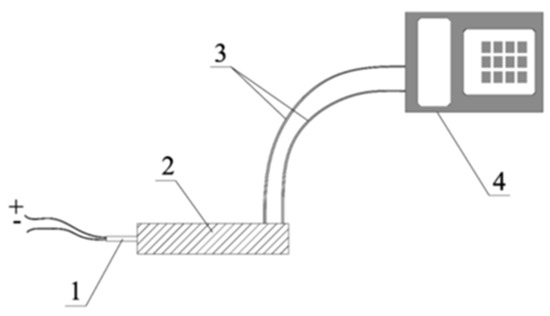
Figure 4.
Burst velocity measurement setup [32]. 1—electric detonator; 2—steel tube with test charge; 3—optical probes; 4—electronic watch.
2.5. Thermochemical Calculation
The detonation properties of the studied AN mixtures were calculated using the EXPLO5 thermochemical code [33,34]. The code predicts the detonation properties (e.g., velocity, pressure, energy, heat, temperature, etc.) of explosives by applying the chemical equilibrium steady-state Chapman–Jouguet (C-J) detonation model. The code calculates the equilibrium composition of detonation products by applying the free energy minimisation technique. EXPLO5 has the possibility of using several equations of the state of gaseous detonation products, but for this study, we used the modified Becker–Kistiakowsky–Wilson (MBKW) equation of state because previous research has shown that it best describes the behaviour of low-density explosives [34].
As already mentioned, AN-based explosives exhibit highly non-ideal behaviour, and their detonation properties depend strongly on the diameter of the explosive charge and the properties of the charge confinement. In addition, non-ideal explosives are characterised by a curved shock front, wide reaction zone (due to much slower chemical reactions compared to typical military brisant explosives), and the existence of a certain amount of unreacted explosive in the Chapman–Jouguet state. Due to the aforementioned reason, the detonation properties of non-ideal explosives cannot be accurately predicted using the Chapman–Jouguet detonation theory. Non-ideal detonation can be modelled using the Wood–Kirkwood (W-K) detonation model [35]. However, its application is complicated by the fact that the model requires a number of input parameters that must be determined experimentally. Due to the lack of the input parameters for AN/Rubber and AN/Hay mixtures (reaction rate parameters, equations of state of the unreacted materials, etc.), we could not use the W-K detonation model. Instead, we performed thermochemical calculations using the Chapman–Jouguet (C-J) detonation model. This model assumes that all materials react up to the C-J point and provides so-called ideal detonation parameters. These parameters correspond to the infinite diameter of the explosive charge.
3. Results and Discussion
Input information for the studied substances needed for the thermochemical calculation is given in Table 1. The experimentally measured detonation velocities of the AN/Hay and AN/Rubber mixtures are given in Table 2.

Table 1.
Input data for reactants (taken from EXPLO5 database).

Table 2.
Measured detonation velocities and calculated detonation parameters of studied compositions.
By comparing the experimental and the calculated detonation velocities (Table 2), it becomes clear that the experimental detonation velocities are about 50% lower than the calculated ones. For illustration, the maximum calculated value for the AN/Rubber mixture is 5002 m/s, while the maximum experimental value equals 2551 m/s. Such a large difference between the calculated and experimental detonation velocities is due to the effect of the charge size (the ideal detonation velocity corresponds to an infinite charge radius, and the experimental values were obtained for confined charges with a radius of 10.8 mm), which confirms the non-ideal behaviour of the studied mixtures.
To gain a better insight into the behaviour of the AN mixtures used in our small-scale experiments, we modelled the propagation of detonation in a standard ANFO explosive (which is a mixture of prilled AN and fuel oil) using the AUTODYN hydrocode. The reason why we used ANFO for modelling and not the studied mixtures are that there is no necessary input data (equation of state and reaction rate parameters) for these. On the other hand, considering that both ANFO and the studied mixtures contain dominantly AN, it is realistic to assume they behave in very similar way.
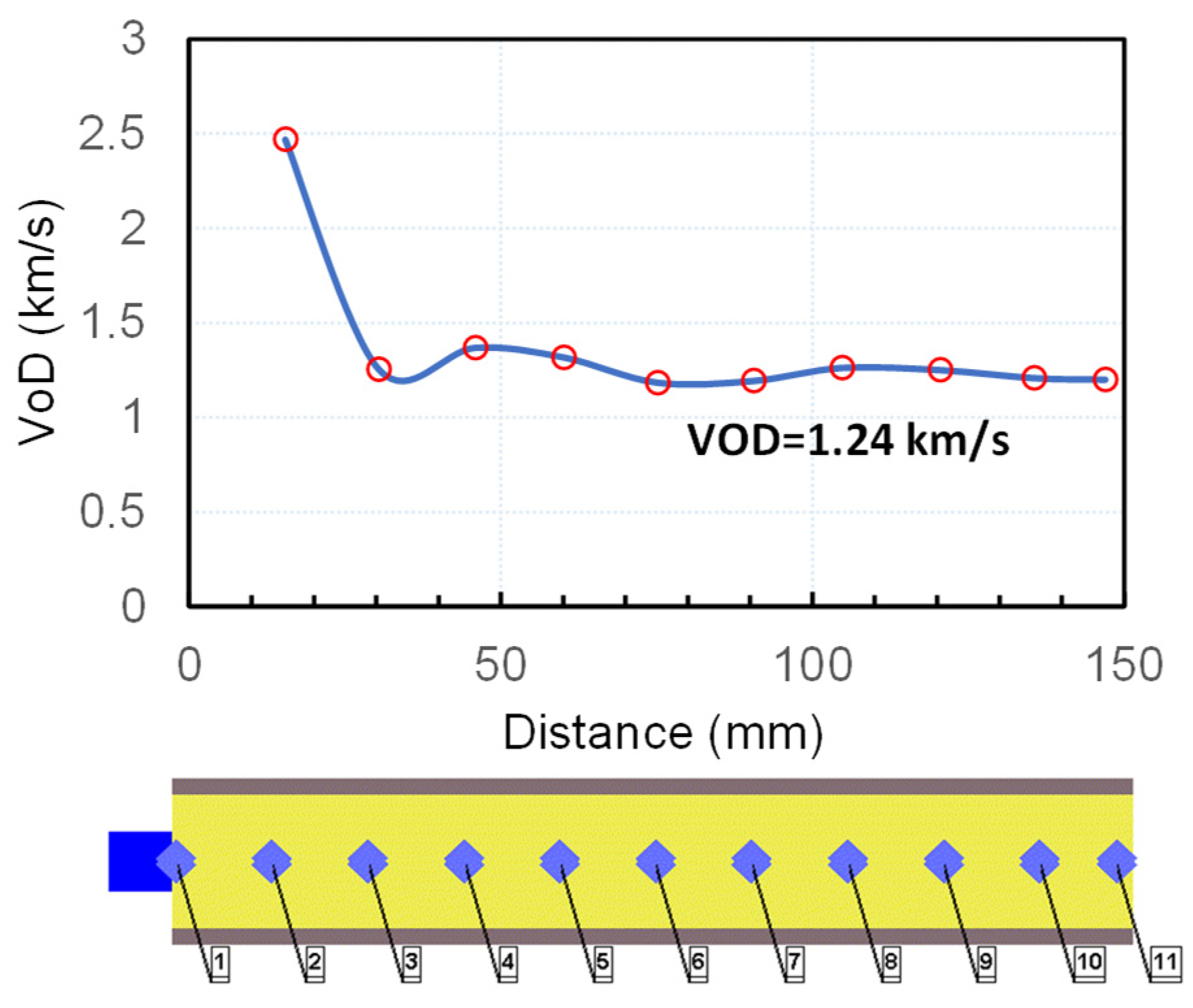
The simulation was carried out by applying the Lee–Tarver Ignition and Growth (I&G) reactive flow model [37]. The parameters for the reaction rate and the equations of state for the reacted and unreacted ANFO were taken from [38]. Steel confinement was modelled using the material properties of steel (4340 alloy steel) taken from the AUTODYN material library [39]. The charges were initiated with 1.2 g PETN. Eleven gauges were placed at regular intervals within the charge to register the arrival time of the detonation wave, based on which the shock velocity was determined as a function of the distance from the initiation point (Figure 5).
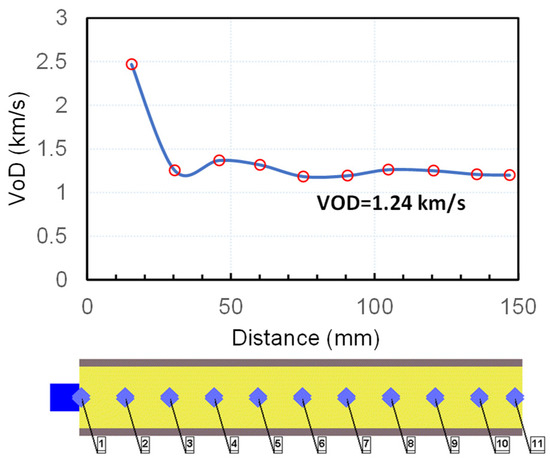
Figure 5.
Calculated detonation velocity–distance profile for ANFO (ρ0 = 0.8 g/cm3).
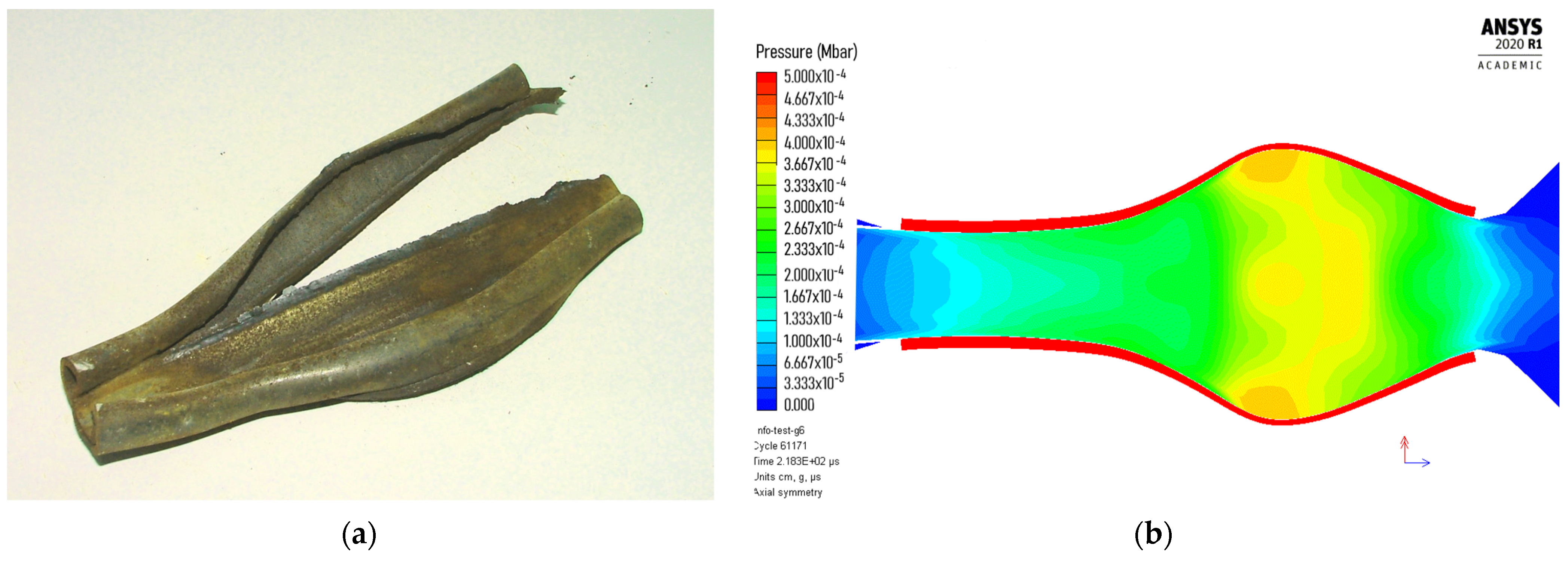
The simulation (Figure 5) showed that the shock velocity and the maximum pressure reach an almost constant value (D = 1.28 km/s, p = 0.3 GPa) after approximately 50 mm distance. The calculated detonation velocity is very close to the value we determined experimentally (D = 1.11 km/s at ρ0 = 0.82 g/cm3). However, the other parameters are highly different along the charge axis. For example, the maximum conversion of ANFO into a detonation product at the moment of arrival of the shock wave to the end of the charge (approximately after 115 μs) equals 25% in the central part of the charge (gauges 5–7) and less than 10% at the end of the charge. We could not precisely determine the sonic point (the point at which the flow is sonic, i.e., C0 = D − u) from the simulation, but based on the density jump in the reaction zone, we estimated that the sonic point reaches approximately 15–20 μs after the arrival of the shock front and changes along the charge axis. The amount of reacted explosive at estimated sonic point also changes along the charge axis and ranges between 10 and 25%. The majority of the explosive from the central part of the charge reacts after the sonic point is reached (i.e., during the expansion process) and reacts completely after approximately 150 μs. This results in larger deformation of the steel tube in the central part (Figure 6).
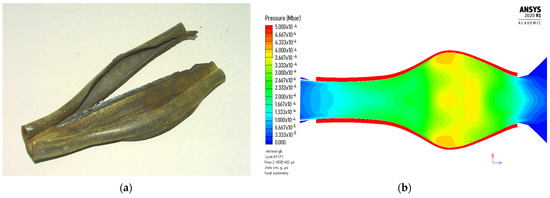
Figure 6.
Appearance of steel tube after detonation: (a) AN/Rubber (90/10, experiment), and (b) ANFO (Autodyn simulation).
Figure 6 shows that the deformation of the steel tube caused by the detonation of the AN/Rubber mixture is similar to that caused by ANFO detonation, which is confirmation that ANFO and the AN/Rubber mixture behave similarly. Although the calculated detonation velocity–distance profile (Figure 5) indicates that it is a steady low-velocity detonation, it is difficult to assert with certainty whether, under the conditions of our experiment, it is a low-velocity detonation or detonation-like explosion. Regardless, we believe that small-scale experiments, along with thermochemical calculations, can provide an answer to the question of what the optimal ratio is between AN and organic material in terms of the optimal detonation performance.
3.1. Effect of Amount of Organic Material on Detonation Properties
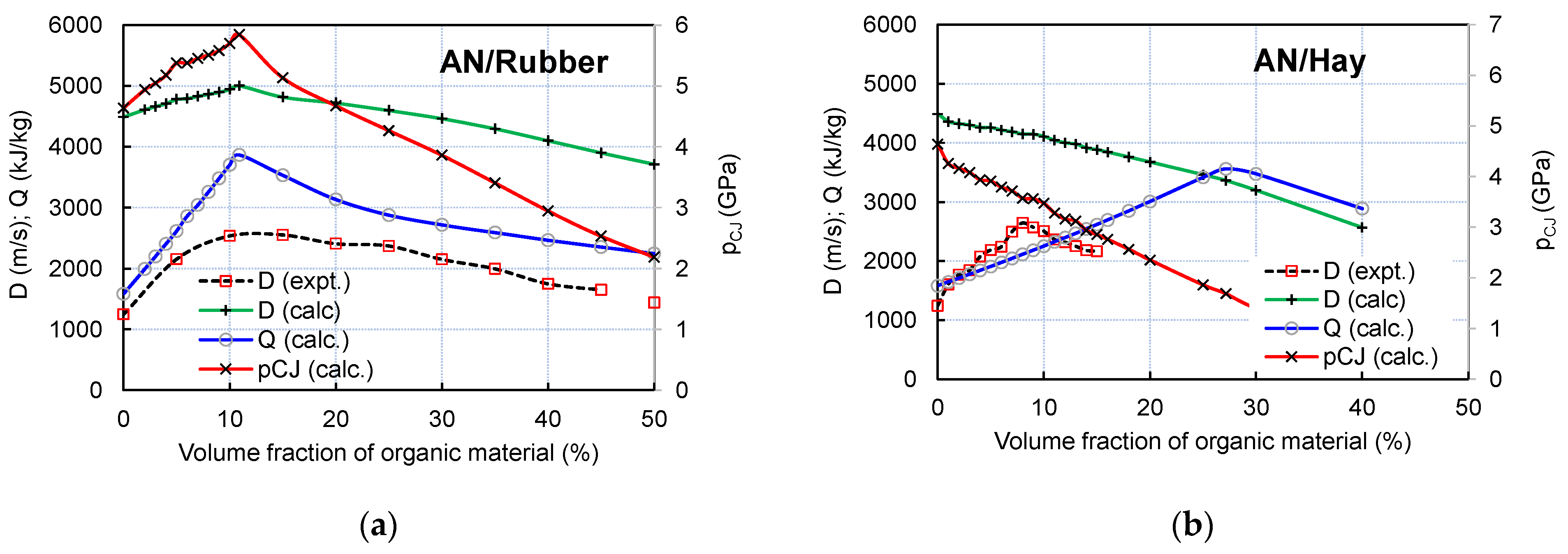
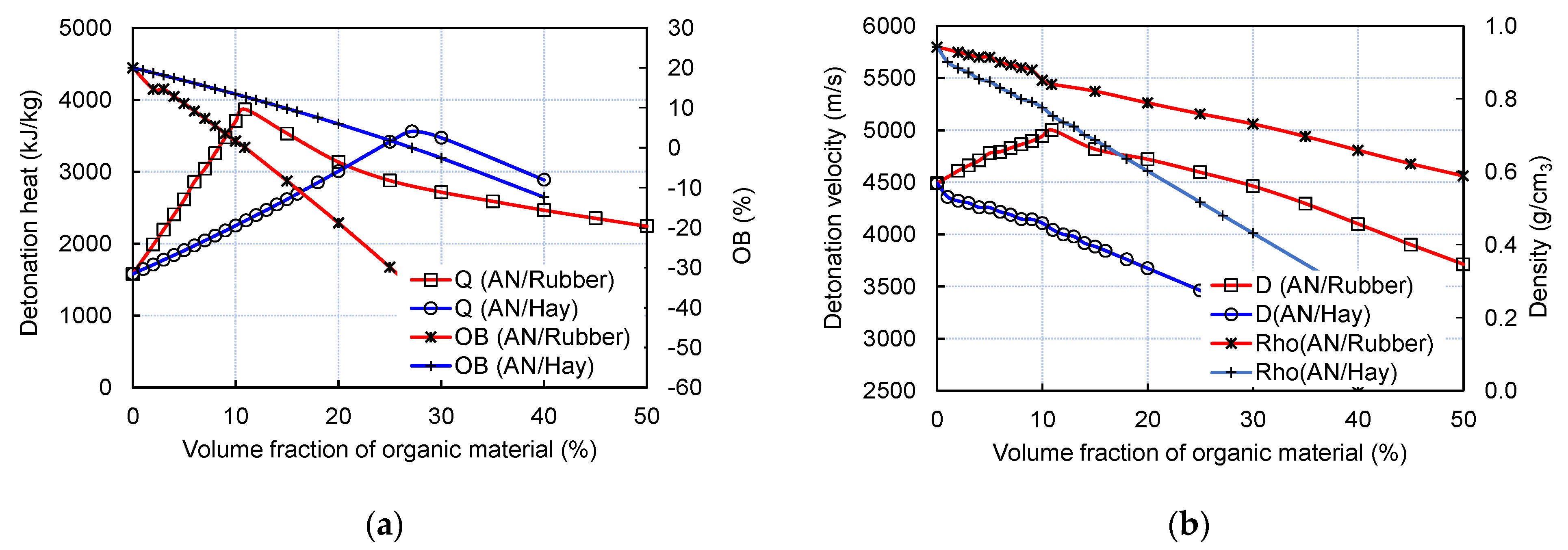
The changes in the experimental and calculated detonation velocities (D) and the calculated detonation heat (Q) and detonation pressure (pCJ) with the variation in the volume fraction of rubber and hay are shown in Figure 7.
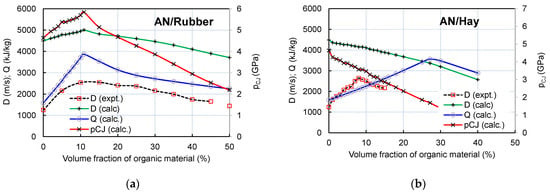
Figure 7.
Changes in calculated detonation velocity, pressure, and heat with variation in volume fraction of rubber (a) and hay (b). Experimental burst velocities are given for comparison.
As visible from Figure 5, the experimental burst velocity of both the AN/Rubber and AN/Hay mixtures increases with the increase in volume fraction of organic material and reaches its maximum values of 2664 m/s at an 8% volume fraction of hay and 2551 m/s at 15% rubber. For the AN/Rubber mixture, the thermochemical calculation predicts the detonation velocity–volume fraction of the rubber profile similar to that obtained by the experiments; this shows an increase in detonation velocity up to 10.9% by volume (D = 5002 m/s), followed by a slow decrease with a further increase in the rubber content. The calculated (ideal) detonation velocity is twice as high as the measured one, which is the consequence of the non-ideal character of detonation under the conditions of this small-cale experiment.
Unlike the AN/Rubber mixture, the experimental and calculated detonation velocity–volume fraction profiles are quite different. The calculation predicts a continuous decrease in detonation velocity and pressure with the hay content, while the experiment predicts an increase in velocity with up to 8% of hay. The analysis showed that two factors simultaneously influence the calculated profile: firstly, the composition, and secondly, the density of the mixtures. Compared to rubber, hay contains twice as much carbon and hydrogen per unit of mass. Furthermore, unlike rubber, in addition to carbon and hydrogen, hay contains a certain amount of oxygen. Therefore, its oxygen balance (OB) is more positive than the OB of rubber (OB of hay equals −129.4%; OB of rubber is −332.4%). The result of the above-mentioned calculation is as follows: for the same volume fraction, the AN/Hay mixture produces less H2O and CO2 than the AN/Rubber mixture, which results in a lower detonation heat, velocity, and pressure (Figure 8a).
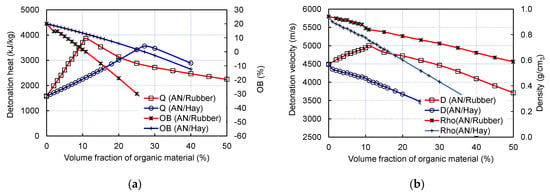
Figure 8.
Change in calculated detonation velocity and oxygen balance (a) and detonation velocity and density with volume fraction of rubber and hay (b).
The effect of another factor, density, is shown in Figure 8b. Since hay has a lower density than rubber (0.391 g/cm3 compared to 0.466 g/cm3), the addition of hay decreases the density of the AN/Hay mixture more than in the case of the AN/Rubber mixture. Ultimately, the simultaneous action of these two factors, density and composition, results in the calculated detonation velocity–volume fraction profiles of AN/Hay given in Figure 8b. For the sake of validation, we performed the calculation, assuming a constant density of the AN/Hay mixture, and found that detonation velocity continuously increases with the hay content and reaches the maximum value at the zero oxygen balance concentration. This confirms the significant effect of the density of organic material on the detonation velocity.
We do not have a sound explanation for why the experimental detonation velocity for the AN/Hay mixture has a very different profile compared to the calculated one. The fact that detonation fails at 15% of hay by volume (6.8% by mass) leads to the conclusion that small-scale experiments are performed at diameters that are close to critical for the AN/Hay mixture, given that a small variation in certain parameters can cause a change in detonation behaviour. For example, the addition of hay may change the failure radius of the mixture or may slow down the decomposition reaction, which will increase or decrease the detonation velocity. In contrast to the AN/Hay mixture, the AN/Rubber mixture detonates even at 50% by volume of rubber (33% by mass), which may indicate that the failure radius is smaller and the reaction rate of rubber is faster. To answer these questions, additional experiments should be conducted with larger-diameter charges.
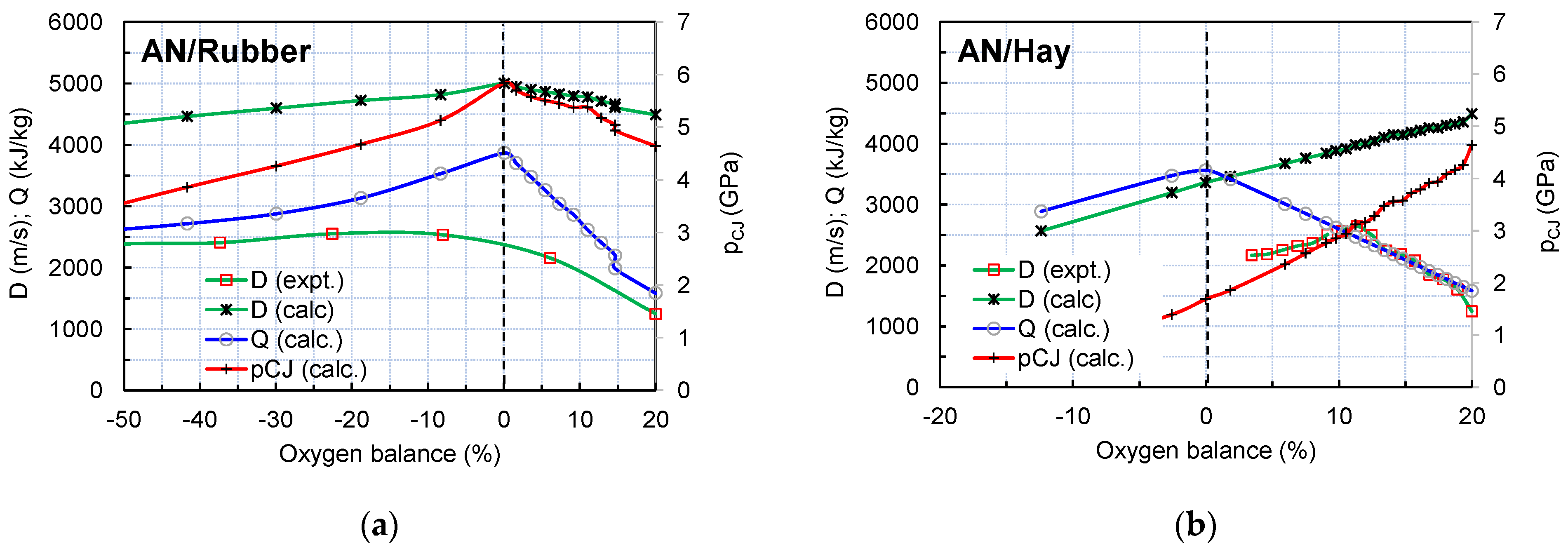
While the maximum value of the detonation velocity and pressure for the AN/organic materials mixtures can be reached at different AN/organic materials ratios, depending on the chemical composition and density of organic material, the maximum value of the detonation heat is reached in all cases at the zero oxygen balance (Figure 9).
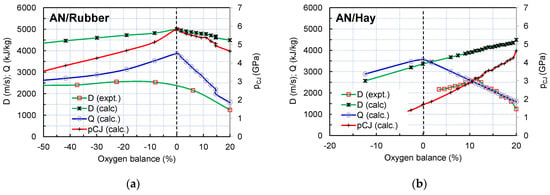
Figure 9.
Variation in detonation parameters with oxygen balance with amounts of rubber (a) and hay (b).
The volume ratio and the mass ratio at which the oxygen balance equals zero and values of detonation heat at the zero oxygen balance are given in Table 3. These ratios correspond to the optimal mixture composition in terms of the heat of detonation, i.e., the energetic characteristics. Along with the data for the studied AN/Rubber and AN/Hay mixture, the data for AN/Fuel oil (ANFO) are also given for comparison.

Table 3.
Values of volume and mass ratios at which OB = 0% and calculated corresponding values of detonation heat and velocity.
It follows from Table 3 that both the detonation heat and velocity for the AN/Rubber mixture are very close to those of ANFO (about 2.3% lower), while the AN/Hay mixture has a significantly lower predicted velocity and heat.
3.2. Concentration of Detonation Products
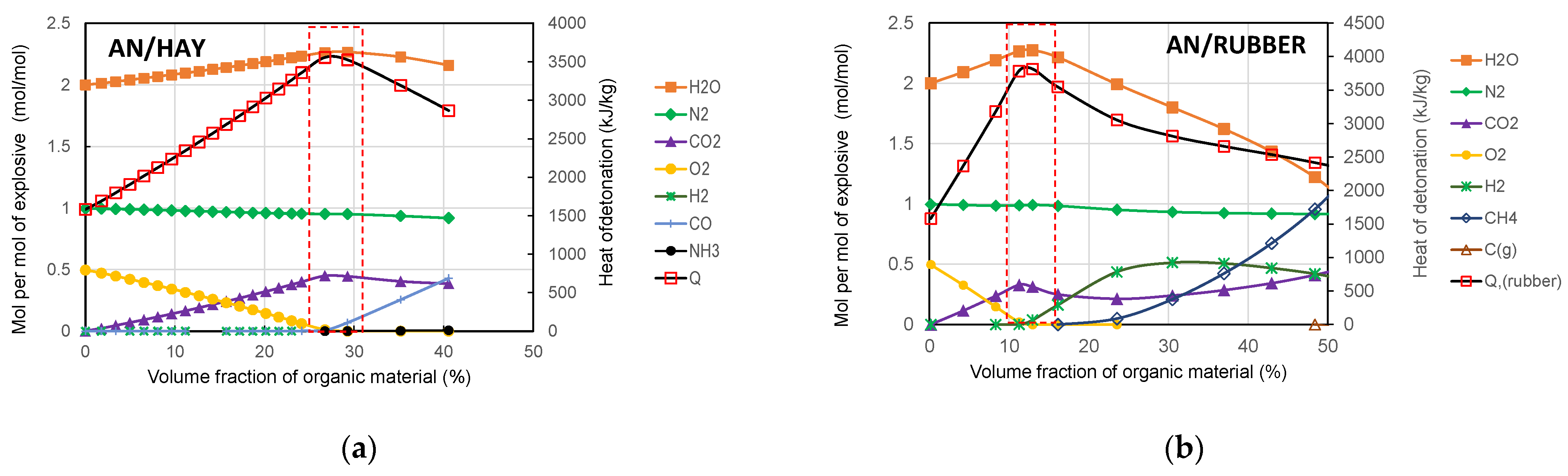
To understand the variation in the detonation properties of the studied AN-based mixtures with the amount of organic material, we analysed how the amount of organic material affects the composition and concentration of the detonation products. The concentration was calculated using the EXPLO5 code, and the results of the calculation are shown graphically in Figure 10.
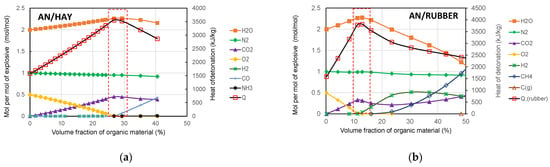
Figure 10.
Change in concentration of detonation products with amounts of hay (a) and rubber (b).
The main detonation products of the studied mixtures are H2O, N2, and CO2, while CO, CH4, NH3, H2, NO, etc., are present in smaller quantities. Since H2O and CO2 have the most negative values of the enthalpy of formation (−241.81 kJ/mol and −393.52 kJ/mol, respectively), these two detonation products contribute the most to the increase in Q. It can be also seen from Figure 10 that for both mixtures, the maximum detonation heat coincides with the maximum concentration of H2O and CO2. As shown in Figure 9, the maximum detonation heat was obtained for the AN/organic material ratio, corresponding to the zero oxygen balance.
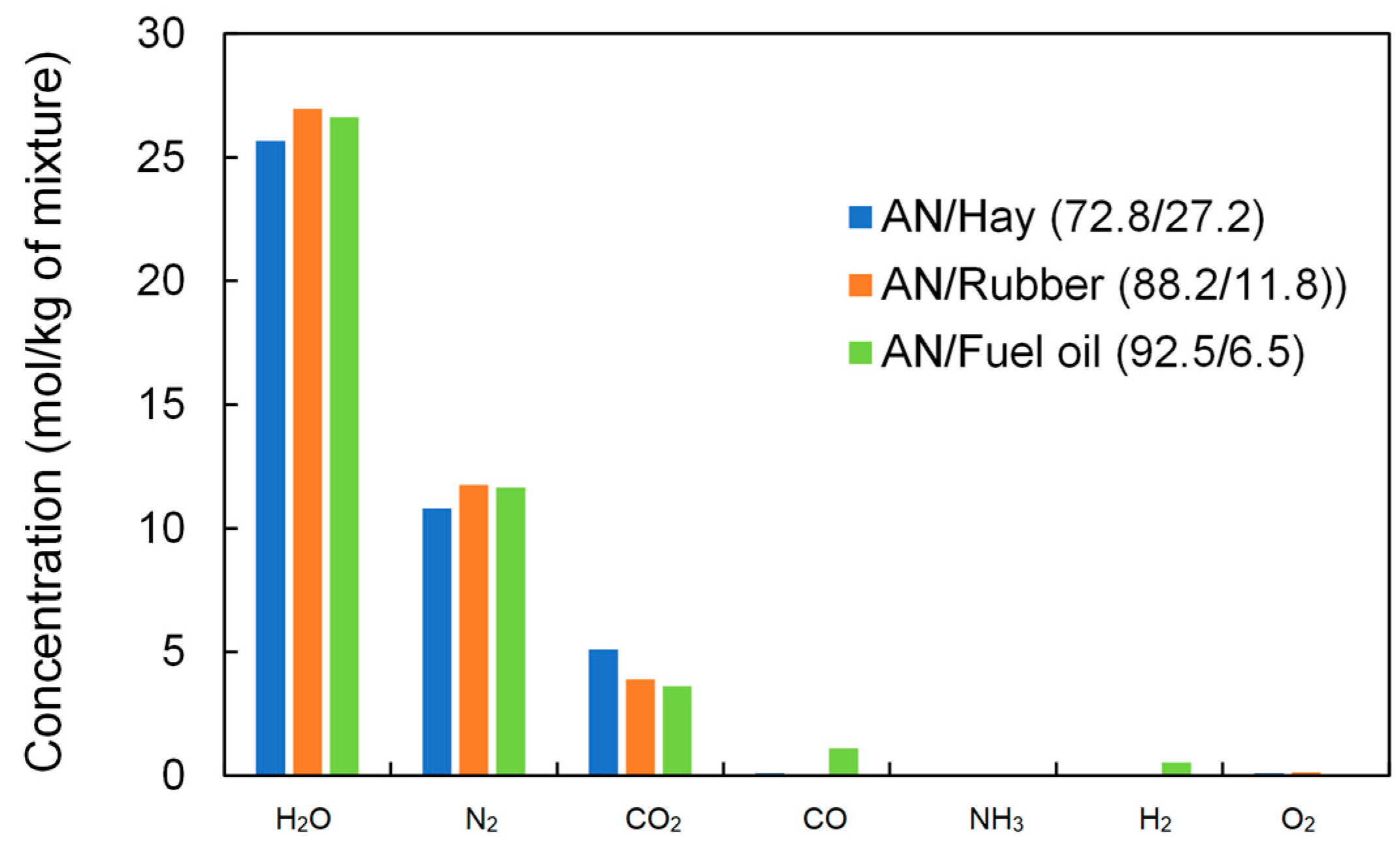
Generally, by adding organic material, the concentrations of H2O and CO2 increase, while the concentration of O2 decreases up to the point corresponding to the zero oxygen balance. With the further addition of organic material, the concentrations of H2O and CO2 decrease, while the concentrations of CO, H2, and CH4 increase because the available oxygen is not sufficient for the complete oxidation of carbon and hydrogen. It should be noted that in the case of the AN/Rubber mixture, the concentration of CH4 increases significantly for volume fractions of rubber above 20%. A comparison of the concentrations of detonation products at the zero oxygen balance is shown in Figure 11. The concentration of the detonation products of ANFO (AN/Fuel oil) is also given for comparison.
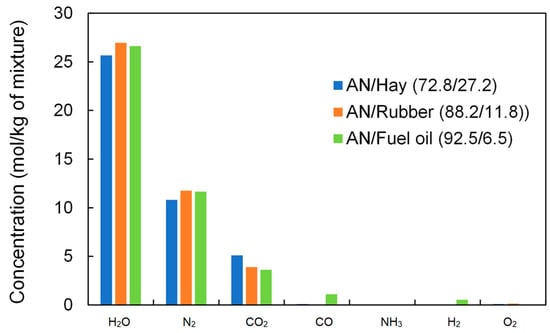
Figure 11.
Calculated concentrations of detonation products of ANFO and studied mixtures at the maximum detonation velocity (products present in a concentration of less than 0.001 mol/kg are ignored).
As can be seen from Figure 11, the product concentrations for both the studied mixture and for ANFO are quite similar. This particularly applies to the AN/Rubber mixture and ANFO, where there is a small difference in concentration. The AN/Hay mixture produces somewhat less H2O (5%) and N2 (8%), but more CO2 (30%) than AN/Rubber. This explains why the calculated heats of detonation for these three mixtures (Table 3) do not differ significantly.
4. Conclusions
Mixtures of ground ammonium nitrate with ground recycled rubber and hay were investigated using a thermochemical calculation and experimentally by determining the burst velocity, with the intention of finding the optimal composition in terms of the best performance (i.e., working capacity).
It was shown that under the conditions of a small-scale experiment (around 50 g of the mixture in a steel tube having 21.5 mm inner diameter), the maximum burst velocity was achieved at 8% of hay by volume (with D = 2644 m/s), while for the AN/rubber mixture, the maximum burst velocity was achieved at 15% of rubber by volume (with D = 2551 m/s).
The thermochemical calculations have shown that the maximum heat of detonation was achieved at a composition that corresponds to the zero oxygen balance; for AN/Rubber mixture, it is at the 89.2/10.8 volume ratio (Q = 3867 kJ/kg), and for AN/Hay, it is at the 72.85/27.15 volume ratio (Q = 3560 kJ/kg). These values are close to the values for ANFO (Q = 3903 kJ/kg) at the zero oxygen balance.
The main detonation products for both mixtures at the zero oxygen balance include H2O, N2, and CO2. The AN/Hay mixture produces less H2O (5%) and N2 (8%), but more CO2 (30%) than the AN/Rubber mixture.
In this research, the experiments were performed on small samples (≈50 g), so the detonation of such charges had an extremely non-ideal behaviour. In the continuation of this research, tests will be performed on larger samples and under the conditions of mine boreholes to obtain the best possible insight into the performance of the studied mixtures.
Author Contributions
Conceptualization, V.Š. and M.D.; methodology, V.Š. and M.D.; software, M.S.; validation, V.Š. and M.S.; experiment, V.Š., M.D. and J.V.; investigation, V.Š., M.D. and J.V.; writing—original draft preparation, V.Š.; writing—review and editing, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Croatian Science Foundation (HRZZ) under the projects IP-2019-04-1618.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This work has been supported by the Croatian Science Foundation (HRZZ) under the projects IP-2019-04-1618 “An improved nonideal detonation model of commercial explosives” (NEIDEMO).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rao, P.S. Ammonium Nitrate. In Encyclopedia of Toxicology: Third Edition; Elsevier: Amsterdam, The Netherlands, 2014; pp. 209–211. ISBN 9780123864543. [Google Scholar]
- Baranov, E.G.; Vedin, A.T.; Bondarenko, I.F. Mining and Industrial Applications of Low—Density Explosives; A.A. Balkema: Cape Town, South Affrica, 1996. [Google Scholar]
- Rock, J.; Maurer, A.; Pereira, N. Coming of Age for Low-Density Explosives. In Proceedings of the 2005 Coal Operators’ Conference; Aziz, N., Ed.; University of Wollongong & the Australasian Institute of Mining and Metallurgy: Wollongong, Australia, 2005; pp. 175–17982. [Google Scholar]
- Nielsen, K.; Heltzen, A.M. Recent Norwegian Experiance with Polystyrene Diluted ANFO (ISANOL). In Proceedings of the 2nd International Symposium on Rock Fragmentation by Blasting, Keystone, CO, USA, 23–26 August 1987; pp. 231–238. [Google Scholar]
- Heltzen, A.M.; Kure, K. Blasting with ANFO/Polystyrene Mixtures. In Proceedings of the 6th Conference of Explosives and Blasting Techniques (ISEE), Clevland, OH, USA, February 1980; pp. 105–116. [Google Scholar]
- Rock, J. Improving Blasting Outcomes Using SoftLOAD Low-Density Explosives. In Proceedings of the EXPLO 2004 Conference, Perth, Australia, 26–28 July 2004; pp. 153–158. [Google Scholar]
- Wilson, J.M.; Moxon, N.T. The Development of a Low Shock Energy Ammonium Nitrate Based Explosive. In Proceedings of the Second Large Open Pit Mining Conference, Latrobe Valley, Australia, 3–6 April 1989; pp. 39–43. [Google Scholar]
- Hunter, C.; Fedak, K.; Todoeschuck, J. Development of Low Density Explosives with Wall Control Applications. In Proceedings of the ISEE Annual Conference, Clevlend, OH, USA, 1993; pp. 549–554. [Google Scholar]
- Silva, G.C.O. Development, Characterization and Application of a Reactive Bulking Agent for Wall Control. Ph.D. Thesis, Queen’s University Kingston, Kingston, ON, Canada, 2007; p. 370. [Google Scholar]
- Sheahan, R.M.; Beattie, T.A. Effect of Explosive Type on Fines Generation in Blasting. In Proceedings of the Third International Symposium on Rock Fragmentation by Blasting, Brisbane, Australia, 26–31 August 1990. [Google Scholar]
- Johnson, R.J. SANFO: The Missing Link in Explosives Technology. In Proceedings of the Twenty-Second Annual Conference on Explosives and Blasting Technique, Orlando, FL, USA, 4–8 February 1996. [Google Scholar]
- Pal Roy, P.; Sawmliana, C.; Singh, R.K.; Chakunde, V.K. Effective Blasting Using Mixture of Ammonium Nitrate, Fuel Oil, Sawdust and Used Oil at Limestone Mine. Min. Technol. 2012, 121, 46–51. [Google Scholar] [CrossRef]
- Mousavi, A.A.A.; Burley, S.J.; Al-Hassani, S.T.S.; Byers Brown, W. Simulation of Explosive Welding with ANFO Mixtures. Propellants Explos. Pyrotech. 2004, 29, 188–196. [Google Scholar] [CrossRef]
- Mousavi, A.A.A.; Burley, S.J.; Al-Hassani, S.T.S. Simulation of Explosive Welding Using the Williamsburg Equation of State to Model Low Detonation Velocity Explosives. Int. J. Impact Eng. 2005, 31, 719–734. [Google Scholar] [CrossRef]
- Forsyth, W.; Deen, J.; Sterk, P. Assessment of Perimeter Blasting at Homestake Mine. In Proceedings of the 23rd Annual Conference on Explosives and Blasting Techniques, ISEE, Cleveland, OH, USA, 2–5 February 1977. [Google Scholar]
- Harries, G.; Gribble, D.P. The Development of a Low Shock Energy Explosive—ANRUB. In Rock Fragmentation by Blasting; Balkema: Rotterdam, The Netherland, 1993; pp. 379–386. [Google Scholar]
- Curtis, M.E. Regulating the Velocity of ANFO Utilizing Blends of Non-Explosive Materials. In Proceedings of the 13th Annual Symposium on Explosives and Blasting Research, Las Vegas, NV, USA, 2–5 February 1997; 1997; pp. 47–54. [Google Scholar]
- Beach, F.; Gribble, D.; Littlefair, M.; Roundely, R.; Testrow, I.; Wiggin, M. BlastLite—The Practical Low-Density Solution; The Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 2004; pp. 147–151. [Google Scholar]
- Miyake, A.; Takahara, K.; Ogawa, T.; Ogata, Y.; Wada, Y.; Arai, H. Influence of Physical Properties of Ammonium Nitrate on the Detonation Behaviour of ANFO. J. Loss Prev. Process Ind. 2001, 14, 533–538. [Google Scholar] [CrossRef]
- Zygmunt, B.; Buczkowski, D. Influence of Ammonium Nitrate Prills’ Properties on Detonation Velocity of ANFO. Propellants Explos. Pyrotech. 2007, 32, 411–414. [Google Scholar] [CrossRef]
- Buczkowski, D.; Zygmunt, B. Influence of Ammonium Nitrate Prills Porosity and Dimensions on Detonation Velocity of ANFO Explosives. In Proceedings of the 5th International Seminar New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 24–25 April 2002; pp. 45–51. [Google Scholar]
- Catanach, R.A.; Hill, L.G. Diameter Effect Curve and Detonation Front Curvature Measurements for ANFO. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2002; pp. 906–909. [Google Scholar]
- Bohanek, V.; Dobrilović, M.; Škrlec, V. Influence of the Initiation Energy on TheVelocity of Detonation of ANFO Explosive. Cent. Eur. J. Energetic Mater. 2013, 10, 555–568. [Google Scholar]
- Dobrilović, M.; Bohanek, V.; Žganec, S. Influence of Explosive Charge Temperature on the Velocity of Detonation. Cent. Eur. J. Energetic Mater. 2014, 11, 191–197. [Google Scholar]
- Zygmunt, B.; Buczkowski, D. Agriculture Grade Ammonium Nitrate as the Basic Ingredient of Massive Explosive Charges. Propellants Explos. Pyrotech. 2012, 37, 685–690. [Google Scholar] [CrossRef]
- Jackson, S.I.; Kiyanda, C.B.; Short, M. Precursor Detonation Wave Development in ANFO Due to Aluminum Confinement. In Proceedings of the 14th International Detonation Symposium, Coeur d’Alene, ID, USA, 11 April 2010; pp. 740–749. [Google Scholar]
- Bohanek, V.; Sućeska, M.; Dobrilović, M.; Hartlieb, P. Effect of Confinement on Detonation Velocity and Plate Dent Test Results for ANFO Explosive. Energies 2022, 15, 4404. [Google Scholar] [CrossRef]
- Ester, Z. Miniranje I: Eksplozivne Tvari, Svojstva i Metode Ispitivanja, 1st ed.; Rudarsko-Geološko-Naftni Fakultet: Zagreb, Croatia, 2005. [Google Scholar]
- Enciklopedija Guma. Hrvatska Enciklopedija, Mrežno Izdanje; Leksikografski zavod Miroslav Krleža: Zagreb, Croatia, 2023. [Google Scholar]
- Baček, I.; Hanaček, K.; Kanjir, I. Karakterizacija Gorivih Svojstava Trave Miscanthus & Giganteus Uzgojene u Republici Hrvatskoj; Agronomski fakultet, Sveučilište u Zagrebu: Zagreb, Croatia, 2012. [Google Scholar]
- EN 13763-15:2005; Explosives for Civil Uses—Detonators and Relays—Part 15: Determination of Equivalent Initiating Capability. CEN: Brussels, Belgium, 2005.
- Stanković, S.; Škrlec, V.; Dobrilović, M.; Bohanek, V. Velocity of Detonation of AN Base Blasting Agent with Addition of Hay and Recycled Rubber. In Proceedings of the 21st seminar New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 18–20 April 2018; pp. 1042–1051. [Google Scholar]
- Sućeska, M. EXPLO5 User’s Guide; OZM Research Publisher: Hrochuv Tynec, Czech Republic, 2023. [Google Scholar]
- Suceska, M.; Stimac Tumara, B.; Künzel, M. Using Thermochemical Code EXPLO5 to Predict the Performance Parameters of Explosives. Mater. Wysokoenergetyczne/High Energy Mater. 2021, 13, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Štimac, B.; Škrlec, V.; Dobrilović, M.; Sućeska, M. Numerical Modelling of Non-Ideal Detonation in ANFO Explosives Applying Wood-Kirkwood Theory Coupled with EXPLO5 Thermochemical Code. Def. Technol. 2021, 17, 1740–1752. [Google Scholar] [CrossRef]
- Fried, L.E.; Howard, W.M.; Souers, P.C. CHEETAH 2.0 User’s Manual, Lawrence Livermore National Laboratory Report UCRL-MA-117541 Rev. 5; LLNL: Livermore, CA, USA, 1998. [Google Scholar]
- Lee, E.L.; Tarver, C.M. Phenomenological Model of Shock Initiation in Heterogeneous Explosives. Phys. Fluids 1980, 23, 2362–2372. [Google Scholar] [CrossRef]
- Bohanek, V.; Štimac Tumara, B.; Serene, C.H.Y.; Sućeska, M. Shock Initiation and Propagation of Detonation in ANFO. Energies 2023, 16, 1744. [Google Scholar] [CrossRef]
- ANSYS, Inc. ANSYS Autodyn: User’s Manual Release 13.0; ANSYS, Inc.: Canonsburg, PA, USA, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).