1. Introduction
Osteoarthritis (OA) is mainly considered a disease of older people, as over one-third of the population over 65 years old present OA in at least one joint [
1]. However, factors such as obesity, sports, genetic factors, work, previous injuries, or socioeconomic status can influence osteoarthritic changes in joints [
2,
3] in younger populations. Knee osteoarthritis (KOA) was believed to be a mere cartilage degeneration disease caused by cartilage wear; however, it is now considered a complex articular metabolic disease associated with biomechanics, inflammation, and complex biological responses that affect the immune system (the cartilage, subchondral bone, and synovial membrane) [
4,
5].
In addition, during the process of inflammation in OA, inflammatory mediators stimulate chondrocytes to produce lysis enzymes, maintaining a vicious cycle consisting of inflammation, enzymes, chondrolysis, and inflammation [
6].
Hence, treatment for knee OA has aimed to improve the anatomical, metabolic, and physiological abnormalities that cause a vicious cycle. There are several potential treatments for this disease, including surgical administration, prescription drugs, physical activity, and traditional and new physical therapy methods [
7]. Various treatment methods are currently being attempted. Orthologics, such as platelet-rich plasma (PRP) therapy, mesenchymal stem cell (MSC) therapy, and extracorporeal shock wave therapy (ESWT), are among these new treatments that are gradually gaining attention [
8]. In particular, PRP injections have consistently outperformed HA injections in treating knee osteoarthritis, offering pain relief and improved function [
9]. As a result, PRP is recommended for cases where HA has not been effective.
According to a report by Aso et al. [
10], OA pain in the knee is dominated by pain from the subchondral bone and synovitis. The presence of a bone marrow lesion (BML), which is a lesion of the subchondral bone, is a risk factor for the structural progression of the disease; the presence of synovitis and joint effusion are also pathologies closely related to pain and disease progression. When considering the treatment of knee OA pain, it is important to understand the cause and pathology of pain and take an appropriate approach. In recent years, magnetic resonance imaging (MRI) has been used to diagnose knee joint pain, and it has become possible to observe BMLs in the subchondral bone or synovitis in detail. Therefore, the cause of knee OA pain can now be easily identified by MRI.
APS, which is a condensed and processed form of PRP, has been developed for OA. In OA, the inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)-α exacerbate cartilage destruction and OA progression. APS contains components that block the action of these inflammatory cytokines [
11]. APS reportedly reduces knee OA pain and prevents OA progression [
12]. It is effective in patients with synovitis-derived intra-articular hydrops and is recommended for patients with persistent intra-articular hydrops.
On the other hand, ESWT has become a new conservative treatment for OA in recent years [
8,
11,
13]. ESWT reduces pain and improves motor function. Gao et al. [
14] and Sansone et al. [
15] reported ESWT as an effective and reliable non-invasive treatment that rapidly reduces BMLs on pain-related MRI and improves knee function. In 2018, Kang et al. reported the first use of targeted ESWT for BMLs in knee OA [
16]. This treatment method is based on the speculation that physical stimulation of bone marrow induced by ESWT may improve bone remodeling abnormalities and promote the healing of microfractures [
16]. Bone and cartilage regeneration reduces inflammation, induces angiogenesis, stimulates stem cell activity, and improves tissue regeneration and healing [
13]. In addition, ESWT promotes osteoblast growth, differentiation, and transforming growth factor (TGF)-β1 expression. Furthermore, OA treatment reportedly controls subchondral bone remodeling and improves the trabeculae [
13].
We previously performed MRI on patients with knee OA whose symptoms (pain and swelling) did not improve even after intra-articular injection of hyaluronic acid. As ESWT acts directly on the bone, it is effective against BMLs of the subchondral bone [
17]. Conversely, APS is absorbed through the synovial membrane and acts primarily on the synovial membrane; thus, it is an effective treatment for synovitis. Therefore, ESWT is recommended for patients with BMLs based on MRI findings and APS therapy is recommended for patients with synovitis and intra-articular hydrops.
Some patients had both BML and synovitis, and they were treated with a combination of APS and ESWT to improve their condition. For the first year, we used both APS and PRP, but APS therapy was overwhelmingly better at improving intra-articular edema, and thus, we now recommend APS therapy to most patients, except for those who strongly hope to receive PRP.
There are three reasons why we used a combination of APS and ESWT for severe knee OA.
First, we thought that a single treatment would not be sufficient to deal with both synovial pain and subchondral bone pain, which are the causes of knee pain. In other words, APS is effective for synovitis, but ESWT is effective for BML, and thus, we thought that severe knee OA with both synovitis and BML must be treated with both APS and ESWT.
Second, many cases of severe knee OA, such as Kellgren–Lawrence classification grade 4 (KL4), have fewer synovitis symptoms, such as joint fluid, which may reduce the effectiveness of APS than KL2 or KL3. This was because we thought that applying some kind of stimulus to ESWT would make it easier for APS to respond.
Third, for severe KOA, such as KL4, there are some reports that ESWT and APS alone were inefficient, and thus, we thought that it would be better if we combined both of them. In 2013, Zhao et al. reported that ESWT was effective for KL2 and KL3 knee OA [
8]. Moreover, in 2022, Ishihara et al. reported that APS therapy was administered for KL4 knee OA; the effectiveness rate was 47.4% after 6 months of follow-up [
18]. In addition, Nakajima et al. performed APS on KL4 knee OA and reported that a few cases improved after 3 months [
19]. APS and ESWT alone were reported to be effective for mild-to-moderate knee OA, such as KL2 and KL3, but were less effective and short-lasting for more severe OA, such as KL4. Kuwasawa et al. performed a 12-month follow-up study of 148 knees with KL2, 3, and 4 OA that received APS injections and reported that the Knee Injury and Osteoarthritis Outcome Score (KOOS) was significantly lower in KL4 than KL2 [
20]. These reports showed that both ESWT and APS were less effective for KL4 severe knee OA.
Since 2019, a combination of APS therapy and ESWT for knee OA has been used to increase the success rate and duration in cases with synovitis and BML on MRI. The effectiveness of these combination treatments for severe knee OA has not been reported.
Therefore, this study aimed to examine the effects (success rate and duration) of a combination of APS and ESWT on knee OA by severity.
2. Materials and Methods
2.1. Study Design
We used a retrospective study design. APS therapy was performed from December 2019 to November 2022 in patients diagnosed with knee OA at our clinic or another clinic. Of the 39 patients (13 male individuals, 16 knees; 26 female individuals, 35 knees) who completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire at 3 and 6 months, 24 with KL4 (totaling 33 knees) were included. The KL classification was used to determine the OA severity of the knee. The mean age of patients was 71.0 (52–89) years.
After the APS injection, the A + E group that underwent ESWT after APS therapy had 26 knees, with 1, 5, and 20 knees scored as KL2, KL3, and KL4, respectively. Those who did not do ESWT after APS therapy (A-alone group) had 25 knees, with 6, 6, and 13 knees scored as KL2, KL3, and KL4, respectively. In the revision, KL2 and KL3 were excluded and only KL4 was included, which is more severe. As a result, the A + E group had 20 knees and the A-alone group had 13 knees. In the A + E group, an average of 4.5 ESWTs were performed post-APS therapy (
Table 1).
All patients underwent an ESWT session before the APS infusion and tried to continue ESWT once every 2 weeks for the first three times after APS therapy and once every 4–8 weeks thereafter. However, there were cases when patients did not attend the clinic for social reasons. The group that underwent ESWT after APS therapy (A + E group) included 20 knees, averaging 4.5 ESWTs. The group that did not undergo ESWT after APS therapy (group A-alone) had 13 knees. There was no significant difference in age or number of cases between the two groups. This study adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
2.2. ESWT Methodology
At our clinic, hyaluronic acid intra-articular injection therapy was performed under the diagnosis of varus knee OA, but there was no improvement in symptoms, and ESWT was indicated for patients with BML identified by MRI. The irradiation site was the medial condyle of the femur and the medial plateau of the tibia, or both, where the BML was observed and the area where the pain was felt.
The shock wave was produced using a Duolith SD1 (Storz Medical, Tägerwilen, Switzerland). First, MRI confirmed the areas where the BML was found in the medial condyle of the femur and the medial plateau of the tibia. The patient’s knee was maximally flexed, and the ultrasound probe was directed to the site where the BML was believed to be present to identify areas of cartilage thinning. The same part was considered to be the site where the BML existed, and the position of the irradiation target was determined. The operator’s free hand gripped the handpiece vertically against the target and began irradiation, gradually increasing the power level to the limit of the patient’s pain tolerance. The maximum output was up to 0.25 mJ/mm2. Since the pain stimulus weakened and disappeared if the same area was irradiated continuously, we tried to irradiate the entire lesion in three dimensions from multiple directions while listening to the degree of pain of the examinee in detail. As a general rule, ESWT should be irradiated at 2500 shots at 4 Hz at least 3 times every 2 weeks. Afterward, if the improvement of symptoms was insufficient, irradiation was continued once every 2–4 weeks until the 3-month questionnaire.
2.3. APS Methodology
Intra-articular APS injections have been reported to improve pain and function in patients with knee OA. APS is made by extracting growth factors and anti-inflammatory molecules in PRP, including IL-1-receptor antagonist, soluble IL-1 receptor II, and soluble tissue necrotic factor (TNF) receptor, through dehydration with polyacrylamide beads.
APS was prepared using the nSTRIDE APS Kit (Zimmer Biomet, Warsaw, IN, USA). In the first step, 55 mL of blood and 4 mL of anticoagulant citrate dextrose solution A (Citra Labs, Braintree, MA, USA) were injected into the nSTRIDE Cell Separator, and approximately 6 mL of PRP was separated after centrifugation at 3200 rpm for 15 min. The prepared PRP was then transferred to the nSTRIDE Concentrator, where it was exposed to polyacrylamide beads and filtered by centrifugation at 2000 rpm for 2 min to produce approximately 2–3 mL of APS. About 10 min after creating the APS, it was injected under ultrasound guidance. If there was intra-articular effusion after the joint puncture, joint fluid was first aspirated as much as possible and then the APS was injected.
2.4. Outcomes
First, we investigated the mean KOOSs before the APS injection, 3 months after the APS injection, and 6 months after the APS injection in both the A + E and A-alone groups.
Next, to study the success rates, we examined the rates of 20% improvement and 50% improvement in the KOOS in each group from the pre-APS score at 3 and 6 months.
The mean KOOS (improvement value [IV]) between the A + E and A-alone groups 3 months after APS injection (Pre-3M) and from 3 to 6 months (3–6M) were compared as a study of the duration of effects.
2.5. Statistical Analyses
A paired t-test was used to compare the KOOSs before and 3 and 6 months after the APS injection and before and after implementation, and an unpaired t-test was used for the comparisons between the A and A + E groups. Statistical significance was set at p < 0.05. Microsoft Excel 2016 (Microsoft, Redmond, WA, USA) was used for the analysis.
2.6. Ethical Procedures
Therapeutics performed in this study were reviewed and approved by the Technical and Legal Advisory Committee for Regenerative Medicine of the Japan Advanced Medical Association and received by the Ministry of Health, Labour and Welfare (approval no. PB5180014) based on the Act on Securing Safety of Regenerative Medicine.
Informed consent to use the clinical data for study purposes was obtained from all patients who were eligible for the treatments described in this paper.
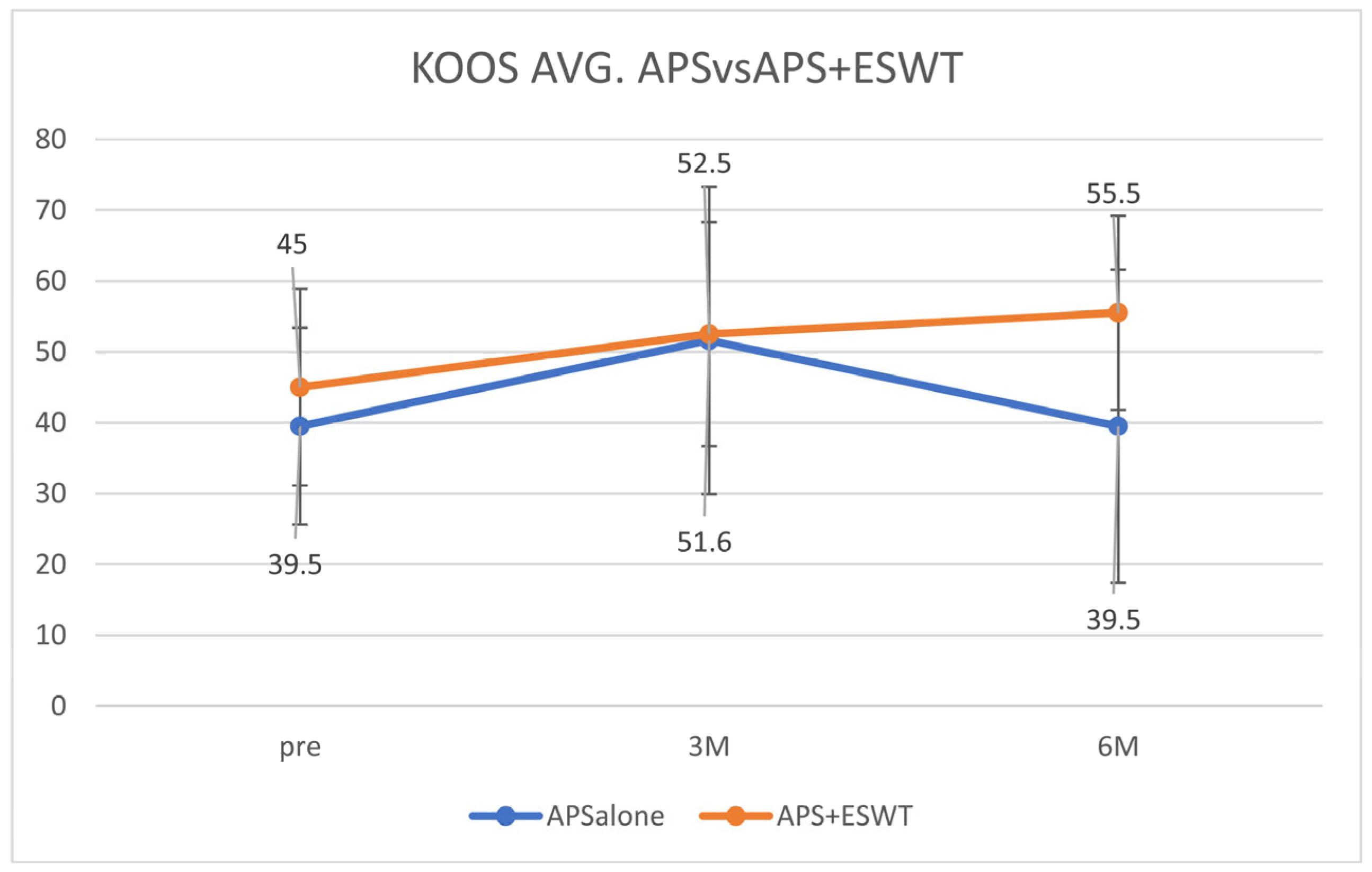
The average KOOS values were 39.5 ± 11.5 before the APS therapy, 51.6 ± 20.8 after 3 months, and 39.5 ± 20.5 after 6 months in the A-alone group. In contrast, in the A + E group, the values were 45.0 ± 12.7 before the APS therapy, 52.5 ± 15.3 at 3 months, and 55.5 ± 13.2 after 6 months. The average KOOS decreased from 3 to 6 months in the A-alone group but increased in the A + E group (
Figure 1).
Table 2.
Result (KOOS avg., KOOS pain, KOOS symp, KOOS ADL, KOOS sport, KOOS QOL).
Table 2.
Result (KOOS avg., KOOS pain, KOOS symp, KOOS ADL, KOOS sport, KOOS QOL).
| | KOOS Avg. (Figure 1) | KOOS Pain (Figure 2A) | KOOS Symp (Figure 2B) | KOOS ADL (Figure 2C) | KOOS Sport (Figure 2D) | KOOS QOL (Figure 2E) |
|---|
Pre
A-alone | 39.5
±11.5 | 46.0
±13.6 | 47.6
±15.0 | 57.7
±16.2 | 24.3
±15.4 | 40.4
±11.5 |
Pre
A + E | 45.0
±12.7 | 53.6
±16.3 | 51.6
±13.1 | 67.9
±15.1 | 26.8
±14.3 | 44.1
±12.7 |
3 months
A-alone | 51.6
±20.8 | 59.6
±23.6 | 63.2
±21.4 | 65.5
±20.9 | 38.9
±20.3 | 51.6
±20.8 |
3 months
A + E | 52.5
±15.3 | 63.6
±19.0 | 57.5
±17,9 | 74.6
±17.8 | 37.7
±15.7 | 52.5
±15.3 |
6 months
A-alone | 39.5
±20.5 | 44.0
±24.2 | 46.9
±29.0 | 55.6
±20.4 | 26.0
±14.5 | 39.5
±20.5 |
6 months
A + E | 55.5
±13.2 | 66.0
±12.8 | 62.4
±11.3 | 72.7
±15.7 | 44.3
±21.9 | 55.5
±13.2 |
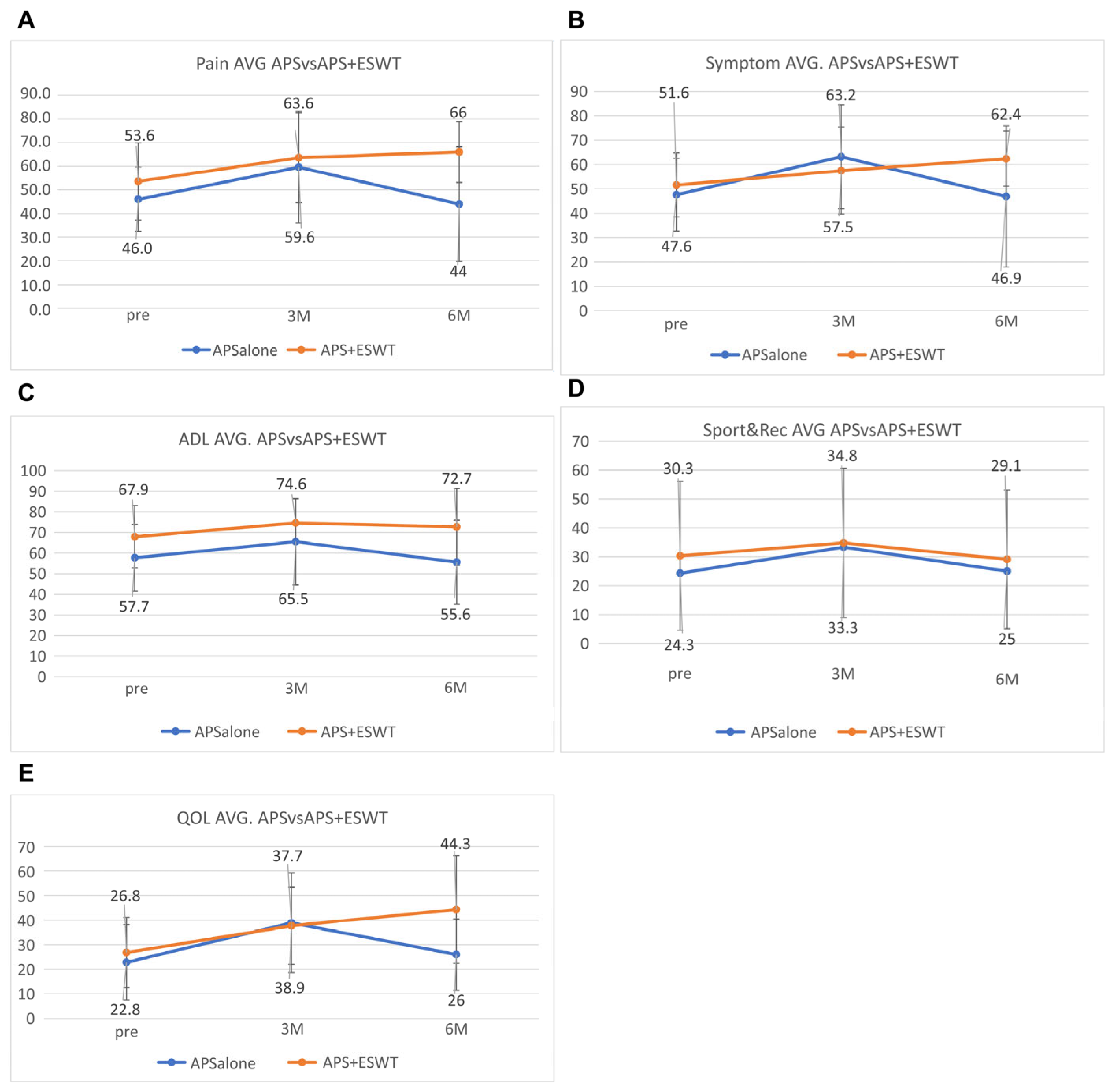
The mean KOOS pain subscore was 46.0 ± 13.6 before the APS therapy, 59.6 ± 23.6 after 3 months, and 44.0 ± 24.2 after 6 months in the A-alone group. In contrast, in the A + E group, the values were 53.8 ± 16.3 before the APS therapy, 63.6 ± 19.0 at 3 months, and 66.0 ± 12.8 after 6 months (
Figure 2A). The mean other symptoms subscore was 47.6 ± 15.0 before the APS therapy, 63,2 ± 21.4 after 3 months, and 46.9 ± 29.0 after 6 months in the A-alone group. In contrast, in the A + E group, the values were 51.6 ± 13.1 before the APS therapy, 57.5 ± 17.9 at 3 months, and 62.4 ± 11.3 after 6 months (
Figure 2B). The mean activities of daily living (ADL) subscore was 57.7 ± 16.2 before the APS therapy, 65.5 ± 20.9 after 3 months, and 55.6 ± 20.4 after 6 months in the A-alone group. In contrast, in the A + E group, the values were 67.9 ± 15.1 before the APS therapy, 74.6 ± 17.8 at 3 months, and 72.7 ± 15.7 after 6 months (
Figure 2C). The mean sports and recreation subscore was 24.3 ± 30.9 before the APS therapy, 33.3 ± 32.1 after 3 months, and 25.0 ± 30.7 after 6 months in the A-alone group. In contrast, in the A + E group, the values were 30.3 ± 25.7 before the APS therapy, 34.8 ± 25.8 at 3 months, and 29.1 ± 24.0 after 6 months (
Figure 2D). The mean quality of life (QOL) subscore was 22.8 ± 15.4 before the APS therapy, 38.9 ± 20.3 after 3 months, and 26.0 ± 14.5 after 6 months in the A-alone group. In contrast, in the A + E group, the values were 26.8 ± 14.3 before the APS therapy, 37.7 ± 15.7 at 3 months, and 44.3 ± 21.9 after 6 months (
Figure 2E). Most KOOS subscores decreased from 3 months to 6 months in the A-alone group but increased in the A + E group.
Regarding success rates, in the A-alone group, six patients (46.2%) had an improvement of ≥20% in their KOOSs after 3 months and five (38.5%) had an improvement of ≥50%. In contrast, in the A + E group, 11 patients (55.0%) had an improvement of ≥20% in their KOOSs and 4 (20.0%) had an improvement of ≥50% in their KOOSs. After 6 months, two (28.6%) patients had an improvement of ≥20% in their KOOSs in the A-alone group and one (14.3%) had an improvement of ≥50%. In the A + E group, seven (58.3%) patients had an improvement of ≥20% in their KOOSs and five (35.7%) of ≥50%. The success rate in the A-alone group was higher at 3 months than at 6 months for both 50% and 20% improvements. In the A + E groups, the success rate was higher at 6 months than at 3 months (
Table 3).
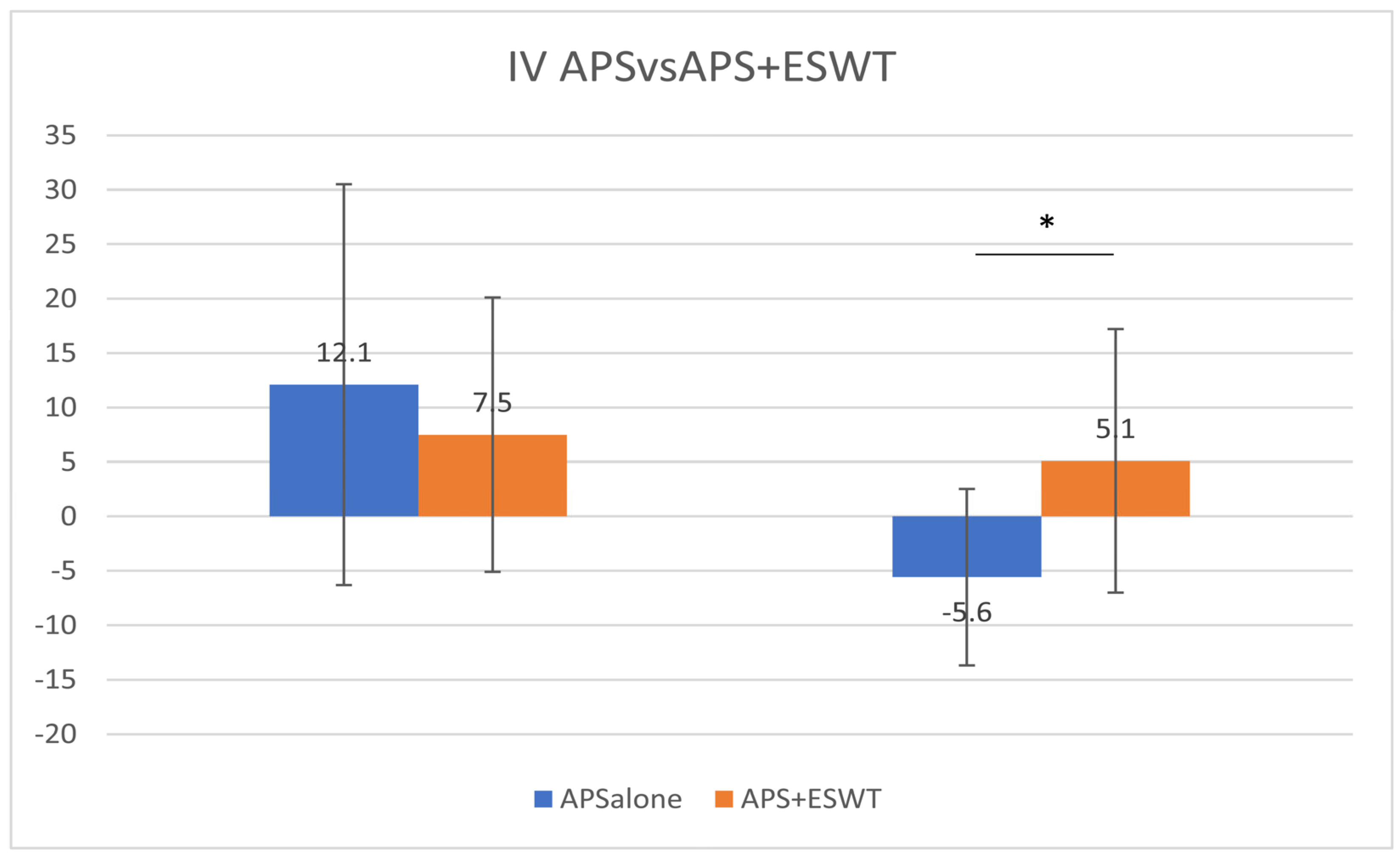
Regarding the duration, the IVs at Pre-3M were 12.1 ± 18.4 and 7.5 ± 12.6 in the A-alone and A + E groups, respectively, and −5.6 ± 8.1 and 5.1 ± 12.1 at 3–6M, respectively. The mean KOOS at Pre-3M showed no significant difference in improvement between the two groups; however, the improvement was significant in the A + E group compared with that in the A-alone group at 3–6M (unpaired
t-test,
p = 0.026;
Figure 3). There were no complications or side effects in the two study groups. Immediately after the APS injection, there were cases of minor fever or swelling due to pain, which is common in the case of APS only. As expected, the fever and swelling were reduced after a few days.
4. Discussion
The mean KOOS of the A-alone group decreased between 3 and 6 months, and the success rate in the A-alone group was higher at 3 months than at 6 months for both 50% and 20% improvement in the A-alone group. These results suggest that the effect of APS therapy alone on severe knee OA, such as KL4, lasted for at most 3 months. This result is consistent with those reported by Nakajima et al. [
19].
However, the average KOOS in the A + E group increased between 3 and 6 months, and the success rates in the A + E group were higher after 6 months than after 3 months for both 50% and 20% improvement. These results suggest that ESWT after the APS injection improved the duration of the effects and the performance rate.
In addition, the IV at 3–6M was significantly higher in the A + E group than in the A-alone group, suggesting that ESWT contributed significantly to improved performance from 3 to 6 months. Furthermore, the IV at 3–6M in the A + E group was 5.1 ± 12.1 compared with 7.5 ± 12.6 at Pre-3M; however, it was still a positive change. Further improvement could be expected if irradiation continued after 6 months by adding ESWT. Therefore, additional irradiation (booster irradiation) is considered effective in improving performance.
In more severe cases of KL4, the duration and success rate of ESWT after APS injection were higher than those of APS alone, suggesting that the combination of APS and ESWT was more synergistic than ESWT or APS alone. However, the mechanism underlying the synergistic effect remains unknown, although several hypotheses were proposed. The mechanism can be inferred from highly severe knee OA characteristics, such as KL4.
According to Miller et al., increased pathological free nerve endings in knee joints with advanced OA are important when considering the mechanism underlying chronic pain generation [
21]. In the present study, all patients were irradiated with ESWT once before APS injection; however, in highly severe OA, such as KL4, BMLs are often enlarged or advanced, and it is believed that there are more pathological free nerve endings. Consequently, a single irradiation was not sufficient, and it is thought that multiple additional ESWT irradiations are required to destroy the free nerve endings present in BMLs. Additionally, as symptoms and living standards improve, activity levels naturally increase. OA may worsen over time compared with before the APS injection. Therefore, multiple and additional ESWT irradiations contribute to symptom or pathological improvement.
In addition, KL4 knee OA has less synovitis than KL2 and KL3, and fewer inflammatory cytokines are expressed [
22]. APS targeting inflammatory cytokines may inevitably be less effective for severe knee OA, such as KL4. However, if ESWT stimulation increases inflammatory cytokines, such as TNFα, even temporarily, it may make it easier for APS to work effectively in response to TNFα [
23]. Consequently, ESWT causes pseudo-inflammation, which facilitates the action of APS-containing anti-inflammatory cytokines. In addition, cell adhesion factors that have an affinity for APS and PRP may be stimulated by ESWT, facilitating the effects of APS.
In addition, combined treatment may be expected to produce anti-inflammatory and cell proliferation effects, as seen on radiographs of the KL4 front-of-knee OA of a 64-year-old woman before APS therapy and 48 months after APS therapy. This patient continued ESWT approximately once or twice a month after the APS injection but showed a slight opening of the joint dehiscence compared with that before the APS injection (
Figure 4). MRI of the same patient 48 months later showed the reduction in BML in T1 (upper photo), and the disappearance of bone cysts in T2 (lower photo) was confirmed (
Figure 5). Since APS is a PRP product, it originally contains a large amount of TGFβ; however, the increased TGFβ level due to ESWT stimulation may have contributed to the anti-inflammatory effect and the regulation of cell proliferation and differentiation [
24,
25].
If ESWT stimulates endogenous stem cells, the interaction between the stimulated endogenous stem cells and the injected APS solution may promote cell proliferation [
26,
27]. However, further studies are required to confirm this hypothesis.
In addition, in severe knee OA, surgery, such as joint replacement, may be recommended, but it can be presented as a transition or alternative therapy for patients who cannot undergo surgery due to age restrictions or various social backgrounds. Regenerative medicine for knee osteoarthritis has recently attracted attention, and orthologics, such as PRP or MSC therapy, have attracted attention in recent years, but most of them are administered intra-articularly. When intra-articular administration is performed, most of the material is absorbed into the synovial membrane, except in severe cases of the rupture of some subchondral bone plates. The synovial membrane nourishes the cartilage, but even if there is an improvement in pain, it cannot directly improve the BML-derived pain from the bone marrow. However, not all BML-induced bone pain is resolved with ESWT. In particular, there seems to be a limit to pain with bone cysts and BML that contains a large amount of osteonecrotic tissue. In addition, in BMLs where the subchondral bone plate is ruptured, joint fluid easily enters the bone marrow and conservative treatment may be limited.
In this study, we recognize that further treatments other than ESWT that can repair BMLs, bone cysts, necrotic tissues, or subchondral plates will be needed in the future.
This study had some limitations. First, the only endpoint was the KOOS, and thus, the results are limited. The New Knee Society Score was to be used to evaluate patient-reported outcome measures and objective knee joint function. However, studies in one clinic were limited in assessing the effect of treatment using the KOOS, which is most often used, from the patient’s point of view. Second, image evaluation should not only be through KL classification but it should also include the relationship with BMLs. In this study, ESWT was applied on patients with knee OA with BMLs observed on MRI, but the type of BML irradiated and how it is done should be determined. Third, it is unclear whether ESWT irradiation irradiates the BML precisely or whether other areas are also irradiated. In addition, in this series, the number of shock waves was set at 2500 per session, but the shock wave strength and number were not constant and depended on the patient. While these were supposed to be constant, the shock wave level chosen was the maximum strength the patient could tolerate; therefore, we had to observe the patient’s reaction during and after the shock wave irradiation and determine the strength and irradiation time of the shock wave. In addition, the number of irradiations was the same regardless of whether there was one large BML or multiple, and thus, the energy irradiated per unit area varied depending on the size and number of BMLs. This was also different from clinical trials, and it was a limitation of the actual treatment. Fourth, there were differences in how patients spent their time after APS or ESWT. It cannot be ruled out that some patients may worsen due to differences in life, work, or sports activities after APS or ESWT. Unlike clinical trials, we believe that this is a limitation of research using actual patients. Fifth, as these are case studies, they were not compared with controls. Sixth, a well-formulated research plan was absent, but the APS treatments followed a research protocol. Whether or not to do ESWT depends on whether it is easy to go to my clinic. It is recommended that all patients continue irradiation once every 2 weeks after the APS injection for a total of three times and once every 4–8 weeks thereafter. However, whether to use ESWT after the APS injection was determined by the patient. Some patients were subjected to additional shock wave irradiation because their symptoms did not improve during treatment. However, some patients were not subjected to additional irradiation because they felt unwell after the APS injection. We believe that these are the limitations of case reports rather than clinical trials.