2.1. Reagents and Apparatus
Reagents: hydrochloric acid [
29], anhydrous sodium carbonate [
30], calcium carbonate [
31], sodium hydroxide [
32], potassium hydroxide [
33], Lithium hydroxide monohydrate [
34], manganese dioxide [
35], deionized water [
36]. All reagents are analytical grade.
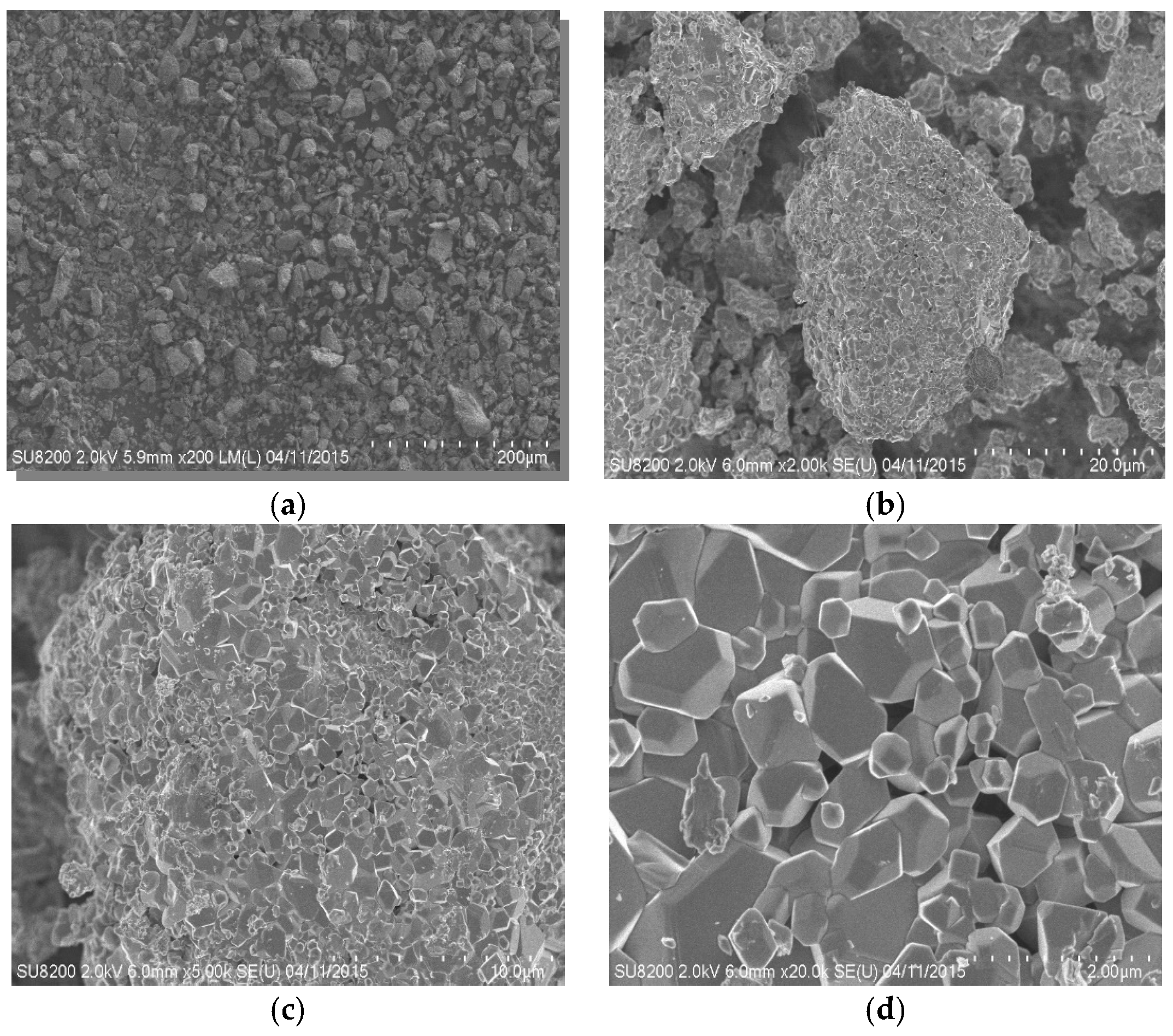
The following equipment was used: ball mill (Nanjing Nanda Instrument Co., Ltd., Nanjing, China), standard sampling sieve (Xinxiang Dahan Vibration Machinery Co., Ltd., Xinxiang, China), 1000 mL corundum crucible (Zibo huisen ceramic co., ltd., Zibo, China), magnetic heating stirrer (Heidolph instrument equipment co., ltd., Shanghai, China), 15 L high-pressure reaction kettle (Kaiyuan Chemical Machinery Manufacturing Co., Ltd., Kaiyuan, China), atomic absorption spectrometer (AAS, AA700, American PerkinElmer Company, Waltham, MA, USA), scanning electron microscope (SEM, SU-8220, Hitachi, Ltd., Tokyo, Japan).
2.2. Preparation of Lithium Extraction Mother Liquor from DCFA
The DCFA used in this study was obtained from coal combustion products in the Pingshuo mining area, Shanxi, China, following a desilication process.
(1) We ground the DCFA with a ball mill; sieved it to 200 mesh with a sample sieve; weighed the DCFA, Na
2CO
3, and CaCO
3 in a mass ratio of 1.0:0.8:1.0; mixed them evenly; and put them into a corundum crucible. These were baked in a muffle furnace at 1200 °C for 1.5 h, and cooled naturally to 750 °C. A constant temperature was maintained for 20 min, then the samples were removed and ground when they were cool. The reaction is as follows:
The roasted DCFA was further activated and the lithium was converted from an aluminosilicate to an aluminate, which is more conducive to the next reaction.
(2) The processed DCFA was ground into a paste with 5% Na
2CO
3 solution and placed in an autoclave to be heated at 150 °C for 1 h. The reaction is as follows:
At this heating temperature and time, Na2CO3 can replace lithium in the solution, leaving lithium to exist in the form of Li2CO3. The solution obtained is filtered, followed by the extraction of aluminum, to give the lithium extraction mother liquor from the DCFA. The lithium extraction mother liquor derived from DCFA mentioned in this paper is prepared using the above method.
2.3. Experimental Design
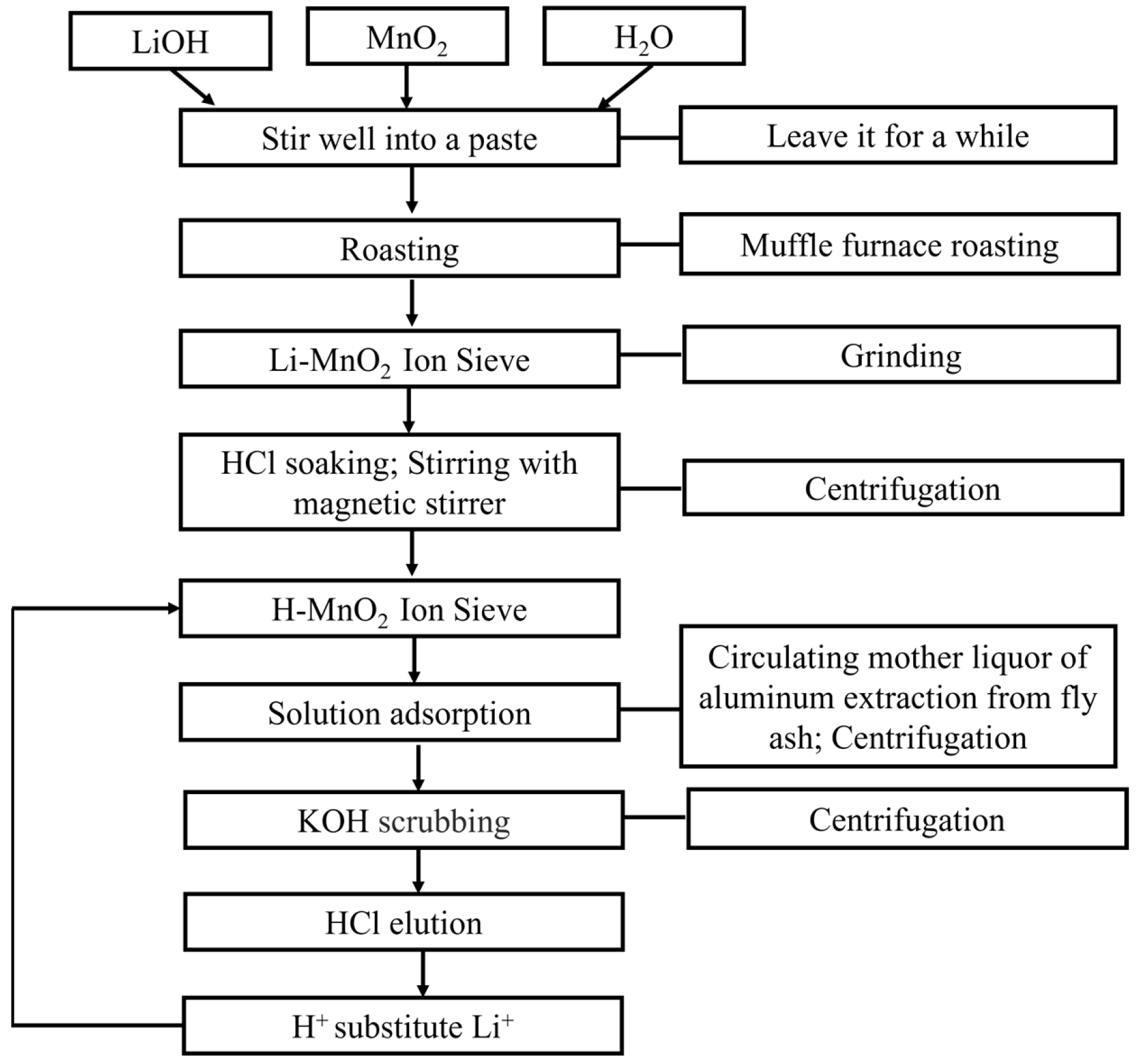
Preparation principle of manganese dioxide lithium ion sieve: the precursor of lithium manganese with X structure is synthesized by mixed sintering of manganese compound and lithium compound, and then subsequently Li+ is eluted by acid washing, which makes it have a memory of lithium. In the present study, a manganese dioxide lithium ion sieve was obtained by mixed sintering of manganese dioxide and lithium hydroxide, followed by elution with hydrochloric acid.
In this experiment, firstly, a preparation experiment of the manganese dioxide lithium ion sieve was carried out to identify the optimum preparation conditions. Secondly, an adsorption experiment of the manganese dioxide lithium ion sieve was performed to ascertain the most effective adsorption parameters; and finally, an acid elution experiment was carried out to determine the optimum elution conditions.
2.3.1. Orthogonal Experimental Design for Manganese Dioxide Lithium Ion Sieve Preparation
The study was conducted using a five-level and six-factor experimental design to investigate the effect of various parameters on the optimum preparation conditions of a manganese dioxide lithium ion sieve. The six factors considered were the dioxide-to-lithium hydroxide mass ratio (A), roasting temperature (B), roasting time (C), HCl concentration (D), HCl soaking time (E) and stirring time (F). The specific levels of each factor are outlined in
Table 1.
Additional conditions were outlined as follows: the liquid–solid ratio of the lithium extraction mother liquor derived from DCFA to the manganese dioxide lithium ion sieve was maintained at 100:0.1. The adsorption time was 30 min, while the adsorption temperature was controlled in a range of 45–50 °C. Too high or too low a temperature may reduce the selectivity of the ion sieve to lithium ions, and a high temperature may lead to deactivation of the ion sieve, while a low temperature may slow down the adsorption rate. The rotation speed was set at 500 r/min, and centrifugation was performed for 2 min. Additionally, the pH of KOH was set at 8. The purpose of KOH was to serve as a washing agent for the lithium ion-adsorbing ion sieve, effectively eliminating impurities such as Na+, Ca2+ and Al3+. KOH washing time was set at 30 min, while centrifugation was carried out for 2 min.
Experiments were arranged in an L25(5
6) orthogonal array, and 25 groups of experiments were needed, as shown in
Table 2.
2.3.2. Orthogonal Experimental Design of Adsorption
A study employing four levels and three factors was conducted to investigate the effect of adsorption time (A), KOH pH (B) and KOH scrubbing time (C) on the efficiency of lithium adsorption by the manganese dioxide lithium ion sieve. The corresponding factor values are presented in
Table 3.
Other conditions were as follows: the manganese dioxide lithium ion sieve was prepared using the above optimized procedure. The liquid–solid ratio of the lithium extraction mother liquor derived from DCFA to the manganese dioxide lithium ion sieve was set at 100:0.1. Stirring was carried out at a controlled temperature of 45–50 °C, with a stirring speed of 500 r/min, followed by centrifugation for 2 min.
An L16(4
3) orthogonal array was used to arrange experiments, and a total of 16 groups of experiments were needed, as seen in
Table 4.
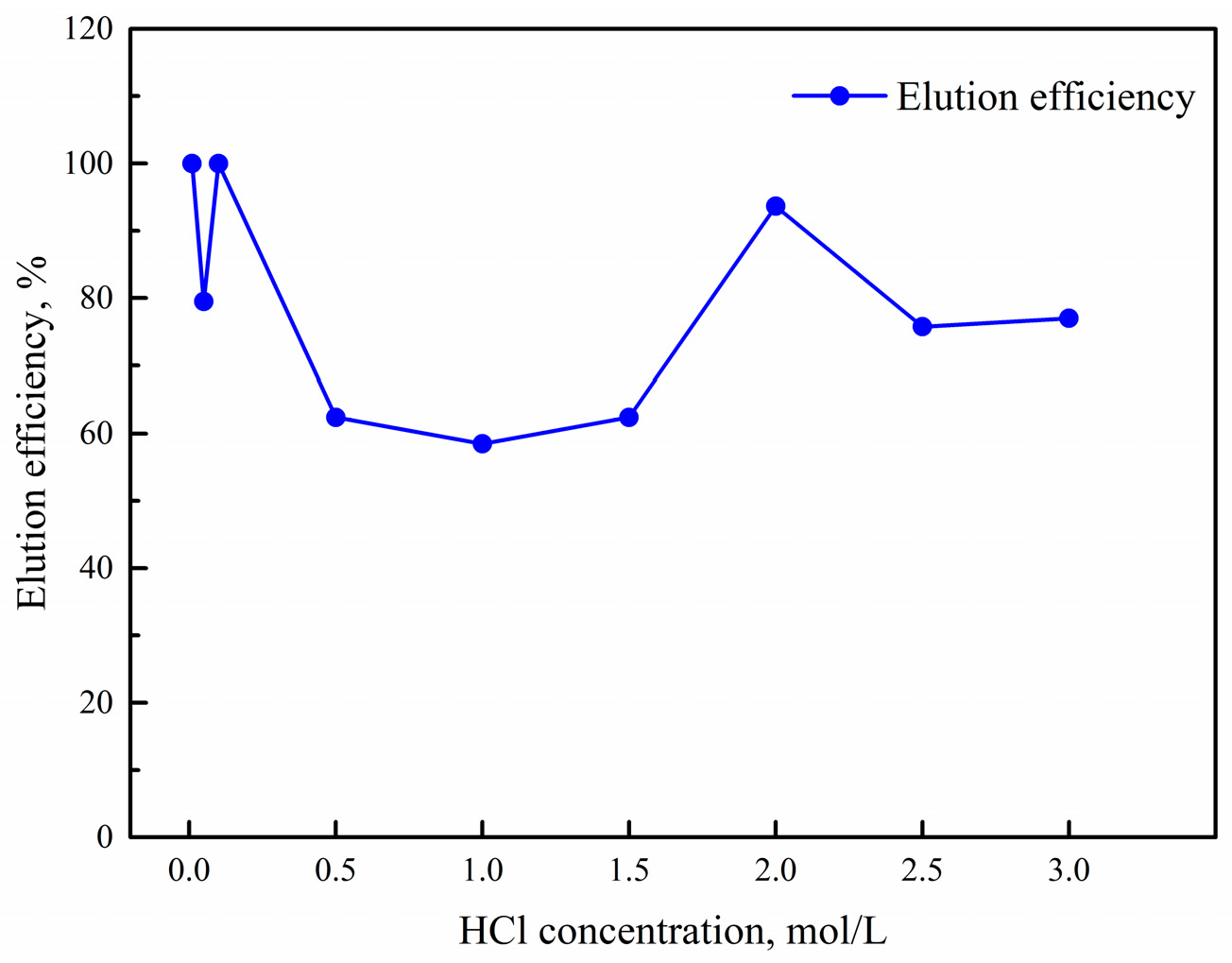
2.3.3. Elution Experiment Analysis
The parameters impacting the elution efficiency were HCl concentration and elution time. A single factorial experiment was applied to investigate the effect of these parameters on elution efficiency.
2.4. Experimental Procedure
A certain amount of lithium hydroxide and manganese dioxide was placed in a corundum crucible. A certain amount of water was added, and the mixture was stirred into a paste, which was then left for a period of time to allow the lithium hydroxide and manganese dioxide to mix completely. The mixture was then sintered in a muffle furnace at a specified temperature for a specified time.
During high-temperature sintering, the sample tended to experience agglomeration. After a certain period of sintering, gradual cooling was implemented within a muffle furnace at approximately 200–250 °C. The sample was maintained at this constant temperature for 20 min before being extracted from the furnace for subsequent grinding, resulting in the formation of a lithium ion sieve.
A specific quantity of pre-treated lithium ion sieve was measured and placed in a 250 mL conical flask. HCl at a specified concentration was added for a specified soaking time. The flask was placed on a rotary stirrer to allow rotation to ensure the thorough progress of the H+ and Li+ displacement reaction. Centrifugation was then used to separate and remove the lithium ions, followed by a drying process to finally obtain the hydrogen ion sieve.
A certain amount of the prepared hydrogen–lithium ion sieve was placed into a 250 mL conical flask. A specified amount of prepared lithium extraction mother liquor derived from DCFA was added. The flask was placed on a rotary stirrer where it was rotated and stirred to complete the displacement reaction of H
+ and Li
+. During this phase, lithium ions in the solution were adsorbed onto the ion sieve. The ion sieve was then separated by centrifugation. The lithium content in the solution after adsorption was measured using AAS, which allowed for the determination of the lithium adsorption efficiency, which is calculated by Formula (1):
where
represents the concentration of Li
+ in the solution,
the remaining concentration of Li
+ in the solution after adsorption by the adsorbent, and
the volume of the solution adsorbed by the adsorbent.
The ion sieve with lithium ions adsorbed was placed into a 250 mL conical flask containing KOH solution with a specific pH. The flask was placed on a rotary magnetic stirrer and stirred to remove interference from ions such as Na+, Ca2+, and Al3+ in the solution, which could potentially affect the behavior of the lithium ions.
HCl at a specific concentration was added to the lithium-ion-adsorbed ion sieve within a 250 mL conical flask. The flask was positioned on a rotary stirrer and set into rotation to facilitate the completion of the H
+ and Li
+ displacement reaction. During this process, the lithium ions adsorbed by the ion sieve were eluted into the solution; at the same time, the ion sieve was transferred into a hydrogen ion sieve which could be repeatedly utilized to adsorb lithium ions. Subsequently, the lithium content in the solution was measured using AAS to ascertain the elution efficiency of lithium, which is calculated by Formula (2):
where
and
are the Li
+ concentrations in the solution, in the residual solution after adsorption by the adsorbent and in the solution after pickling, respectively;
and
represent the volume of the adsorption solution of the adsorbent and the volume of the pickling solution, respectively.
The experimental procedure schematic is shown in
Figure 1.