Efficiency of Biofortification with Zn and Se in Soybean: Yield and Overall Mineral Content in Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trail and Soil Properties
2.2. Biofortification Treatments
- Control (without Se or Zn solutions): water was applied;
- Se_1 treatment: 10 g/ha Se;
- Se_2 treatment: 20 g/ha Se;
- Se_3 treatment: 30 g/ha Se;
- Zn_1 treatment: 3 kg/ha Zn;
- Zn_2 treatment: 6 kg/ha Zn;
- Se_3 Zn_2 treatment: 30 g/ha Se + 6 kg ha Zn.
2.3. Plant Material Sampling and Harvest
2.4. Analysis of Plant Material
2.5. Calculations of Removal Amount of the Nutrients and Biofortification Efficiency
2.6. Statistical Data Processing
3. Results
3.1. Macro- and Micronutrient Status in the Soybean Grain
3.1.1. The ANOVA of the Macro- and Micronutrient Status in the Soybean Grain
3.1.2. Macro- and Microelement Accumulation in the Grain
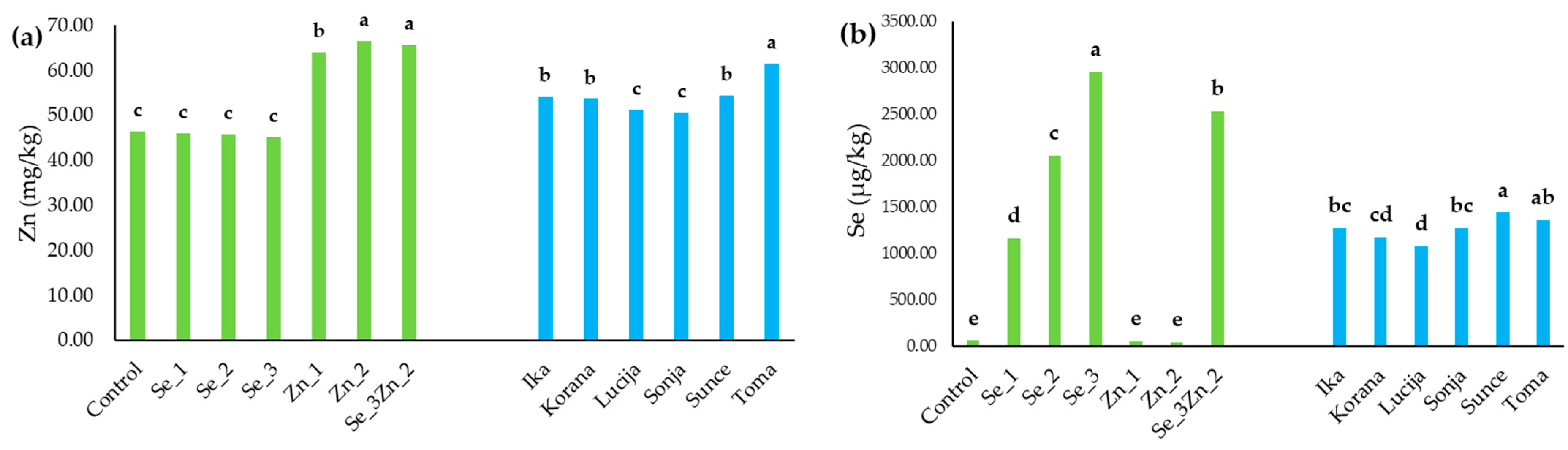
3.1.3. Zn and Se Accumulation in the Grain
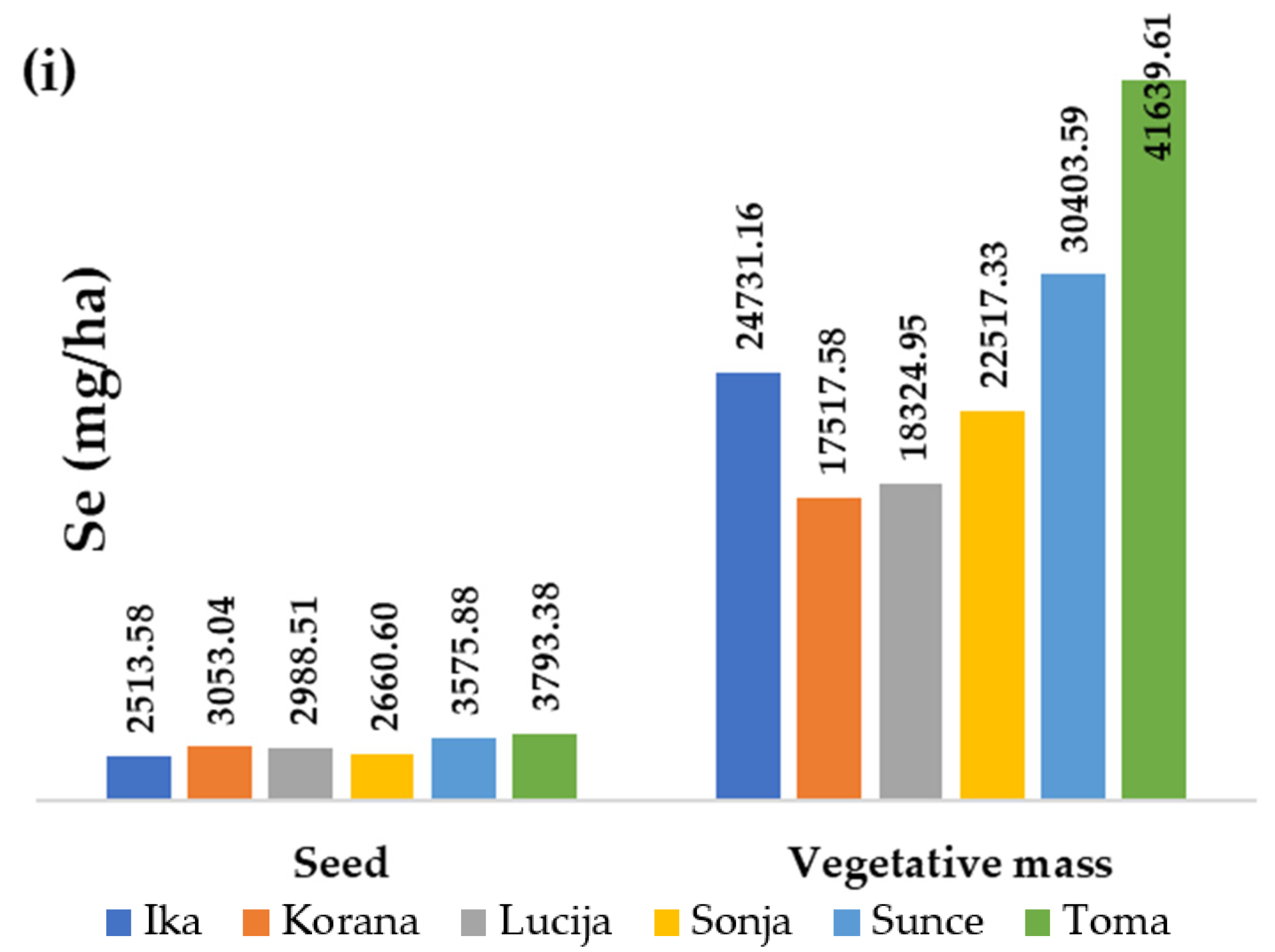
3.2. Distribution of Zn and Se in Above-Ground Organs
3.3. Linear Regression of Biofortification and Grain Zn and Se
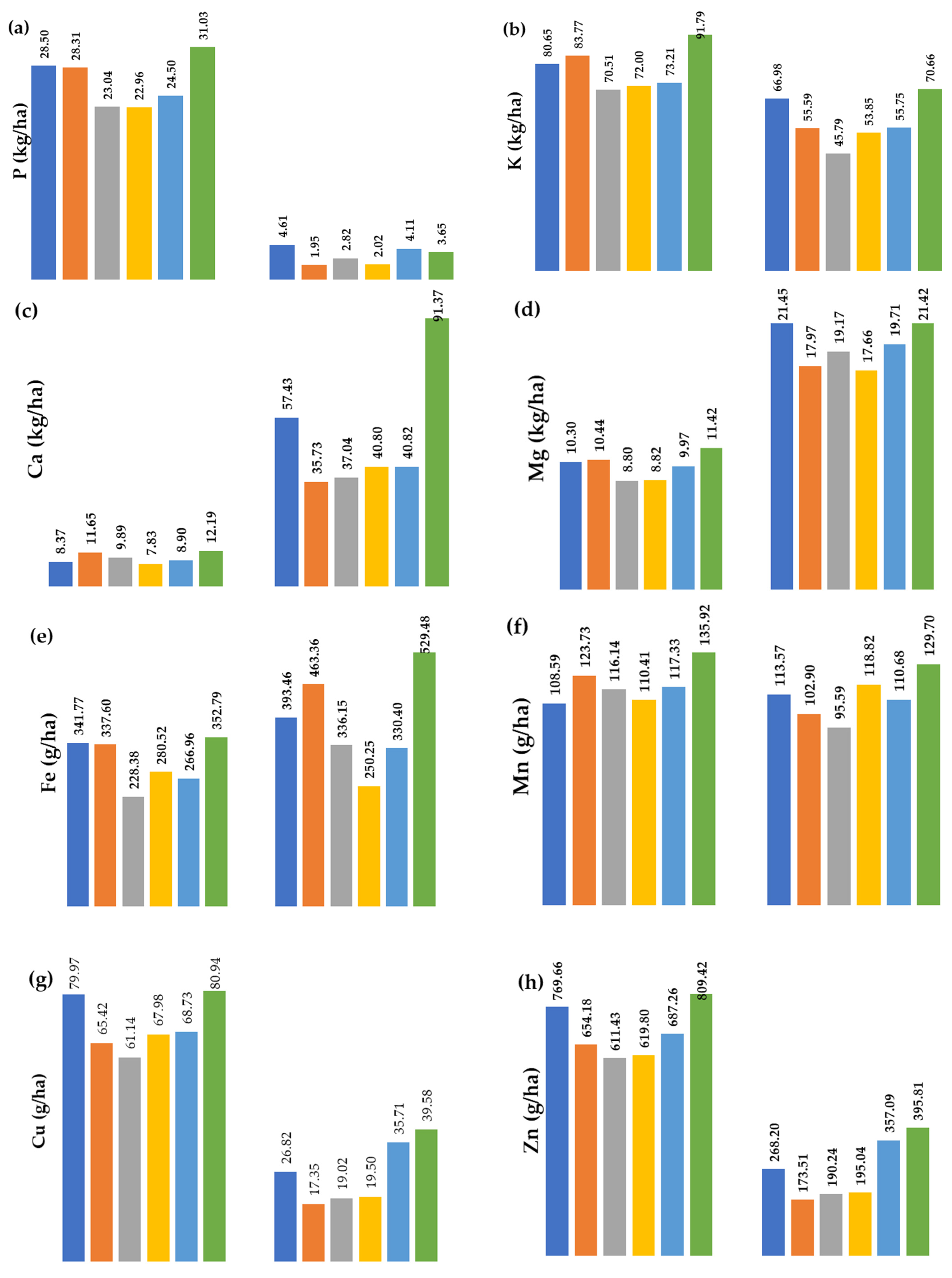
3.4. Macro- and Micronutrient Removal by the Soybean
3.4.1. The ANOVA of the Macro- and Micronutrient Rem Oval by the Plant
3.4.2. Macro- and Micronutrient Removal by the Plant
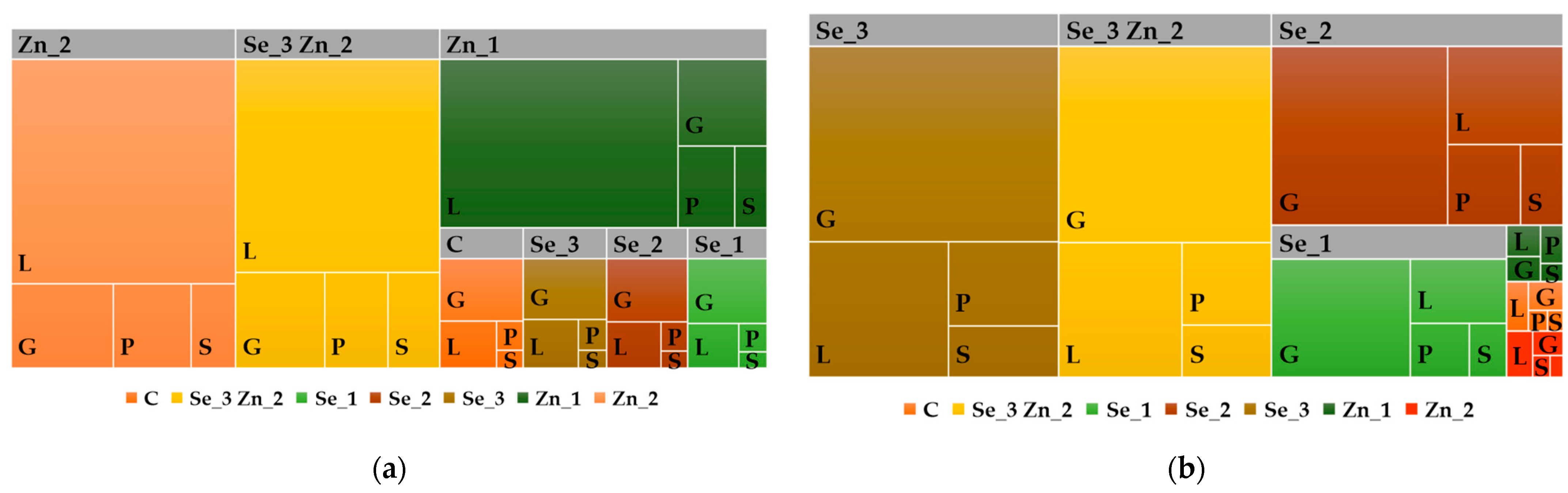
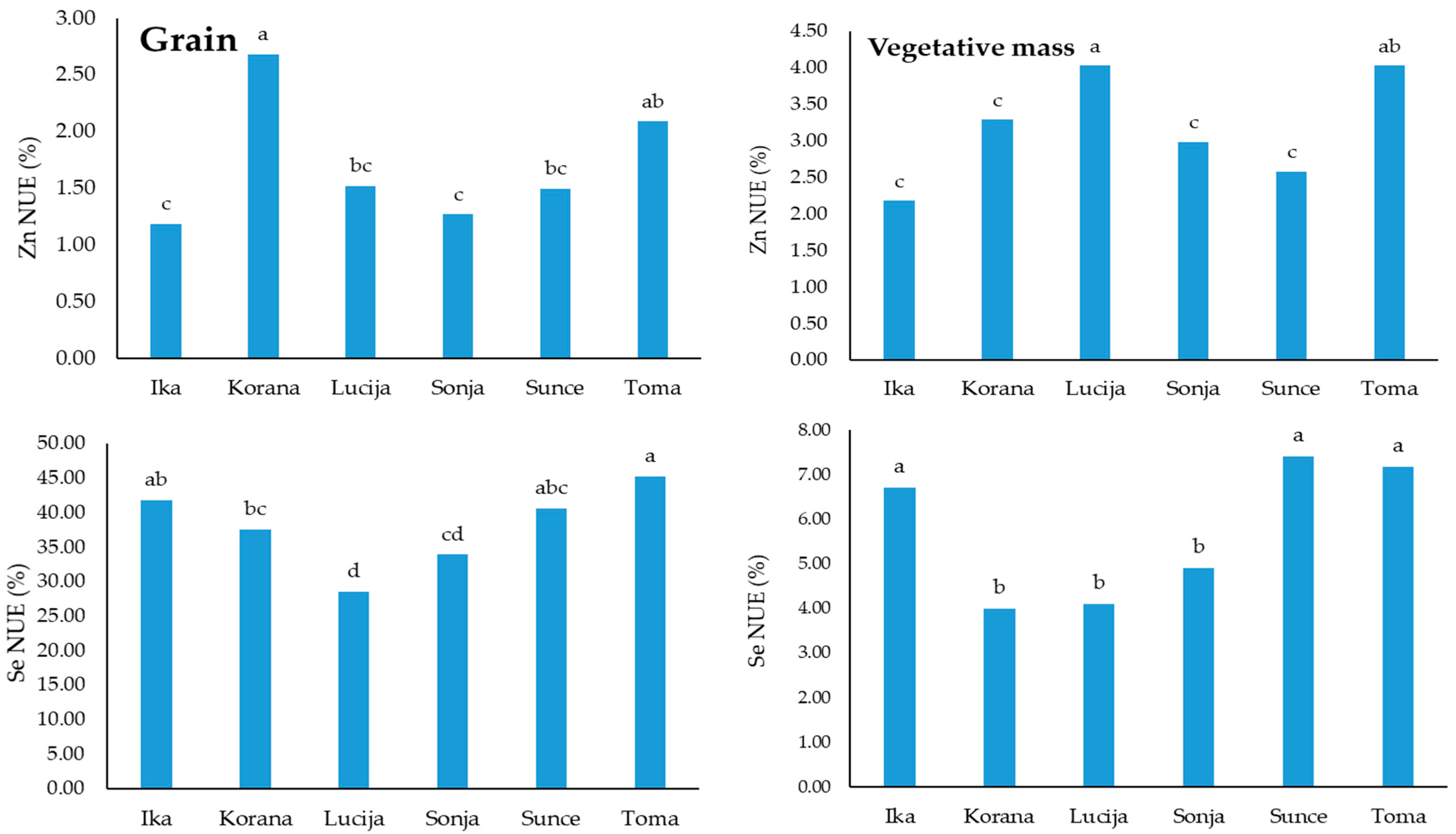
3.5. Nutrient Use Efficiency of Zn and Se Treatments
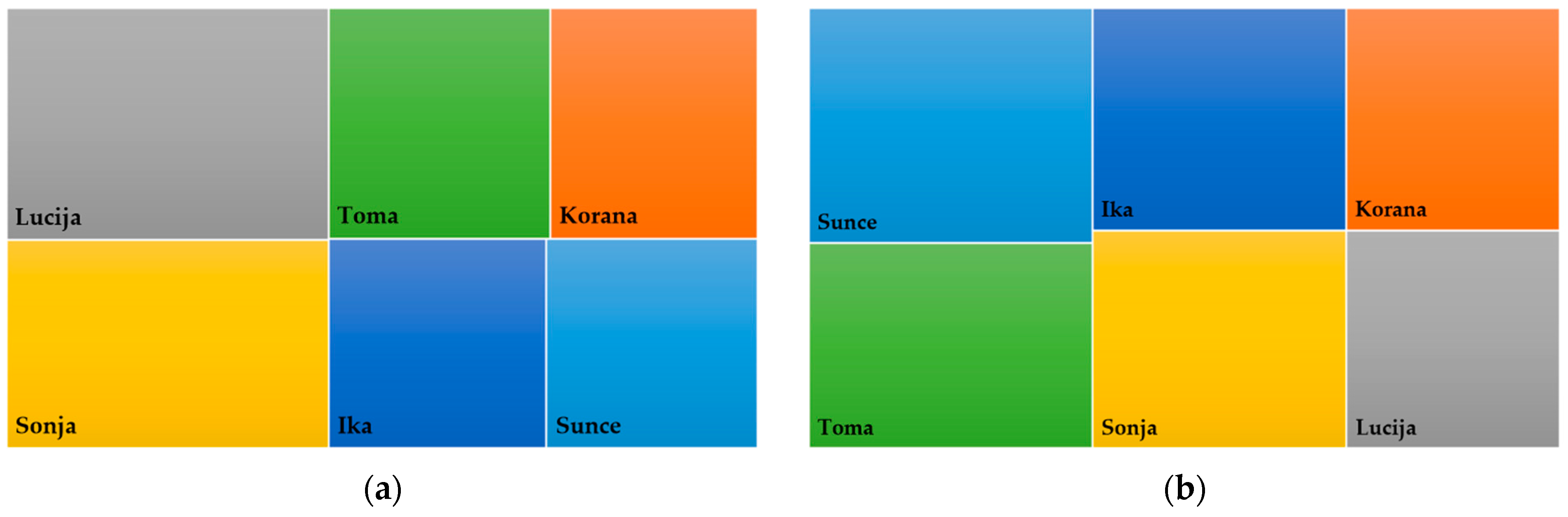
3.6. Categorization of Soybean Varieties for NUE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations, FAO; International financial institution and a specialized agency of the United Nations, IFAD; United Nations International Children’s Emergency Fund, UNICEF; World Food Programme, WFP; World Health Organization, WHO. The State of Food SECURITY and Nutrition in The World 2019. Safeguarding Against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019. [Google Scholar]
- Lopes, S.O.; Abrantes, L.C.S.; Azevedo, F.M.; Morais, N.D.S.D.; Morais, D.D.C.; Gonçalves, V.S.S.; Fontes, E.A.F.; Franceschini, S.C.C.; Priore, S.E. Food insecurity and micronutrient deficiency in adults: A systematic review and meta-analysis. Nutrients 2023, 15, 1074. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Rosado, P.; Roser, M. Hunger and undernourishment. Our World in Data. 2023. Available online: https://ourworldindata.org/hunger-and-undernourishment (accessed on 3 October 2024).
- Ganguly, R.; Sarkar, A.; Dasgupta, D.; Acharya, K.; Keswani, C.; Popova, V.; Minkina, T.; Maksimov, A.Y.; Chakraborty, N. Unravelling the Efficient Applications of Zinc and Selenium for Mitigation of Abiotic Stresses in Plants. Agriculture 2022, 12, 1551. [Google Scholar] [CrossRef]
- National Institutes of Health, Strengthening Knowledge and Understanding of Dietary Supplements. 2024. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 3 October 2024).
- Zavros, A.; Andreou, E.; Aphamis, G.; Bogdanis, G.C.; Sakkas, G.K.; Roupa, Z.; Giannaki, C.D. The effects of zinc and selenium Co-supplementation on resting metabolic rate, thyroid function, physical fitness, and functional capacity in overweight and obese people under a hypocaloric diet: A randomized, double-blind, and placebo-controlled trial. Nutrients 2023, 15, 3133. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Hall, A.G.; Broadley, M.R.; Foley, J.; Boy, E.; Bhutta, Z.A. Preventing and controlling zinc deficiency across the life course: A call to action. Adv. Nutr. 2024, 15, 100181. [Google Scholar] [CrossRef] [PubMed]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.Á. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic biofortification with Se, Zn, and Fe: An effective strategy to enhance crop nutritional quality and stress defense—A review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Galić, L.; Špoljarević, M.; Auriga, A.; Ravnjak, B.; Vinković, T.; Lončarić, Z. Combining Selenium Biofortification with Vermicompost Growing Media in Lamb’s Lettuce (Valerianella locusta L. Laterr). Agriculture 2021, 11, 1072. [Google Scholar] [CrossRef]
- Yağmur, B.; Okur, B.; Okur, N. The Effect of Nitrogen, Magnesium, and Iron Applications on the Nutrient Content of Parsley (Petroselinum crispum). Poljoprivreda 2022, 28, 3–8. [Google Scholar] [CrossRef]
- Xue, Y.F.; Li, X.J.; Yan, W.; Miao, Q.; Zhang, C.Y.; Huang, M.; Sun, J.B.; Qi, S.J.; Ding, Z.H.; Cui, Z.L. Biofortification of different maize cultivars with zinc, iron and selenium by foliar fertilizer applications. Front. Plant Sci. 2023, 14, 1144514. [Google Scholar] [CrossRef]
- Manojlović, M.S.; Lončarić, Z.; Cabilovski, R.R.; Popović, B.; Karalić, K.; Ivezić, V.; Ademi, A.; Singh, B.R. Biofortification of wheat cultivars with selenium. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 715–724. [Google Scholar] [CrossRef]
- Hu, F.Q.; Jiang, S.C.; Wang, Z.; Hu, K.; Xie, Y.M.; Zhou, L.; Zhu, J.Q.; Xing, D.-Y.; Du, B. Seed priming with selenium: Effects on germination, seedling growth, biochemical attributes, and grain yield in rice growing under flooding conditions. Plant Direct 2022, 6, e378. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Usman, M.; Nadeem, F.; ur Rehman, H.; Wahid, A.; Basra, S.M.; Siddique, K.H. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Mangueze, A.V.D.J.; Pessoa, M.F.; Silva, M.J.; Ndayiragije, A.; Magaia, H.E.; Cossa, V.S.; Reboredo, F.H.; Carvalho, M.L.; Santos, J.P.; Guerra, M.; et al. Simultaneous zinc and selenium biofortification in rice. Accumulation, localization and implications on the overall mineral content of the flour. J. Cereal Sci. 2018, 82, 34–41. [Google Scholar] [CrossRef]
- Klognerová, K.; Vosmanská, M.; Száková, J.; Mestek, O. Vliv pěstebních podmínek na speciaci selenu v tkáních řepky olejky. Chem. Listy 2015, 109, 216–222. [Google Scholar]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Tomei, F.A.; Barton, L.L.; Lemanski, C.L.; Zocco, T.G.; Fink, N.H.; Sillerud, L.O. Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J. Ind. Microbiol. 1995, 14, 329–336. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.-R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 17, pp. 225–241. [Google Scholar] [CrossRef]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Mrština, T.; Praus, L.; Száková, J.; Kaplan, L.; Tlustoš, P. Foliar selenium biofortification of soybean: The potential for transformation of mineral selenium into organic forms. Front. Plant Sci. 2024, 15, 1379877. [Google Scholar] [CrossRef]
- Van Dael, P.; Davidsson, L.; Ziegler, E.E.; Fay, L.B.; Barclay, D. Comparison of selenite and selenate apparent absorption and retention in infants using stable isotope methodology. Pediatr. Res. 2002, 51, 71–75. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Younas, N.; Fatima, I.; Ahmad, I.A.; Ayyaz, M.K. Alleviation of zinc deficiency in plants and humans through an effective technique; biofortification: A detailed review. Acta Ecol. Sin. 2023, 43, 419–425. [Google Scholar] [CrossRef]
- Cerkal, R.; Hřivna, L.; Ryant, P.; Prokeš, J.; Březinova Belcredi, N.; Vejražka, K.; Michnová, M.; Gregor, T. Zinc-effect on the spring barley’s plant and roots growth, grain technological quality, and yeast fermentation. Kvas. Prum. 2010, 56, 152–159. [Google Scholar] [CrossRef][Green Version]
- Zebec, V.; Lisjak, M.; Jović, J.; Kujundžić, T.; Rastija, D.; Lončarić, Z. Vineyard Fertilization Management for Iron Deficiency and Chlorosis Prevention on Carbonate Soil. Horticulturae 2021, 7, 285. [Google Scholar] [CrossRef]
- Vrsaljko, A. Dinamika nakupljanja olova u listu i dijelovima ploda bajama. Poljoprivreda 2023, 29, 35–42. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Norouzi, M.; Afyuni, M.; Schulin, R. Zinc biofortification of wheat through preceding crop residue incorporation into the soil. Eur. J. Agron. 2017, 89, 131–139. [Google Scholar] [CrossRef]
- FAOStat. 2024. Available online: https://www.fao.org/faostat/en/ (accessed on 24 September 2024).
- Galić Subašić, D.; Jurišić, M.; Rebekić, A.; Josipović, M.; Radočaj, D.; Rapčan, I. Relationship Between the Soybean (Glycine max L. Merr.) Yield Components and Seed Yield Under Irrigation Conditions. Poljoprivreda 2022, 28, 32–38. [Google Scholar] [CrossRef]
- Pospišil, A.; Ivanović, K.; Pospišil, M. The Potential of White Lupin (Lupinus albus L.) Seed and Biomass Yield in organic Farming. Poljoprivreda 2022, 28, 18–23. [Google Scholar] [CrossRef]
- Krizmanić, G.; Ćupić, T.; Tucak, M.; Horvat, D.; Brkić, A.; Beraković, I.; Marković, M. Procjena agronomskih vrijednosti i stabilnost komponenti prinosa novostvorenih linija jaroga stočnog graška (Pisum sativum L.). Poljoprivreda 2022, 28, 9–16. [Google Scholar] [CrossRef]
- Andrade, J.F.; Ermacora, M.; De Grazia, J.; Rodríguez, H.; Mc Grech, E.; Satorre, E.H. Soybean seed yield and protein response to crop rotation and fertilization strategies in previous seasons. Eur. J. Agron. 2023, 149, 126915. [Google Scholar] [CrossRef]
- Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy 2021, 11, 1015. [Google Scholar] [CrossRef]
- Vollmann, J. Introduction to the Soybean Topical Issue and the upcoming World Soybean Research Conference 11. OCL 2023, 30, 8. [Google Scholar] [CrossRef]
- Matoša Kočar, M.; Vila, S.; Petrović, S.; Rebekić, A.; Sudarić, A.; Duvnjak, T.; Markulj Kulundžić, A. Variability of fatty acid profiles, oxidative stability and nutritive quality of oil in selected soybean genotypes. Poljoprivreda 2020, 26, 11–20. [Google Scholar] [CrossRef]
- Radočaj, D.; Jurišić, M.; Gašparović, M.; Plaščak, I. Optimal Soybean (Glycine max L.) Land Suitability Using GIS-Based Multicriteria Analysis and Sentinel-2 Multitemporal Images. Remote Sens. 2020, 12, 1463. [Google Scholar] [CrossRef]
- Uher, D. Utjecaj komercijalnih inokulanata na kemijski sastav i fermentaciju silaže od lucerne. Poljoprivreda 2024, 30, 75–80. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Feed demand landscape and implications of food-not feed strategy for food security and climate change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef]
- Novoselec, J.; Klir, Ž.; Domaćinović, M.; Lončarić, Z.; Antunović, Z. Biofortification of feedstuffs with microelements in animal nutrition. Poljoprivreda 2018, 24, 25–34. [Google Scholar] [CrossRef]
- Moorby, J.M.; Fraser, M.D. New feeds and new feeding systems in intensive and semi-intensive forage-fed ruminant livestock systems. Animal 2021, 15, 100297. [Google Scholar] [CrossRef]
- Gulin, J.; Florijančić, T.; Bilandžić, N.; Ozimec, S.; Bošković, I.; Lončarić, Z. Heavy Metals (As, Cd, Hg and Pb) in Hare Tissues: A Survey. Poljoprivreda 2023, 29, 86–96. [Google Scholar] [CrossRef]
- Antunović, Z.; Novoselec, J.; Mioč, B.; Širić, I.; Držaić, V.; Klir Šalavardić, Ž. Macroelements in the Milk of the Lacaune Dairy Sheep Depending on the Stage of Lactation. Poljoprivreda 2024, 30, 54–59. [Google Scholar] [CrossRef]
- Kralik, Z.; Kralik, G.; Radanović, A. The Sensory Characteristics of Eggs Enriched With the fish and Linseed Oil. Poljoprivreda 2024, 30, 60–66. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Ivanov, K. Accumulation of heavy metals in leguminous crops (bean, soybean, peas, lentils and gram). J. Environ. Prot. Ecol 2003, 4, 787–795. [Google Scholar]
- Salaić, M.; Galić, V.; Jambrović, A.; Zdunić, Z.; Šimić, D.; Brkić, A.; Petrović, S. Assessing Genetic Variability For NUE in Maize Lines from Agricultural Institute Osijek. Poljoprivreda 2024, 30, 13–20. [Google Scholar] [CrossRef]
- Radočaj, D.; Jurišić, M.; Zebec, V.; Plaščak, I. Delineation of Soil Texture Suitability Zones for Soybean Cultivation: A Case Study in Continental Croatia. Agronomy 2020, 10, 823. [Google Scholar] [CrossRef]
- Agyenim-Boateng, K.G.; Zhang, S.; Shohag, M.J.I.; Shaibu, A.S.; Li, J.; Li, B.; Sun, J. Folate Biofortification in Soybean: Challenges and Prospects. Agronomy 2023, 13, 241. [Google Scholar] [CrossRef]
- Kobraee, S.; NoorMohamadi, G.; Heidari Sharifabad, H.; DarvishKajori, F.; Delkhosh, B. Influence of micronutrient fertilizer on soybean nutrient composition. Indian J. Sci. Technol. 2011, 4, 763–769. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Furlan, T.; Heinrichs, R. Effect of glyphosate and zinc application on yield, soil fertility, yield components, and nutritional status of soybean. Commun. Soil Sci. Plant Anal. 2016, 47, 1033–1047. [Google Scholar] [CrossRef][Green Version]
- Han, Ş.; Sönmez, İ.; Qureshi, M.; Güden, B.; Gangurde, S.S.; Yol, E. The effects of foliar amino acid and Zn applications on agronomic traits and Zn biofortification in soybean (Glycine max L.). Front. Plant Sci. 2024, 15, 1382397. [Google Scholar] [CrossRef]
- ISO 10390: 2005; Soil Quality—Determination of pH. In International Standard. Croatian Standards Institute: Zagreb, Croatia, 2005.
- ISO 14235: 1998; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. In International Standard. Croatian Standards Institute: Zagreb, Croatia, 1998.
- Egnér, H.; Riehm, H.; Domingo, W. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- ISO 11466:1995; Extraction of Trace Elements Soluble in Aqua Regia. International Standard Organization: Geneva, Switzerland, 1995.
- Trierweiler, F.; Lindsay, W. EDTA-ammonium carbonate soil test for Zn. ProcSoil Sci. Soc. Am. 1969, 33, 49–54. [Google Scholar] [CrossRef]
- Lončarić, Z.; Ivezić, V.; Kerovec, D.; Rebekić, A. Foliar Zinc-Selenium and Nitrogen Fertilization Affects Content of Zn, Fe, Se, P, and Cd in Wheat Grain. Plants 2021, 10, 1549. [Google Scholar] [CrossRef]
- Kingston, H.M.; Jassie, L.B. Microwave energy for acid decomposition at elevated temperatures and pressures using biological and botanical samples. Anal. Chem. 1986, 58, 2534–2541. [Google Scholar] [CrossRef]
- SAS Institute Inc., Cary, NC, USA. Available online: https://www.sas.com/en_us/home.html (accessed on 5 November 2024).
- Shanmugavel, D.; Rusyn, I.; Solorza-Feria, O.; Kamaraj, S.K. Sustainable SMART fertilizers in agriculture systems: A review on fundamentals to in-field applications. Sci. Total Environ. 2023, 904, 166729. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J.; Konvalina, P.; Kopecký, M. Impacts of Environmental Factors and Nutrients Management on Tomato Grown under Controlled and Open Field Conditions. Agronomy 2023, 13, 916. [Google Scholar] [CrossRef]
- Radočaj, D.; Tuno, N.; Mulahusić, A.; Jurišić, M. Evaluation of Ensemble Machine Learning for Geospatial Prediction of Soil Iron in Croatia. Poljoprivreda 2023, 29, 53–61. [Google Scholar] [CrossRef]
- Roberts, D.P.; Mattoo, A.K. Sustainable crop production systems and human nutrition. Front. Sustain. Food Syst. 2019, 3, 72. [Google Scholar] [CrossRef]
- Esper Neto, M.; Lara, L.M.; Maciel de Oliveira, S.; Santos, R.F.D.; Braccini, A.L.; Inoue, T.T.; Batista, M.A. Nutrient removal by grain in modern soybean varieties. Front. Plant Sci. 2021, 12, 615019. [Google Scholar] [CrossRef] [PubMed]
- Matoša Kočar, M.; Sudarić, A.; Sudar, R.; Duvnjak, T.; Zdunić, Z. Screening of early maturing soybean genotypes for production of high-quality edible oil. Zemdirb.-Agric. 2018, 105, 55–62. [Google Scholar] [CrossRef]
- Singer, W.M.; Lee, Y.C.; Shea, Z.; Vieira, C.C.; Lee, D.; Li, X.; Cunicelli, M.; Kadam, S.S.; Waseem Khan, M.A.; Shannon, G.; et al. Soybean genetics, genomics, and breeding for improving nutritional value and reducing antinutritional traits in food and feed. Plant Genome 2023, 16, e20415. [Google Scholar] [CrossRef]
- Altaf, M.T.; Liaqat, W.; Jamil, A.; Jan, M.F.; Baloch, F.S.; Barutçular, C.; Nadeem, M.A.; Mohamed, H.I. Strategies and bibliometric analysis of legumes biofortification to address malnutrition. Planta 2024, 260, 85. [Google Scholar] [CrossRef]
- Krueger, K.; Goggi, A.S.; Mallarino, A.P.; Mullen, R.E. Phosphorus and potassium fertilization effects on soybean seed quality and composition. Crop Sci. 2013, 53, 602–610. [Google Scholar] [CrossRef]
- Taliman, N.A.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of Phosphorus Fertilization on the Growth, Photosynthesis, Nitrogen Fixation, Mineral Accumulation, Seed Yield, and Seed Quality of a Soybean Low-Phytate Line. Plants 2019, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Radočaj, D.; Jurišić, M.; Kulundžić, A.M.; Antunović, M. Prediction of sugar beet yield and quality parameters with varying nitrogen fertilization using ensemble decision trees and artificial neural networks. Comput. Electron. Agric. 2023, 212, 108076. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Portugal, J.R.; Bossolani, J.W.; Moretti, L.G.; Fernandes, A.M.; Garcia, J.L.N.; Garcia, G.L.d.B.; Pilon, C.; Cantarella, H. Dynamics of Macronutrient Uptake and Removal by Modern Peanut Cultivars. Plants 2021, 10, 2167. [Google Scholar] [CrossRef] [PubMed]
- Filippi, D.; Denardin, L.G.D.O.; Ambrosini, V.G.; Alves, L.A.; Flores, J.P.M.; Martins, A.P.; de Castro Pias, O.H.; Tiecher, T. Concentration and removal of macronutrients by soybean seeds over 45 years in Brazil: A meta-analysis. Rev. Bras. Ciência Solo 2021, 45, e0200186. [Google Scholar] [CrossRef]
- Santos, E.F.; Pongrac, P.; Reis, A.R.; Rabêlo, F.H.S.; Azevedo, R.A.; White, P.J.; Lavres, J. Unravelling homeostasis effects of phosphorus and zinc nutrition by leaf photochemistry and metabolic adjustment in cotton plants. Sci. Rep. 2021, 11, 13746. [Google Scholar] [CrossRef]
- Nath, S.; Dey, S.; Kundu, R.; Paul, S. Phosphate and zinc interaction in soil and plants: A reciprocal cross-talk. Plant Growth Regul. 2024, 104, 591–615. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, W.; Dai, J.; Guo, X.; Lu, Q.; Wang, T.; Li, S.; Liu, T.; Zuo, Y. AhNRAMP1 enhances manganese and zinc uptake in plants. Front. Plant Sci. 2019, 10, 415. [Google Scholar] [CrossRef]
- Muhammad, I.; Volker, R.; Günter, N. Accumulation and distribution of Zn and Mn in soybean seeds after nutrient seed priming and its contribution to plant growth under Zn-and Mn-deficient conditions. J. Plant Nutr. 2017, 40, 695–708. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Rahman, Z.A.; Ishak, C.F.; Musa, H.M.; Zakikhani, H. Interaction effects of zinc and manganese on growth, uptake response and chlorophyll content of sweet corn (Zea mays var. saccharata). Asian J. Plant Sci. 2014, 13, 26–33. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Behera, S.K.; Verma, V.; Kaur, M.; Singh, P.; Alamri, S.; Skalicky, M.; Hossain, A. Biofortification of wheat (Triticum aestivum L.) genotypes with zinc and manganese lead to improve the grain yield and quality in sandy loam soil. Front. Sustain. Food Syst. 2023, 7, 1164011. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and Principles of Environmentally Sustainable Food and Agriculture Systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Galić, L.; Špoljarević, M.; Jakovac, E.; Ravnjak, B.; Teklić, T.; Lisjak, M.; Perić, K.; Nemet, F.; Lončarić, Z. Selenium Biofortification of Soybean Seeds Influences Physiological Responses of Seedlings to Osmotic Stress. Plants 2021, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Rose, I.A.; Felton, W.L.; Banks, L.W. Responses of four soybean varieties to foliar zinc fertilizer. Aust. J. Exp. Agric. Anim. Husb. 1981, 21, 236–240. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Twardowska, I. Biofortification of soybean (Glycine max L.) with Se and Zn, and enhancing its physiological functions by spiking these elements to soil during flowering phase. Sci. Total Environ. 2020, 740, 139648. [Google Scholar] [CrossRef]
- Silva, M.A.; de Sousa, G.F.; Corguinha, A.P.B.; de Lima Lessa, J.H.; Dinali, G.S.; Oliveira, C.; Lopes, G.; Amaral, D.; Brown, P.; Guilherme, L.R.G. Selenium biofortification of soybean genotypes in a tropical soil via Se-enriched phosphate fertilizers. Front. Plant Sci. 2022, 13, 988140. [Google Scholar] [CrossRef]
- Kong, L.; Tao, Y.; Xu, Y.; Zhou, X.; Fu, G.; Zhao, L.; Wang, Q.; Li, H.; Wan, Y. Simultaneous Biofortification: Interaction between Zinc and Selenium Regarding Their Accumulation in Wheat. Agronomy 2024, 14, 1513. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Torma, S.; Vilček, J. Potential of nutrients use from plant residues after oil plants harvesting. J. Cent. Eur. Agric. 2024, 25, 342–351. [Google Scholar] [CrossRef]
- Uher, D.; Horvatić, I. Utjecaj konsocijacije kukuruza s grahom na kakvoću i prinos krme. Poljoprivreda 2023, 29, 3–8. [Google Scholar] [CrossRef]
- Gantner, R.; Steiner, Z.; Gantner, V.; Zmaić, L. Grass-Fed Cattle as an Option to Improve the Sustainability of Cattle Industry in Croatia. Poljoprivreda 2024, 30, 81–90. [Google Scholar] [CrossRef]
- Risi, F.G.E.; Hüther, C.M.; Righi, C.A.; Umburanas, R.C.; Tezotto, T.; Dourado Neto, D.; Reichardt, K.; Pereira, C.R. Sustainability Analysis of Nitrogen Use Efficiency in Soybean-Corn Succession Crops of Midwest Brazil. Nitrogen 2024, 5, 232–253. [Google Scholar] [CrossRef]


| Variety | Maturity Group | Sowing Rate (Plants/ha) |
|---|---|---|
| Ika | 0–I | 560.000 |
| Korana | 00 | 620.000 |
| Lucija | 00–0 | 600.000 |
| Sonja | 0 | 580.000 |
| Sunce | 0–I | 520.000 |
| Toma | 0 | 610.000 |
| Source of Variation | P | K | Ca | Mg | Fe | Mn | Cu | Zn | Se |
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | μ kg−1 | ||||||||
| Y | <0.0001 | <0.0001 | <0.0001 | ns | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| V | <0.0001 | ns | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ns | <0.0001 | <0.0001 |
| B | <0.0001 | ns | ns | ns | * | <0.0001 | ns | <0.0001 | <0.0001 |
| Y × V | <0.0001 | <0.0001 | <0.0001 | ** | * | <0.0001 | <0.0001 | <0.0001 | ns |
| V × B | ns | ns | <0.0001 | ns | ns | <0.0001 | ns | <0.0001 | <0.0001 |
| Y × B | * | ns | ns | ns | ns | <0.0001 | ** | <0.0001 | <0.0001 |
| Y × V × B | ns | ns | ns | ns | ns | <0.0001 | ** | <0.0001 | <0.0001 |
| Source of Variation | Plant Part Yield | Removal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Fe | Mn | Cu | Zn | Se | ||
| kg ha−1 | g ha−1 | mg ha−1 | ||||||||
| Y | * | <0.0001 | <0.0001 | ns | ** | <0.0001 | <0.0001 | ns | <0.0001 | <0.0001 |
| V | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| B | ns | ns | ns | ** | ns | ns | <0.0001 | ns | <0.0001 | <0.0001 |
| P | ns | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ** | <0.0001 | <0.0001 | <0.0001 |
| Y × V | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ** | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Y × B | ns | ns | ns | ** | ns | ns | ** | * | <0.0001 | <0.0001 |
| Y × P | ns | ** | <0.0001 | * | ns | <0.0001 | <0.0001 | <0.0001 | ** | <0.0001 |
| V × B | ns | ns | ns | <0.0001 | ns | ns | ns | ns | <0.0001 | <0.0001 |
| V × P | <0.0001 | <0.0001 | ns | <0.0001 | ** | ns | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| B × P | ns | ns | ns | ** | ns | ns | ns | ns | <0.0001 | <0.0001 |
| Y × V × B | ** | ns | ** | <0.0001 | ** | ns | ** | ** | <0.0001 | <0.0001 |
| Y × V × P | ** | <0.0001 | ** | <0.0001 | * | ** | ** | ** | <0.0001 | <0.0001 |
| Y × B × P | ns | ns | ns | * | ns | ns | ns | ns | <0.0001 | <0.0001 |
| V × B × P | ns | ns | ns | <0.0001 | ns | ns | ns | ns | * | <0.0001 |
| Y × V × B × P | ns | ns | ns | <0.0001 | ns | ns | ns | ns | ns | <0.0001 |
| Applied Foliar Fertilizer | Yield (t/ha) | Zn Removal (g/ha) | Se Removal (mg/ha) | Zn Use Efficiency (%) | Se Use Efficiency (%) |
|---|---|---|---|---|---|
| Grain | |||||
| Control | 3.9 | 180.1 b | 212.0 e | - | - |
| Se_1 (10 g/ha Se) | 4.0 | 253.0 b | 4527.1 d | - | 43.2 a |
| Se_2 (20 g/ha Se) | 4.0 | 184.7 b | 8136.3 c | - | 39.6 a |
| Se_3 (30 g/ha Se) | 3.9 | 176.7 a | 11391.1 a | - | 37.3 ab |
| Zn_1 (3 kg/ha Zn) | 4.0 | 257.7 a | 200.8 e | 2.6 a | - |
| Zn_2 (6 kg/ha Zn) | 3.9 | 259.3 a | 165.0 e | 1.3 b | - |
| Se_3 Zn_2 (30 g/ha Se + 6 kg ha Zn) | 3.8 | 184.0 a | 9700.3 b | 1.2 b | 31.6 c |
| Average | 3.9 | 213.6 | 4904.7 | 2.6 | 37.9 |
| Vegetative mass | |||||
| Control | 3.7 | 23.4 c | 77.0 e | - | - |
| Se_1 (10 g/ha Se) | 4.0 | 24.4 c | 745.1 d | - | 6.7 a |
| Se_2 (20 g/ha Se) | 4.0 | 24.4 c | 1240.0 c | - | 5.8 ab |
| Se_3 (30 g/ha Se) | 3.8 | 25.6 c | 1788.2 a | - | 5.7 ab |
| Zn_1 (3 kg/ha Zn) | 4.0 | 156.8 b | 146.5 d | 4.4 a | - |
| Zn_2 (6 kg/ha Zn) | 3.9 | 187.14 a | 81.0 d | 2.7 b | - |
| Se_3 Zn_2 (30 g/ha Se + 6 kg ha Zn) | 3.8 | 196.8 a | 1472.3 b | 2.9 b | 4.7 b |
| Average | 3.9 | 91.2 | 792.9 | 3.6 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarić, Z.; Varga, I.; Nemet, F.; Perić, K.; Jović, J.; Zebec, V.; Ivezić, V.; Iljkić, D.; Galić, L.; Sudarić, A. Efficiency of Biofortification with Zn and Se in Soybean: Yield and Overall Mineral Content in Plant. Appl. Sci. 2024, 14, 11349. https://doi.org/10.3390/app142311349
Lončarić Z, Varga I, Nemet F, Perić K, Jović J, Zebec V, Ivezić V, Iljkić D, Galić L, Sudarić A. Efficiency of Biofortification with Zn and Se in Soybean: Yield and Overall Mineral Content in Plant. Applied Sciences. 2024; 14(23):11349. https://doi.org/10.3390/app142311349
Chicago/Turabian StyleLončarić, Zdenko, Ivana Varga, Franjo Nemet, Katarina Perić, Jurica Jović, Vladimir Zebec, Vladimir Ivezić, Dario Iljkić, Lucija Galić, and Aleksandra Sudarić. 2024. "Efficiency of Biofortification with Zn and Se in Soybean: Yield and Overall Mineral Content in Plant" Applied Sciences 14, no. 23: 11349. https://doi.org/10.3390/app142311349
APA StyleLončarić, Z., Varga, I., Nemet, F., Perić, K., Jović, J., Zebec, V., Ivezić, V., Iljkić, D., Galić, L., & Sudarić, A. (2024). Efficiency of Biofortification with Zn and Se in Soybean: Yield and Overall Mineral Content in Plant. Applied Sciences, 14(23), 11349. https://doi.org/10.3390/app142311349









