Mechanical and Physicochemical Properties of Ti6Al4V Alloy After Plastic Working and 3D Printing Intended for Orthopedics Implants
Abstract
1. Introduction
2. Materials and Methods
- -
- Heating of the furnace to a temperature of 820 ± 10 °C;
- -
- Annealing of the samples for 120 min;
- -
- Tempering the material to 500 °C with spontaneous cooling of the furnace from 820 °C to room temperature ± 20 °C.
2.1. Material Structure
2.2. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy
2.3. Surface Roughness
2.4. Wettability and Surface Energy
2.5. Corrosion Resistance and Macroscopic Observations
2.6. Hardness
2.7. Statistical Analysis
3. Results and Discussion
3.1. Material Structure
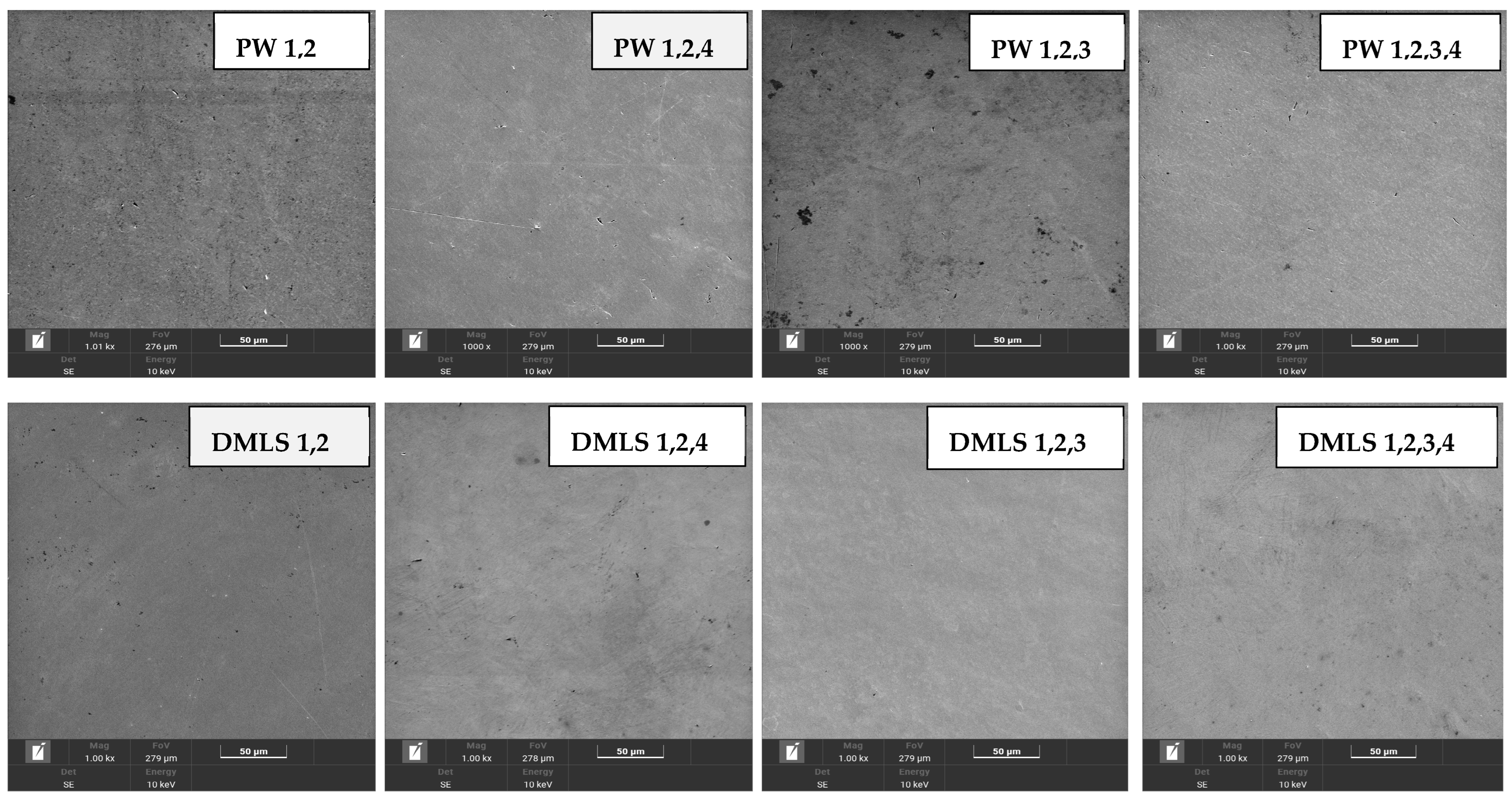
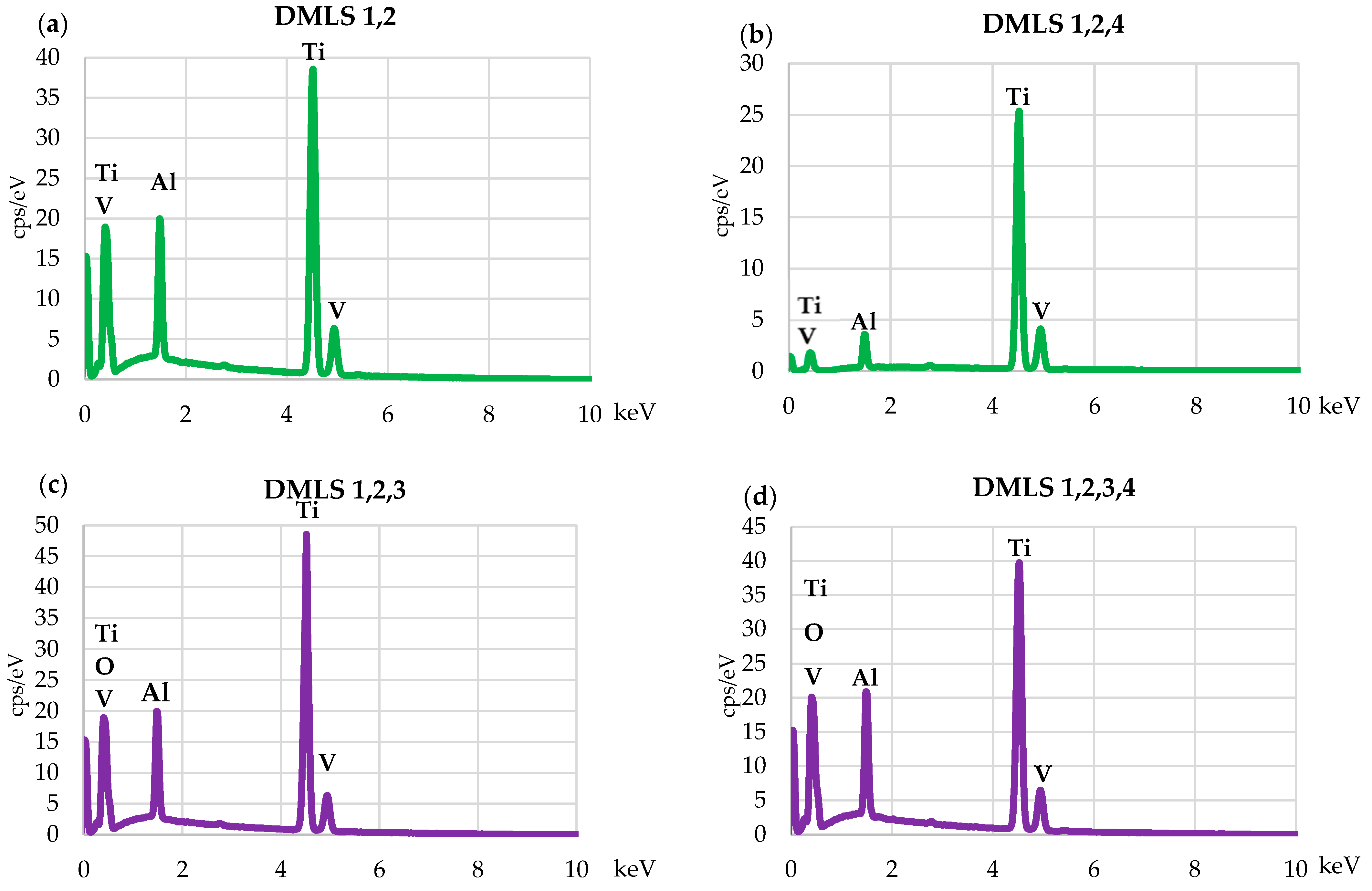
3.2. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy
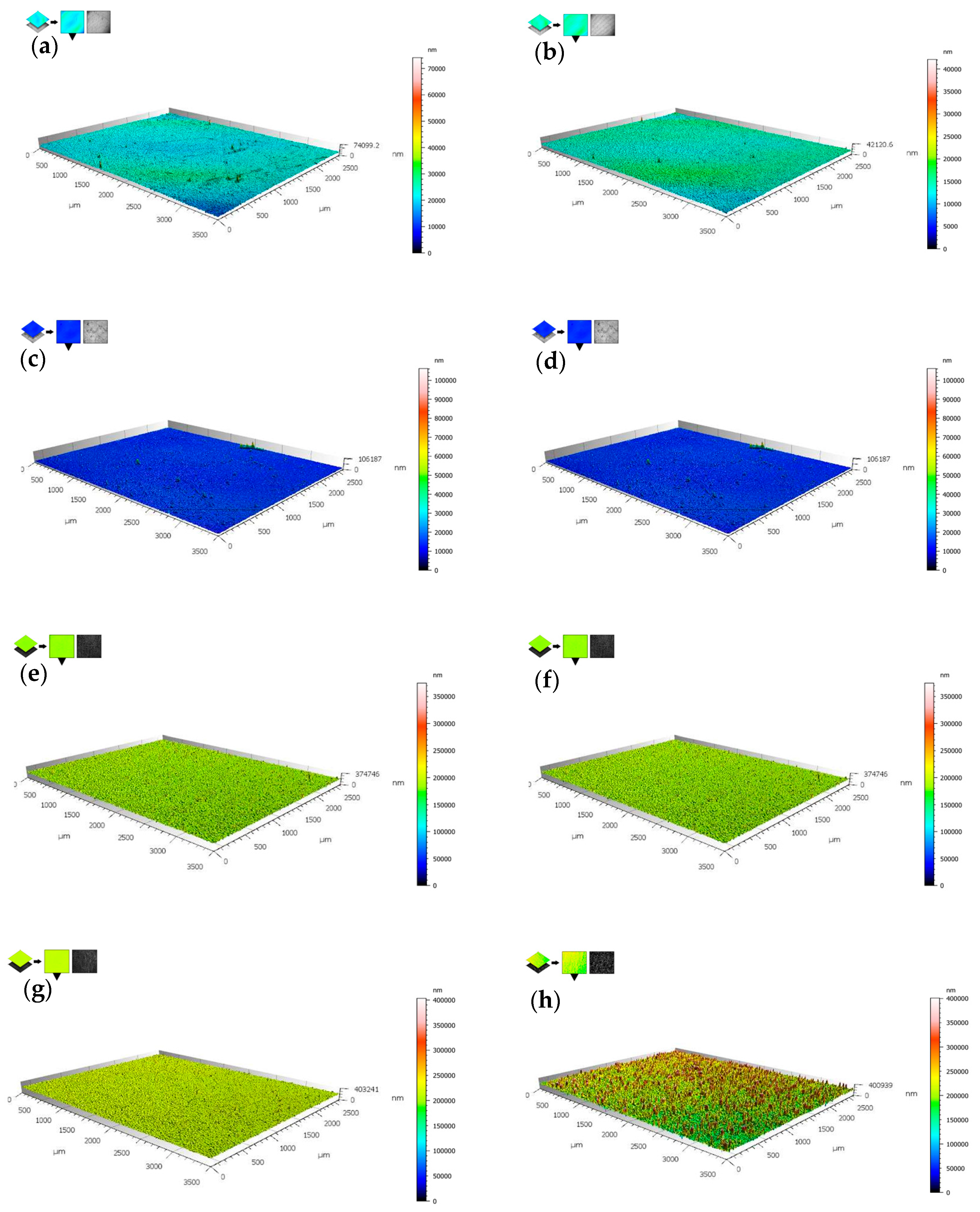
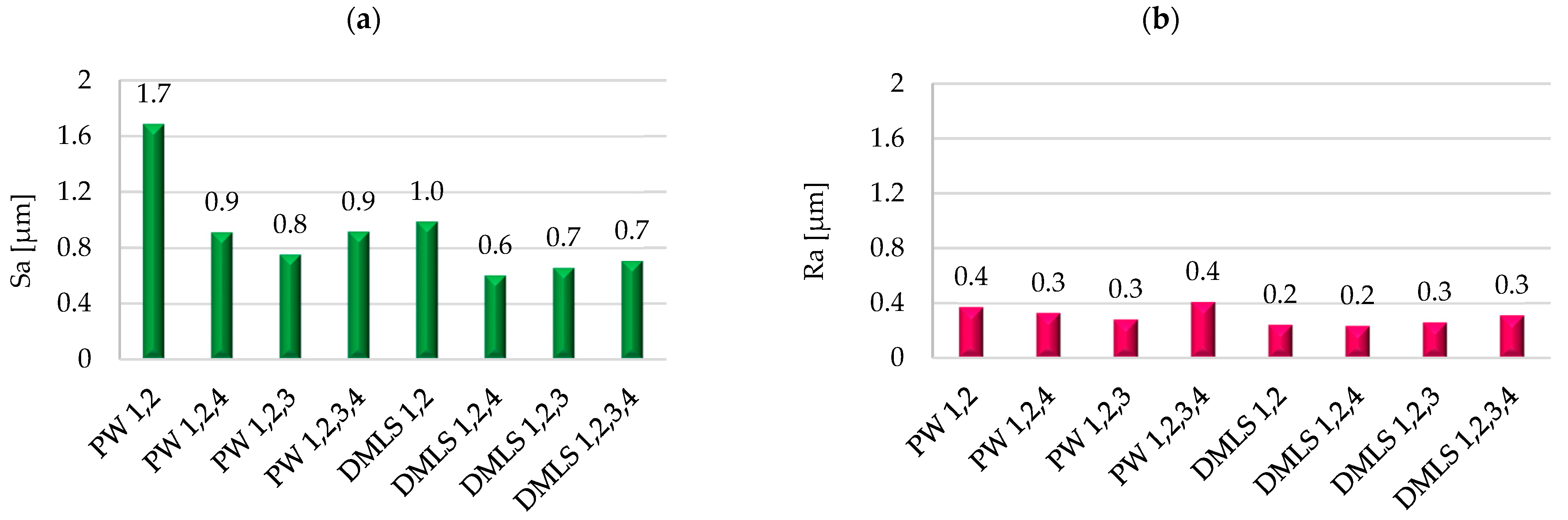
3.3. Surface Roughness
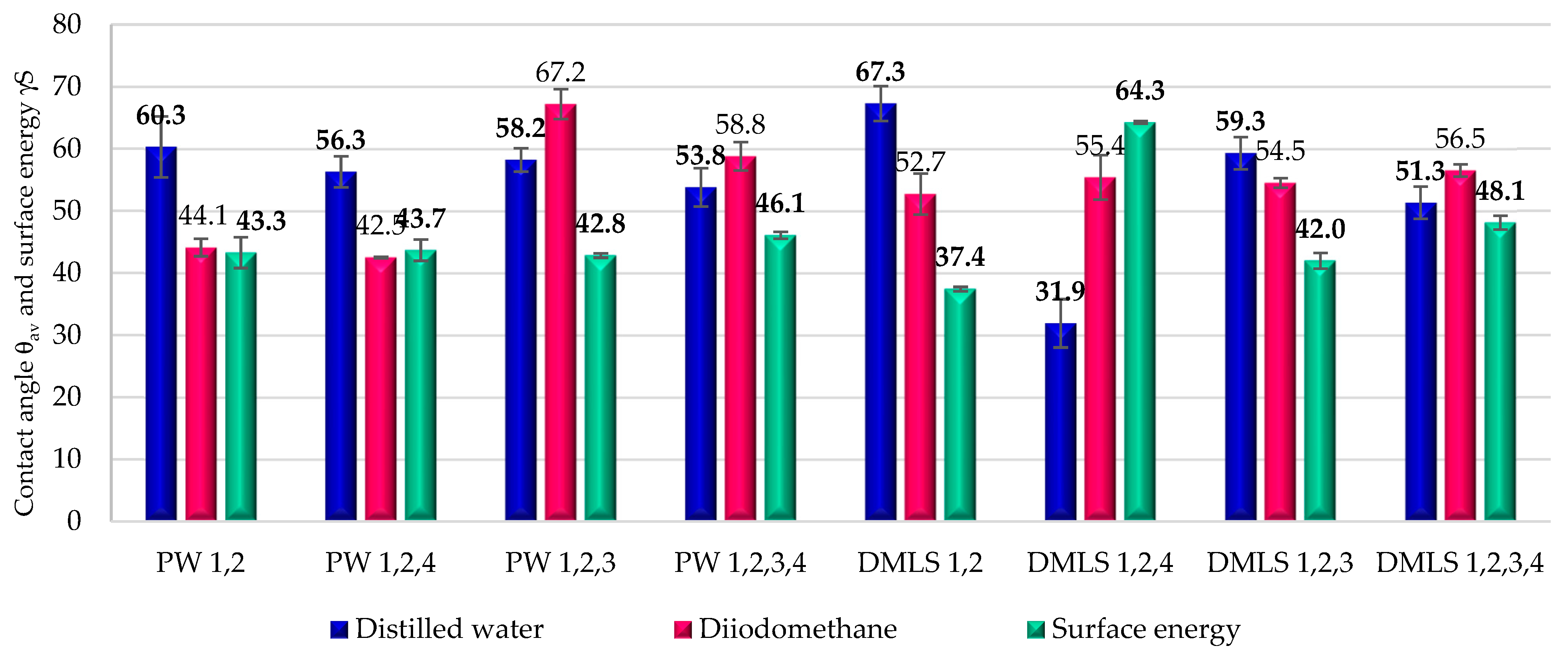
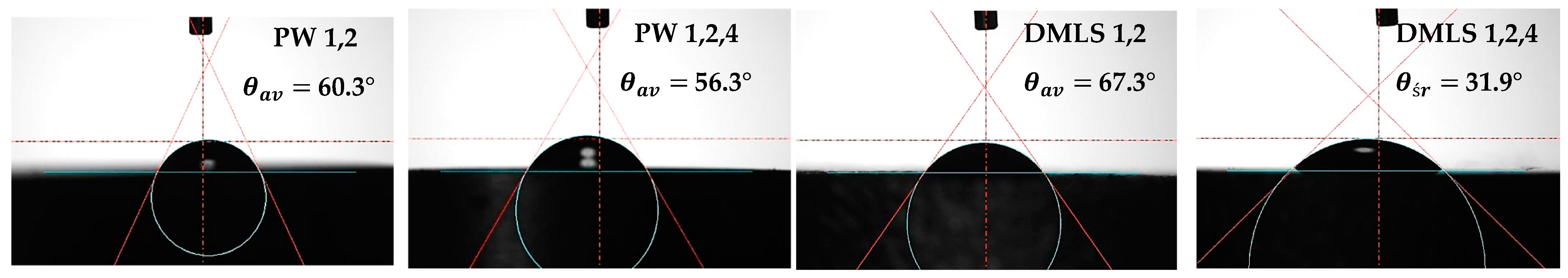
3.4. Wettability and Surface Energy
3.5. Corrosion Resistance and Macroscopic Observations
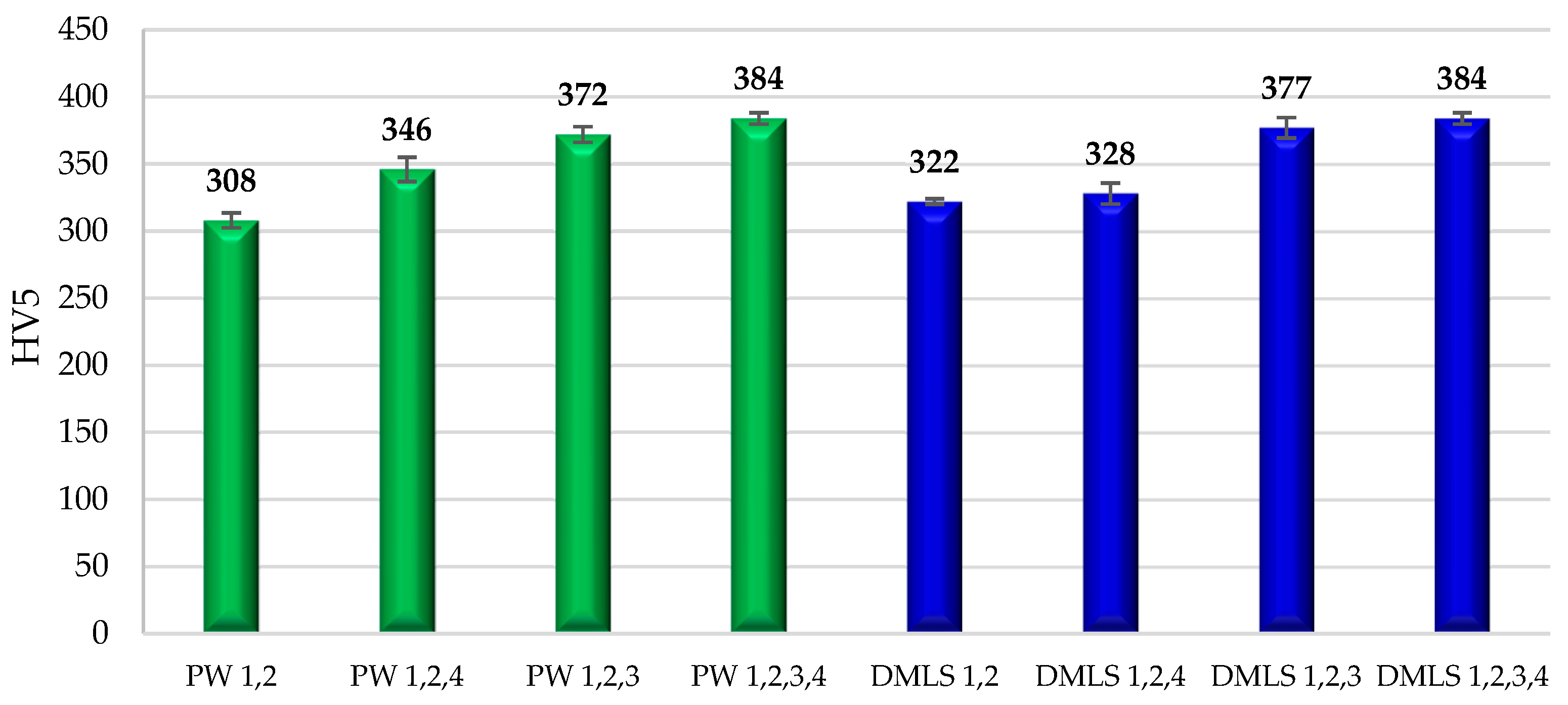
3.6. Hardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savini, A.; Savini, G.G. A short history of 3D printing, a technological revolution just started. In Proceedings of the Conference: 2015 ICOHTEC/IEEE International History of High-Technologies and their Socio-Cultural Contexts Conference (HISTELCON), Tel-Aviv, Israel, 18–19 August 2015. [Google Scholar]
- Tuli, N.T.; Khatun, S.; Rashid, A.B. Unlocking the future of precision manufacturing: A comprehensive exploration of 3D printing with fiber-reinforced composites in aerospace, automotive, medical, and consumer industries. Heliyon 2024, 10, e27328. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A. Chapter 1—An Abbreviated History of Medical 3D Printing. In 3D Printing for the Radiologist; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–10. [Google Scholar] [CrossRef]
- Paramasivam, V.; Sindhu Singh, G.; Santhanakrishnan, S. 3D Printing of Human Anatomical Models for Preoperative Surgical Planning. Procedia Manuf. 2020, 48, 684–690. [Google Scholar] [CrossRef]
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J. Orthop. Transl. 2023, 42, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, J.; Kang LTian, J.; Zhang, X.; Hu, J.; Huang, Y.; Liu, F.; Wang, H.; Wu, Z. An overview of 3D printed metal implants in orthopedic applications: Present and future perspectives. Heliyon 2023, 9, e17718. [Google Scholar] [CrossRef]
- Memarian, P.; Pishavar, E.; Zanotti, F.; Trentini, M.; Camponogara, F.; Soliani, E.; Gargiulo, P.; Isola, M.; Zavan, B. Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 1045. [Google Scholar] [CrossRef]
- Fashanu, F.F.; Marcellin-Little, D.J.; Linke, B.S. Review of surface finishing of additively manufactured metal implants. In Proceedings of the ASME 2020 15th International Manufacturing Science and Engineering Conference MSEC2020, Virtual, 3 September 2020; pp. 2–8. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Alghauli, M.A.; Aljohani, M.H.A.; Zafar, M.S. Advanced additive manufacturing in implant dentistry: 3D printing technologies, printable materials, current applications and future requirements. Bioprinting 2024, 42, e00356. [Google Scholar] [CrossRef]
- Murr, L.E. Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: An overview. J. Mater. Res. Technol. 2020, 9, 1087–1103. [Google Scholar] [CrossRef]
- Brandl, B.; Eder, S.; Palanisamy, A.; Heupl, S.; Terzic, I.; Katschnig, M.; Nguyen, T.; Senck, S.; Roblegg, E.; Spoerk, M. Toward high-resolution 3D-printing of pharmaceutical implants—A holistic analysis of relevant material properties and process parameters. Int. J. Pharm. 2024, 660, 124356. [Google Scholar] [CrossRef]
- Singh, H.N.; Agrawal, S.; Kuthe, A.M. Design of customized implants and 3D printing of symmetric and asymmetric cranial cavities. J. Mech. Behav. Biomed. Mater. 2023, 146, 106061. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Wang, J.; Zhao, D. Research progress of metal-based additive manufacturing in medical implants. Rev. Adv. Mater. Sci. 2023, 62, 20230148. [Google Scholar] [CrossRef]
- Mehboob, H.; Tarlochan, F.; Mehboob, A.; Chang, S.-H.; Ramesh, S.; Harun, W.S.W.; Kadirgama, K. A novel design, analysis and 3D printing of Ti-6Al-4V alloy bio-inspired porous femoral stem. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Z.C.; Tsai, P.I.; Kuo, M.Y.P.; Sun, J.S.; Chen, S.Y.; Shen, H.H. Augmentation of DMLS Biomimetic Dental Implants with Weight-Bearing Strut to Balance of Biologic and Mechanical Demands: From Bench to Animal. Materials 2019, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Humnabad, P.; Das, I.; Tarun, R. An overview of direct metal laser sintering (DMLS) technology for metal 3D printing. J. Mines Met. Fuels 2022, 70, 127–133. [Google Scholar] [CrossRef]
- Longhitano, G.A.; Larosa, M.A.; Munhoz, A.L.J.; de Carvalho Zavagliaa, C.A.; Ierardi, M.C.F. Surface Finishes for Ti-6Al-4V Alloy Produced by Direct Metal Laser Sintering. Mater. Res. 2015, 18, 838–842. [Google Scholar] [CrossRef]
- Larosa, M.A.; Jardini, A.L.; de Carvalho Zavaglia, C.A.; Kharmandayan, P.; Calderoni, D.R.; Filho, R.M. Microstructural and Mechanical Characterization of a Custom-Built Implant Manufactured in Titanium Alloy by Direct Metal Laser Sintering. Adv. Mech. Eng. 2014, 6, 945819. [Google Scholar] [CrossRef]
- Danielli, F.; Berti, F.; Nespoli, A.; Presti, V.L.; Sironi, E.; Ninarello, D.; Villa, T.; Petrini, L. Additive manufacturing for orthopedic implants: Morphological and material characterization of SLM thin Ti6Al4V samples. Procedia Struct. Integr. 2024, 56, 82–89. [Google Scholar] [CrossRef]
- Walker, P.; Malz, S.; Trudel, E.; Nosir, S.; ElSayed, M.S.A.; Kok, L. Effects of Ultrasonic Impact Treatment on the Stress-Controlled Fatigue Performance of Additively Manufactured DMLS Ti-6Al-4V Alloy. Appl. Sci. 2019, 92, 787. [Google Scholar] [CrossRef]
- Koju, N.; Niraula, S.; Fotovvati, B. Additively Manufactured Porous Ti6Al4V for Bone Implants: A Review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- Nelson, K.; Kelly, C.N.; Gall, K. Effect of stress state on the mechanical behavior of 3D printed porous Ti6Al4V scaffolds produced by laser powder bed fusion. Mater. Sci. Eng. B 2022, 286, 116013. [Google Scholar] [CrossRef]
- Gao, P.; Fan, B.; Yu, X.; Liu, W.; Wu, J.; Shi, L.; Yang, D.; Tan, L.; Wan, P.; Hao, Y.; et al. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioact. Mater. 2020, 5, 680–693. [Google Scholar] [CrossRef]
- Peia, X.; Wang, L.; Zhou, C.; Wu, L.; Lei, H.; Fan, S.; Zeng, Z.; Deng, Z.; Kong, Q.; Jiang, Q.; et al. Ti6Al4V orthopedic implant with biomimetic heterogeneous structure via 3D printing for improving osteogenesis. Mater. Des. 2022, 211, 110964. [Google Scholar] [CrossRef]
- Orłowska, A.; Kajzer, W.; Goldsztajn, K.; Gawron, A.; Godzierz, M.; Nowińska, K.; Basiaga, M.; Simka, W.; Szewczenko, J. Functionalization of 3D printed Ti6Al4V high-porous spinal implant surface with use of plasma electrolytic oxidation. Appl. Surf. Sci. 2024, 659, 159948. [Google Scholar] [CrossRef]
- Orłowska, A.; Szewczenko, J.; Kajzer, W.; Goldsztajn, K.; Basiaga, M. Study of the Effect of Anodic Oxidation on the Corrosion Properties of the Ti6Al4V Implant Produced from SLM. J. Funct. Biomater. 2023, 14, 191. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F. Review of 3D-PrintedTitanium-BasedImplants:Materials and Post-Processing. ChemBioEng Rev. 2024, e202400032. [Google Scholar] [CrossRef]
- PN-EN ISO 5832-3; Implanty Dla Chirurgii—Materiały Metalowe—Część 3 Stop Tytanu 6−Aluminium 4−Wanad do Przeróbki Plastycznej. ISO: Geneva, Switzerland, 2021.
- Zhang, C.; Zhang, H.; Peng, W.; Feng, A.; Hu, J.; Wang, W.; Yuan, H.; Li, Q.; Fu, Q. 3D-printed Ti6Al4V thoracic fusion cage: Biomechanical behavior and strengthening mechanism. J. Mater. Res. Technol. 2024, 31, 2685–2695. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, W.; Yang, W.; Bi, J.; Li, H.; Gao, X.; Zhang, B.; Shi, G.; Li, K.; Wei, Z.; et al. Vancomycin-encapsulated hydrogel loaded microarc-oxidized 3D-printed porous Ti6Al4V implant for infected bone defects: Reconstruction, anti-infection, and osseointegration. Bioact. Mater. 2024, 42, 18–31. [Google Scholar] [CrossRef]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based alloys and composites for orthopedic implants Applications: A comprehensive review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Hadad, H.; Boos Lima, F.B.D.J.; Shirinbak, I.; Porto, T.S.; Chen, J.E.; Guastaldi, F.P.S. The impact of 3D printing on oral and maxillofacial surgery. J. 3D Print. Med. 2023, 7, 3DP007. [Google Scholar] [CrossRef]
- EOS Titanium Ti64 Grade 23; Material Data Sheet, Metal Solutions; EOS GmbHEOS GmbH—Electro Optical Systems: Krailling, Germany, 2022.
- EOS Titanium Ti64 for EOS M 300-4; Material Data Sheet; EOS GmbHEOS GmbH—Electro Optical Systems: Krailling, Germany, 2022.
- Instruction EOS M 100; User Manual EOS M100; EOS GmbHEOS GmbH—Electro Optical Systems: Krailling, Germany, 2019.
- EOS M100; Parameter Sheet; EOS GmbHEOS GmbH—Electro Optical Systems: Krailling, Germany, 2015.
- PN-EN ISO 25178-1:2016-08; Specyfikacje Geometrii Wyrobów (GPS)—Struktura Geometryczna Powierzchni: Przestrzenna—Część 6: Klasyfikacja Metod Pomiaru Struktury Geometrycznej Powierzchni. ISO: Geneva, Switzerland, 2016.
- PN-EN ISO 21920-2:2022-06; Specyfikacje Geometrii Wyrobów (GPS)—Struktura Geometryczna Powierzchni: Profil—Część 2: Terminy, Definicje i Parametry Struktury Geometrycznej Powierzchni. ISO: Geneva, Switzerland, 2022.
- PN-EN ISO 10993-15; Biologiczna Ocena Wyrobów Medycznych—Część 1: Ocena i Badanie w Procesie Zarządzania Ryzykiem. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 6507-1; Metale. Pomiar Twardości Sposobem Vickersa. Część 1 Metoda Badań. ISO: Geneva, Switzerland, 2018.
- Garbacz, H.; Ossowski, M.; Wiecieński PWierzchoń, T.; Kurzydłowski, K.J. Mikrostruktura i właściwości warstw międzymetalicznych na stopie Ti-6Al-4V. Politech. Warsz. 2007, 1, 45–55. [Google Scholar]
- Mahlobo, M.G.R.; Chikosha, L.; Olubambi, A. Study of the corrosion properties of powder rolledTi6Al4V alloy applied in the biomedical implants. J. Mater. Res. Technol. 2022, 18, 3631–3639. [Google Scholar] [CrossRef]
- Cabrini, M.; Carrozza ALorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Fino, P.; Scenini, F. Influence of surface finishing and heat treatments on the corrosion resistance of LPBF-produced Ti-6Al-4V alloy for biomedical applications. J. Mater. Process. Technol. 2022, 308, 117730. [Google Scholar] [CrossRef]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of cleaning and sterilisation on titanium implant surface properties and cellular response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Bociąga, D.; Jastrzębski, K.; Olejnik, A.; Świątek, L.; Marchwicka, M. Wpływ wielokrotnej sterylizacji na właściwości biomateriałów. Eng. Biomater. 2016, 19, 11–20. [Google Scholar]
- Lekoadi, P.; Tlotleng, M.; Annan, K.; Maledi, N.; Masina, B. Evaluation of Heat Treatment Parameters on Microstructure and Hardness Properties of High-Speed Selective Laser Melted Ti6Al4V. Metals 2021, 11, 255. [Google Scholar] [CrossRef]





| 1—Sandblasting | 2—Mechanical Polishing | 3—Heat Treatment | 4—Steam Sterilization | |
|---|---|---|---|---|
| PW 1,2 | x | x | ||
| PW 1,2,4 | x | x | x | |
| PW 1,2,3 | x | x | x | |
| PW 1,2,3,4 | x | x | x | x |
| DMLS 1,2 | x | x | ||
| DMLS 1,2,4 | x | x | x | |
| DMLS 1,2,3 | x | x | x | |
| DMLS 1,2,3,4 | x | x | x | x |
| Samples | Outer Surface [wt%] | |||
|---|---|---|---|---|
| Ti | Al | V | O | |
| PW 1,2 | 86.4 | 5.1 | 3.8 | 4.7 |
| PW 1,2,4 | 90.6 | 5.3 | 4.1 | - |
| PW 1,2,3 | 86.8 | 5.3 | 3.7 | 4.2 |
| PW 1,2,3,4 | 87.2 | 5.1 | 3.6 | 4.1 |
| DMSL 1,2 | 90.4 | 5.7 | 3.9 | - |
| DMSL 1,2,4 | 90.6 | 5.5 | 3.9 | - |
| DMSL 1,2,3 | 87.1 | 5.4 | 3.7 | 3.8 |
| DMSL 1,2,3,4 | 86.4 | 5.5 | 3.8 | 4.3 |
| Sample Name | icore [mA/cm2] | Ecorr [V] | Rp [kΩ·cm2] | |||
|---|---|---|---|---|---|---|
| Av. | SD | Av. | SD | Av. | SD | |
| PW 1,2 | 275 | 20 | −0.17 | 0.07 | 120 | 20 |
| PW 1,2,4 | 181 | 2 | −0.15 | 0.03 | 149 | 86 |
| PW 1,2,3 | 365 | 148 | −0.05 | 0.01 | 235 | 2 |
| PW 1,2,3,4 | 286 | 89 | −0.03 | 0.04 | 129 | 66 |
| DMLS 1,2 | 238 | 22 | −0.133 | 0.05 | 81 | 7 |
| DMLS 1,2,4 | 473 | 65 | −0.135 | 0.04 | 65 | 16 |
| DMLS 1,2,3 | 162 | 45 | −0.120 | 0.02 | 66 | 43 |
| DMLS 1,2,3,4 | 249 | 82 | −0.116 | 0.01 | 78 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajzer, W.; Wielgus, G.; Kajzer, A. Mechanical and Physicochemical Properties of Ti6Al4V Alloy After Plastic Working and 3D Printing Intended for Orthopedics Implants. Appl. Sci. 2024, 14, 11181. https://doi.org/10.3390/app142311181
Kajzer W, Wielgus G, Kajzer A. Mechanical and Physicochemical Properties of Ti6Al4V Alloy After Plastic Working and 3D Printing Intended for Orthopedics Implants. Applied Sciences. 2024; 14(23):11181. https://doi.org/10.3390/app142311181
Chicago/Turabian StyleKajzer, Wojciech, Gabriela Wielgus, and Anita Kajzer. 2024. "Mechanical and Physicochemical Properties of Ti6Al4V Alloy After Plastic Working and 3D Printing Intended for Orthopedics Implants" Applied Sciences 14, no. 23: 11181. https://doi.org/10.3390/app142311181
APA StyleKajzer, W., Wielgus, G., & Kajzer, A. (2024). Mechanical and Physicochemical Properties of Ti6Al4V Alloy After Plastic Working and 3D Printing Intended for Orthopedics Implants. Applied Sciences, 14(23), 11181. https://doi.org/10.3390/app142311181










