Ionic Character and Alkyl Chain Length of Surfactants Affect Titanium Dioxide Dispersion and Its UV-Blocking Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
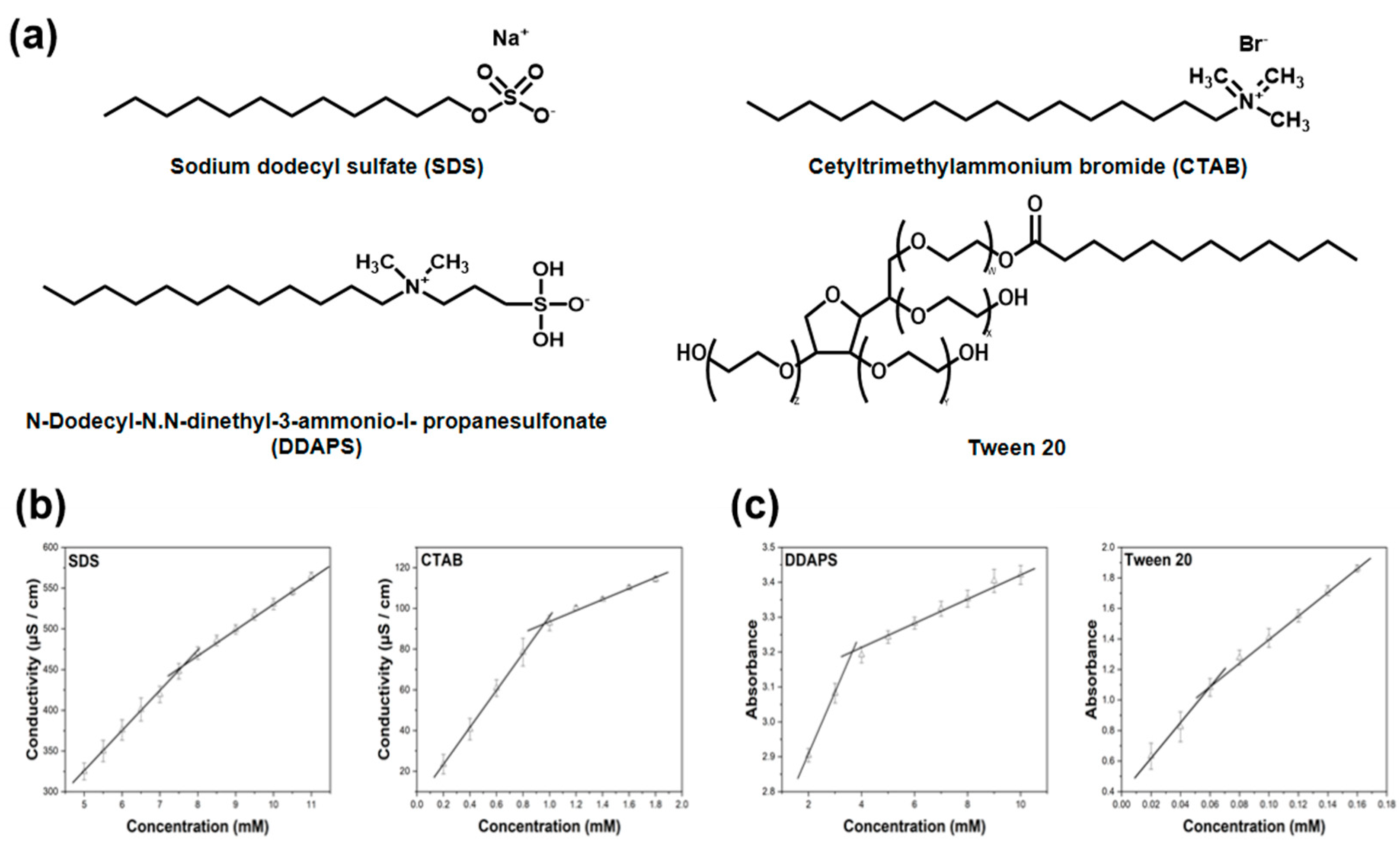
2.2. Determination of Critical Micelle Concentration (CMC)
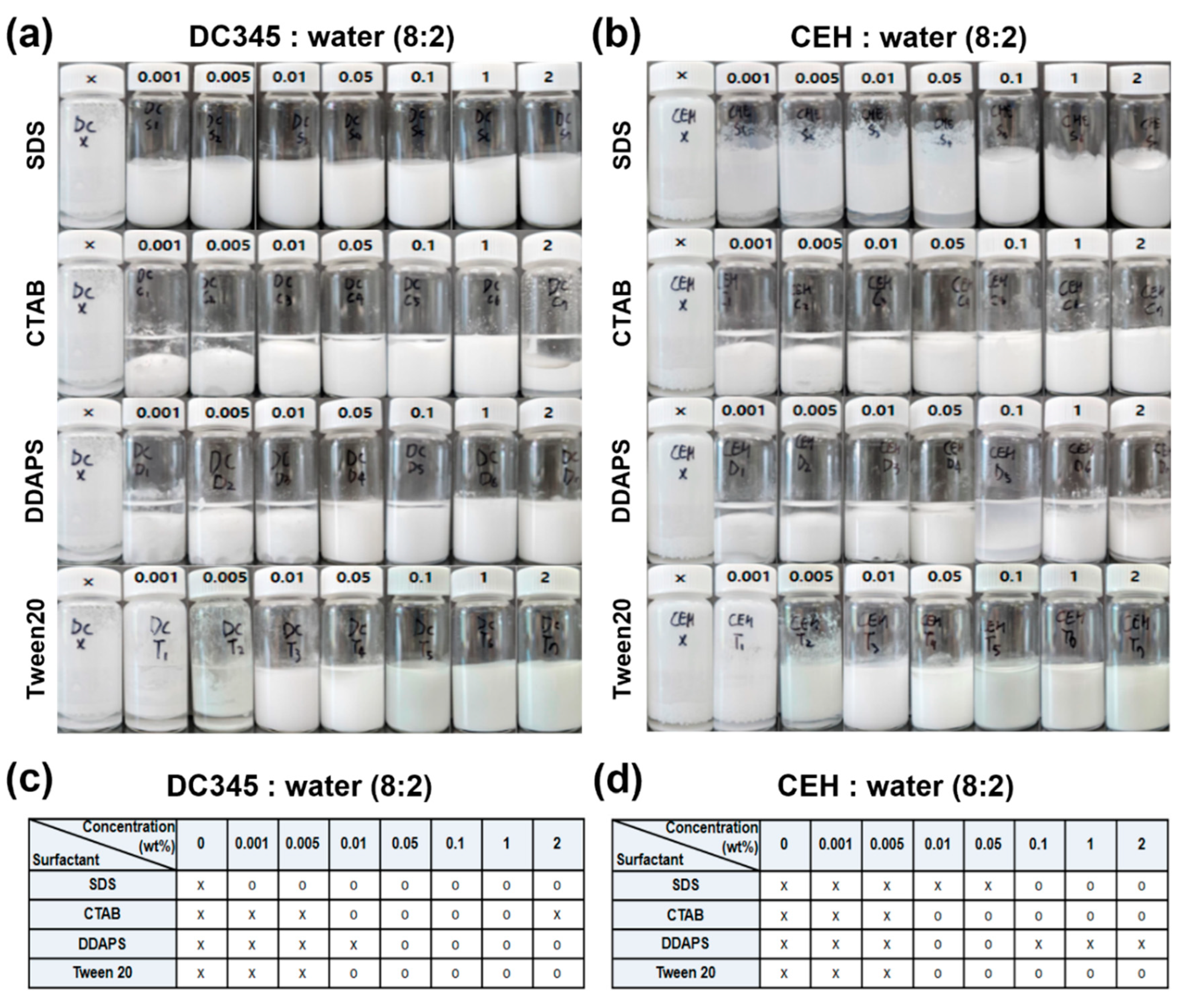
2.3. Preparation of TiO2 Dispersion in W/O Formulation
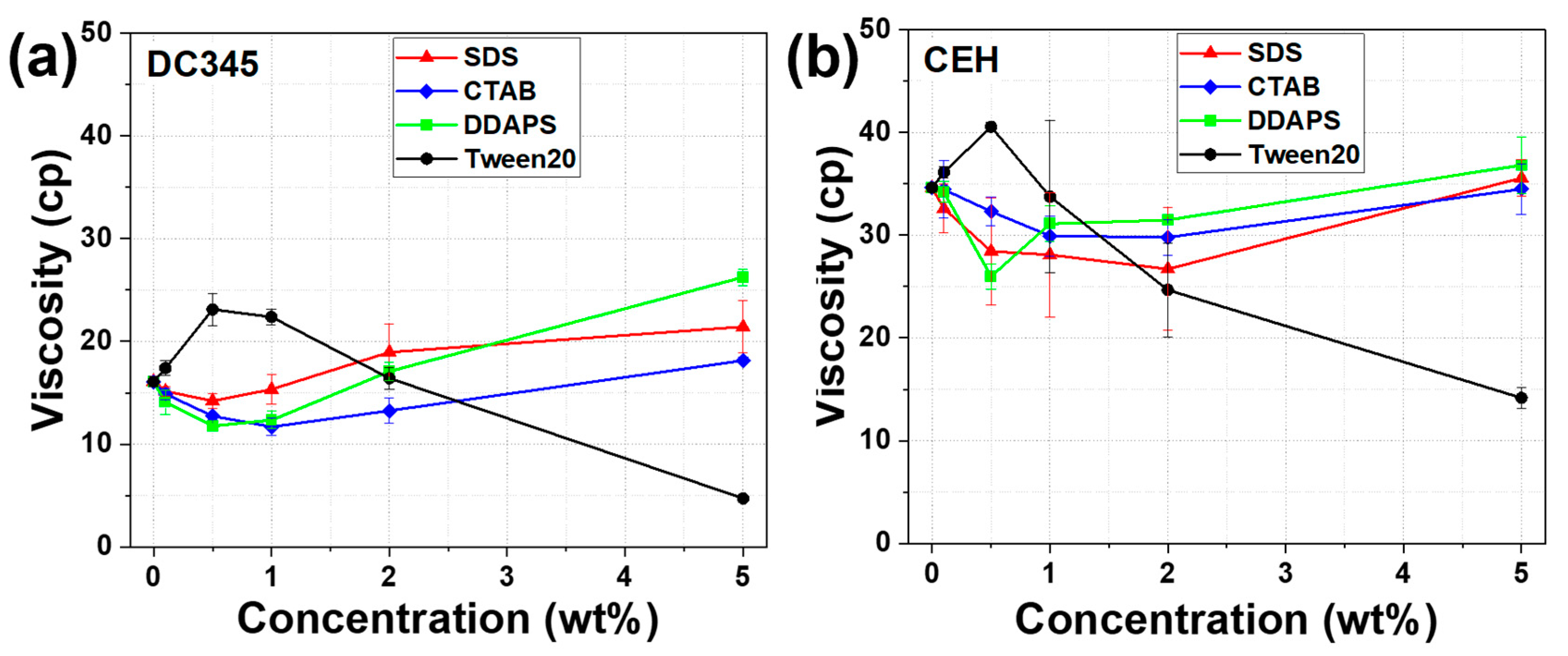
2.4. Viscosity Measurement of TiO2 Dispersion
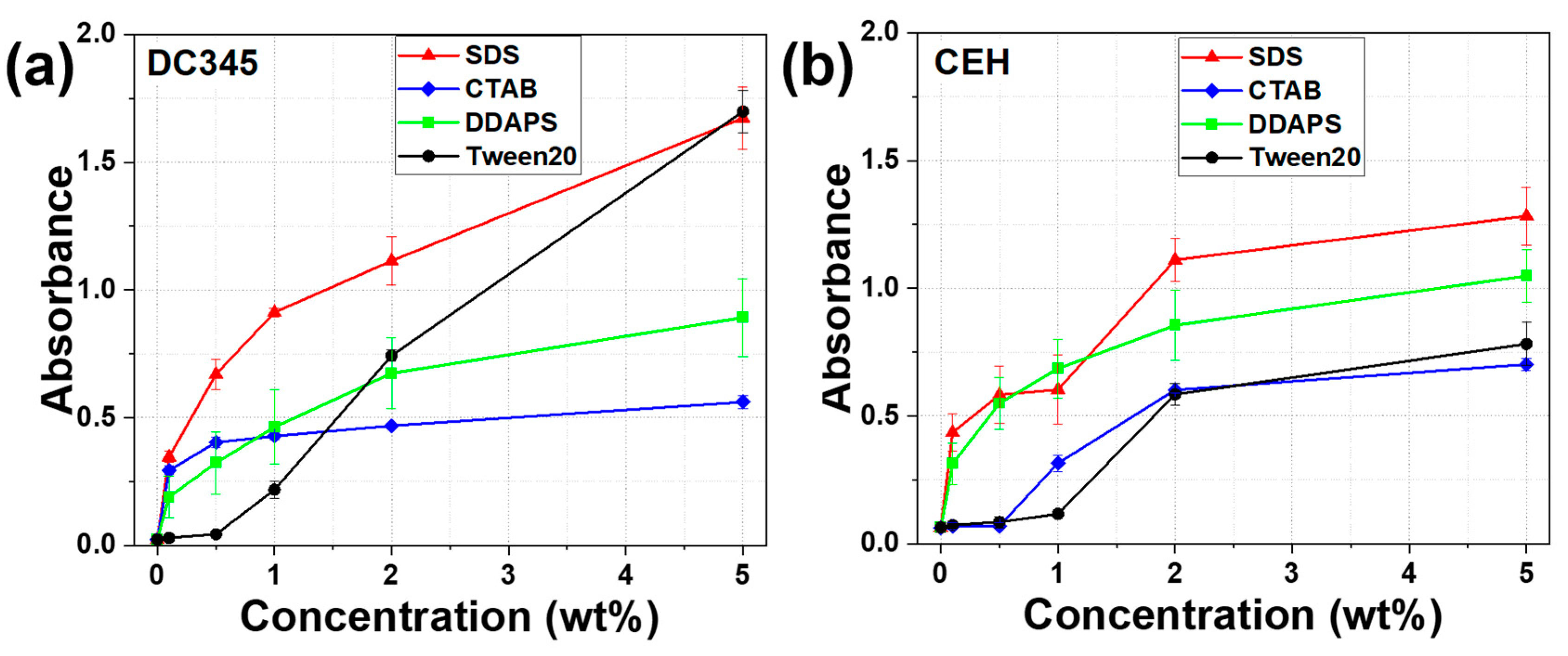
2.5. Assessment of UV-Blocking Efficacy
3. Results and Discussion
3.1. CMC Determination of Surfactants
3.2. Stability of TiO2 Dispersion upon Adding Surfactants
3.3. Effects of Surfactants on the UV-Blocking Efficacy of TiO2 Dispersion
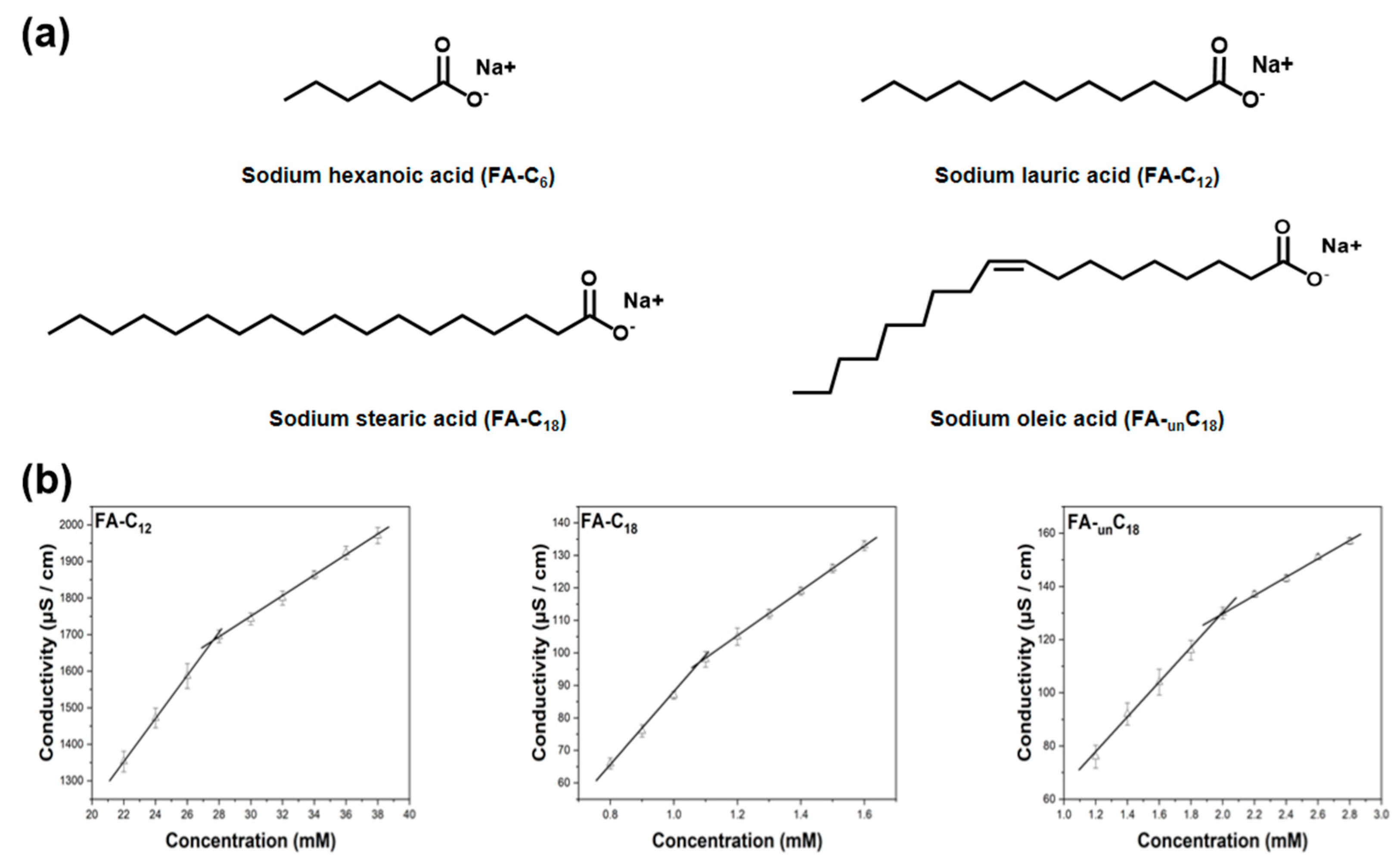
3.4. CMC Determination of Fatty Acids with Different Alkyl Chains
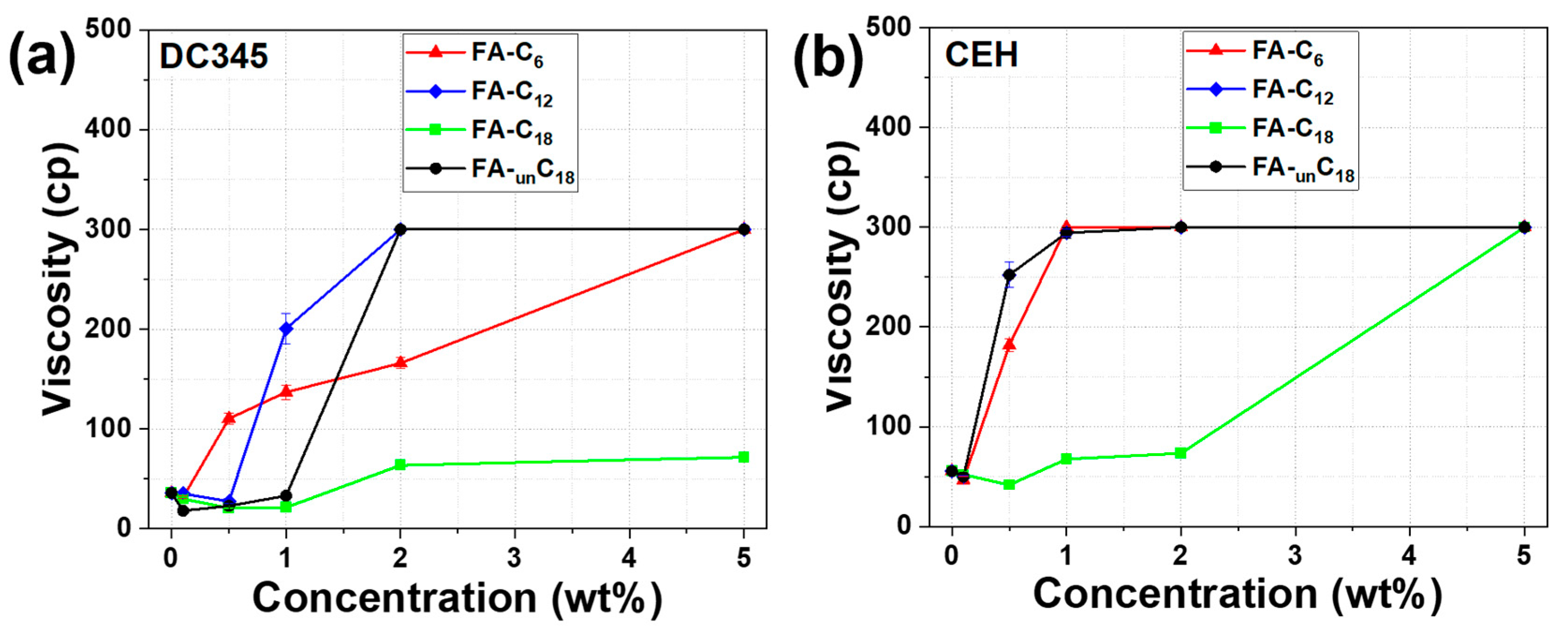
3.5. Effect of Addition of Fatty Acids with Different Alkyl Chains on the Stability of TiO2 Dispersion
3.6. Effect of Fatty Acids with Different Alkyl Chains on the UV-Blocking Efficacy of TiO2 Dispersion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filipe, P.; Silva, J.N.; Silva, R.; Cirne De Castro, J.L.; Marques Gomes, M.; Alves, L.C.; Santus, R.; Pinheiro, T. Stratum Corneum Is an Effective Barrier to TiO2 and ZnO Nanoparticle Percutaneous Absorption. Ski. Pharmacol. Physiol. 2009, 22, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The Banned Sunscreen Ingredients and Their Impact on Human Health: A Systematic Review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Ben Hlima, H.; Ben Chobba, I.; Kadri, A. Development and Stability Studies of Sunscreen Cream Formulations Containing Three Photo-Protective Filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef]
- Powar, N.S.; Chaeeun, A.; In, S.I. A Comprehensive Exploration of Nanomaterials in Cosmetics. IEEE Nanotechnol. Mag. 2024, 18, 15–26. [Google Scholar] [CrossRef]
- Faure, B.; Salazar-Alvarez, G.; Ahniyaz, A.; Villaluenga, I.; Berriozabal, G.; De Miguel, Y.R.; Bergström, L. Dispersion and Surface Functionalization of Oxide Nanoparticles for Transparent Photocatalytic and UV-Protecting Coatings and Sunscreens. Sci. Technol. Adv. Mater. 2013, 14, 23001. [Google Scholar] [CrossRef]
- Tseng, S.; Huang, C.H.; Hsu, J.P. Electrophoresis of Two Spheres: Influence of Double Layer and van Der Waals Interactions. J. Colloid Interface Sci. 2015, 451, 170–176. [Google Scholar] [CrossRef]
- Pu, Z.; Fan, X.; Su, J.; Zhu, M.; Jiang, Z. Aqueous Dispersing Mechanism Study of Nonionic Polymeric Dispersant for Organic Pigments. Colloid Polym. Sci. 2022, 300, 167–176. [Google Scholar] [CrossRef]
- Dange, C.; Phan, T.N.T.; André, V.; Rieger, J.; Persello, J.; Foissy, A. Adsorption Mechanism and Dispersion Efficiency of Three Anionic Additives [Poly(Acrylic Acid), Poly(Styrene Sulfonate) and HEDP] on Zinc Oxide. J. Colloid Interface Sci. 2007, 315, 107–115. [Google Scholar] [CrossRef]
- Deiss, J.L.; Anizan, P.; El Hadigui, S.; Wecker, C. Steric Stability of TiO2 Nanoparticles in Aqueous Dispersions. Colloids Surf. A Physicochem. Eng. Asp. 1996, 106, 59–62. [Google Scholar] [CrossRef]
- Liufu, S.; Xiao, H.; Li, Y. Adsorption of Poly(Acrylic Acid) onto the Surface of Titanium Dioxide and the Colloidal Stability of Aqueous Suspension. J. Colloid Interface Sci. 2005, 281, 155–163. [Google Scholar] [CrossRef]
- Lunn, D.J.; Seo, S.; Lee, S.H.; Zerdan, R.B.; Mattson, K.M.; Treat, N.J.; McGrath, A.J.; Gutekunst, W.R.; Lawrence, J.; Abdilla, A.; et al. Scalable Synthesis of an Architectural Library of Well-Defined Poly(Acrylic Acid) Derivatives: Role of Structure on Dispersant Performance. J. Polym. Sci. A Polym. Chem. 2019, 57, 716–725. [Google Scholar] [CrossRef]
- Mabrouk, M.M.; Hamed, N.A.; Mansour, F.R. Simple Spectrophotometric Method to Measure Surfactant CMC by Employing the Optical Properties of Curcumin’s Tautomers. J. Chem. Educ. 2021, 98, 2603–2609. [Google Scholar] [CrossRef]
- Ponphaiboon, J.; Limmatvapirat, S.; Limmatvapirat, C. Development and Evaluation of a Stable Oil-in-Water Emulsion with High Ostrich Oil Concentration for Skincare Applications. Molecules 2024, 29, 982. [Google Scholar] [CrossRef] [PubMed]
- Biswal, N.R.; Singh, J.K. Interfacial Behavior of Nonionic Tween 20 Surfactant at Oil–Water Interfaces in the Presence of Different Types of Nanoparticles. RSC Adv. 2016, 6, 113307–113314. [Google Scholar] [CrossRef]
- Kim, B.J.; Oh, S.G.; Han, M.G.; Im, S.S. Synthesis and Characterization of Polyaniline Nanoparticles in SDS Micellar Solutions. Synth. Met. 2001, 122, 297–304. [Google Scholar] [CrossRef]
- Liu, Y.; Tourbin, M.; Lachaize, S.; Guiraud, P. Silica Nanoparticles Separation from Water: Aggregation by Cetyltrimethylammonium Bromide (CTAB). Chemosphere 2013, 92, 681–687. [Google Scholar] [CrossRef]
- Wu, X.-J.; Jiang, Y.; Xu, D. A Unique Transformation Route for Synthesis of Rodlike Hollow Mesoporous Silica Particles. J. Phys. Chem. C 2011, 115, 11342–11347. [Google Scholar] [CrossRef]
- Yang, J.; Lee, T.I.; Lee, J.; Lim, E.K.; Hyung, W.; Lee, C.H.; Song, Y.J.; Suh, J.S.; Yoon, H.G.; Huh, Y.-M.; et al. Synthesis of Ultrasensitive Magnetic Resonance Contrast Agents for Cancer Imaging Using PEG-Fatty Acid. Chem. Mater. 2007, 19, 3870–3876. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.K.; Jeon, S.I. Study on the Critical Micelle Concentration Changes of Surfactants in Magnetized Water. Korean Chem. Soc. 2009, 53, 125–132. [Google Scholar] [CrossRef]
- Woodland, M.A.; Lucy, C.A. Altering the Selectivity of Inorganic Anion Separations Using Electrostatic Capillary Electrophoresis. Analyst 2001, 126, 28–32. [Google Scholar] [CrossRef]
- Bak, A.; Podgórska, W. Investigation of Drop Breakage and Coalescence in the Liquid-Liquid System with Nonionic Surfactants Tween 20 and Tween 80. Chem. Eng. Sci. 2012, 74, 181–191. [Google Scholar] [CrossRef]
- Cholewa, E.; Burgess, I.; Kunze, J.; Lipkowski, J. Adsorption of N-Dodecyl-N,N-Dimethyl-3-Ammonio-1-Propanesulfonate (DDAPS), a Model Zwitterionic Surfactant, on the Au(111) Electrode Surface. J. Solid State Electrochem. 2004, 8, 693–705. [Google Scholar] [CrossRef]
- Schulz, J.; Hohenberg, H.; Pflucker, F.; Gartner, E.; Will, T.; Pfeiffer, S.; Wepf, R.; Wendel, V.; Gers-Barlag, H.; Wittern, K. DIstribution of Sunscreens on Skin. Adv. Drug Deliv. Rev. 2002, 54, 157–163. [Google Scholar] [CrossRef]
- Jun, H.; Le Kim, T.H.; Han, S.W.; Seo, M.; Kim, J.W.; Nam, Y.S. Polyglycerol-Poly(ε-Caprolactone) Block Copolymer as a New Semi-Solid Polymeric Emulsifier to Stabilize O/W Nanoemulsions. Colloid. Polym. Sci. 2015, 293, 2949–2956. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of Titanium Dioxide Nanoparticles in Cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Dussert, A.-S.; Gooris, E.; Hemmerle, J. Characterization of the Mineral Content of a Physical Sunscreen Emulsion and Its Distribution onto Human Stratum Corneum. Int. J. Cosmet. Sci. 1997, 19, 119–129. [Google Scholar] [CrossRef]
- Mavon, A.; Miquel, C.; Lejeune, O.; Payre, B.; Moretto, P. In Vitro Percutaneous Absorption and in Vivo Stratum Corneum Distribution of an Organic and a Mineral Sunscreen. Ski. Pharmacol. Physiol. 2007, 20, 10–20. [Google Scholar] [CrossRef]
- Beasley, D.G.; Meyer, T.A. Characterization of the UVA Protection Provided by Avobenzone, Zinc Oxide, and Titanium Dioxide in Broad-Spectrum Sunscreen Products. Am. J. Clin. Dermatol. 2010, 11, 413–421. [Google Scholar] [CrossRef]
- Warson. Waterborne Coatings and Additives. Edited by D. R. Karsa and W. D. Davies. Royal Society of Chemistry, Cambridge, 1995. Pp. Ix + 257, Price £49.50. ISBN 0-85404-740-9. Polym. Int. 1996, 40, 75. [Google Scholar] [CrossRef]
- Núñez-Rojas, E.; Domínguez, H. Computational Studies on the Behavior of Sodium Dodecyl Sulfate (SDS) at TiO2(Rutile)/Water Interfaces. J. Colloid Interface Sci. 2011, 364, 417–427. [Google Scholar] [CrossRef]
- Kawazoe, A.; Kawaguchi, M. Characterization of Silicone Oil Emulsions Stabilized by TiO2 Suspensions Pre-Adsorbed SDS. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 283–287. [Google Scholar] [CrossRef]
- Poorsargol, M.; Alimohammadian, M.; Sohrabi, B.; Dehestani, M. Dispersion of Graphene Using Surfactant Mixtures: Experimental and Molecular Dynamics Simulation Studies. Appl. Surf. Sci. 2019, 464, 440–450. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Pu, N.-W.; Wang, C.-A.; Liu, Y.-M.; Sung, Y.; Wang, D.-S.; Ger, M.-D. Dispersion of Graphene in Aqueous Solutions with Different Types of Surfactants and the Production of Graphene Films by Spray or Drop Coating. J. Taiwan Inst. Chem. Eng. 2012, 43, 140–146. [Google Scholar] [CrossRef]
- Amirtham, V.A.; Dhinesh, D.K.; Khan, I.A. Experimental Investigation of Thermal Conductivity and Stability of TiO2-Ag/Water Nanocompositefluid with SDBS and SDS Surfactants. Thermochim. Acta 2019, 678, 178308. [Google Scholar] [CrossRef]
- Li, X.; Yoneda, M.; Shimada, Y.; Matsui, Y. Effect of Surfactants on the Aggregation and Stability of TiO2 Nanomaterial in Environmental Aqueous Matrices. Sci. Total Environ. 2017, 574, 176–182. [Google Scholar] [CrossRef]
- Loosli, F.; Stoll, S. Effect of Surfactants, PH and Water Hardness on the Surface Properties and Agglomeration Behavior of Engineered TiO2 Nanoparticles. Environ. Sci. Nano 2017, 4, 203–211. [Google Scholar] [CrossRef]
- Imae, T.; Muto, K.; Ikeda, S. The PH Dependence of Dispersion of TiO2 Particles in Aqueous Surfactant Solutions. Colloid Polym. Sci. 1991, 269, 43–48. [Google Scholar] [CrossRef]
- EN ISO 24443:2012; Cosmetics—Sun Protection Test Methods—In Vitro Determination of Sunscreen UVA Photoprotection. ISO: Geneva, Switzerland, 2012.
- Lee, S.Y.; Welbourn, R.; Clarke, S.M.; Skoda, M.W.A.; Clifton, L.; Zarbakhsh, A. Adsorption of Sodium Hexanoate on α-Alumina. J. Colloid Interface Sci. 2013, 407, 348–353. [Google Scholar] [CrossRef]
- Al-Busaidi, I.K.; Al-Maamari, R.S.; Karimi, M.; Naser, J. Effect of Different Polar Organic Compounds on Wettability of Calcite Surfaces. J. Pet. Sci. Eng. 2019, 180, 569–583. [Google Scholar] [CrossRef]
- Shafiei, M.; Kazemzadeh, Y.; Martyushev, D.A.; Dai, Z.; Riazi, M. Effect of Chemicals on the Phase and Viscosity Behavior of Water in Oil Emulsions. Sci. Rep. 2023, 13, 4100. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, J.; Bai, S.; Kubelka, J.; Piri, M. Wettability Reversal on Oil-Wet Calcite Surfaces: Experimental and Computational Investigations of the Effect of the Hydrophobic Chain Length of Cationic Surfactants. J. Colloid Interface. Sci. 2022, 619, 168–178. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, J.; Jeon, Y.; Jeong, S.; Lim, J.M.; Kim, Y.; Myeong, H.; Han, J.; Choi, Y.; Jo, S.-M.; Yang, S.Y.; et al. Ionic Character and Alkyl Chain Length of Surfactants Affect Titanium Dioxide Dispersion and Its UV-Blocking Efficacy. Appl. Sci. 2024, 14, 11035. https://doi.org/10.3390/app142311035
Mun J, Jeon Y, Jeong S, Lim JM, Kim Y, Myeong H, Han J, Choi Y, Jo S-M, Yang SY, et al. Ionic Character and Alkyl Chain Length of Surfactants Affect Titanium Dioxide Dispersion and Its UV-Blocking Efficacy. Applied Sciences. 2024; 14(23):11035. https://doi.org/10.3390/app142311035
Chicago/Turabian StyleMun, Jaehun, Yeji Jeon, Suhui Jeong, Jeong Min Lim, Yeojin Kim, Hwain Myeong, Jeongwoo Han, Youngwoo Choi, Seong-Min Jo, Seung Yun Yang, and et al. 2024. "Ionic Character and Alkyl Chain Length of Surfactants Affect Titanium Dioxide Dispersion and Its UV-Blocking Efficacy" Applied Sciences 14, no. 23: 11035. https://doi.org/10.3390/app142311035
APA StyleMun, J., Jeon, Y., Jeong, S., Lim, J. M., Kim, Y., Myeong, H., Han, J., Choi, Y., Jo, S.-M., Yang, S. Y., An, B.-S., Hwang, D. Y., & Seo, S. (2024). Ionic Character and Alkyl Chain Length of Surfactants Affect Titanium Dioxide Dispersion and Its UV-Blocking Efficacy. Applied Sciences, 14(23), 11035. https://doi.org/10.3390/app142311035







