Featured Application
Heavy metal sequestration using fixed-bed columns for wastewater decontamination.
Abstract
This study evaluated the adsorbent capacity of the Ecuadorian avocado (Persea americana Hass.) seed and peel wastes as an alternative method for cadmium (Cd), mercury (Hg), lead (Pb), and nickel (Ni) ion removal from aqueous solutions. The laboratory microscale process was performed using fixed-bed columns containing 1 g of 600 μm particles of biomaterial pretreated with ethanol and ethylene glycol. Subsequently, metal solutions of different concentrations were eluted and measured by flame atomic absorption spectroscopy. Results showed that fixed-bed columns allow efficient adsorption of Pb (2.6 mg/g) with ethanol pretreatment. Lower adsorption capacity was achieved for Cd, Hg, and Ni ions. Favorable adsorption with high retention capacity was found for Pb+2 for the ethanol pretreated bio-adsorbent at higher concentrations (120 mg/L). Lower removal percentages were found for Cd+2, Hg+2, and Ni+2; Ni showed the lowest adsorption capacities and negative RL values, suggesting inefficient adsorbent development. Regeneration of Cd, Hg, and Pb ions from avocado peel and seed showed the highest recovery when 1 mol/L HCl solution was used. Regarding the adsorption isotherms, the Langmuir model was the one that best fit our data, demonstrating that adsorption takes place in a uniform monolayer and that each contaminant ion occupies a single site.
Keywords:
biosorption; bio-adsorbent; cadmium; desorption; mercury; micro-scale process; nickel; lead 1. Introduction
Different industrial and domestic processes release large amounts of wastewater containing substances such as metals, suspended solids, organic and inorganic compounds, among others. This wastewater contaminates aquatic and terrestrial ecosystems [1,2,3]. Even though metals are natural components of the Earth’s surface [4,5], the main sources of their pollution are associated with anthropogenic activities, principally from the metal–mechanical industries, manufacture of batteries, construction elements, pigments, electrical accumulators, coal and oil combustion, galvanoplastic processes, continuous use of pesticides and fertilizer, and waste incineration [6,7,8].
Pollution by certain metals is one of the most important environmental problems since, depending on the organism, they produce different adverse effects even at low concentrations [8]. In addition, these contaminants are persistent in the environment [9,10,11,12], can bioaccumulate, and, in some cases, biomagnify through the food chain [13,14,15].
Among the most harmful non-essential metals and metalloids are lead (Pb), mercury (Hg), and cadmium (Cd), which have carcinogenic effects on living organisms [1,16]. Other metals, such as nickel (Ni), are considered oligo-elements that have biological functions. However, they can produce negative effects on human health when ingested in high amounts [8,17,18].
In Ecuador, the maximum permissible limits for polluting metals in wastewater are established according to their different receiving bodies in the Unified Text of Secondary Environmental Legislation (TULSMA, for its acronym in Spanish), where the respective limits for Cd are 0.02 mg/L for sewage and fresh water and 0.2 mg/L for marine water, for Hg 0.01 mg/L for sewage and marine water and 0.005 mg/L for fresh water, for Pb 0.5 mg/L for sewage and marine water and 0.2 mg/L for fresh water and for Ni 2.0 mg/L in all types of receiving bodies [19].
At present, different technologies and methods have been used to remediate heavy metals in contaminated environments [20,21,22,23]. Among these, physicochemical and biological processes have been developed. The former includes reverse osmosis [24,25,26], filtration [27,28,29], chemical oxidation [30,31,32], chemical leaching [31,33,34], nanotechnology [35], and electrokinetic remediation [36,37,38]. However, some of these methods use solvents or have some limitations regarding the amounts of matrixes that can be remediated, but in general, they require a lot of effort and are limited by their high costs [11,39]. Based on the immobilization of metals using microorganisms, the biological alternatives can include activated sludge with microorganisms [36,40,41]. These processes have certain disadvantages because they require the management of microorganisms, they are ineffective on non-degradable compounds, and also there is the possibility for accumulation of sludge foam and generation of biological sludge and by-products [41].
Besides, low-cost biomaterials with high adsorption capacities can act as high-performance retainers of contaminating metals [42] and as an alternative to costly commercial activated carbon adsorbents [43,44,45], including radioisotopes [46]. The biosorption process for the treatment of contaminated effluents [47,48] is a technique that involves the removal of contaminants using inert biomass [49,50,51]. Among the bio-adsorbent materials that have been most studied for the uptake of toxic metals are bacteria [52], fungi [53], algae [54], yeast biomass [55], agro-industrial waste such as wheat bran [56,57,58], rice husk [59,60,61,62], and fruits and vegetables [63,64,65,66,67,68,69,70,71], among others. Avocado seeds and peel tissue wastes have demonstrated an important capacity for toxic trace metals retention [43,72,73,74,75,76]. The high consumption of avocados produces a significant availability of these residues that can be processed and used for metal sequestration [43].
In this regard, the present study aimed to (a) design microscale fixed-bed columns based on Ecuadorian avocado (Persea americana Hass.) seed and peel tissues; (b) analyze the concentration of Cd, Hg, Ni, and Pb adsorbed in the biomass using atomic absorption spectrophotometry; (c) determine the maximum retention rate of metals in the columns for the removal of Cd, Hg, Ni, and Pb from aqueous solutions; (d) summarize the thermodynamic equilibrium relationship between adsorbate and adsorbent at constant temperatures, using the Langmuir and Freundlich isotherm models, and finally (e) evaluate the percentage of desorption of the metals using different pH values in desorption solutions to achieve the regeneration and reuse of the adsorbent columns.
2. Materials and Methods
2.1. Obtention and Treatment of Bio-Adsorbent Material
In the present study, the material used as bio-adsorbent was prepared with avocado seed and peel waste that had previously been dehydrated and ground. The seeds and peel wastes were donated by MIRA Avocado Oil (Cumbayá-Ecuador), and came from the province of Carchi, Ecuador.
The treatment of the bio-adsorbent material was prepared as described in a method by Aiyesanmi et al. [77] with some modifications. The raw material was ground (Hamilton Beach, Glen Allen, VA, USA) and sieved through a 600 μm mesh. Then, 10 g of this were used for each test. Each amount of bio-adsorbent was stirred in a vortex (Heathrow Scientific, Vernon Hills, IL, USA, at 3000 rpm, for 1 min) with the addition of different pretreatment solvents in proportions 1:10 w/v, as follows: 40% v/v ethanol (Merck, Darmstadt, Germany) and 40% ethylene glycol (Merck, Darmstadt, Germany). The pretreatment solvents were diluted in high-quality reagent-grade water (resistivity 18.2 MΩ·cm at 25 °C).
Both treatments were left to macerate for 4 days and shaken for 5 min each day. After the maceration time had elapsed, the bio-adsorbent materials were filtered through qualitative filter paper in a Buchner funnel using a vacuum pump (Millipore, Burlington, MA, USA) and dried at 60 °C for 24 h in an oven (Memmert, Schwabach, Germany). Finally, the material was sieved again at 600 µm to obtain particle homogeneity and stored in self-sealing bags inside a desiccator.
In this study, the bio-adsorbent particle size (600 µm) and the maceration time (4 days) were not variables. The concentration of each metal in the initial solution was the only variable considered.
2.2. Characterization of the Bio-Adsorbent Material
The water and mineral (ash) content was measured by triplicate through drying and calcination according to the test method proposed by Carter and Gregorich [78]. Additionally, the porosity was assessed by determining the real density using the pycnometer method and the apparent density, using the test tube method, of the bio-adsorbent material [79]. The pH of the material was estimated in an aqueous medium (1:10 w/v) of the original material by stirring for 15 min and using a pH-meter Session 1 (Hach, Ames, IA, USA). Finally, the identification of the functional groups was carried out using Fourier Transform Infrared Spectroscopy, with an Attenuated Reflection Accessory (FTIR-ATR; Spectrum BX, Perkin Elmer, Waltham, MA, USA) at 400–4000 cm−1, and 10 readings were taken according to the method proposed by García-González et al. [80].
2.3. Preparation and Testing of Fixed-Bed Columns
To prepare the microscale adsorbent columns, approximately 1 g of the dried bio-adsorbent (for both pretreatments) was introduced into a 5 mL syringe with a 1 cm column diameter and compacted with the syringe plunger to 2 cm in length by applying normal hand pressure for 10 s. Individual aqueous solutions of the metals under study were prepared at different initial concentrations (Ci., Table S1) from certified standards of Cd, Hg, Ni, and Pb at 1000 mg/L (Inorganic Ventures, Christiansburg, VA, USA). Twenty-five milliliters of the solutions of each metal were channeled into the adsorber using a Manifold vacuum chamber at 20 mmHg. Finally, the percolated liquid was collected in hermetically sealed plastic tubes, and the solutions were stored until subsequent analysis was performed by atomic absorption spectroscopy. The process was performed in triplicate for each metal concentration, using separate columns for the unmodified adsorbent and both pretreated adsorbents (ethanol and ethylene glycol).
2.4. Quantification of Metals by Atomic Absorption Spectroscopy
Quantification of Cd, Pb, and Ni was performed using flame atomic absorption spectrophotometry (FAAS) using an AAnalyst 400 spectrometer (Perkin Elmer, Waltham, MA, USA), following the standard methods 3030B and 3111A [81]. Hg was determined using cold vapor atomic absorption spectrophotometry (CVAAS), according to the reference methods 3030B and 3112B [81].
For both techniques, external standard calibration curves were used to interpolate the content of the samples, and blanks and recovery of external standards were analyzed as quality controls.
2.5. Determination of Adsorption Isotherms
The Langmuir and Freundlich isothermal models were used to analyze the experimental equilibrium measurements for the adsorption of ions of the studied metals in the bio-adsorbent material. The Langmuir model describes the adsorption process when a monolayer of contaminant is adsorbed onto a uniform adsorbent surface. The equilibrium constant controls the distribution of the compounds between the two phases; therefore, both adsorption and desorption rates are equal at equilibrium [82,83,84]. The estimation of the Langmuir model is carried out using the relationship between the equilibrium concentration (Ce, mg/L) and the equilibrium adsorbent capacity (q, mg/g), considering the maximum adsorption capacity (qmax, mg/g) and the Langmuir constant (KL, L/mg, Equation (1)).
The Langmuir model can be evaluated from a dimensionless factor (separation factor, RL) to show that the experimental data fully belong to this model. The RL is calculated using the initial adsorbate concentration (Ci, mg/L) and the KL (Equation (2)).
An RL equal to zero demonstrates an irreversible adsorption. In the case that 0 < RL < 1, it belongs to a favorable isothermal system. If RL is equal to one, it means linear desorption. Finally, 1 < RL corresponds to an unfavorable isothermal system [85].
The Freundlich model, unlike the Langmuir model, is not restricted to monolayer adsorption and can also be applied to multilayer adsorption, where the adsorbent has a heterogeneous surface composed of several types of adsorption sites, and the adsorbed amount is the sum of adsorption on all sites until the adsorption energy decreases exponentially at the end of the adsorption process [83,86].
In the linear form of the Freundlich isotherm model, the constant Kf is an approximate indicator of adsorption capacity, while 1/n is a function of the strength of adsorption in the adsorption process [84] (Equation (3)).
The values of n and 1/n depend on temperature and indicate adsorption conditions such as adsorption intensity or surface heterogeneity [83]. The magnitude 1/n is a measure of the adsorption intensity directly associated with the adsorption efficiency; 0 < 1/n < 1 indicates favorable adsorption, 1/n > 1 unfavorable adsorption, and 1/n = 1 irreversible adsorption. The removal efficiency is expressed as the percent of sorption [87], according to Equation (4):
2.6. Percentage of Desorption Calculations
After the adsorption process, the biomass was rinsed with an acid solution, following the method proposed by Carro de Diego [88] with certain modifications. Different eluting solutions were used through the designed columns: (1) 25 mL of medium quality reagent grade water, (2) 25 mL hydrochloric acid 0.01 mol/L (HCl, analytical grade, Merck, Rahway, NJ, USA), and (3) 25 mL of HCl 1.00 mol/L. The process was carried out using a Manifold vacuum, and the eluting solutions were collected to determine the content of desorbed metals by atomic absorption spectroscopy. The percentage of desorption efficiency was calculated according to Equation (5).
where Cdes is the concentration of adsorbate desorbed in mg/g, and Cads is the concentration of adsorbate adsorbed in mg/g [89].
3. Results and Discussion
The potential application of avocado biomass for the adsorption of contaminants, including toxic metals, has been previously described [43,72,73,74,75,76]. Ahmad and Danish [43] published a review considering several pretreatments of raw avocado fiber (including sodium hydroxide and phosphoric acid, among others) but did not mention that ethylene glycol or ethanol pretreatments were used. However, efficiency testing of avocado fibers for contaminant removal is widespread. Bassareh [90] compared avocado with two other materials (sour orange and walnut) and found that the avocado leaf-based fiber adsorbed the most Pb. The activated carbon obtained from avocado was more efficient than that obtained from the other fibers. Chimdessa and Ejeta [91] evaluated the adsorption of a mixture of metals on avocado-based activated carbon. Copper (Cu), Cd, and Pb were adsorbed in the highest quantities (up to 700 mg for Cu). For Cd, the efficiency was the highest, reaching 99.5%. Boeykens et al. [73] also mentioned the high performance of modified avocado biomass for Pb and Cr retention in water samples. In addition, Mahmoud et al. [92] managed to remove hexavalent chromium using modified avocado seeds.
These studies were performed using a batch method (simple agitation and maceration) and showed promising results indicating that avocado seed and peel biomass can effectively retain and immobilize metal ions such as Cd+2, Cu+2, Cr+6, Ni+2, Pb+2, and Zn+2. Nevertheless, studies regarding fixed-bed columns as filters made of avocado peel and seed waste have not been found. This is particularly important considering that, in developing countries, high costs often limit the use of conventional treatment methods on a large scale. Therefore, the application of the technical-economic feasibility of the remediation process based on the adsorbent properties of locally available materials at a small scale is the first step to the recurring challenge of removing toxic metals from aqueous solutions, with possible future applications in wastewater decontamination.
3.1. Bio-Adsorbent’s Physicochemical Properties
Previous authors determined the individual composition of the avocado peel and seeds of the same variety as those used in the present study [93,94,95]. The composition of avocado seeds previously had a wide range of parameters. Among these, 52.7–54.1% were water, 97.7–98.8% organic matter, 2.4–8.4% protein, 0.5–17.4% total fat, 27.5–29.7% carbohydrates, approximately 4% crude fiber, and vitamin A, vitamin C, phytosterols, triterpenes, vitamins K, B5, B6, and E, potassium, and folic acid were minority components. The peel is composed of 65.7–76.9% water., 93.4–99.3% organic matter, 1.5–7.42% protein, 11.0–13.7% total fat, 6.9–46.59% crude fiber, and around 16.24% carbohydrates; also, it contains bioactive compounds such as chlorophylls, carotenoids, and phenolic compounds.
In the present study, a mixture of avocado peel and seed waste from the oil extraction process was used. This bio-adsorbent showed a water content between 7.63–7.76%, similar to the reported by Sánchez-Quezada and Lorca-Piña [96] in seed flour of avocado dried by oven at 60 °C with values between 7.94–9.28%.
Regarding the mineral content (ashes), our bio-adsorbent had between 8.82–8.92%, while Sánchez-Quezada and Loarca-Piña [96] reported lower values (2.22–2.33%). It has been observed that low mineral content favors contaminant adsorption processes since adsorbents can only provide a finite number of binding sites on their surface, reducing their removal capacity when there are other minerals adsorbed [83,97].
The real and apparent density of the bio-adsorbent was between 0.96–0.98 g/mL and 0.295–0.323 g/mL, respectively, and showed a porosity between 0.630–0.712 g/mL. No previously reported values have been found for avocado peel and seeds biomass. Higher apparent density values favor the biosorption of metal ions [98]. Regarding porosity, studies have shown that the porous structure of the bio-adsorbents with a higher specific surface area will favor the diffusion of metal ions [99]. The solution of the studied bio-adsorbent has a pH of 5.1 ± 0.0. In the bio-adsorption of metals from wastewater, the degree of ionization of the adsorbate molecules and the presence of the functional groups on the adsorption surface depend on this, which is a determining factor in the efficiency of the process [43]. Mallampati et al. [100] verified the effect of pH on the retention of Pb in avocado peel biomass, determining that the percentage of cationic Pb removal increased with increasing pH due to the efficient exchange of cations with H+ ions of carboxylic functional groups present on the surface of the adsorbent as it can affect the adsorbent’s surface charge. In the present study, the percentage of the highest adsorption was tested between a pH of 2 and 10, and the maximum retention was found at a pH value of 5 in all cases. The pH of the aqueous medium also influences the metal species distribution and functional group dissociation on active sites [83]. Moreover, the adsorption efficiency of the adsorbents depends on the degree of ionization of adsorbate molecules, and the surface properties of the adsorbent depend on the pH of the solution [43].
3.2. Identification of Functional Groups by FTIR-ATR
Differentiation of the functional groups present in the biomaterial was then carried out between the pretreatment processes with ethylene glycol and ethanol (Figure 1).
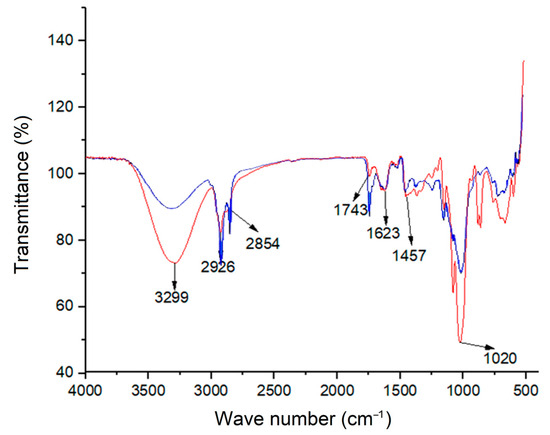
Figure 1.
Infrared spectra (FTIR-ATR) of the biomass pretreated with ethanol (blue) and ethylene glycol (red) for the identification of present functional groups.
Similar bands were found in both pretreatment processes; however, differences could be identified in the transmission peaks of the functional groups. The main functional groups in the material (after pretreatment processes) are described in Table 1.

Table 1.
The main bands of functional groups identified by FTIR-ATR on both pretreated avocado biomasses.
The presence of carbonyl and hydroxyl groups confirmed by the bond vibrations observed at 1743 cm−1 could be associated with carboxyl groups [43]. These functional groups can interact with metal ions by donating electron pairs from oxygen, allowing the formation of stable complexes. Similar results have been previously reported [101]. The strong peak found at 3299 cm−1 for ethylene glycol pretreatment is related to the hydroxyl groups due to ethylene glycol having two hydroxyl radicals in the molecule. That is why its signal is higher than the material pretreated with ethanol, which has just one hydroxyl group. Thus, the residual solvent could have been present in the bio-adsorbent.
The bands appearing between 1500–500 cm−1 are the products of several types of bond vibrations, which is why it is often difficult to assign their origin to a particular band. In addition, these bond vibrations originate from a strong interaction between neighboring bonds. This complex region is often known as the fingerprint and can vary from one subsample to another [102]. However, characteristic bands of certain families of compounds can be identified. In this case, a high band between 1100 and 1000 cm−1 is commonly associated with the presence of complex carbohydrates (cellulose) [102], which was observed in the samples from the present study (Figure 1).
Previous studies have used avocado seed powder [72,103,104] and applied several pretreatment processes to the bio-adsorption material using acids, bases, and salts [73,100,105]. However, to the best of our knowledge, pretreatment processes using ethanol or ethylene glycol have not been found. Both ethanol and ethylene glycol are industrially used as solvents. Both also have the same 2-carbon structure linked to one or two hydroxyl groups, respectively. Ethanol has degreasing properties [106], which would allow a partial elimination of the lipid content present on the surface of the biomaterial that hinders its interaction with metal ions. It could also act by dissolving other impurities that may be retained on the surface. On the other hand, although ethylene glycol does not have these degreasing properties, the presence of two hydroxyl ions could allow it to interact with the external molecular branches present in the biomaterial and, in this way, open or release them, preparing them to receive metal ions. Previous studies have used other compounds, such as alkalis and acids, to pretreat samples [43,73,76,100,105]. However, one purpose of this study was to find alternative reagents with lower toxicities and non-corrosiveness that are more easily accessible, particularly in developing countries.
After the pretreatments, changes in the architecture of the material were visually evident (Figure 2). The untreated bio-adsorbent (Figure 2a) shows agglomerations and a low contact surface. In addition, the material’s color has an orange tone, probably due to the presence of lipids in the material. In the case of the bio-adsorbent pretreated with ethanol, a change in the architecture is notable (Figure 2b): the bio-adsorbent particles are looser, and their color is less orange. The degreasing ethanol’s capacity may allow a change in the structural conformation of the individual particles. In the case of the bio-adsorbent pretreated with ethylene glycol (Figure 2c), it could be said that it is intermediate in architecture between the two previous ones because although its particles are loose, they do not present the same distribution as those corresponding to the pretreatment with ethanol. As for the color, although the orange has been somewhat lost, it does not reach the tone of the bio-adsorbent pretreated with ethanol. Because ethylene glycol is non-polar and is not considered a degreaser, it cannot eliminate the lipid content of the material.
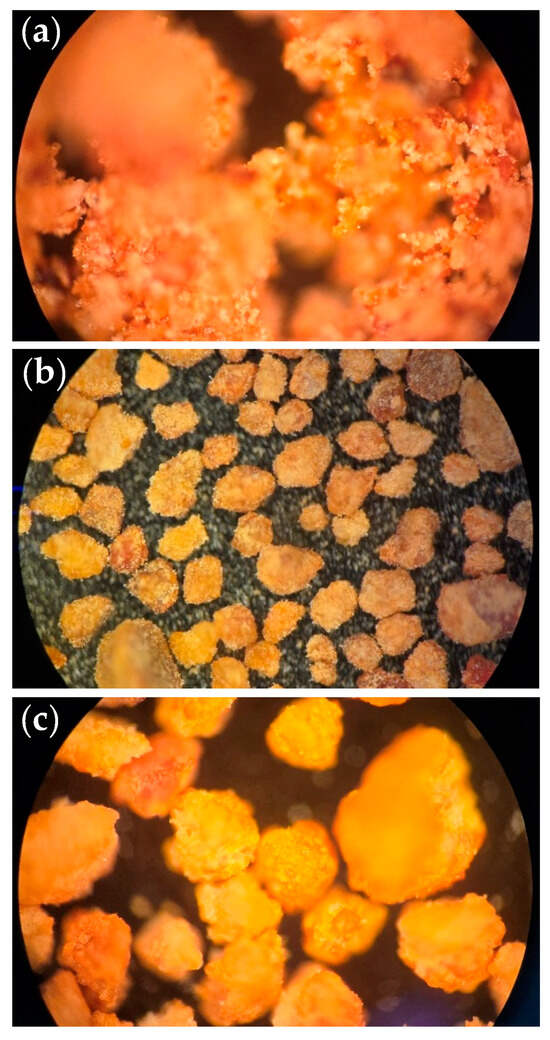
Figure 2.
Photographs taken on a Nikon TU Plan Fluor 5X0.15 A/0 EPI D contrast microscope Nikon (Tokyo, Japan) of the bio-adsorbents (a) untreated and with the pretreatments (b) ethanol and (c) ethylene glycol.
Additionally, only a batch mode for the adsorption process has been reported (agitation and maceration) [43]. Thus, the functional groups identified in the avocado peel and seed biomass, under the proposed pretreatment processes, can achieve promising adsorption results for metal retention using fixed-bed columns.
3.3. Bio-Adsorption Percentage
The initial contaminant metals’ content was evaluated in the raw material before each pretreatment process. Results were below the quantification limit for Cd and Hg, and 0.51 ± 0.02 and 0.39 ± 0.02 mg/kg for Pb and Ni, respectively. After the pretreatments, the contaminant metal content of the biomaterials was below the quantification limits for all tested metals. Thus, these pretreatments with ethanol and ethylene glycol not only help to open the surface structure of the bio-adsorbent, allowing it to receive the contaminant cations, but also to remove the cations that were bonded before.
Multipoint calibration curves at different concentrations were used for quantification in both FAAS and CVAAS techniques. Linear regression coefficients (R2) were evaluated (above 0.99) (Table 2).

Table 2.
Calibration curve parameters for the determination of metals using FAAS for Cd, Ni, Pb, and CVAAS for Hg.
Unlike previous studies where the percentage of removal increased with the increasing concentration of the analyte [76,100], in our study, metal adsorption decreased when metal concentration increased for both pretreatment processes (Figure 3). For Pb, it was observed that the biomaterial had higher remotion percentages at concentrations up to 125 mg/L than the other three metals tested (Cd, Hg, and Ni), in which the optimal adsorption occurs at values lower than 35 mg/L (Figure 3). As metal concentration increased, lower retention by the material was observed due to the reduction in, and an eventual competition for, the available active binding sites on the adsorbent surface [43,107]. Furthermore, a relatively high concentration of metal ions in the solution, compared to the possible adsorption sites available, saturates the surface, leading to a decrease or even desorption of the metal.
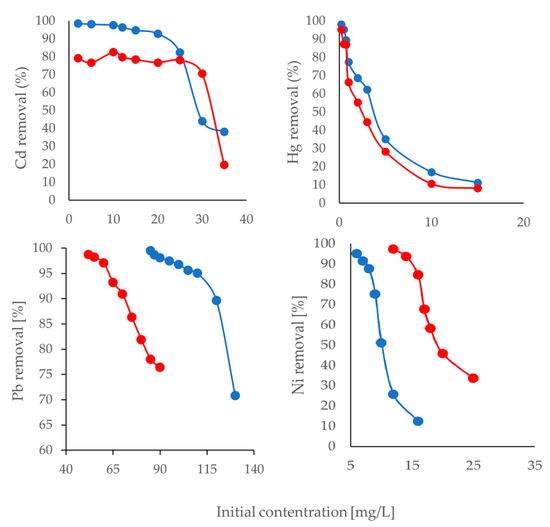
Figure 3.
Removal percentages for initial concentrations of Cd, Hg, Pb, and Ni pretreated with ethanol (blue) and ethylene glycol (red).
The avocado bio-adsorbent showed the highest retention capacity for Pb in ethanol pretreatment. The maximum adsorption percentage was 99.50% at an initial concentration of 85 mg/L. This decreased slightly to 95.00% at 110 mg/L, followed by a considerable decrease of up to 70.77% at 130 mg/L. The maximum removal percentage using the ethylene glycol pretreatment was 98.73% at a concentration of 52 mg/L; however, retention drastically decreased to 76.36% at 90 mg/L. Other studies have found a maximum removal percentage of 98% for Pb using pretreated biomass of avocado seeds treated with 85% phosphoric acid [73,108], while 83% removal has been achieved with the same non-pretreated bio-adsorbent [108] when using Pb solutions with a concentration of 30 mg/L.
Cd showed stable and promising removal percentages when using ethanol pretreatment. However, concentrations of the metal were not higher than 20 mg/L. Muluh [109] reported a 98.03% removal efficiency of Cd using avocado seed-pretreated carbon at concentrations of 200 mg/L, with an adsorbent dosage of 0.10 g, a pH of 3.37, and a contact time of 100 min.
The affinity of the avocado bio-adsorbent for the retention of Pb compared to the other metals tested (Cd, Hg, and Ni) was evident as higher concentrations were adsorbed. This could be related to the solubility of each metal ion, considering that Pb has a substantially lower solubility in aqueous media when it forms different salts. It has been demonstrated that the adsorption process for metals in different sorbents is affected by the initial pH of the solution since the pH determines the surface charge of the adsorbent and the degree of speciation and ionization of the adsorbate [87,110]. Moreover, other factors such as temperature, initial metal concentration, coexisting ions, and adsorbent dosage also affect the adsorption equilibrium [83].
Studies have shown the efficient removal of Cd and Pb using modified avocado seed at a maximum pH of 6, whereas, at lower pH values, the presence of carboxyl groups (-COOH) in the avocado seed retains protons, reducing the probability of binding to any positively charged metal ions [76]. Mqehe-Nedzivhe [101] achieved a higher removal percentage by increasing the initial concentration from 5 to 30 mg/L when solutions were adjusted to a pH of 6 at 25 °C.
For Hg, less than 80% removal percentage was obtained at lower concentrations of 1 mg/L for both pretreatments. Finally, for Ni, the ethanol-pretreated bio-adsorbent showed a slightly higher retention than the ethylene glycol-pretreated one. However, the limited adsorption did not show that avocado biomass was a good adsorber for this metal.
In all cases, the relative standard deviations of the replicates were less than 10%, demonstrating acceptable reproducibility of metal sequestration.
3.4. Determination of Bio-Adsorption Isotherms
The Langmuir and Freundlich isotherm models were applied in the present study (Table 3). For the Langmuir model, adsorbents with the highest possible qm and a high value of KL are the most desirable for their use in metal sequestration [83].

Table 3.
Calculated maximum adsorption capacity (qm) values for Cd, Hg, Pb, and Ni ions and parameters of Langmuir and Freundlich models using the peel and seed avocado adsorbent.
The adsorption isotherms for Cd, Hg, Pb, and Ni ions on the avocado adsorbent are plotted in Figure 4. The experimental data fitted the Langmuir model better than the Freundlich model in all cases.
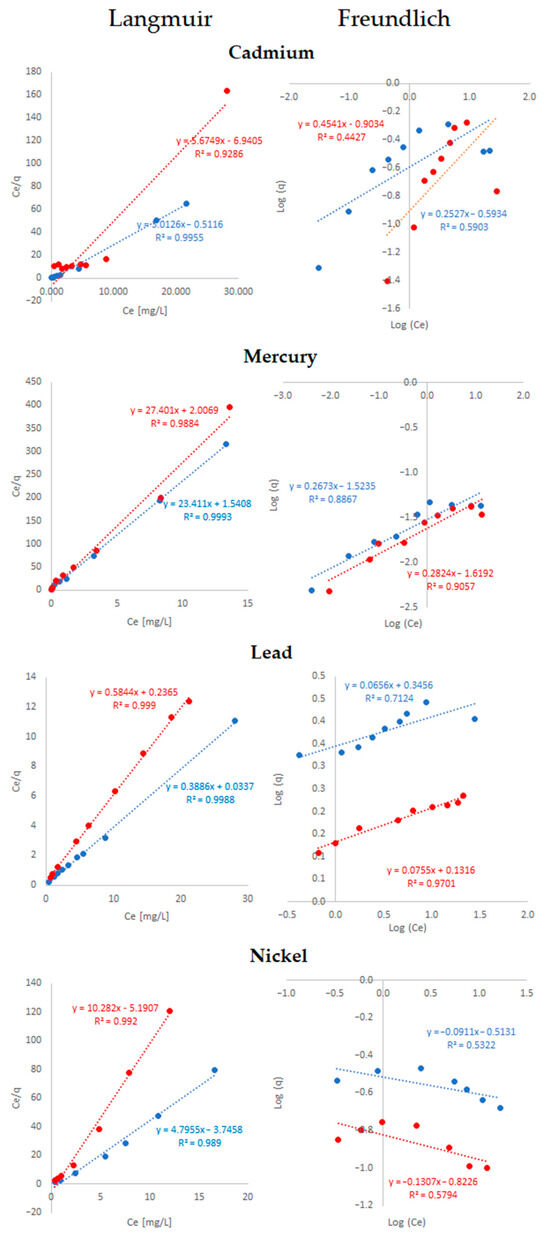
Figure 4.
Adsorption isotherm linearity (R2) for cadmium, mercury, lead, and nickel ions on the avocado peel and seed avocado bio-adsorbent using ethanol (blue) and ethylene glycol (red) as pretreatments.
Our results showed that the avocado bio-adsorbent performed differently for each metal adsorption, regardless of pretreatment. From Langmuir model calculations, Cd and Ni showed the lowest adsorption capacities (qm) and negative RL values, which suggest an inefficient development of the adsorbent to these metals’ retention. Aside from the qm values for Hg being low, the RL values demonstrate a favorable adsorption process [111]. Pb had the highest qm values, and its RL was in the favorable range for adsorption. Regression values (R2) indicate that the adsorption process for the four metals studied fits the Langmuir model more accurately. (Table 3 and Figure 4), in which all R2 values were above 0.99. The higher R2 values in the Langmuir isotherm model show the monolayer adsorption of these metals onto the surface with a finite number of identical sites [87]. Therefore, it can be assumed that a single metal molecule is adsorbed at each site, and each site might fit a single adsorbed molecule [82]. This kind of adsorption process has homogeneous and constant energy [112,113]. Contrary to our results, Wanja et al. [76] showed significantly higher adsorption capacities for Cd and Pb with the Langmuir model when using unmodified and acid-modified powdered avocado seed. Results were 162.58–177.99 mg/g and 802.23–932.08 mg/g for Cd and Pb, respectively. Other composite mineral adsorbents composed of clinoptilolite, feldspars, and Portland cement have been used for the removal of Cd and Pb from contaminated water, showing maximum adsorption capacities of 932.08 mg/g for Pb and 177.99 mg/g for Cd [87]. The Langmuir isotherm model is based on the hypothesis that all adsorption active sites are equivalent and that the ability of a molecule to bind to the surface is independent of whether there are nearby occupied positions. Furthermore, adsorption is restricted to a monolayer, and there are no lateral interactions between the adsorbate molecules [114].
In the analysis of Freundlich model calculations, 1/n values demonstrated probably favorable adsorptions for all metals except for Ni. Other studies using avocado seed adsorbent, in which unfavorable adsorption was obtained for Cd and Pb, have reported values for 1/n greater than 1 [76]. The surface of the adsorbent is assumed to be energetically heterogeneous, consisting of groups of adsorption sites with characteristic energies. Freundlich isotherm also assumes that there are no lateral interactions between the adsorbed molecules and that only one monolayer is adsorbed [114].
Previous studies have reported varying results for Cd, Hg, Pb, and Ni removal using different bio-adsorbents (Table 4). However, in the present study, a micro-scale model was used to simulate the adsorption of metals using avocado peel and seed with promising results for possible real-world applications in discharge waters.

Table 4.
Comparison of adsorption capacities using different types of bio-adsorbents.
3.5. Desorption Process
Adsorption must be an efficient and economical process, which is why the regeneration and reuse of the bio-adsorbent is sought, as well as the recovery of metal ions. In this regard, a subsequent efficient desorption process would allow the regeneration and at least one reuse cycle of the bio-adsorbent. According to Aiyesanmi et al. [77], the most efficient method for metal ion desorption is the treatment with dilute acid, as the presence of acid in the system (low pH) increases ionic strength and, therefore, raises the competition between ions due to the protonation of the active sites of the bio-adsorbent. This process breaks the ionic forces and liberates metal ions from the surface of the material [70]. For the desorption process, different eluent parameters are important to consider, such as type of eluent, concentration, type of desorption (batch or column), volume, pH, etc. [89]. It has been reported that Cd, Pb, Ni, Zn, and Al were desorbed at low pH using 0.1 N HCl, confirming that acids are suitable desorbing agents [115]. However, the pH and concentration of the eluent must be tested. On the one hand, pH affects the charges on both the eluent and solute ions (adsorbate–adsorbent system). On the other hand, higher concentrations may damage the structure of exhausted adsorbent, while lower concentrations could result in slower desorption rates, requiring higher eluent volumes [89].
In the present study, for the four metals tested and the two pretreatment methods applied, the desorption percentages using different eluents showed a high desorption percentage when using HCl 1 M, except in Ni, where the highest percentages were when using HCl 0.01 M (Table 5).

Table 5.
Desorption test developed with water, HCl 0,01 mol/L, and HCl 1mol/L.
Wanja et al. [76] studied the individual desorption process of Cu, Cd, and Pb ions from acid-modified avocado seed, where the highest recovery was achieved with 0.1 M sulfuric acid (>98%). Other studies have reported a maximum percentage recovery of 100% for Cd (II) using 0.075 M HCl solution in pretreated carbon prepared from coir pith [116].
4. Conclusions
Our study showed the adsorption efficiency of laboratory-prepared micro-scale fixed-bed columns using 1 g of avocado seed and peel tissues to remove Cd, Pb, Hg, and Ni, and this bio-adsorbent was pretreated with ethanol and ethylene glycol solutions before the retention process. Favorable adsorption with high retention capacity was found for Pb+2 for the ethanol pretreatment process at higher concentrations (120 mg/L). Even though lower removal percentages were found for Cd+2, Hg+2, and Ni+2, the adsorption equilibrium data obtained best fit the Langmuir isotherm model for the four metals under study, with regression values (R2) over 0.98. Ni showed the lowest adsorption capacities and negative RL values, suggesting deficient adsorbent development. Regeneration testing of the bio-adsorbent showed the highest recovery when using 1 mol/L HCl solution. However, testing is needed to determine adsorption–desorption efficiencies and maximum regeneration and reuse since, nowadays, it is important to use eco-friendly and cost-effective adsorbents for the remediation of contaminants.
As far as we know, this is the first study carried out using fixed bed columns with avocado seed and peel wastes for the removal of metal contamination in aqueous solutions. Nevertheless, more experimental factors are still needed, including temperature, adsorbent dose, particle size, or even other material pretreatment processes, to evaluate the adsorption capacity of this bio-adsorbent.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app142310851/s1. Table S1: Results of Cd, Hg, Ni, and Pb adsorption isotherm tests using microscale fixed bed columns with pretreated avocado seeds and peel.
Author Contributions
Conceptualization: D.R.-E. and J.V.-G.; Methodology: A.G.-N., C.M.-N. and P.Y.V.-T.; Software: A.G.-N., C.M.-N. and P.Y.V.-T.; Validation: A.G.-N., C.M.-N. and P.Y.V.-T.; Formal Analysis: A.G.-N., C.M.-N. and P.Y.V.-T.; Investigation: A.G.-N., C.M.-N. and D.R.-E.; Resources: J.V.-G., A.O.-C. and H.N.; Data Curation: A.G.-N., C.M.-N., G.S.Y.-J., J.V.-G. and D.R.-E.; Writing—Original Draft: A.G.-N., C.M.-N., G.S.Y.-J. and D.R.-E.; Writing—Review and Editing: G.S.Y.-J., D.R.-E., A.O.-C. and H.N.; Visualization: D.R.-E., J.V.-G. and A.O.-C.; Supervision, P.Y.V.-T.; Project Administration: J.V.-G.; Funding Acquisition, J.V.-G., A.O.-C. and H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The authors also thank PUCE for the financial support to carry out the experiments.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Mira Avocado Oil for donating the ground avocado peel and seed waste used in this research. In addition, the authors express their gratitude to William Basantes for his support in calculating and interpreting the results, and Kyla Hoggins for editing the English text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Cardona Gutiérrez, A.F.; Cabañas Vargas, D.D.; Zepeda Pedreguera, A. Evaluación del poder biosorbente de cáscara de naranja para la eliminación de metales pesados, Pb (II) y Zn (II). Ingeniería 2013, 17, 1–9. [Google Scholar]
- Holt, M.S. Sources of Chemical Contaminants and Routes into the Freshwater Environment. Food Chem. Toxicol. 2000, 38, S21–S27. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Chapter 4—Heavy Metals. In An Introduction to Nuclear Waste Immobilisation; Ojovan, M.I., Lee, W.E., Eds.; Elsevier: Oxford, UK, 2005; pp. 35–41. ISBN 978-0-08-044462-8. [Google Scholar]
- Romero-Estévez, D.; Yánez-Jácome, G.S.; Navarrete, H. Non-Essential Metal Contamination in Ecuadorian Agricultural Production: A Critical Review. J. Food Compos. Anal. 2023, 115, 104932. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments—A Review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Caviedes Rubio, D.I.; Muñoz Calderón, R.A.; Perdomo Gualtero, A.; Rodríguez Acosta, D.; Sandoval Rojas, I.J. Tratamientos para la Remoción de Metales Pesados Comúnmente Presentes en Aguas Residuales Industriales. Una Revisión. Ing. Región 2015, 13, 73–90. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ.—Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Danovaro, R.; Cocozza di Montanara, A.; Corinaldesi, C.; Dell’Anno, A.; Illuminati, S.; Willis, T.J.; Gambi, C. Bioaccumulation and Biomagnification of Heavy Metals in Marine Micro-Predators. Commun. Biol. 2023, 6, 1206. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.P.M.; Ma, W.C.; Van Straalen, N.M. Biomagnification of Metals in Terrestrial Ecosystems. Sci. Total Environ. 1993, 134, 511–524. [Google Scholar] [CrossRef]
- Madgett, A.S.; Yates, K.; Webster, L.; McKenzie, C.; Moffat, C.F. The Concentration and Biomagnification of Trace Metals and Metalloids across Four Trophic Levels in a Marine Food Web. Mar. Pollut. Bull. 2021, 173, 112929. [Google Scholar] [CrossRef]
- Rafique, S.; Gillani, S.S.; Nazir, R. Lead and Cadmium Toxic Effects on Human Health: A Review. J. Nutr. Food Sci. 2021, 11, 459. [Google Scholar]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Hossain Mondal, M.; Bhattarai, A.; Saha, B. A Comprehensive Review on the Sources, Essentiality and Toxicological Profile of Nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, E.; Moraes, M.F. Nickel—From Toxic to Essential Nutrient. Better Crops 2007, 91, 26–27. [Google Scholar]
- MAATE Libro VI Anexo I del Texto Unificado de Legislacion Secundaria de Medio Ambiente 2017. Available online: https://www.ambiente.gob.ec/wp-content/uploads/downloads/2018/05/TULSMA.pdf (accessed on 20 November 2024).
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Kumar, M.; Seth, A.; Singh, A.K.; Rajput, M.S.; Sikandar, M. Remediation Strategies for Heavy Metals Contaminated Ecosystem: A Review. Environ. Sustain. Indic. 2021, 12, 100155. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A Critical Review on Various Remediation Approaches for Heavy Metal Contaminants Removal from Contaminated Soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent Advances in Conventional and Contemporary Methods for Remediation of Heavy Metal-Contaminated Soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Aljendeel, H.A. Removal of Heavy Metals Using Reverse Osmosis. J. Eng. 2011, 17, 647–658. [Google Scholar] [CrossRef]
- Benito, Y.; Ruíz, M.L. Reverse Osmosis Applied to Metal Finishing Wastewater. Desalination 2002, 142, 229–234. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel Technologies for Reverse Osmosis Concentrate Treatment: A Review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef] [PubMed]
- d’Halluin, M.; Rull-Barrull, J.; Bretel, G.; Labrugère, C.; Le Grognec, E.; Felpin, F.-X. Chemically Modified Cellulose Filter Paper for Heavy Metal Remediation in Water. ACS Sustain. Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Ghobadi, R.; Altaee, A.; Zhou, J.L.; Karbassiyazdi, E.; Ganbat, N. Effective Remediation of Heavy Metals in Contaminated Soil by Electrokinetic Technology Incorporating Reactive Filter Media. Sci. Total Environ. 2021, 794, 148668. [Google Scholar] [CrossRef]
- Xiao, H.; Low, Z.-X.; Gore, D.B.; Kumar, R.; Asadnia, M.; Zhong, Z. Porous Metal–Organic Framework-Based Filters: Synthesis Methods and Applications for Environmental Remediation. Chem. Eng. J. 2022, 430, 133160. [Google Scholar] [CrossRef]
- Bennedsen, L.R. Chapter 2—In Situ Chemical Oxidation: The Mechanisms and Applications of Chemical Oxidants for Remediation Purposes. In Chemistry of Advanced Environmental Purification Processes of Water; Søgaard, E.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 13–74. ISBN 978-0-444-53178-0. [Google Scholar]
- Koul, B.; Taak, P. Chemical Methods of Soil Remediation. In Biotechnological Strategies for Effective Remediation of Polluted Soils; Koul, B., Taak, P., Eds.; Springer: Singapore, 2018; pp. 77–84. ISBN 9789811324208. [Google Scholar]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent Advances in Soil Remediation Technology for Heavy Metal Contaminated Sites: A Critical Review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Bisone, S.; Blais, J.-F.; Mercier, G. Counter-Current Metal Leaching and Precipitation for Soil Remediation. Soil Sediment Contam. Int. J. 2013, 22, 856–875. [Google Scholar] [CrossRef]
- Fedje, K.K.; Yillin, L.; Strömvall, A.-M. Remediation of Metal Polluted Hotspot Areas through Enhanced Soil Washing—Evaluation of Leaching Methods. J. Environ. Manag. 2013, 128, 489–496. [Google Scholar] [CrossRef]
- Ekrami, E.; Pouresmaieli, M.; sadat Hashemiyoon, E.; Noorbakhsh, N.; Mahmoudifard, M. Nanotechnology: A Sustainable Solution for Heavy Metals Remediation. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100718. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, Y.; Wan, Y. Remediation of Cadmium-Contaminated Soil Using Modified Activated Carbon Fiber Combined with Electrodynamic Remediation Technology. Rendiconti Lincei Sci. Fis. E Nat. 2024, 35, 655–671. [Google Scholar] [CrossRef]
- Muthuraman, R.M.; Murugappan, A.; Soundharajan, B. A Sustainable Material for Removal of Heavy Metals from Water: Adsorption of Cd(II), Pb(II) and Cu(II) Using Kinetic Mechanism. Desalination Water Treat. 2021, 220, 192–198. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Lens, P.N.; Vochten, P.M.; Speleers, L.; Verstraete, W.H. Direct Treatment of Domestic Wastewater by Percolation over Peat, Bark and Woodchips. Water Res. 1994, 28, 17–26. [Google Scholar] [CrossRef]
- Löser, C.; Seidel, H.; Hoffmann, P.; Zehnsdorf, A. Remediation of Heavy-Metal-Polluted River Sediments by Bioleaching Using the Percolation Principle; Technologisch Instituut vzw: Antwerpen, Belgium, 1999; pp. 213–222. ISBN 978-90-76019-11-6. Available online: https://www.researchgate.net/profile/Christian-Loeser-2/publication/263366180_Remediation_of_heavy-metal-polluted_river_sediments_by_bioleaching_using_the_percolation_principle/links/00b4953b669289f0ca000000/Remediation-of-heavy-metal-polluted-river-sediments-by-bioleaching-using-the-percolation-principle.pdf (accessed on 20 November 2024).
- Dissanayake, D.M.R.E.A.; Chathuranga, P.K.D.; Perera, P.I.; Vithanage, M.; Iqbal, M.C.M. Modeling of Pb(II) Adsorption by a Fixed-Bed Column. Bioremediation J. 2016, 20, 194–208. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M. A Review of Avocado Waste-Derived Adsorbents: Characterizations, Adsorption Characteristics, and Surface Mechanism. Chemosphere 2022, 296, 134036. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Driss Alami, S.B. Aprovechamiento de Hueso de Aceituna Biosorción de Iones Metálicos. Doctoral Thesis, Universidad de Granada, Granada, Spain, 2010. [Google Scholar]
- Abdelhamid, A.A.; Badr, M.H.; Mohamed, R.A.; Saleh, H.M. Using Agricultural Mixed Waste as a Sustainable Technique for Removing Stable Isotopes and Radioisotopes from the Aquatic Environment. Sustainability 2023, 15, 1600. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an Efficient Method for Removing Heavy Metals from Industrial Effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Saini, S.; Gill, J.K.; Kaur, J.; Saikia, H.R.; Singh, N.; Kaur, I.; Katnoria, J.K. Biosorption as Environmentally Friendly Technique for Heavy Metal Removal from Wastewater. In Fresh Water Pollution Dynamics and Remediation; Qadri, H., Bhat, R.A., Mehmood, M.A., Dar, G.H., Eds.; Springer: Singapore, 2020; pp. 167–181. ISBN 9789811382772. [Google Scholar]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Saravanan, A.; Vo, D.-V.N. Advances in Biosorbents for Removal of Environmental Pollutants: A Review on Pretreatment, Removal Mechanism and Future Outlook. J. Hazard. Mater. 2021, 420, 126596. [Google Scholar] [CrossRef]
- Aryal, M.; Liakopoulou-Kyriakides, M. Bioremoval of Heavy Metals by Bacterial Biomass. Environ. Monit. Assess. 2015, 187, 4173. [Google Scholar] [CrossRef]
- Naseem Akthar, M.; Sivarama Sastry, K.; Maruthi Mohan, P. Mechanism of Metal Ion Biosorption by Fungal Biomass. Biometals 1996, 9, 21–28. [Google Scholar] [CrossRef]
- Lucaci, A.-R.; Bulgariu, L. Biosorption of Technologically Valuable Metal Ions on Algae Wastes: Laboratory Studies and Applicability. Water 2024, 16, 512. [Google Scholar] [CrossRef]
- van Wyk, C.S. Removal of Heavy Metals from Metal-Containing Effluent by Yeast Biomass. Afr. J. Biotechnol. 2011, 10, 11557–11561. [Google Scholar]
- Mathew, B.B.; Jaishankar, M.; Biju, V.G.; Beeregowda, K.N. Role of Bioadsorbents in Reducing Toxic Metals. J. Toxicol. 2016, 2016, 4369604. [Google Scholar] [CrossRef]
- Özer, A.; Özer, D.; Özer, A. The Adsorption of Copper(II) Ions on to Dehydrated Wheat Bran (DWB): Determination of the Equilibrium and Thermodynamic Parameters. Process Biochem. 2004, 39, 2183–2191. [Google Scholar] [CrossRef]
- Özer, A.; Pirinççi, H.B. The Adsorption of Cd(II) Ions on Sulphuric Acid-Treated Wheat Bran. J. Hazard. Mater. 2006, 137, 849–855. [Google Scholar] [CrossRef]
- Kumar, U.; Bandyopadhyay, M. Sorption of Cadmium from Aqueous Solution Using Pretreated Rice Husk. Bioresour. Technol. 2006, 97, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Thomas Choong, S.Y. Rice Husk as a Potentially Low-Cost Biosorbent for Heavy Metal and Dye Removal: An Overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Song, S.-T.; Hau, Y.-F.; Saman, N.; Johari, K.; Cheu, S.-C.; Kong, H.; Mat, H. Process Analysis of Mercury Adsorption onto Chemically Modified Rice Straw in a Fixed-Bed Adsorber. J. Environ. Chem. Eng. 2016, 4, 1685–1697. [Google Scholar] [CrossRef]
- Loukidou, M.X.; Zouboulis, A.I. Biosorption of Toxic Metals. In Water Encyclopedia; Lehr, J.H., Keeley, J., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Adsorption of Heavy Metals from Water Using Banana and Orange Peels. Water Sci. Technol. 2003, 47, 185–190. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Senturk, E.; Ozkan, T. Adsorption Kinetics for the Removal of Chromium (VI) from Aqueous Solutions on the Activated Carbons Prepared from Agricultural Wastes. Water SA 2004, 30, 533–539. [Google Scholar] [CrossRef]
- Dhakal, R.P.; Ghimire, K.N.; Inoue, K. Adsorptive Separation of Heavy Metals from an Aquatic Environment Using Orange Waste. Hydrometallurgy 2005, 79, 182–190. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Bernal-Jacome, L.A.; Acosta-Rodriguez, I. Adsorption of Cadmium(II) from Aqueous Solution on Natural and Oxidized Corncob. Sep. Purif. Technol. 2005, 45, 41–49. [Google Scholar] [CrossRef]
- Marshall, W.E.; Wartelle, L.H.; Boler, D.E.; Johns, M.M.; Toles, C.A. Enhanced Metal Adsorption by Soybean Hulls Modified with Citric Acid. Bioresour. Technol. 1999, 69, 263–268. [Google Scholar] [CrossRef]
- Nasiruddin Khan, M.; Farooq Wahab, M. Characterization of Chemically Modified Corncobs and Its Application in the Removal of Metal Ions from Aqueous Solution. J. Hazard. Mater. 2007, 141, 237–244. [Google Scholar] [CrossRef]
- Sarin, V.; Pant, K.K. Removal of Chromium from Industrial Waste by Using Eucalyptus Bark. Bioresour. Technol. 2006, 97, 15–20. [Google Scholar] [CrossRef]
- Miralles, N.; Valderrama, C.; Casas, I.; Martínez, M.; Florido, A. Cadmium and Lead Removal from Aqueous Solution by Grape Stalk Wastes: Modeling of a Fixed-Bed Column. J. Chem. Eng. Data 2010, 55, 3548–3554. [Google Scholar] [CrossRef]
- Raulino, G.S.C.; Vidal, C.B.; Lima, A.C.A.; Melo, D.Q.; Oliveira, J.T.; Nascimento, R.F. Treatment Influence on Green Coconut Shells for Removal of Metal Ions: Pilot-Scale Fixed-Bed Column. Environ. Technol. 2014, 35, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Bazzo, A.; Adebayo, M.A.; Dias, S.L.P.; Lima, E.C.; Vaghetti, J.C.P.; De Oliveira, E.R.; Leite, A.J.B.; Pavan, F.A. Avocado Seed Powder: Characterization and Its Application for Crystal Violet Dye Removal from Aqueous Solutions. Desalination Water Treat. 2016, 57, 15873–15888. [Google Scholar] [CrossRef]
- Boeykens, S.P.; Redondo, N.; Obeso, R.A.; Caracciolo, N.; Vázquez, C. Chromium and Lead Adsorption by Avocado Seed Biomass Study through the Use of Total Reflection X-Ray Fluorescence Analysis. Appl. Radiat. Isot. 2019, 153, 108809. [Google Scholar] [CrossRef]
- Díaz-Muñoz, L.L.; Bonilla-Petriciolet, A.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I. Sorption of Heavy Metal Ions from Aqueous Solution Using Acid-Treated Avocado Kernel Seeds and Its FTIR Spectroscopy Characterization. J. Mol. Liq. 2016, 215, 555–564. [Google Scholar] [CrossRef]
- Rangel, A.V.; Becerra, M.G.; Guerrero-Amaya, H.; Ballesteros, L.M.; Mercado, D.F. Sulfate Radical Anion Activated Agro-Industrial Residues for Cr(VI) Adsorption: Is This Activation Process Technically and Economically Feasible? J. Clean. Prod. 2021, 289, 125793. [Google Scholar] [CrossRef]
- Wanja, N.E.; Murungi, J.; Wanjau, R.; Hassanali, A. Application of Chemically Modified Avocado Seed for Removal of Copper (II), Lead(II), and Cadmium(II) Ions from Aqueous Solutions. Int. J. Res. Eng. Appl. Sci. 2016, 6, 1–15. [Google Scholar]
- Aiyesanmi, A.F.; Adebayo, M.A.; Arowojobe, Y. Biosorption of Lead and Cadmium from Aqueous Solution in Single and Binary Systems Using Avocado Pear Exocarp: Effects of Competing Ions. Anal. Lett. 2020, 53, 2868–2885. [Google Scholar] [CrossRef]
- Canadian Society of Soil Science. Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Pinawa, MB, Canada; Boca Raton, FL, USA, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- Cabrera Andrade, L.F. Bioadsorción de Iones de Plomo y Cromo Procedentes de Aguas Residuales Utilizando la Cáscara del Tomate de Árbol (Solanum betaceum). Bachelor’s Thesis, Universidad Politécnica Salesiana, Cuenca, Ecuador, 2017. [Google Scholar]
- García-González, R.; Gomez-Espinosa, R.M.; Ávila-Pérez, P.; García-Gaitán, B.; García-Rivas, J.L.; Zavala-Arce, R.E. Estudio de biosorción de Cu2+ en el criogel quitosano-celulosa. Rev. Mex. Ing. Quím. 2016, 15, 311–322. [Google Scholar] [CrossRef]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 24th ed.; American Public Health Association, American Water Works Association, and Water Environment Federation: Washington, DC, USA, 2023. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm Models for Adsorption of Heavy Metals from Water—A Review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn 2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Larenas Uría, C.; Andrango, D.; Inga, P. Estudio isotérmico de biosorción de plomo en aguas utilizando residuos vegetales. La Granja 2008, 8, 3. [Google Scholar] [CrossRef]
- Souza, R.S.; Chaves, L.H.G.; Fernandes, J.D. Isotermas de Langmuir e de Freundlich Na Descrição Da Adsorção de Zinco Em Solos Do Estado Da Paraíba. Rev. Bras. Ciênc. Agrár. 2007, 2, 123–127. [Google Scholar] [CrossRef][Green Version]
- Lim, W.-R.; Kim, S.W.; Lee, C.-H.; Choi, E.-K.; Oh, M.H.; Seo, S.N.; Park, H.-J.; Hamm, S.-Y. Performance of Composite Mineral Adsorbents for Removing Cu, Cd, and Pb Ions from Polluted Water. Sci. Rep. 2019, 9, 13598. [Google Scholar] [CrossRef] [PubMed]
- Carro de Diego, L.M. Eliminación de Mercurio de Efluentes Acuosos con Materiales de Bajo Coste: Proceso Combinado de Bioadsorción-Reducción. Doctoral Thesis, Universidade da Coruña, A Coruña, Spain, 2012. [Google Scholar]
- Patel, H. Review on Solvent Desorption Study from Exhausted Adsorbent. J. Saudi Chem. Soc. 2021, 25, 101302. [Google Scholar] [CrossRef]
- Bassareh, H.; Karamzadeh, M.; Movahedirad, S. Synthesis and Characterization of Cost-Effective and High-Efficiency Biochar for the Adsorption of Pb2+ from Wastewater. Sci. Rep. Nat. Publ. Group 2023, 13, 15608. [Google Scholar] [CrossRef] [PubMed]
- Chimdessa, M.A.; Ejeta, B.A. Removal of Cadmium, Copper and Lead from Aqueous Solution Using Activated Carbon Prepared from Avocado Kernel. Orient. J. Chem. 2022, 38, 65–71. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Said, G.F.; Ibrahim, G.A.A.; Elnashar, A.A.S. Effective Removal of Hexavalent Chromium from Water by Sustainable Nano-Scaled Waste Avocado Seeds: Adsorption Isotherm, Thermodynamics, Kinetics, and Error Function. Biomass Convers. Biorefinery 2024, 14, 14725–14743. [Google Scholar] [CrossRef]
- Aymacaña Albán, A.E. Caracterización Bromatológica de La Cáscara de Aguacate (Persea americana) y Posterior Extracción e Identificación de La Fracción Con Mayor Actividad Antimicrobiana y Antioxidante. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2018. [Google Scholar]
- Salmerón Ruiz, M.L. Fracción Indigestible, Bioaccesibilidad in Vitro y Actividad Antioxidante, de Compuestos Fenólicos de La Cáscara de Aguacate Cv. “Hass”. Master’s Thesis, Centro de Investigación en Alimentación y Desarrollo, Hermosillo, Mexico, 2014. [Google Scholar]
- Zaldivar-Ortega, A.K.; Barrera-Jiménez, J.A.; Cenobio-Galindo, A.D.J.; Pérez-Soto, E.; Franco-Fernández, M.J.; Campos-Montiel, R.G. Potencial Uso de La Cáscara y Semilla de Aguacate Como Fuente de Compuestos Bioactivos Con Actividades Funcionales Para Un Desarrollo Sustentable. Bol. Cienc. Agropecu. ICAP 2023, 9, 30–33. [Google Scholar] [CrossRef]
- Sánchez-Quezada, V.; Loarca-Piña, G. Caracterización química, fisicoquímica y nutracéutica de la semilla del aguacate (Persea americana Mill) para el uso en la industria. Sánchez-Quezada Loarca-Piña 2022, 7, 297–303. [Google Scholar]
- Zamora Velazco, G.N. Obtención de Carbón Activado a Partir de Semillas de dos Palmeras de la Amazonía Peruana “Shapaja” (Attalea phalerata) y “Aguaje” (Mauritia flexuosa). Bachelor’s Thesis, Universidad Nacional Agraria la Molina, Lima, Peru, 2010. [Google Scholar]
- Ordóñez Ochoa, A.E. Determinación de la Capacidad Adsorbente de los Residuos de la Industria de la papa (Solanum tuberosum) para Remoción de Metales Pesados en Aguas Contaminadas. Bachelor’s Thesis, Universidad Politécnica Saleciana, Cuenca, Ecuador, 2017. [Google Scholar]
- Zhong, L.; Peng, X.; Yang, D.; Sun, R. Adsorption of Heavy Metals by a Porous Bioadsorbent from Lignocellulosic Biomass Reconstructed in an Ionic Liquid. J. Agric. Food Chem. 2012, 60, 5621–5628. [Google Scholar] [CrossRef] [PubMed]
- Mallampati, R.; Xuanjun, L.; Adin, A.; Valiyaveettil, S. Fruit Peels as Efficient Renewable Adsorbents for Removal of Dissolved Heavy Metals and Dyes from Water. ACS Sustain. Chem. Eng. 2015, 3, 1117–1124. [Google Scholar] [CrossRef]
- Mqehe-Nedzivhe, K.C.; Makhado, K.; Olorundare, O.F.; Arotiba, O.A.; Makhatha, E.; Nomngongo, P.N.; Mabuba, N. Bio-Adsorbents for the Removal of Heavy Metals from Water. In Arsenic—Analytical and Toxicological Studies; Stoytcheva, M., Zlatev, R., Eds.; InTech: London, UK, 2018; ISBN 978-1-78923-516-6. [Google Scholar]
- Cortez, P. Análisis de Los Espectros de Infrarrojo; Instituto de Investigaciones en Guadalajara: Guadalajara, Mexico, 2020. [Google Scholar]
- Aranda-García, E.; Cristiani-Urbina, E. Effect of PH on Hexavalent and Total Chromium Removal from Aqueous Solutions by Avocado Shell Using Batch and Continuous Systems. Environ. Sci. Pollut. Res. 2019, 26, 3157–3173. [Google Scholar] [CrossRef]
- Vazquez-Palma, D.E.; Netzahuatl-Munoz, A.R.; Pineda-Camacho, G.; Cristiani-Urbina, E. Biosorptive Removal of Nickel(II) Ions from Aqueous Solutions by Hass Avocado (Persea americana Mill. Var. Hass) Shell as an Effective and Low-Cost Biosorbent. Fresenius Environ. Bull. 2017, 26, 3501–3513. [Google Scholar]
- Devi, R.; Singh, V.; Kumar, A. COD and BOD Reduction from Coffee Processing Wastewater Using Avacado Peel Carbon. Bioresour. Technol. 2008, 99, 1853–1860. [Google Scholar] [CrossRef]
- Said, M.I.; Abustam, E.; Wahab, A.W.; Taba, P.; Gani, A.; Wahid, A.M. Effect of Ethanol Used in a Degreasing Process on Bali Cattle Bones on the Physicochemical Properties of Extracted Collagen. Bulg. J. Agric. Sci. 2019, 25, 418–423. [Google Scholar]
- Olasehinde, E.F.; Adegunloye, A.V.; Adebayo, M.A.; Oshodi, A.A. Sequestration of Aqueous Lead(II) Using Modified and Unmodified Red Onion Skin. Anal. Lett. 2018, 51, 2710–2732. [Google Scholar] [CrossRef]
- Boeykens, S.P.; Saralegui, A.; Caracciolo, N.; Piol, M.N. Agroindustrial Waste for Lead and Chromium Biosorption. J. Sustain. Dev. Energy Water Environ. Syst. 2018, 6, 341–350. [Google Scholar] [CrossRef]
- Muluh, N.S. Central Composite Design Analysis and Optimization of Cadmium Adsorption from Synthetic Wastewater by Avocado Seed Activated Carbon. Int. J. Adv. Res. Dev. 2017, 2, 652–661. [Google Scholar]
- Fernández Villalón, L.M.; Calzado Lamela, O.; Adrian, D.; Carmenaty, C. Factores de mayor influencia en la adsorción de metales pesados por biomasa seca de Kluyveromyces Marxianus CCEBI 201. Tecnol. Quím. 2018, 38, 335–345. [Google Scholar]
- Abbas, S.H.; Ismail, I.M.; Mostafa, T.M.; Sulaymon, A.H. Biosorption of Heavy Metals: A Review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Astudillo, S.; Vera, L. Evaluación del poder biosorbente de la hoja de maíz en la remoción de metales pesados. Afinidad 2019, 77, 182–188. [Google Scholar]
- Romero Cano, L.A. Preparación y uso de Cáscaras de Naranja Como Biosorbente para la Remoción de Compuestos Orgánicos. Bachelor’s Thesis, CIDETEQ, Santiago de Querétaro, Mexico, 2013. [Google Scholar]
- Jung, M.-W.; Ahn, K.-H.; Lee, Y.; Kim, K.-P.; Rhee, J.-S.; Tae Park, J.; Paeng, K.-J. Adsorption Characteristics of Phenol and Chlorophenols on Granular Activated Carbons (GAC). Microchem. J. 2001, 70, 123–131. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Desorption of Heavy Metals from Metal Loaded Sorbents and E-Wastes: A Review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Namasivayam, C. Activated Carbon from Coconut Coirpith as Metal Adsorbent: Adsorption of Cd(II) from Aqueous Solution. Adv. Environ. Res. 2003, 7, 471–478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).