Mineral Powder Extraction by the Natural Drying of Water from the Public Springs in Borsec
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sample Collection and Storage
2.2. Investigation Methods
3. Results
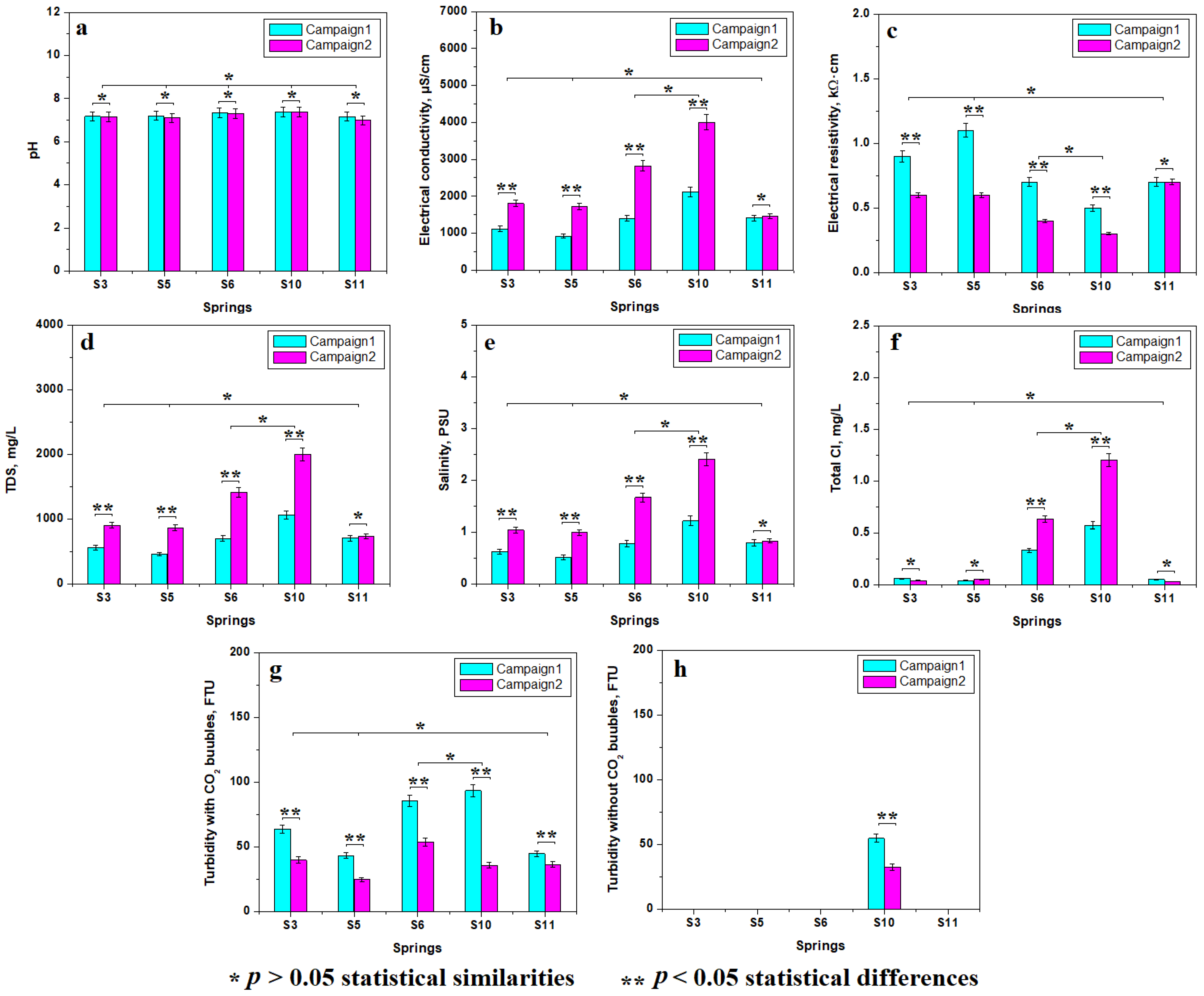
3.1. Water Sample Properties
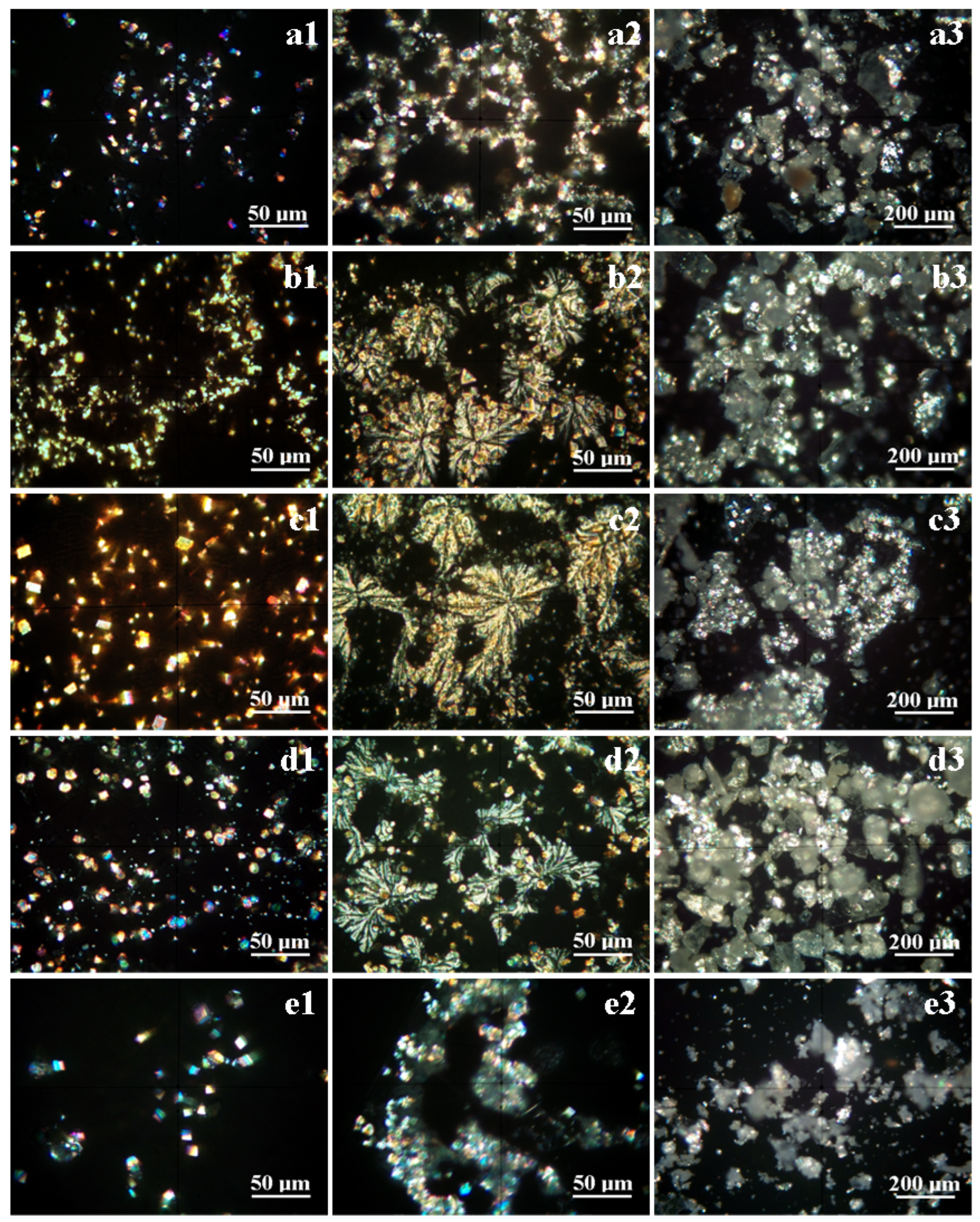
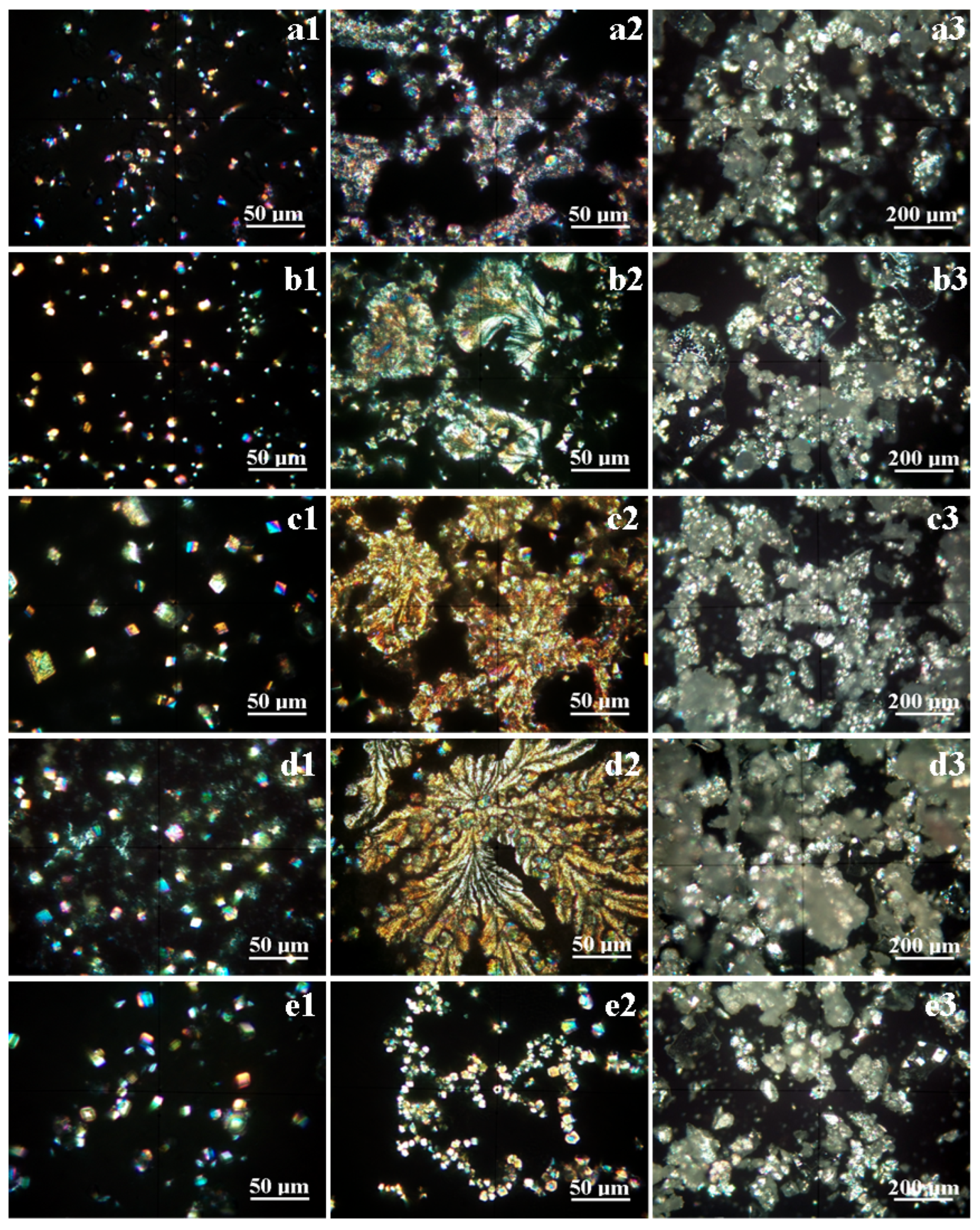
3.2. Mineral Crystallization Assessment
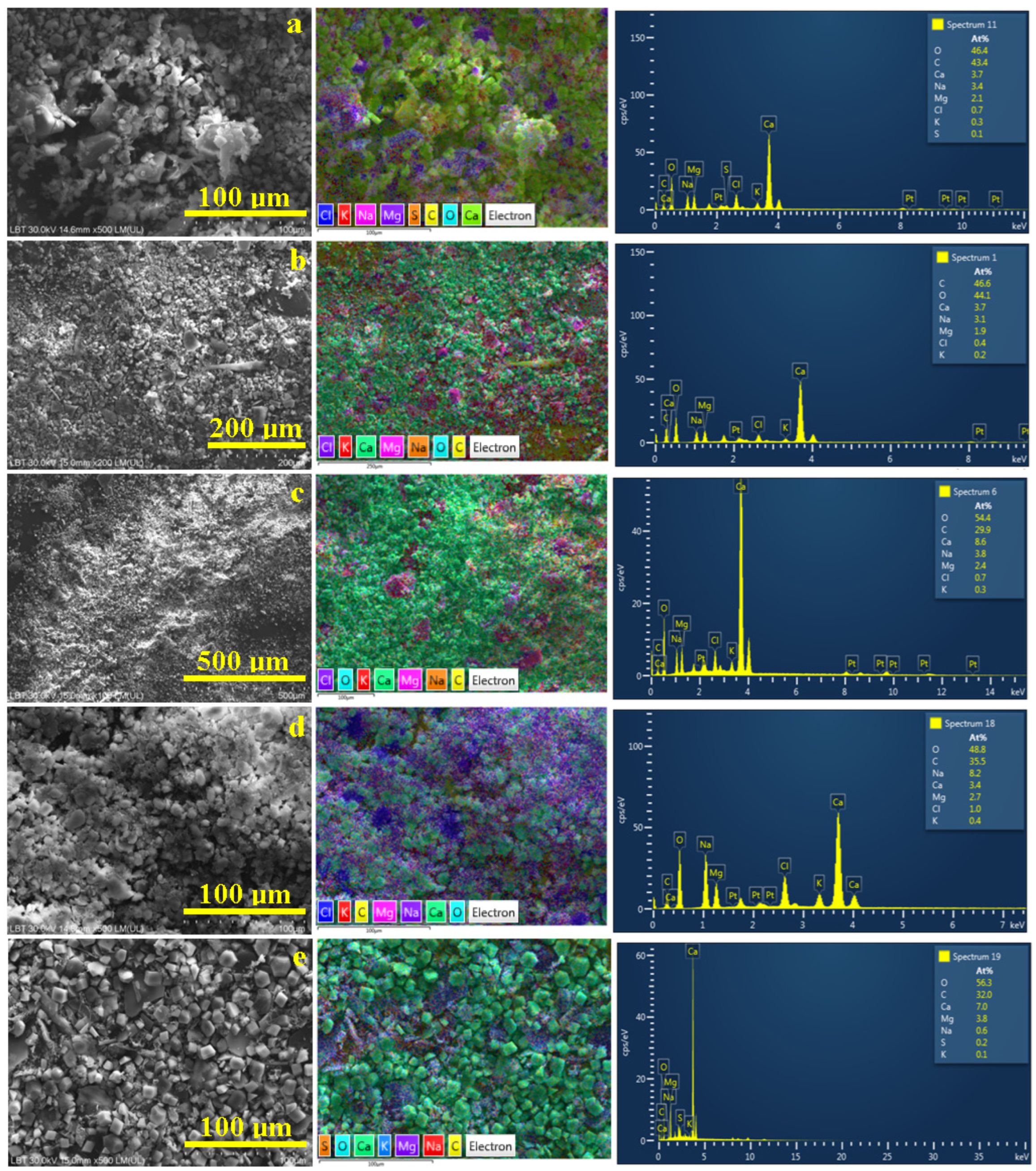
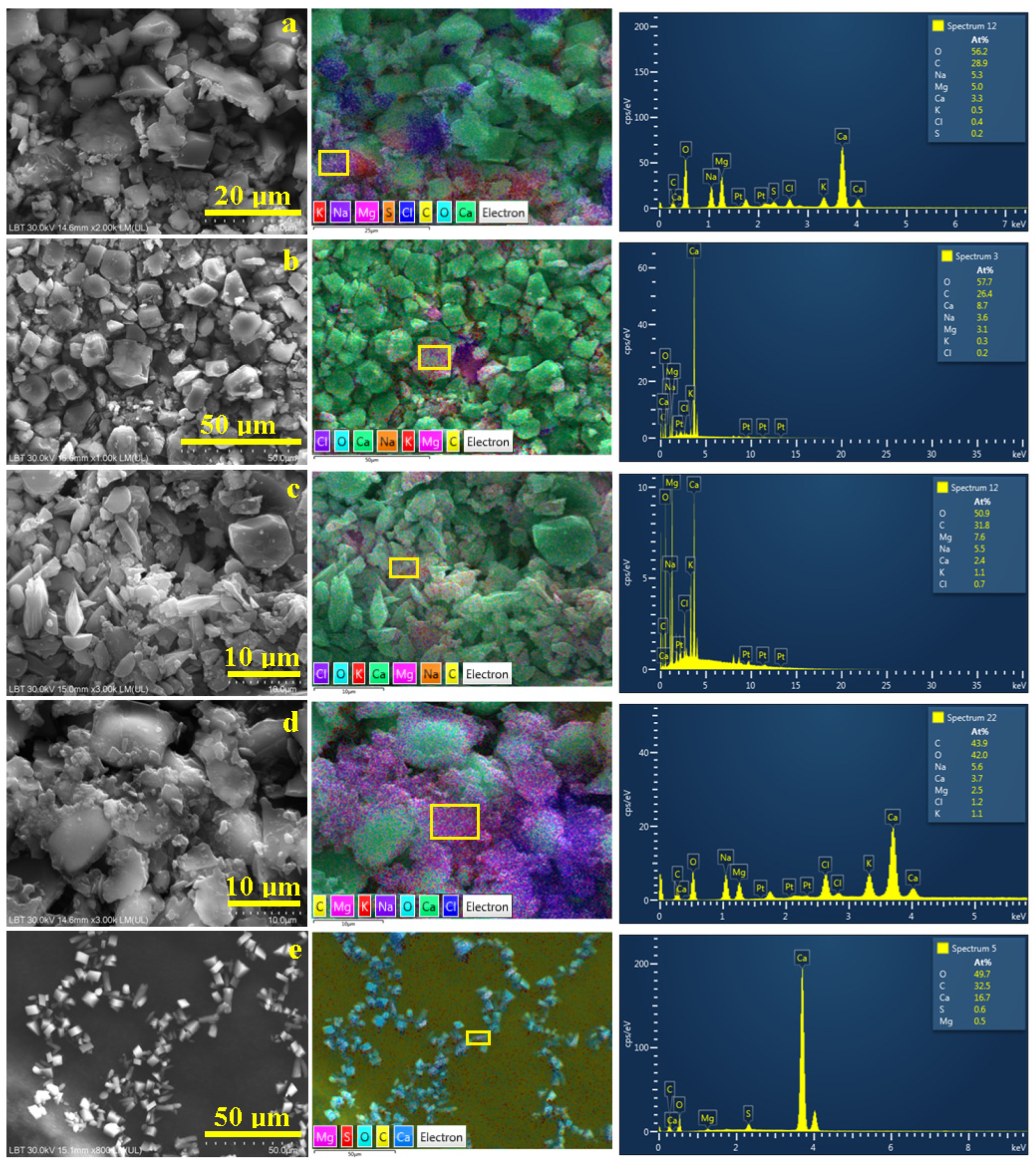
3.3. Microstructural Observation and Elemental Spectroscopy
4. Discussion
- -
- Spring 3 for the following: hyperacid gastritis, chronic colitis, chronic renal afflictions, biliary lithiasis, post hepatic sequels;
- -
- Spring 5 for the following: hypertension in stages I and II, coronary afflictions, hypo-acid gastritis, afflictions of the colon, and renal afflictions;
- -
- Spring 6: hypo- and normal acid chronic colitis, and enterocolitis;
- -
- Spring 10: thyroid afflictions, enterocolitis insufficiency, diabetes, gastrointestinal inarticulateness;
- -
- Spring 11: chronic gastrointestinal diseases, hypo- or normal acid gastritis, dironic post hepatitis (jaundice), bilious complaints, nutrition disorders, external applications.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iosipescu, R.; Iosipescu, S. Historical documentary regarding Borsec. Rev. Monum. Istorice 2010, 79, 90–97. [Google Scholar]
- Fielitz, W.; Seghedi, I. Late Miocene–Quaternary volcanism, tectonics and drainage system evolution in the East Carpathians, Romania. Tectonophysics 2005, 410, 111–136. [Google Scholar] [CrossRef]
- Bodor, K.; Bodor, Z.; Szép, A.; Szép, R. Classification and hierarchical cluster analysis of principal Romanian bottled mineral waters. J. Food Compos. Anal. 2021, 100, 103903. [Google Scholar] [CrossRef]
- Pricajan, A. Mineral and Thermal Water from Romania; Technical Publishing House: Bucharest, Romania, 1972; 296p. [Google Scholar]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Mahmud, M.; Abdi, C.; Rosadi, R.; Yanto, D.H.Y.; Bilad, M.R. Combination of Coagulation, Adsorption, and Ultrafiltration Processes for Organic Matter Removal from Peat Water. Sustainability 2022, 14, 370. [Google Scholar] [CrossRef]
- Manasypov, R.M.; Lim, A.G.; Krickov, I.V.; Raudina, T.V.; Kurashev, D.G.; Shirokova, L.S.; Pokrovsky, O.S. Colloids in Thermokarst Lakes along a Permafrost and Climate Gradient of Permafrost Peatlands in Western Siberia Using In Situ Dialysis Procedure. Water 2023, 15, 1783. [Google Scholar] [CrossRef]
- Ledésert, B.A. Application of Minerals for the Characterization of Geothermal Reservoirs and Cap Rock in Intracontinental Extensional Basins and Volcanic Islands in the Context of Subduction. Minerals 2024, 14, 263. [Google Scholar] [CrossRef]
- Yang, M.; Tan, L.; Batchelor-McAuley, C.; Compton, R.G. The solubility product controls the rate of calcite dissolution in pure water and seawater. Chem. Sci. 2024, 15, 2464–2472. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Y.; Wang, G.; Li, T.; Liu, F.; Wei, S.; Yan, X.; Gan, H.; Zhang, W. Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water. Water 2022, 14, 4041. [Google Scholar] [CrossRef]
- Lange, T.P.; Palcsu, L.; Szakács, A.; Kővágó, A.; Gelencsér, O.; Gál, A.; Gyila, S.; Tóth, T.M.; Mațenco, L.; Krézsek, C.; et al. The link between lithospheric scale deformations and deep fluid emanations: Inferences from the Southeastern Carpathians, Romania. Evol. Earth 2023, 1, 100013. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Touret, J.L.R. CO2, carbonate-rich melts, and brines in the mantle. Geosci. Front. 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Marieni, C.; Voigt, M.; Clark, D.E.; Gíslason, S.R.; Oelkers, E.H. Mineralization potential of water-dissolved CO2 and H2S injected into basalts as function of temperature: Freshwater versus Seawater. Int. J. Greenh. Gas Control 2011, 109, 103357. [Google Scholar] [CrossRef]
- Borsec Public Springs Composition Declaration Displayed on the Entrance Front. National Mineral Water Company of Romania. Available online: https://www.snam.ro/ (accessed on 21 February 2024).
- Metzger, M.; Konrad, A.; Blendinger, F.; Modler, A.; Meixner, A.J.; Bucher, V.; Brecht, M. Low-Cost GRIN-Lens-Based Nephelometric Turbidity Sensing in the Range of 0.1–1000 NTU. Sensors 2018, 18, 1115. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.; Ahmad, A.; Sendra, S.; Lloret, J.; Lorenz, P. Combination of Machine Learning and RGB Sensors to Quantify and Classify Water Turbidity. Chemosensors 2024, 12, 34. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Physicochemical Parameters and Terroir Assessment of Mineral Water from Mount Smolikas in Greece: A Two-Year Study. Analytica 2023, 4, 280–299. [Google Scholar] [CrossRef]
- Brika, B.; Dévora-Isiordia, G.E.; Alturki, E. Chemical Composition of Selected Brands of Bottled Water Commercilaized in Tripoli, Libya. Environ. Sci. Proc. 2022, 21, 48. [Google Scholar] [CrossRef]
- Avram, S.E.; Birle, B.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water 2024, 16, 1027. [Google Scholar] [CrossRef]
- Petean, I.; Paltinean, G.A.; Taut, A.C.; Avram, S.E.; Pripon, E.; Barbu Tudoran, L.; Borodi, G. Ag and Sn Implications in 3-Polker Coins Forgeries Evidenced by Nondestructive Methods. Materials 2023, 16, 5809. [Google Scholar] [CrossRef]
- Vazquez, P.; Lux, J. Salt Crystallization on Crazannes Limestone in a Long-Term Storage Environment. Minerals 2023, 13, 1282. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Zelek-Pogudz, S.; Solecka, U.; Solecki, M.L.; Szostak, E.; Zborowski, K.K.; Zając, M. Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland. Minerals 2022, 12, 1561. [Google Scholar] [CrossRef]
- Petean, I.; Arghir, G.; Câmpean, R.F.; Bărăian, M.; Hosu Prack, A.G. Crystalographyc relations applied to homogeneous cristalization of badenian salt. Acta Tech. Napoc. Ser. Appl. Mech. Math. 2011, 54, 193–200. [Google Scholar]
- Nie, X.; Wang, Z.; Wan, J.; Wang, G.; Li, Y.; Ouyang, S. Competition between homogeneous and heterogeneous crystallization of CaCO3 during water softening. Water Res. 2024, 250, 121061. [Google Scholar] [CrossRef] [PubMed]
- Bucca, M.; Dietzel, M.; Tang, J.; Leis, A.; Köhler, S.J. Nucleation and crystallization of otavite, witherite, calcite, strontianite, hydrozincite, and hydrocerussite by CO2 membrane diffusion technique. Chem. Geol. 2009, 266, 143–156. [Google Scholar] [CrossRef]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, H.; Wang, X.; Ning, M.; Wang, X.; Ge, Y.; Wang, H.; Tang, R.; Hou, M. Thermodynamic and Kinetic Studies of Dolomite Formation: A Review. Minerals 2023, 13, 1479. [Google Scholar] [CrossRef]
- Land, L.S. Failure to precipitate dolomite at 25 °C from dilute solution despite 1000-fold oversaturation after 32 years. Aquat. Geochem. 1998, 4, 361–368. [Google Scholar] [CrossRef]
- Pimentel, C.; Pina, C.M. Reaction pathways towards the formation of dolomite-analogues at ambient conditions. Geochim. Cosmochim. Acta 2016, 178, 259–267. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Wada, Y.; Hiaki, T.; Onoe, K.; Matsumoto, M. Effects of CO2 fine bubble injection on reactive crystallization of dolomite from concentrated brine. J. Cryst. Growth 2017, 469, 36–41. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Ibañez-Velasco, A.; Ruiz-Agudo, C.; Bonilla-Correa, S.; Elert, K.; Rodríguez-Navarro, C. Damage of porous building stone by sodium carbonate crystallization and the effect of crystallization modifiers. Constr. Build. Mater. 2024, 411, 134591. [Google Scholar] [CrossRef]
- Ali, A.; Mendes, C.E.; de Melo, L.G.T.C.; Wang, J.; Santos, R.M. Production of Sodium Bicarbonate with Saline Brine and CO2 Co-Utilization: Comparing Modified Solvay Approaches. Crystals 2023, 13, 470. [Google Scholar] [CrossRef]
- Gómez-Peralta, J.I.; García-Peña, N.G.; Bokhimi, X. Crystal-Site-Based Artificial Neural Networks for Material Classification. Crystals 2021, 11, 1039. [Google Scholar] [CrossRef]
- Papp, D.C.; Niţoi, E. Isotopic composition and origin of mineral and geothermal waters from Tuşnad Băi Spa, Harghita Mountains, Romania. J. Geochem. Explor. 2006, 89, 314–317. [Google Scholar] [CrossRef]
- Carreira, P.M.; Marques, J.M.; Graça, R.C.; Aires-Barros, L. Radiocarbon application in dating “complex” hot and cold CO2-rich mineral water systems: A review of case studies ascribed to the northern Portugal. Appl. Geochem. 2008, 23, 2817–2828. [Google Scholar] [CrossRef]
- Segura, D.; Cerepi, A.; Loisy, C. Aquifer-CO2 leak project. Effect of CO2-rich water percolation in porous limestone cores: Simulation of a leakage in a shallow carbonate freshwater aquifer. Chem. Geol. 2024, 657, 122105. [Google Scholar] [CrossRef]
- Linc, R.; Pantea, E.; Șerban, E.; Ciurba, A.-P.; Serban, G. Hydrochemical and Microbiological Investigations and the Therapeutic Potential of Some Mineral Waters from Bihor County, Romania. Sustainability 2023, 15, 15640. [Google Scholar] [CrossRef]
- Wangchuk, P.; Yeshi, K.; Ugyen, K.; Dorji, J.; Wangdi, K.; Samten; Tshering, P.; Nugraha, A.S. Water-Based Therapies of Bhutan: Current Practices and the Recorded Clinical Evidence of Balneotherapy. Water 2021, 13, 9. [Google Scholar] [CrossRef]
- Dickson-Gomez, J.; Nyabigambo, A.; Rudd, A.; Ssentongo, J.; Kiconco, A.; Mayega, R.W. Water, Sanitation, and Hygiene Challenges in Informal Settlements in Kampala, Uganda: A Qualitative Study. Int. J. Environ. Res. Public Health 2023, 20, 6181. [Google Scholar] [CrossRef]
- Jovicic, N.; Andjic, M.; Novakovic, J.; Jeremic, N.; Zivkovic, V.; Srejovic, I.; Stanojevic, D.; Ristic, P.; Bolevich, S.; Jakovljevic, V. The effects of low mineral content water on microbiota, metabolic, and oxidative stress parameters in patients with type 2 diabetes mellitus. Heliyon 2023, 9, e18725. [Google Scholar] [CrossRef]
- Injeyan, M.; Bidault, V.; Bacchetta, J.; Bertholet-Thomas, A. Hydration and Nephrolithiasis in Pediatric Populations: Specificities and Current Recommendations. Nutrients 2023, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Calvó, P.; Costa-Bauza, A.; Grases, F. Effect of Phytate (InsP6) and Other Inositol-Phosphates (InsP5, InsP4, InsP3, InsP2) on Crystallization of Calcium Oxalate, Brushite, and Hydroxyapatite. Biomolecules 2023, 13, 1061. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M.; Martín-Rubí, J.A.; Pozo, E.; Maraver, F. Mobility of elements in interaction between artificial sweat and peloids used in Spanish spas. Appl. Clay Sci. 2010, 48, 506–515. [Google Scholar] [CrossRef]
- Karakaya, M.C.; Karakaya, N.; Sarıoğlan, S.; Koral, M. Some properties of thermal muds of some spas in Turkey. Appl. Clay Sci. 2010, 48, 531–537. [Google Scholar] [CrossRef]
- Chelnokov, G.; Kharitonova, N.; Bragin, I.; Chudaev, O. Geochemistry of mineral water and gases of the Razdolnoe Spa (Primorye, Far East of Russia). Appl. Geochem. 2015, 59, 147–154. [Google Scholar] [CrossRef]


| Spring | GPS Coordinates | Relative Distance, m | Specific Information |
|---|---|---|---|
| Spring 3 | 46°97′38.5″ N 25°56′29.7″ E | S3–S5 17.59 | Actual name: Boldizsár; former names: Madonna, Horia Placement: nearby 7 Spring Avenue |
| Water type: mineral sparkling carbonated Daily flow Q = 6600 L/day; water temperature 8.2–10 °C | |||
| Spring 5 | 46°97′37.5″ N 25°56′26.1″ E | S5–S6 43.19 | Actual name: László; former name: Closca Placement: on 7 Spring Avenue close to Spring 3 |
| Water type: mineral sparkling carbonated Daily flow Q = 5100 L/day; water temperature 8.2–10 °C | |||
| Spring 6 | 46°97′33.1″ N 25°56′27.8″ E | S6–S3 44.98 | Actual name Lazar; former name: Crisan Placement: on 7 Spring Avenue close to Spring 3 |
| Water type: mineral sparkling carbonated Daily flow below 500 L/day; water temperature 8.2–10 °C | |||
| Spring 10 | 46°58′25.4″ N 25°33′45.3″ E | S3–S10 334.47 | Actual name: Kossuth Placement: 3 Stadionului Street; at an altitude of 909 m (40 m higher than the other springs) |
| Water type: mineral sparkling carbonated Daily flow below 500 L/day; water temperature 8.2–10 °C | |||
| Spring 11 | 46°97′11.8″ N 25°56′75.5″ E | S10–S11 116.61 | Actual name: Petofi Placement: fairy meadow nearby Spring 10 |
| Water type: mineral sparkling carbonated Daily flow Q = 900 L/day; water temperature 8.2–10 °C |
| Parameter | Measuring Unit | Spring 3 | Spring 5 | Spring 6 | Spring 10 | Spring 11 |
|---|---|---|---|---|---|---|
| pH | pH | 6.25 | 6.25 | 6.43 | 6.53 | 6.19 |
| CO32− | mg/L | 1470 | 1540 | 2430 | 3660 | 1555.5 |
| CO2 free | mg/L | 1310 | 1230 | 1450 | 2200 | 1540 |
| Ca2+ | mg/L | 250 | 267 | 465 | 576 | 266.4 |
| Fe2+ | mg/L | 2.84 | 1.71 | 0.194 | 2.09 | 2.57 |
| Mg2+ | mg/L | 78.4 | 90.4 | 163 | 245 | 127.14 |
| K+ | mg/L | 17.3 | 17.2 | 29 | 41.2 | 7.82 |
| Na+ | mg/L | 64.4 | 68.2 | 111 | 211 | 41.12 |
| Si4+ | mg/L | 18.8 | 18.7 | 28.5 | 41.5 | nd |
| Cl− | mg/L | nd | nd | nd | nd | 12.9 |
| SO42− | mg/L | nd | nd | nd | nd | 16.8 |
| NH4 | mg/L | nd | nd | nd | nd | nd |
| Mn2+ | mg/L | nd | nd | nd | nd | 0.4 |
| Characteristics | Mineral Composition | |||||
|---|---|---|---|---|---|---|
| Aragonite | Calcite | Pseudo-Dolomite | Halite | Natron | ||
| Chemical formula | CaCO3 | CaCO3 | CaMg(CO3)2 | NaCl | Na2CO3·10H2O | |
| Crystal system | Orthorhombic | Trigonal | Trigonal | Cubic | Monoclinic | |
| Habit | rhombic granular | rhombohedral pseudo-hexagonal | columnar granular | rectangular plates | prismatic columnar | |
| POM colors | violet–blue | yellow white –brown | yellow white –brown | transparent pale blue–gray | yellow– yellow gray | |
| Campaign 1 | ||||||
| Spring 3 | Amount, wt.% | 11 | 65 | 13 | 6 | 5 |
| Size range, μm | 3–5 | 5–8 | 5–10 | * | 5–12 | |
| Spring 5 | Amount, wt.% | 9 | 68 | 17 | 3 | 3 |
| Size range, μm | 2.5–5 | 3–8 | 5–10 | * | 5–15 | |
| Spring 6 | Amount, wt.% | 19 | 48 | 20 | 8 | 5 |
| Size range, μm | 3–10 | 5–15 | 5–18 | * | 5–20 | |
| Spring 10 | Amount, wt.% | 15 | 53 | 21 | 7 | 4 |
| Size range, μm | 2–15 | 5–12 | 5 -18 | * | 5–10 | |
| Spring 11 | Amount, wt.% | 7 | 78 | 9 | 3 | 3 |
| Size range, μm | 5–10 | 5–10 | 5–12 | * | 5–10 | |
| Campaign 2 | ||||||
| Spring 3 | Amount, wt.% | 13 | 57 | 17 | 8 | 5 |
| Size range, μm | 2.5–5 | 3–5 | 3–6 | * | 5–10 | |
| Spring 5 | Amount, wt.% | 10 | 62 | 15 | 8 | 5 |
| Size range, μm | 3–10 | 3–12 | 5–15 | * | 5–10 | |
| Spring 6 | Amount, wt.% | 26 | 32 | 21 | 14 | 7 |
| Size range, μm | 5–10 | 5–25 | 5–20 | * | 5–15 | |
| Spring 10 | Amount, wt.% | 25 | 31 | 18 | 17 | 9 |
| Size range, μm | 3–12 | 5–15 | 5–18 | 1.5–2 | 3–10 | |
| Spring 11 | Amount, wt.% | 14 | 67 | 16 | 3 | below detection |
| Size range, μm | 5–13 | 5–15 | 5–15 | * | - | |
| Element, at. % | Spring 3 | Spring 5 | Spring 6 | Spring 10 | Spring 11 |
|---|---|---|---|---|---|
| O | 46.4 | 46.6 | 54.4 | 48.8 | 56.3 |
| C | 43.4 | 44.1 | 29.9 | 35.5 | 32.0 |
| Ca | 3.7 | 3.7 | 8.6 | 8.2 | 7.0 |
| Mg | 2.1 | 1.9 | 2.4 | 2.7 | 3.8 |
| Na | 3.4 | 3.1 | 3.8 | 3.4 | 0.6 |
| Cl | 0.7 | 0.4 | 0.7 | 1.0 | - |
| K | 0.3 | 0.2 | 0.3 | 0.4 | 0.1 |
| S | 0.1 | - | - | - | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, S.E.; Platon, D.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Mineral Powder Extraction by the Natural Drying of Water from the Public Springs in Borsec. Appl. Sci. 2024, 14, 10806. https://doi.org/10.3390/app142310806
Avram SE, Platon DV, Tudoran LB, Borodi G, Petean I. Mineral Powder Extraction by the Natural Drying of Water from the Public Springs in Borsec. Applied Sciences. 2024; 14(23):10806. https://doi.org/10.3390/app142310806
Chicago/Turabian StyleAvram, Simona Elena, Denisa Viviana Platon, Lucian Barbu Tudoran, Gheorghe Borodi, and Ioan Petean. 2024. "Mineral Powder Extraction by the Natural Drying of Water from the Public Springs in Borsec" Applied Sciences 14, no. 23: 10806. https://doi.org/10.3390/app142310806
APA StyleAvram, S. E., Platon, D. V., Tudoran, L. B., Borodi, G., & Petean, I. (2024). Mineral Powder Extraction by the Natural Drying of Water from the Public Springs in Borsec. Applied Sciences, 14(23), 10806. https://doi.org/10.3390/app142310806







