Abstract
Borsec is one of the most important mineral water spa resorts in Romania and is also an important mineral water bottling facility. There are several public springs with significant mineral content. The present paper focuses on mineral powder extraction by the drying of water samples collected from springs no. 3, 5, 6, 10, and 11. These springs have a continuous flow being available for everyone who wants to fill a bottle; meanwhile, the rest of the water is discarded into the river. Thus, the dissolved ions such as Ca2+, Mg2+, Na+, and Cl− are wasted. This study aims to investigate the possibility of mineral content extraction as crystalline powder by drying. The dissolved ions’ reaction with carbonic acid generates carbonates which crystallize progressively with the water evaporation. Mineralogical investigation including X-ray diffraction (XRD) and polarized light optical microscopy (POM) reveal that calcite (rhombohedral and pseudo-hexagonal crystals of about 5–25 µm) is the dominant mineral followed by pseudo-dolomite (columnar crystals of about 5–20 µm), aragonite (rhombic and granular crystals of 2.5–15 µm), and natron (prismatic crystals of about 5–20 µm), in addition to small amounts of halite. Scanning electron microscopy (SEM) investigation combined with energy dispersive (EDS) elemental analysis indicates that traces of K are uniformly distributed in the calcite mass and some S traces for springs 3 and 11 are distributed predominantly into the pseudo-dolomite crystals. The crystalline germs precipitate from the supersaturated solution via homogeneous germination and progressively grow. The latest stage is characterized by the formation of a dendritic crust of calcite mixed with halite that embeds the individually grown crystals. The amount of the formed crystals strongly depends on the water’s total dissolved solids (TDS) and salinity: the springs with high TDS and salinity form a large number of crystals and spectacular dendritic crusts such as spring 10 followed by springs 6 and 5. Lower mineralization was observed in springs 3 and 5, which was related with the S traces. Also, it is evident that mineralization is seasonally dependent: the mineral amount was lower in November 2023 than for the samples collected in March 2024. The obtained mineral powder might be used for spa baths or for the electrolytic balance regulation in dietary supplements due to the high calcium and magnesium content.
1. Introduction
Borsec is a small town situated in a depression of the same name located in the Oriental Carpathian Mountains (Romania) at the eastern side of Giurgeului Mountain at an altitude of 880 m. The depression is influenced by the tectonics that resulted in faults during the post Pliocene sediment displacement during the volcanic and seismic activity that developed after the Pliocene period [1,2]. The geological admixture of sedimentary material with volcanic material within the tectonic faults represents the mineralization sources of the natural springs in the area [2,3]. Some local geological drillings were carried out during the period from 1960 to 1970 revealing the complex stratigraphy of the Borsec depression involving two basic aquifers [4].
The first aquifer has a top layer formed by gray–black peat with clay intercalations with a depth up to 10 m. The following layer is situated between 10 to 20 m in depth. It is formed by a gray clay–sand admixture containing sericite chlorite mixed with crystalline shale based on quartz particles. Between 20 and 30 m in depth, gravel containing crystalline shale mixed with limestone is found, followed by gray clay intercalations from 30 to 45 m in depth. The next geological layer consists of massive deposits of gray–white dolomitic limestone down to 65 m in depth. The aquifer is situated at 65 m in depth sustained by the compact base of a dark dolomitic limestone layer ranging up to 80 m in depth.
The second aquifer has a top layer of peat having a depth of about 5 m followed by a thick layer (5–18 m in depth) containing chlorite green shale intercalation. The following layer is formed by green clay with sand intercalation structured in a dense mass situated up to 30 m in depth where a complex layer begins, consisting of talcose deposits admixed with dolomitic limestone, which merely becomes an intercalation of chlorite and talcose with graphitic intercalation down to 40 m in depth. The layer between 40 and 50 m in depth contains green and gray clay–limestone tuff with silica sand intercalation. The geological layer beneath 50 m in depth is formed by dolomitic limestone which becomes a compact structure in the depth range of 75–80 m sustaining the aquifer at 60 m in depth.
Rainwater infiltrates through all these geological layers interacting with the mineral matter. Literature data show that peat has ion-changing properties making it able to retain certain water impurities [5,6]. Clay layers also help the water purification and enrich its mineral content with several ions such as Mg and Fe from the chlorite structure [7]. The granular matter of the dolomite–crystalline shale admixtures allows for water interaction with calcite and dolomite mineral grains. Calcite has a water solubility of 0.013 g/L at 25 °C [8] and dolomite of 0.013 g/L [9], partly facilitating the dissolution of the rock formations into the infiltrated waters, raising its mineral content. Thus, the mineral composition of the water strongly depends on the water amount infiltrated through the geological layers and on the partial dissolving of the calcite and dolomite formations along with the ion-changing behavior of peat and chlorite. Each of the two aquifers will generate particularized water depending on the spring flow rate and the local mineral interactions. The mineralized water is collected into the deeper aquifer and is subjected to interactions with the volcanic characteristics of the deeper geological formations of compact dolomitic limestone. These are in close connection with the volcanic deposits of andesite and pyroclastic rocks. Literature data reveal that the geological formations within the Borsec area are affected by the volcanic activity regarding the Ciomadu Mare volcano, which induces significant carbonated and sulfurous fumaroles [10]. Thus, carbon dioxide emanations are propagated through the andesite and pyroclastite fissures which conduct them to the adjacent dolomitic formations, acting as an inert gas-lift to the mineralized aquifer where they react with the water molecules causing the formation of carbonic acid specific to the sparkling water [11,12]. The fumaroles’ gas-lifting of CO2 acts as a dynamic pressurizing of the aquifer, which is determined to burst out at the surface forming natural sparkling mineral water springs. These valuable sparkling mineral water springs were noticed from the XVIII century, being mentioned in the Transylvanian Principate mineralogical treaty elaborated by Jan Fridwaldszky in 1767 published in Cluj-Napoca (e.g., Claudiopolis), as was mentioned by Iosipescu [1]. The first commercial approach regarding the Borsec sparkling mineral water was developed by Anton Zimmethhausen in 1804 by a bottling facility using ceramic vials. The health benefit of the Borsec mineral water was rapidly observed and a curing house was built during the XVIII century and continuously developed up to today.
Despite the springs’ celebrity, there were not any studies conducted or any articles published regarding the mineral dissolution and re-crystallization of the Borsec waters. Therefore, the main aim of the present research is the evaluation of mineral extraction during the crystallization processes within the Borsec sparkling mineral water public springs (which did not belong to the bottling company) and their advanced characterization. The mineral amount’s dependence on seasonality requires investigation through two campaigns: one held during the late autumn related to low rainwater and another held in spring related to high rainwater levels. Thus, the water infiltration’s influence on the mineral amount can be considered. The determined physicochemical parameters of the collected water samples require correlation with the mineral formation during natural drying. The crystallized samples were characterized in a complex manner by using X-ray diffraction (XRD) coupled with polarized light microscopy (POM), and their morphological aspects were combined with elemental characterization which was effectuated by scanning electron microscopy (SEM) coupled with elemental analysis (EDX). It is expected that such complex investigations correlated with the water properties will evidence significant aspects regarding the mineral powder extraction and its potential applications. We chose five springs having the greatest water flow among the public springs in Borsec; the other ones have very low flow and are not set up for public access.
2. Materials and Methods
2.1. Water Sample Collection and Storage
Borsec is an important spa resort in Romania with a tradition regarding the mineral water curing of many health afflictions. There were over 15 sparkling mineral springs discovered that have a steady mineral composition and seasonally dependent flow. Five of these springs are owned by the mineral water bottling company, and the other springs are situated in the public domain. As previously mentioned, the main goal of this research is to study the mineral crystallization from the waters of the most representative public springs for the local population as well as for the tourists. Therefore, we chose the following springs: 3, 5, 6, 10, and 11 for the investigation. Their position on the map is displayed in Figure 1 and a description including the exact GPS position is presented in Table 1.
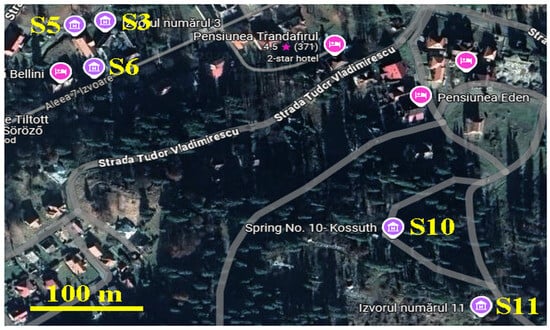
Figure 1.
The investigated springs’ position on the Borsec town map [Google Maps 2024].

Table 1.
The investigated springs’ general characteristics [4].
Springs 3, 5, and 6 are situated in the center of Borsec town and springs 10 and 11 are situated on the eastern side. All considered springs are arranged by wooden buildings with stone bases. The water flows continuously through a metallic grate.
All considered springs have sparkling carbonated mineral water, but they are different due to several particular ions dissolved from the aquifer rocks. Therefore, Table 2 centralizes their chemical compositions according to the public declarations made by the National Mineral Water Company of Romania [13] and displayed on each spring entrance.

Table 2.
Spring mineral composition according to the public declarations [13].
The sample collection schedule was considered for two campaigns: the first one being effectuated during November 2023, which is a representative month for the dry period of the year; and the second campaign was effectuated in March 2024 as a representative period for the wet season due to the abundant precipitation, both rain and snow layer melting.
The water samples were collected in glass vials of 0.5 L with metallic caps. Each vial was properly labeled with the spring number and collection date. Each vial was rinsed with the spring water to remove any eventual impurity and subsequently filled with the water sample and sealed with the metallic cap. They were transported in a cooled bag at 4 °C into the laboratory where they were stored in the refrigerator at 4 °C until their investigation (maximum after 24 h). Before the investigations, the vials were gently brought to room temperature.
2.2. Investigation Methods
The water samples’ physicochemical properties were measured with a HANNA HI 9829 multi-parameter measuring device (Hanna Instruments Co., Leighton Buzzard, UK). We measured the following properties: pH; electrical conductivity and resistivity; total dissolved solids (TDS); salinity; and total chloride ions. The turbidity was measured with a Hanna 93703 turbidimeter having an accuracy range of 0–1000 FTU (NTU) at a resolution of ±5% F.S. The chloride amount was measured with a photo-colorimeter HI 96711 with a measuring range of 0.00 to 2.5 mg/L for the free Cl and 0.00–3.5 mg/L for the total Cl at a resolution of 0.01 mg/L. At least three measurements were performed for each investigated parameter and the mean values were further discussed. The mean values were analyzed with Origin 6.0 software produced by Microcal Co. (Amherst, MA, USA) and plotted in graphic form. The statistical analysis was effectuated using the ANOVA test at a significance level of p = 0.05 followed by Tukey’s post-hoc test.
The polarized light optical microscopy (POM) was performed under polarized light with crossed Nichols with a Laboval 2 Microscope (Carl Zeiss, Oberkochen, Germany) equipped with a digital image-acquiring system with a resolution of 10 mega pixels produced by Samsung Co. (Hangul, Republic of Korea). Further analysis required crystalline powder material. Therefore, the water samples were placed in Petri glasses and subjected to natural drying under ambient conditions. The crystallized crust deposits were individually scratched with a spatula and the resulting powder was placed into glass vials and further subjected to specific analyses.
The X-ray diffraction (XRD) was effectuated on the crystalline powder samples with the Bragg-Brentano diffractometer Bruker D8 Advance produced by Bruker Company (Karlsruhe, Germany) using Cu kα monochromatic radiation having a wavelength of 1.540562 Å. The patterns were obtained at a speed of 1 °/min to ensure a proper development of the peaks including for the small contents. The peak identification was effectuated using the specialized software Match 1.0 produced by Crystal Impact Company (Bonn, Germany) using the PDF 2.0 database: aragonite 71-2396, calcite 86-2339, halite 77-2064, natron 01-0938, pseudo-dolomite (e.g., magnesium calcite) 89-1305.
Scanning Electron Microscopy (SEM) was effectuated with a Hitachi SU8230 Scanning electron Microscope (SEM) (Hitachi Company, Tokyo, Japan) equipped with the Energy-Dispersive Spectroscopy (EDS) detector X-Max 1160 EDX (Oxford Instruments, Oxford, UK). The investigation was effectuated in the high vacuum mode at an acceleration voltage of 30 kV. The powder samples were mounted on the specimen holder using carbon double adhesive bullets and sputtered with a very thin platinum layer for ensuring proper electrical conductivity. The platinum elemental component was subtracted at the elemental analysis. The double adhesive carbon bullets might have a minor influence on the elemental composition measured over large areas and, therefore, this factor must be properly considered at the interpretation of the results.
3. Results
3.1. Water Sample Properties
Physicochemical properties of the water samples are very important for understanding the minerals’ germination and their subsequent growth forming well-developed crystals.
pH plays a key role regarding the water stability and the investigated samples have almost the same pH, as seen in Figure 2a. It is situated in a close range of 7.16 to 7.38 in the first campaign and 6.99 to 7.38 in the second campaign. Thus, the statistical analysis reveals that the pH values indicate a single representative group including all investigated samples. It clearly reveals that the measured pH is independent of the source aquifer and of the seasonality. It proves to be a constant characteristic of the Borsec mineral water established by the stable equilibrium between alkaline ions and the presence of carbonic acid induced by CO32− ions. This fact is of great importance for crystallization chemistry.
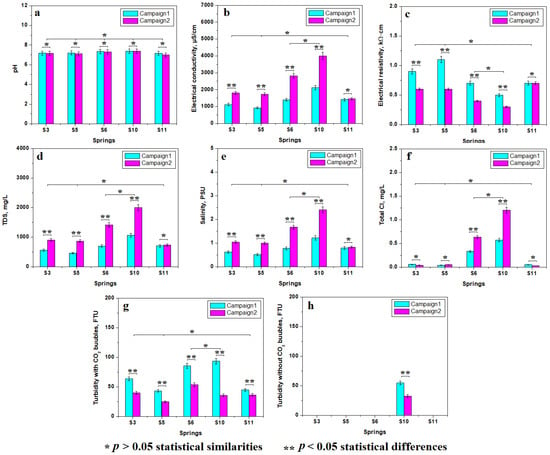
Figure 2.
Mean values of water sample properties: (a) pH, (b) electrical conductivity, (c) electrical resistivity, (d) total dissolved solids, (e) salinity, (f) total Cl, (g) turbidity with CO2 bubbles, and (h) turbidity without CO2 bubbles.
Electrical conductivity and resistivity are complementary properties of the water samples evidencing their ability to conduct electrical currents. Electrical conductivity is facilitated by the ions’ presence while the resistivity is negatively influenced. In other words, the high mineral content induces high electrical conductivity and low resistivity. Thus, Figure 2b,c reveal two statistically relevant groups: the first includes S3, S5, and S11 characterized by relatively low electrical conductivity and relatively high resistivity. On the other hand, the springs S6 and S10 have high conductivity and low resistivity. We also observed some significant statistical differences between the measuring campaigns. The electrical conductivity significantly increases in the second campaign compared to the first one, and the electrical resistivity decreases in the second campaign. This strongly indicates that the mineralization is stronger in the samples collected during March 2024 and relatively weaker in the samples collected during November 2023.
The electrical properties’ variation is explained by the total dissolved solids (TDS) and salinity of the samples. Both the TDS and the salinity are weaker in the samples collected in the first campaign and considerably increased for the second campaign, evidenced by the relevant statistical differences in Figure 2d,e. The statistical analysis reveals two relevant groups: the first is characterized by low TDS and salinity (S3, S5, and S11) and the second group has high values of TDS and salinity (S6 and S10). The samples’ salinity is strongly influenced by the total chloride, shown in Figure 2f, which is low in samples S3, S5, and S11 and high in the S6 and S10 samples.
Turbidity is an important physical parameter of water showing the dispersed particulate matter. It is based on the nephelometric measuring method [14,15]. The CO2 bubbles’ presence in the sparkling mineral water represents a physical problem due to their interference with the light scattering within the nephelometer system generating erroneously high values for the samples’ turbidity; this fact was previously reported in the literature [16,17]. The turbidity measured in our freshwater samples generates bigger values (especially for S6 and S10); see Figure 2g. The value range is situated between 24.62 and 93.27 NTU (Nephelometric Turbidity Units), being too high for drinking water. Therefore, it was necessary to let the water sample lose the gaseous CO2 prior to re-effectuating the turbidity measurements, as shown in Figure 2h. All samples have zero turbidity after the gas bubbles’ removal, except for spring 10 which has remnant turbidity. The values are significantly lower after the gas loss than in the initial samples but still are high. They are related to suspended micro-particles. These could be soil particles or small crystal precipitate due to the high mineral content. Their nature must be further elucidated.
3.2. Mineral Crystallization Assessment
The water property investigation reveals significant differences between the first and the second campaign regarding the mineral content. In consequence, the crystals’ development must be followed distinctly for each campaign. The XRD pattern results for the samples crystallized from the first campaign are presented in Figure 3a and from the second campaign in Figure 3b. Significant differences are observed: the diffraction peaks observed for the first campaign are relatively weaker than those observed for the second campaign, proving that there is higher mineralization in the water samples collected in March 2024 compared to those from November 2023.
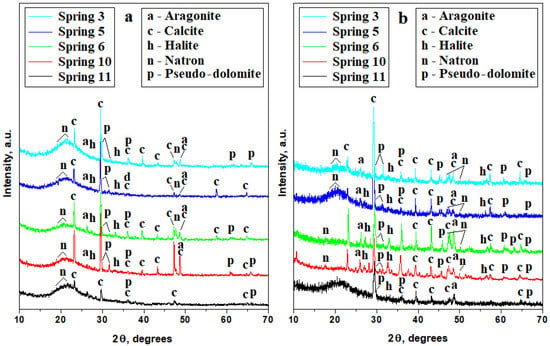
Figure 3.
XRD patterns of the crystals formed from the drying of the water samples collected in (a) the first campaign and (b) the second campaign.
Significant differences for each sample during the first campaign can be observed in Figure 3a. The XRD pattern for the S3 sample has very well-developed peaks for calcite, mainly for the (012) and (104) crystallographic planes, having strong intensities and narrow aspects; the other minerals have less intense peaks due to their particular crystal development and their number compared to the calcite grains. Thus, calcite is followed by pseudo-dolomite, aragonite, and natron. The halite peaks are very weak, being almost constrained to the XRD pattern baseline, but the presence of halite is sustained by the small amount of chloride measured in the water sample. The presence of small peaks indicates that chloride ions are combined with sodium ions generating crystalline halite.
The habit of the formed crystals will be discussed at the mineralogical optical microscopy (POM) investigation. The XRD patterns obtained for the S5 and S11 samples are very similar to those for the S3 sample; only minor variation in the relative intensities is observed. The weaker peaks are developed for the S11 sample due to the relatively low mineralization, in good agreement with its water properties.
Samples S6 and S11 present the most intense and narrow diffraction peaks for calcite, pseudo-dolomite, and aragonite sustaining the development of strong crystals precipitated from the water samples during the drying process. The pseudo-dolomite peak for the (104) crystallographic plane is very well developed in the S6 and S11 samples. The natron and halite peaks are less intense and slightly broadened. Thus, the dominant mineral in S6 and S11 is calcite accompanied by significant amounts of dolomite and aragonite indicating a prolific germination and crystallization from the supersaturated water solution under the drying process.
The weight percent of each mineral was determined by the RIR (Reference Intensity Ratio) method [18,19] and the values are centralized in Table 3 along with the POM results. The crystallization process was more intense in the samples collected in the second campaign, as observed in Figure 3b. Well-developed peaks with strong intensities and narrow aspects predominate. Calcite is the dominant mineral in all samples featuring very vigorous peaks for the (012) and (104) diffracting planes, but its amount strongly depends on the crystallization of the other minerals, mainly pseudo-dolomite and aragonite. The weight percentage of each mineral was determined by the RIR method and centralized in Table 3.

Table 3.
The XRD information correlated with POM observations.
The calcite amount in S3 and S5 certainly exceeds 50% of the composition while in sample S11, it overwhelms the other minerals. This indicates the strong calcium carbonaceous characteristics of these springs.
The pseudo-dolomite amount is rather moderate in the range of 17–18% indicating an average magnesium level during crystallization, but its diffraction peak for (104) is well developed. The halite peaks have low intensities and slightly broadened aspects associated with a low amount and marginal crystallization beside well-developed grains. Also, natron’s presence is moderate due to its relatively low peak intensities indicating the presence of tiny acicular–prismatic crystals. This fact agrees with the composition presented in Table 2 where the Na+ amount is lower for S3 and S5 but average for S11.
The increased levels of Mg2+ and Na+ reported for springs 6 and 10 facilitate the crystallization of pseudo-dolomite along with halite and natron. Therefore, samples S6 and S10 have calcite as the dominant mineral but it is situated in the range of 29–31%, while the amounts of the other minerals, mainly dolomite and aragonite, significantly increase. The pseudo-dolomite crystallization is well sustained by the rich magnesia mineralization of these two springs facilitating the strong development of the peaks for (104) and (202). This indicates a particular growth of the crystals on these crystallographic planes. The relatively increased level of Na facilitates the precipitation of natron and halite. Both XRD patterns feature a strong peak for (200) of halite. It corresponds to the development of large and thin square-based crystals with the height variable on the surface topography [20,21]. Natron crystals are sustained by less intense and relatively broad peaks corresponding to relatively thin crystals.
The formed crystals are investigated under cross-polarized light using the optical mineralogical microscope allowing for their correlation with the XRD data. The POM results for the samples from the first campaign are presented in Figure 4 and the results from the second campaign in Figure 5.
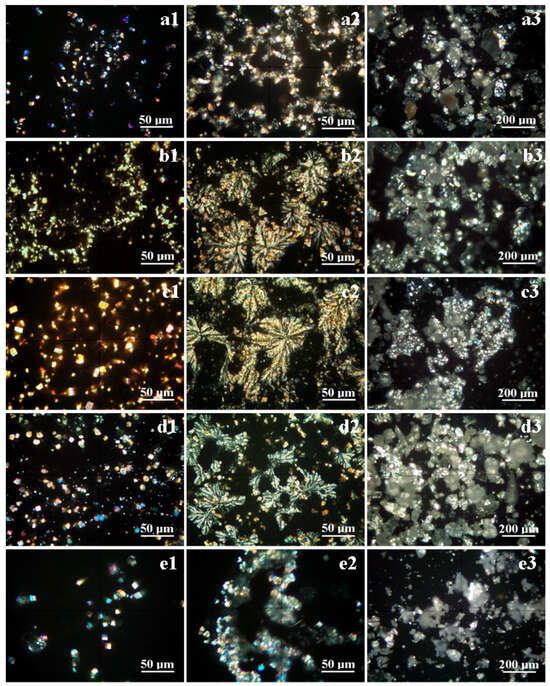
Figure 4.
POM images of the crystals’ development in the water samples collected in the first campaign: (a) Spring 3, (b) Spring 5, (c) Spring 6, (d) Spring 10, and (e) Spring 11; (1) individual grains crystallized from the water samples; (2) grains embedding in the final dendritic crystalline crust; and (3) milled crystalline crust powder which was subjected to the XRD investigation.

Figure 5.
POM images of the crystals’ development in the water samples collected in the second campaign: (a) Spring 3, (b) Spring 5, (c) Spring 6, (d) Spring 10, and (e) Spring 11; (1) individual grains crystallized from the water samples; (2) grains embedding in the final dendritic crystalline crust; and (3) milled crystalline crust powder which was subjected to the XRD investigation.
The specific crystal lattice of each mineral influences the polarized light in a unique manner exhibiting a specific color range that allows for proper identification. Aragonite crystals are colored in violet–blue depending on their position regarding the microscope optical axis. The calcite and pseudo-dolomite crystals are colored in white–yellow at the maximum and brown at the extinction position. The crystal habit is very important for proper identification: calcite crystals are predominantly rhombic and pseudo-hexagonal while dolomite crystals are predominantly columnar prisms. Natron crystals appear yellow at the maximum lighting and gray–yellow at extinction featuring needle shapes due to their monoclinic crystallization.
The early stage of crystallization within the S3 sample features small micro-crystals that are well individualized without coalescence and that were formed by the alkaline ions’ reaction with carbonic acid, as shown in Figure 4(a1). Fine crystals of calcite, pseudo-dolomite, and aragonite appear along with a few fine yellow needles of natron. Halite crystals do not appear in this early stage. The final stage of the crystallization process leads to a supersaturated solution that accelerates the crystallization process, and the individual crystals are covered by additional layers and, finally, when the water is almost completely evaporated, a crystalline crust is formed generating a dendritic chain microstructure; see Figure 4(a2). Halite crystallizes during this stage, the formed crystals being embedded in the crust on the dendrites’ borders; this fact is proved by the pale blue shade observed on their sides, as shown in Figure 4(a2). This indicates that halite crystallizes as thin cubic platelets covering the previously formed crystals. Figure 4(a3) reveals the microstructural aspect of the crystalline powder resulting after the scratching of the crystalline crust. The general aspect is colored pale white due to the large calcite and pseudo-dolomite contents, and the individualized crystals appear embedded into the broken dendrite mass (this powder was used for the XRD).
The S5 sample has more crystallization centers and develops finer crystals, as observed in Figure 4(b1). The calcite and pseudo-dolomite crystals are the most evident on the observation field, and there are some rhombic crystals colored in blue–violet colors belonging to the aragonite (they are most likely observed after the intense blue spots).
The natron crystals are difficult to observe but some finer yellow acicular intercalations can be observed in the central area of the observation field in Figure 4(b1). Halite crystals are not yet formed, and the sodium chloride is still remnant in the supersaturated solution. The intensive evaporation of water during the final stage causes massive crystallization including halite and natron along with the rest of the calcite and pseudo-dolomite forming a dense dendritic structure, seen in Figure 4(b2). The dendrites are grouped in growths of local inflorescences on the corners of some individualized rhombic and pseudo-hexagonal individual calcite crystals. The dendrites contain a fine succession of calcite and dolomite crystallites that will be better observed by the SEM investigation. Their faster growth under the final concentration gradient during drying caused the embedding of most of the free crystals formed in the first stage. The crystalline powder formed after the glass slide scratching reveals finer particles embedded in the crystalline crust of the broken dendrites, as shown in Figure 4(b3).
Spring 6 has a high mineralization that induces the precipitation of a lot of germination centers due to the reaction of the alkaline ions with carbonic acid. These crystals progressively grow as individual well-formed particles during the first stage, shown in Figure 4(c1). The most obvious and representative crystals are calcite (including pseudo-hexagonal and rhombic forms) and prismatic crystals of pseudo-dolomite. The fast increasing of the concentration gradient during the final stage of the drying process induces a rapid crystallization of the remnant liquid, and large dendritic flowers are formed having a span of 50–200 μm, shown in Figure 4(c2). The dendritic structure is formed on the calcite crystals formed in the first phase and embeds the other free crystals in their close vicinity. The dense mineralization is also observed in the broken dendrites’ microstructure in Figure 4(c3). The pale white aspect of the embedding material is induced by the mean overlapping of the light specific to calcite, pseudo-dolomite, and halite. The embedded individual crystals appear as bright spots shining in their specific color range.
Spring 10 has the most intense mineralization compared to the other investigated springs. This leads to a fast development of many crystallization centers as a consequence of Ca2+, Mg2+, and Na+ ions with carbonic acid generating the fast development of calcite, pseudo-dolomite, and aragonite fine crystals, shown in Figure 4(d1). Calcite and pseudo-dolomite crystals (having yellow–brown color) are slightly bigger than aragonite, which appear as small blue–violet spots. The natron crystals are very small and thin with acicular yellow appearance being easily overlooked beside bigger calcite crystals, but some of them are more visible on the left median side of Figure 4(d1). The final stage of crystallization involves the supersaturated solution and subsequent concentration gradient that facilitate dendrite formation. The abundance of growing centers causes the increasing of the number of dendrite flowers and the relative reducing of their size of about 30–100 μm as observed in Figure 4(d2). Similar dendrite is observed in Figure 5(d2).
The dendrite’s borders have a pale blue aspect caused by the significant presence of halite platelets crystallized in the cubic system typically oriented on the (200) crystallographic plane, in agreement with the XRD observation and literature data [22]. The scratched powder reveals the aspect of the broken dendrites; see Figure 4(d3).
Spring 11 is less mineralized compared to the other investigated springs. This fact is sustained by the relatively weaker diffraction peaks observed in Figure 3a. Therefore, the initial crystallization stage occurs with only several germination centers allowing for the progressive growth of relatively bigger crystals, as shown in Figure 4(e1). Most of them belong to the calcite sustaining the XRD observation, and only a few pseudo-dolomite and aragonite crystals are observed. Natron and halite crystals are not observed in the initial stage.
The progressive drying induces a concentration gradient that finally results in the supersaturated solution that allows for the dense precipitation of the remnant minerals due to the reaction of the alkaline ions with the carbonic acid. It consolidates some dendritic chains containing previously formed individual crystals embedded in a crystalline crust having pale blue color; see Figure 4(e2). Figure 4(e3) shows the scarce mineral content compared to the other investigated springs’ samples. The POM results for all investigated samples from the first campaign are centralized in Table 3 along with the XRD results.
The second campaign’s samples have a similar crystallization behavior to the previously observed samples but with more mineral abundance, as seen in Figure 5. The initial stage reveals a significant number of germination centers within S3, S5, and S11 followed by the growth of relatively small and well-formed individual crystals, shown in Figure 5(a1,b1,e1). The samples S6 and S10 having the strongest mineralization generate numerous crystallization centers that rapidly grow into relatively bigger individual crystals, as observed in Figure 5(c1,d1). The final stage of crystallization generates more developed dendrites regarding the crust density and embedded crystals for S3, S5, and S11 than those observed for the first campaign. The dendritic crust evidences the presence of halite as the embedding material towards the dendrites’ borders, as observed in Figure 5(a2,b2,e2).
Springs 6 and 10 have increased mineralization compared to the first campaign and this influences the final stage of crystallization by a large increase in the dendrite span to over 200 μm for S6 in Figure 5(c2) and to over 250 μm for S10 in Figure 5(d2). The dendrites contain a dense package of calcite and pseudo-dolomite crystals along with aragonite traces embedded in a halite and natron base. The previously individualized crystals are now embedded into the developed dendrites. The scratched powders’ aspects for S6 and S10 reveal dense mineralized broken crusts, seen in Figure 5(c3,d3), which lead to the well-developed XRD peaks. The measured crystal sizes are centralized in Table 3 beside the XRD results.
3.3. Microstructural Observation and Elemental Spectroscopy
It is very difficult for the SEM investigation to observe the microstructural aspects of the glass slides previously subjected to the POM investigation due to the lack of electrical conductivity between the developed crystals and the specimen holder. Therefore, the scratched powder containing the formed crystals fits the SEM requirements in some conditions. The powder samples (the crystalline powders resulting from the second campaign were used due to their stronger mineralization) were mounted on the double adhesive carbon bullets which ensure an optimal electrical conduction between crystals and specimen holder and were sputtered with a very thin platinum film to ensure optimal resolution of the electron images. The platinum component was subtracted from the elemental analysis. The obtained values are centralized in Table 4.

Table 4.
The elemental composition of the crystalline powders.
The carbon bullets might influence the elemental analysis on the large areas by quantifying the uncovered spots explaining some abnormally increased values of carbon over the normal stoichiometric composition related to the alkaline elements. Thus, the elemental composition was determined on the large areas observed at low magnifications: 200×–500×, shown in Figure 6.
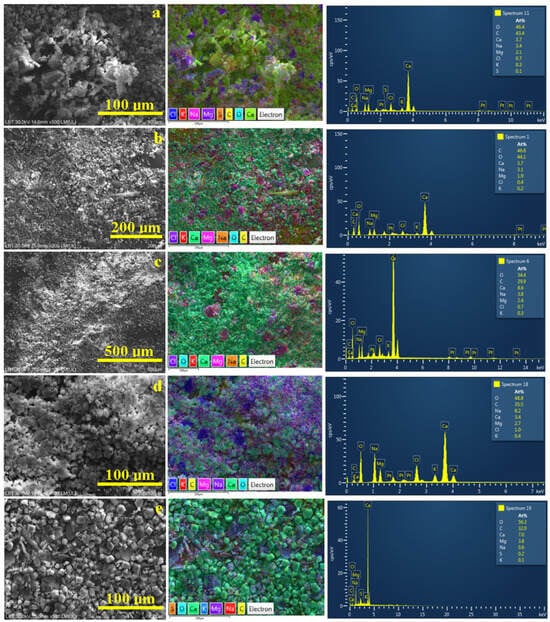
Figure 6.
SEM electron images with the corresponding element distribution map and EDS elemental spectra of the milled crystalline crust powders resulting from the water samples: (a) Spring 3, (b) Spring 5, (c) Spring 6, (d) Spring 10, and (e) Spring 11.
The broken dendrites within the S3 sample are situated in the central side of the electron image in Figure 6a and are surrounded by smaller fractions including individual crystals. The corresponding elemental map features an intense green color for the calcite and aragonite particles (due to the overlapping colors of yellow for C, turquoise for O, and green for Ca). Magnesium is labeled pink, and its distribution is observed inside of some crystalline crusts’ clusters showing the refined distribution of the dolomite crystals. Chloride is labeled dark blue showing its distribution along with the halite components of the crystalline crust. We noticed that the S atoms are predominantly situated around the pseudo-dolomite crystals rather than on the calcite ones (the association between pink and orange spots in the elemental map in Figure 6a). Their occurrence is involved with some sulfur traces in the S3 water below the detection limit in Table 2. The water evaporation concentrates these traces so that they become detectable beside the pseudo-dolomite crystals.
The powder resulting from the S5 broken dendrites is more refined and uniform, as observed in Figure 6b, indicating the advanced fragmentation of the formed dendrites. It is difficult to observe the individual crystals due to the low magnification. The element distribution map within Figure 6b clearly evidences pseudo-dolomite clusters having a pink color surrounded by a dense green mass of calcite and aragonite crystals. The halite fractions appear to be very fragmented; this fact is sustained by the randomly spread dark blue spots. This is a normal situation because of the lower amount of chloride ions detected in the EDX spectrum; see Table 4. The lower right corner of the element distribution map reveals a dark brown area indicating an abnormally higher carbon amount. This area is not fully covered with powder, making visible the carbon bullet, explaining the carbon excess found in the elemental composition.
The S6 powder is finer than the previous one; the microstructural ensemble observed in Figure 6c reveals a compact layer of fine particles. The elemental map evidences bigger clusters containing pseudo-dolomite crystals having a pinkish nuance randomly spread in the calcite particles’ mass. The halite component of the broken crystalline crust appears dark violet according to its specific label. The dense coverage of the bullet with crystal powder ensures the stoichiometric amount of C in the elemental analysis; see Table 4.
The resulting powder from the breaking of the S10 dendrites is also very fine, having well-contoured particles that form a uniform and compact layer that ensures a proper carbon quantification for the elemental analysis; the measured value of 35.5 at.% corresponds to the sample’s stoichiometry. The element distribution map is also very different due to the significantly increased magnesium level depicted by the pink spots spread all over the surrounding greenish calcite and aragonite crystals. There is also a large crust fraction having dark blue color due to the chloride presence, a fact associated to the halite crystals.
In addition to the relatively lower mineralization of spring 11, the formed dendrites are more robust without excessive coalescence between the individually formed crystals. Therefore, the resulting powder contains a lot of well-formed individual crystals as distinct particles. They are spread over the bullet surface in a uniform and compact film ensuring the optimal measurement of the carbon amount. The calcite and aragonite crystals have predominantly rhombic and pseudo-hexagonal shapes and present as green because of the specific element labels overlapping. Their size merely varies in the range of 5–15 µm. We can observe very interesting particles of broken crusts (tabular shape with irregular margins due to the intensive fragmentation) having a planar size of about 25–40 µm and a thickness of about 5 µm. These particles contain both pink and dark blue spots indicating that small pseudo-dolomite crystals were embedded into the halite crust during the last stage of crystallization. The orange spots belonging to the sulfide component are predominantly associated with the pseudo-dolomite particles.
The low magnification SEM observation was necessary for the proper determination of the powder samples’ elemental composition. Crystal morphology requires high magnifications depending on the crystal sizes; therefore, high magnification for the microstructural details ranges from 800× to 2000×.
The microstructural detail in Figure 7a is dominated by the pseudo-hexagonal and rhombic crystals of calcite and aragonite which appear green in the adjacent element distribution map. Their size is situated between 5 to 10 µm in good agreement with the POM investigation.
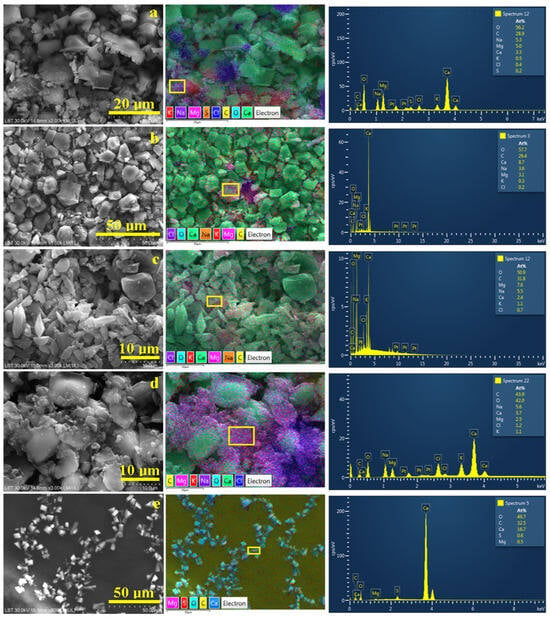
Figure 7.
SEM electron images of the crystals’ microstructural details with their corresponding element distribution map and the EDS elemental spectra of the milled crystalline crust powders resulting from the water samples: (a) Spring 3, (b) Spring 5, (c) Spring 6, (d) Spring 10, and (e) Spring 11.
On the upper right corner of Figure 7a, pseudo-hexagonal crystals appear with the broken remains of the embedding crusts observed from its lateral side revealing a crust thickness of about 5 µm. The left side of the image reveals the top surface of these crust remains with a planar size of about 17 µm. They have a dark blue color with slightly pink dots indicating a halite base which embeds pseudo-dolomite fractions. The pseudo-dolomite prismatic crystals were very affected by the scratching process which mostly disintegrates them into fine particles of 3–5 µm found nearby the crust remains, mainly on the lower side of Figure 6a.
Figure 7b reveals a spectacular mixture of pseudo-hexagonal calcite with rhombic aragonite crystals in the size range of 5–15 µm, in good agreement with the POM observation. Unfortunately, the pseudo-dolomite crystals were damaged by the powder scratching, being found as small fractions associated with the remains of the dendritic crust mostly observed in the center of Figure 7b. Their belonging to the pseudo-dolomite is proved by the pink spots in the adjacent elemental map. Small traces of chloride are observed at the margin of the broken crusts, proving the halite presence.
The powder within the S6 sample has a very interesting morphological detail in Figure 7c. The left upper side of the image is surprising with a nice pseudo-hexagonal calcite crystal of 10 µm being very well individualized, while in the lower left corner the sides of some rhombic aragonite crystals appear. These are surrounded by pseudo-dolomite prismatic crystals with a length of 10 µm and a diameter ranging from 3 to 5 µm. Their belonging to the dolomite class is sustained by their pink spots observed in the element distribution map. This fact is also sustained by the EDS spectrum taken on the surface of a single particle. Some of the elongated particles situated in the center of the image have a needle shape and present orange spots in the element distribution map relating to the natron crystal presence. The prism’s end is fractured because of the mechanical stress exerted during the powder scratching.
Figure 7d reveals the microstructural detail for the S10 powder. It is based on the large pseudo-hexagonal calcite crystals (green in the adjacent element distribution map) surrounded by the fine pink fractions of pseudo-dolomite prisms that were broken during powder collection from the crystallization recipient.
They are intercalated with fine fractions of having a violet aspect related to the Na presence indicating the fragmentation of the natron needle-like crystals. The EDS spectrum taken on the right side of the elemental image reveals the locally increased level of magnesium. The left side of the element distribution map in Figure 7d has a dark blue color due to the significant presence of chloride. This fact is in good agreement with the pale blue haloing observed on the dendrite sides on the POM observation for sample S10.
The microstructural detail observed for the S11 sample, in Figure 7e, is very interesting because it catches an intact dendrite without any breaking marks. There is a succession of calcite crystals with pseudo-hexagonal form and size ranging from 5 to 10 µm and rhombic aragonite crystals ranging from 3 to 10 µm. Their appearance in the element distribution map is greenish blue. The pseudo-dolomite crystals have a prismatic shape with a length of 10–15 µm and feature pink spots in the elemental map. They are interconnected with the other crystals forming the chain dendrites. It is very interesting that sulfur spots accompany the dolomite crystals during the crystallization process and avoid contact with the calcite.
4. Discussion
As previously mentioned, Borsec sparkling mineral water has a long history up to today. Its high quality was highly appreciated at the World Exhibit in Wien in 1873 receiving the title of the Queen of Mineral Water; it also received an Honor Award Diploma at Trieste in 1878 and at the World Exhibit in Paris in 1878. Nowadays, it is found in the Romanian markets under the brand Borsec—The Queen of Mineral Water. The commercially bottled mineral water is exploited from the company’s specific springs. They have moderate mineralization and a constant CO2 level making them optimal for daily consumption. The public springs situated in the Borsec town present a higher mineralization having the precise therapeutic indication to be used in short-term, controlled cures [1,4].
The mineral wonder found in the Borsec waters is formed in the deeper geological layers by the reaction of the infiltrated water with the specific minerals. The first (and most representative) aquifer is formed by the underground water mineralized into the dolomitic crystalline shale found on the northwestern side of the depression [4]. The infiltrated water intensively circulates through the rock fissures moved by the fumarole gas-lift generating local eddies facilitating the mineral dissolution. The dolomitic structure enriches the water with Ca2+ and Mg2+ ions. The peat layer situated on the surface ensures a proper filtration of the water, a fact mentioned in the literature [5,6], while the green clay based on chlorite also brings calcium and magnesium ions into the water along with Na+ and K+. The chloride presence is very low, almost at a trace level as reported in [4], and did not appear in the current public declaration. This aquifer is characterized by a high daily flow and average mineralization. This fact is confirmed by the relatively low TDS and salinity observed for S3, S5, and S11.
The second aquifer is mineralized by the dolomitic rocks’ interactions with the fumarole CO2, which induces the precipitation of calcium carbonate as limestone tuff. These tuff deposits might warp the spring far out from the ground causing frequent changes in their place of surface bursting [4]. This behavior affects the water flow which is moderate to low and presents a decreasing tendency. The springs S6 and S11 fit this typology. The mineralization process also brings Ca2+ and Mg2+ ions into the water along with potassium. The gray-green clay intercalations within the dolomitic crystalline shale ensure the mineralization with alkaline ions such as Na+ and K+. The intercalation of the sedimentary bed with local volcanic rocks such as andesite and pyroclastite ensures a significant level of chloride ions. The low water flow combined with the rich mineralization explains the high TDS and salinity measured for S6 and S10.
The elemental composition of the investigated samples obtained by SEM–EDX investigation confirms the S3, S5, and S11 source as the first aquifer and the S6 and S10 source as the second aquifer. The elemental composition of the crystallized powder has a strong resemblance to the public declaration of the composition excepting chloride. We found that all springs have chloride amounts, but the most representative salted spring is S10, a fact sustained by its increased salinity.
Both aquifers and their subsequent mineralization method induce dissolved anions and cations in the water, the most representative being CO32−. These ions are sensitive to the water solution concentration. The water evaporation increases the solution concentration that facilitates the anion and cation interactions such as the following:
Ca2+(aq) + CO32−(aq) → CaCO3 (s)↓
Ca2+(aq) + Mg2+(aq) + 2CO32−(aq) → CaMg(CO3)2 (s)↓
2Na+(aq) + CO32−(aq) → Na2CO3 (s)↓
Na+(aq) + Cl−(aq) → NaCl (s)↓
The increasing concentration gradient leads to the formation of the supersaturated solution which facilitates the precipitation of solid salt germs via homogeneous crystallization [22,23]. Literature data show that there is a competition between homogeneous and heterogeneous germination of the calcite from the hard waters during the softening process [23]. The heterogeneous crystallization presumes the pre-existence of fine small solid microscopic particles that ensure an optimal site for crystal growth from a supersaturated solution. In our specific case, there would be some soil particles such as quartz, kaolinite, chlorite, or pyroxenes. These solid mineral inclusions would be detected by the XRD investigation and observed at the POM inspection during the early stage of crystallization. The obtained results on our samples did not evidence any soil particle inclusion. This fact is confirmed by the zero turbidity measured in the water samples after the complete evaporation of the CO2 bubbles.
The crystals’ growth is stimulated by continuous water evaporation facilitating solid material deposition onto the homogeneous germination centers. This leads to the formation of well-individualized crystals of fine micro-sizes around 5–20 µm at the early stage of crystallization, a fact evidenced by POM investigation. Calcite crystals are more numerous and have a predominantly pseudo-hexagonal habit (rare rhombic crystals are observed) while aragonite has predominantly rhombic crystals, a fact being in good agreement with SEM observations in the literature [24].
It is obvious that the rich dolomite reported for the geological layers within both aquifers is the main source of the Mg2+ ions in the mineral waters. Literature data clearly show that the reaction (2) did not form dolomite because of its lack of crystalline order among the magnesium and calcium crystallographic planes [25,26,27]. Thus, the magnesium ions randomly replace calcium ions within the calcite structure becoming pseudo-dolomite (e.g., rich magnesium calcite), a fact in good agreement with our XRD data. One of the most relevant peaks of pseudo-dolomite occurs at 29.72° instead of at 29.17° becoming a shoulder of the most representative calcite peak. The relevant peaks for pseudo-dolomite were found according to the powder diffraction file PDF 89-1305.
The pseudo-dolomite germs precipitate due to the combined actions of Ca2+ and Mg2+ ions reacting with the carbonate ions, and the columnar crystals appear shortly after the development of the calcite and aragonite crystals. The EDS spectra in Figure 6 show a lower amount of Mg than would have been normal for dolomite, the stoichiometry being altered by the random placement of the Mg2+ ions within the formed crystals, which is consistent with the data reported for pseudo-dolomite [28]. It was observed that concentrated brine with Ca2+ and Mg2+ injected with a fine bubble CO2 flow induces the reactive crystallization of ordered dolomite at room temperature [29]. However, it requires a micro-controlled process where the fine gas bubbles induce partial re-crystallization over the growing crystals, allowing for the addition of ordered Mg and Ca layers. This is not our case when the CO2 bubbles have randomly emanated from the mineral water, having less ability for modeling crystal growth. Thus, the pseudo-dolomite is formed, a fact confirmed by the elemental observations which agree with the XRD data.
The small amounts of sulfur detected by SEM–EDS for S3 and S11 precipitate towards the pseudo-dolomite prismatic crystals and do not form any detectable mineralization on their own. The successive intercalation of these crystals into the dendritic branches was evidenced by the SEM investigation coupled with the elemental map in Figure 6e. The rich abundance of Ca and Mg as the main mineralizers of the Borsec springs facilitates the fast formation of their specific crystals.
The lesser sodium amount led to a weaker crystallization of the natron particles following Equation (3), a fact proved by the few well-individualized natron crystals observed in the first stage by the POM images. The individualized natron crystals are also proved by the SEM image in Figure 7c. It is a possibility that the presence of already formed small calcite grains facilitates a partial heterogeneous crystallization for the natron crystals, a fact in accordance with the data in the literature reporting natron formation on porous building stones [30]. On the other hand, Ali et al. shows natron precipitation using saline brine and CO2 [31], a fact of great importance especially for spring 10 which has an increased salinity.
The progressive accumulation of the well-individualized crystals ensures a relative organization of their relative positions to one another as observed in the POM images. It is a step forward to the final crystallization stage. The supersaturated solution loses its last water inducing a faster crystallization which forms a dendritic crust embedding the previously formed crystals (a fact confirmed by the MOM images of the last crystallization stage and the SEM microstructural details). In such a condition, the Cl− ions react with the remnant Na+ ions forming halite crusts. This crystallization scenario is sustained by the POM observation of the halite deposits at the margins of the dendrites, a fact confirmed by the SEM elemental maps in Figure 7 and sustained by the halite crystallization modeling in the literature [32].
The amount of the formed crystals along with their precipitation mode is strongly dependent on the water’s physicochemical properties, especially TDS and salinity. The relevant statistical group formed by S3, S5, and S11 leads to a weaker mineralization with smaller dendrites than those formed by the other relevant statistical group containing S6 and S10. On the other hand, both XRD and POM clearly show a greater level of mineralization for the samples collected in March 2024 compared to the samples from November 2023. The seasonality of the mineralization depends on the precipitation level, a fact reported in the literature for Borsec and its neighboring mineral waters [3,33] and for other mineral waters all over the world [34,35].
The water of these springs flows continuously, being available to those who want to drink or fill their bottle. Unused water flows into the nearby river. Its high mineral amount is not desirable for the freshwater river, influencing its ecological parameters. Such aspects could be improved if a small self-sustaining desalinization station is provided for each spring. The spilled water would obtain the desired parameters and the extracted mineral powder could be used for several applications.
The crystallization process during water evaporation ensures an advanced mineral extraction from all investigated springs. Nevertheless, the higher mineralization found in S6 and S10 related to the constant flow of about 500 L/day is more favorable for mineral powder production than that of S3, S5, and S11. It will ensure a daily production of 0.75 kg/day for S6 and 1 kg/day based on the total dissolved solids (TDS) in Figure 2d. Such amounts might be produced by an integrated water evaporation system operated with solar cells ensuring a low carbon footprint. The vaporization facility might be placed nearby the spring location, playing a key role in the transformation of the mineral water into common river water which is better for the local environment.
The novelty of the current research has two goals that could be achieved: the collected mineral powder could be used to prepare specific diet supplements for the electrolytic balance regulation or adjuvant for various treatments. The other goal is the mineral amount normalization of the water spilled into the river, a fact which improves the local environmental aspects of the hydrographic basin.
The therapeutic effect of mineral water is renowned from antiquity, but nowadays there are specialized pathways of treatments depending on each mineral water’s characteristics. Borsec mineral waters from the investigated public springs have sparkling bicarbonate characteristics with significant magnesium content induced by pseudo-dolomite. As previously discussed, the main difference between the investigated springs is the mineral amount and the crystal distribution which certainly influences the curing potential. Therefore, the current therapeutic practices are recommended according to the literature data [1,4]:
- -
- Spring 3 for the following: hyperacid gastritis, chronic colitis, chronic renal afflictions, biliary lithiasis, post hepatic sequels;
- -
- Spring 5 for the following: hypertension in stages I and II, coronary afflictions, hypo-acid gastritis, afflictions of the colon, and renal afflictions;
- -
- Spring 6: hypo- and normal acid chronic colitis, and enterocolitis;
- -
- Spring 10: thyroid afflictions, enterocolitis insufficiency, diabetes, gastrointestinal inarticulateness;
- -
- Spring 11: chronic gastrointestinal diseases, hypo- or normal acid gastritis, dironic post hepatitis (jaundice), bilious complaints, nutrition disorders, external applications.
The high amounts of calcite and pseudo-dolomite associated with the mineral water have a good effect in the neutralization of gastric acids [36] and the increased level of salinity has an antimicrobial effect improving the well-being of patients with chronic colitis and acute enterocolitis. The troublemaker bacteria are annihilated by the mineral water’s salinity [37,38]; thus it is also efficient for ameliorating diarrhea symptoms. Jovicic et al. presents the benefit of relatively low mineralized water on metabolic and oxidative stress amelioration in patients having type II diabetes [39].
Hence, all treatments must be effectuated under controlled guidance and should not be effectuated for longer time spans due to the risk of renal lithiasis [40,41]; the extracted powders could be prescribed by therapists for patients’ home-curing. This is an open path for further research regarding the obtained micro-crystallized powders’ applications for patient treatment.
Another path which could be developed is their use as bath salts for spa treatments. Literature data reveal that micro-crystallized calcite, aragonite, and pseudo-dolomite are used as micro-dispersions for spa treatments [42,43]. The observed amount of sodium chloride identified in the obtained powder is able to enhance the peloid effect [44].
5. Conclusions
The investigated mineral water collected from the Borsec public springs has a high mineral content caused by water infiltration through the porous geological formations and rock fissures that dissolve ions from the occurring minerals. Based on data materialized in the present research, some conclusions can be summarized as follows:
The dissolved ion amount depends on the infiltration rate and on the spring’s flow. The obtained results show that springs 3, 5, and 11 have a moderate mineralization formed by the dolomitic limestone aquifer while springs 6 and 10 have an increased level of mineralization.
The dominant minerals formed in S3, S5, and S11 are as follows: calcite (over 50% wt.) followed by pseudo-dolomite, aragonite, and natron generating relatively weak and small dendrite structures with halite deposits on their sides. Significant differences were found for the springs S6 and S10 such as the following: calcite as the dominant mineral has a 30% wt. The pseudo-dolomite and aragonite amounts are significantly increased due to the high magnesium amount.
The obtained results prove that water mineralization is season-dependent because of the precipitation level. The samples collected during November 2023 in a relatively dry season feature a lower mineralization than that observed in the samples collected during March 2024.
The mineral amount within the water from the investigated Borsec public springs is very high compared to the bottled Borsec mineral drinking water and therefore is recommended only for therapeutic purposes in short-term cures effectuated under therapist prescription. The extracted mineral powder opens two paths: for the further research regarding its use for patient home-curing and for spa treatments.
Author Contributions
Conceptualization, S.E.A. and I.P.; methodology, S.E.A. and I.P.; software, G.B.; validation, S.E.A. and I.P.; formal analysis, D.V.P.; investigation, S.E.A., L.B.T., G.B., D.V.P. and I.P.; resources, S.E.A. and D.V.P.; data curation, I.P.; writing—original draft preparation, S.E.A. and I.P.; writing—review and editing, I.P.; visualization, L.B.T. and I.P.; supervision, S.E.A. and I.P.; project administration, S.E.A. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article.
Acknowledgments
The authors gratefully acknowledge that the XRD diffractometer maintenance was supported by the Ministry of Research, Innovation, and Digitization through Program 1-Development of the National Research and Development System, Subprogram 1.2—Institutional Performance-Funding Projects for Excellence in RDI, Contract No. 37PFE/30.12.2021.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iosipescu, R.; Iosipescu, S. Historical documentary regarding Borsec. Rev. Monum. Istorice 2010, 79, 90–97. [Google Scholar]
- Fielitz, W.; Seghedi, I. Late Miocene–Quaternary volcanism, tectonics and drainage system evolution in the East Carpathians, Romania. Tectonophysics 2005, 410, 111–136. [Google Scholar] [CrossRef]
- Bodor, K.; Bodor, Z.; Szép, A.; Szép, R. Classification and hierarchical cluster analysis of principal Romanian bottled mineral waters. J. Food Compos. Anal. 2021, 100, 103903. [Google Scholar] [CrossRef]
- Pricajan, A. Mineral and Thermal Water from Romania; Technical Publishing House: Bucharest, Romania, 1972; 296p. [Google Scholar]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Mahmud, M.; Abdi, C.; Rosadi, R.; Yanto, D.H.Y.; Bilad, M.R. Combination of Coagulation, Adsorption, and Ultrafiltration Processes for Organic Matter Removal from Peat Water. Sustainability 2022, 14, 370. [Google Scholar] [CrossRef]
- Manasypov, R.M.; Lim, A.G.; Krickov, I.V.; Raudina, T.V.; Kurashev, D.G.; Shirokova, L.S.; Pokrovsky, O.S. Colloids in Thermokarst Lakes along a Permafrost and Climate Gradient of Permafrost Peatlands in Western Siberia Using In Situ Dialysis Procedure. Water 2023, 15, 1783. [Google Scholar] [CrossRef]
- Ledésert, B.A. Application of Minerals for the Characterization of Geothermal Reservoirs and Cap Rock in Intracontinental Extensional Basins and Volcanic Islands in the Context of Subduction. Minerals 2024, 14, 263. [Google Scholar] [CrossRef]
- Yang, M.; Tan, L.; Batchelor-McAuley, C.; Compton, R.G. The solubility product controls the rate of calcite dissolution in pure water and seawater. Chem. Sci. 2024, 15, 2464–2472. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Y.; Wang, G.; Li, T.; Liu, F.; Wei, S.; Yan, X.; Gan, H.; Zhang, W. Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water. Water 2022, 14, 4041. [Google Scholar] [CrossRef]
- Lange, T.P.; Palcsu, L.; Szakács, A.; Kővágó, A.; Gelencsér, O.; Gál, A.; Gyila, S.; Tóth, T.M.; Mațenco, L.; Krézsek, C.; et al. The link between lithospheric scale deformations and deep fluid emanations: Inferences from the Southeastern Carpathians, Romania. Evol. Earth 2023, 1, 100013. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Touret, J.L.R. CO2, carbonate-rich melts, and brines in the mantle. Geosci. Front. 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Marieni, C.; Voigt, M.; Clark, D.E.; Gíslason, S.R.; Oelkers, E.H. Mineralization potential of water-dissolved CO2 and H2S injected into basalts as function of temperature: Freshwater versus Seawater. Int. J. Greenh. Gas Control 2011, 109, 103357. [Google Scholar] [CrossRef]
- Borsec Public Springs Composition Declaration Displayed on the Entrance Front. National Mineral Water Company of Romania. Available online: https://www.snam.ro/ (accessed on 21 February 2024).
- Metzger, M.; Konrad, A.; Blendinger, F.; Modler, A.; Meixner, A.J.; Bucher, V.; Brecht, M. Low-Cost GRIN-Lens-Based Nephelometric Turbidity Sensing in the Range of 0.1–1000 NTU. Sensors 2018, 18, 1115. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.; Ahmad, A.; Sendra, S.; Lloret, J.; Lorenz, P. Combination of Machine Learning and RGB Sensors to Quantify and Classify Water Turbidity. Chemosensors 2024, 12, 34. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Physicochemical Parameters and Terroir Assessment of Mineral Water from Mount Smolikas in Greece: A Two-Year Study. Analytica 2023, 4, 280–299. [Google Scholar] [CrossRef]
- Brika, B.; Dévora-Isiordia, G.E.; Alturki, E. Chemical Composition of Selected Brands of Bottled Water Commercilaized in Tripoli, Libya. Environ. Sci. Proc. 2022, 21, 48. [Google Scholar] [CrossRef]
- Avram, S.E.; Birle, B.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water 2024, 16, 1027. [Google Scholar] [CrossRef]
- Petean, I.; Paltinean, G.A.; Taut, A.C.; Avram, S.E.; Pripon, E.; Barbu Tudoran, L.; Borodi, G. Ag and Sn Implications in 3-Polker Coins Forgeries Evidenced by Nondestructive Methods. Materials 2023, 16, 5809. [Google Scholar] [CrossRef]
- Vazquez, P.; Lux, J. Salt Crystallization on Crazannes Limestone in a Long-Term Storage Environment. Minerals 2023, 13, 1282. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Zelek-Pogudz, S.; Solecka, U.; Solecki, M.L.; Szostak, E.; Zborowski, K.K.; Zając, M. Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland. Minerals 2022, 12, 1561. [Google Scholar] [CrossRef]
- Petean, I.; Arghir, G.; Câmpean, R.F.; Bărăian, M.; Hosu Prack, A.G. Crystalographyc relations applied to homogeneous cristalization of badenian salt. Acta Tech. Napoc. Ser. Appl. Mech. Math. 2011, 54, 193–200. [Google Scholar]
- Nie, X.; Wang, Z.; Wan, J.; Wang, G.; Li, Y.; Ouyang, S. Competition between homogeneous and heterogeneous crystallization of CaCO3 during water softening. Water Res. 2024, 250, 121061. [Google Scholar] [CrossRef] [PubMed]
- Bucca, M.; Dietzel, M.; Tang, J.; Leis, A.; Köhler, S.J. Nucleation and crystallization of otavite, witherite, calcite, strontianite, hydrozincite, and hydrocerussite by CO2 membrane diffusion technique. Chem. Geol. 2009, 266, 143–156. [Google Scholar] [CrossRef]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, H.; Wang, X.; Ning, M.; Wang, X.; Ge, Y.; Wang, H.; Tang, R.; Hou, M. Thermodynamic and Kinetic Studies of Dolomite Formation: A Review. Minerals 2023, 13, 1479. [Google Scholar] [CrossRef]
- Land, L.S. Failure to precipitate dolomite at 25 °C from dilute solution despite 1000-fold oversaturation after 32 years. Aquat. Geochem. 1998, 4, 361–368. [Google Scholar] [CrossRef]
- Pimentel, C.; Pina, C.M. Reaction pathways towards the formation of dolomite-analogues at ambient conditions. Geochim. Cosmochim. Acta 2016, 178, 259–267. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Wada, Y.; Hiaki, T.; Onoe, K.; Matsumoto, M. Effects of CO2 fine bubble injection on reactive crystallization of dolomite from concentrated brine. J. Cryst. Growth 2017, 469, 36–41. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Ibañez-Velasco, A.; Ruiz-Agudo, C.; Bonilla-Correa, S.; Elert, K.; Rodríguez-Navarro, C. Damage of porous building stone by sodium carbonate crystallization and the effect of crystallization modifiers. Constr. Build. Mater. 2024, 411, 134591. [Google Scholar] [CrossRef]
- Ali, A.; Mendes, C.E.; de Melo, L.G.T.C.; Wang, J.; Santos, R.M. Production of Sodium Bicarbonate with Saline Brine and CO2 Co-Utilization: Comparing Modified Solvay Approaches. Crystals 2023, 13, 470. [Google Scholar] [CrossRef]
- Gómez-Peralta, J.I.; García-Peña, N.G.; Bokhimi, X. Crystal-Site-Based Artificial Neural Networks for Material Classification. Crystals 2021, 11, 1039. [Google Scholar] [CrossRef]
- Papp, D.C.; Niţoi, E. Isotopic composition and origin of mineral and geothermal waters from Tuşnad Băi Spa, Harghita Mountains, Romania. J. Geochem. Explor. 2006, 89, 314–317. [Google Scholar] [CrossRef]
- Carreira, P.M.; Marques, J.M.; Graça, R.C.; Aires-Barros, L. Radiocarbon application in dating “complex” hot and cold CO2-rich mineral water systems: A review of case studies ascribed to the northern Portugal. Appl. Geochem. 2008, 23, 2817–2828. [Google Scholar] [CrossRef]
- Segura, D.; Cerepi, A.; Loisy, C. Aquifer-CO2 leak project. Effect of CO2-rich water percolation in porous limestone cores: Simulation of a leakage in a shallow carbonate freshwater aquifer. Chem. Geol. 2024, 657, 122105. [Google Scholar] [CrossRef]
- Linc, R.; Pantea, E.; Șerban, E.; Ciurba, A.-P.; Serban, G. Hydrochemical and Microbiological Investigations and the Therapeutic Potential of Some Mineral Waters from Bihor County, Romania. Sustainability 2023, 15, 15640. [Google Scholar] [CrossRef]
- Wangchuk, P.; Yeshi, K.; Ugyen, K.; Dorji, J.; Wangdi, K.; Samten; Tshering, P.; Nugraha, A.S. Water-Based Therapies of Bhutan: Current Practices and the Recorded Clinical Evidence of Balneotherapy. Water 2021, 13, 9. [Google Scholar] [CrossRef]
- Dickson-Gomez, J.; Nyabigambo, A.; Rudd, A.; Ssentongo, J.; Kiconco, A.; Mayega, R.W. Water, Sanitation, and Hygiene Challenges in Informal Settlements in Kampala, Uganda: A Qualitative Study. Int. J. Environ. Res. Public Health 2023, 20, 6181. [Google Scholar] [CrossRef]
- Jovicic, N.; Andjic, M.; Novakovic, J.; Jeremic, N.; Zivkovic, V.; Srejovic, I.; Stanojevic, D.; Ristic, P.; Bolevich, S.; Jakovljevic, V. The effects of low mineral content water on microbiota, metabolic, and oxidative stress parameters in patients with type 2 diabetes mellitus. Heliyon 2023, 9, e18725. [Google Scholar] [CrossRef]
- Injeyan, M.; Bidault, V.; Bacchetta, J.; Bertholet-Thomas, A. Hydration and Nephrolithiasis in Pediatric Populations: Specificities and Current Recommendations. Nutrients 2023, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Calvó, P.; Costa-Bauza, A.; Grases, F. Effect of Phytate (InsP6) and Other Inositol-Phosphates (InsP5, InsP4, InsP3, InsP2) on Crystallization of Calcium Oxalate, Brushite, and Hydroxyapatite. Biomolecules 2023, 13, 1061. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M.; Martín-Rubí, J.A.; Pozo, E.; Maraver, F. Mobility of elements in interaction between artificial sweat and peloids used in Spanish spas. Appl. Clay Sci. 2010, 48, 506–515. [Google Scholar] [CrossRef]
- Karakaya, M.C.; Karakaya, N.; Sarıoğlan, S.; Koral, M. Some properties of thermal muds of some spas in Turkey. Appl. Clay Sci. 2010, 48, 531–537. [Google Scholar] [CrossRef]
- Chelnokov, G.; Kharitonova, N.; Bragin, I.; Chudaev, O. Geochemistry of mineral water and gases of the Razdolnoe Spa (Primorye, Far East of Russia). Appl. Geochem. 2015, 59, 147–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).