Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production
Abstract
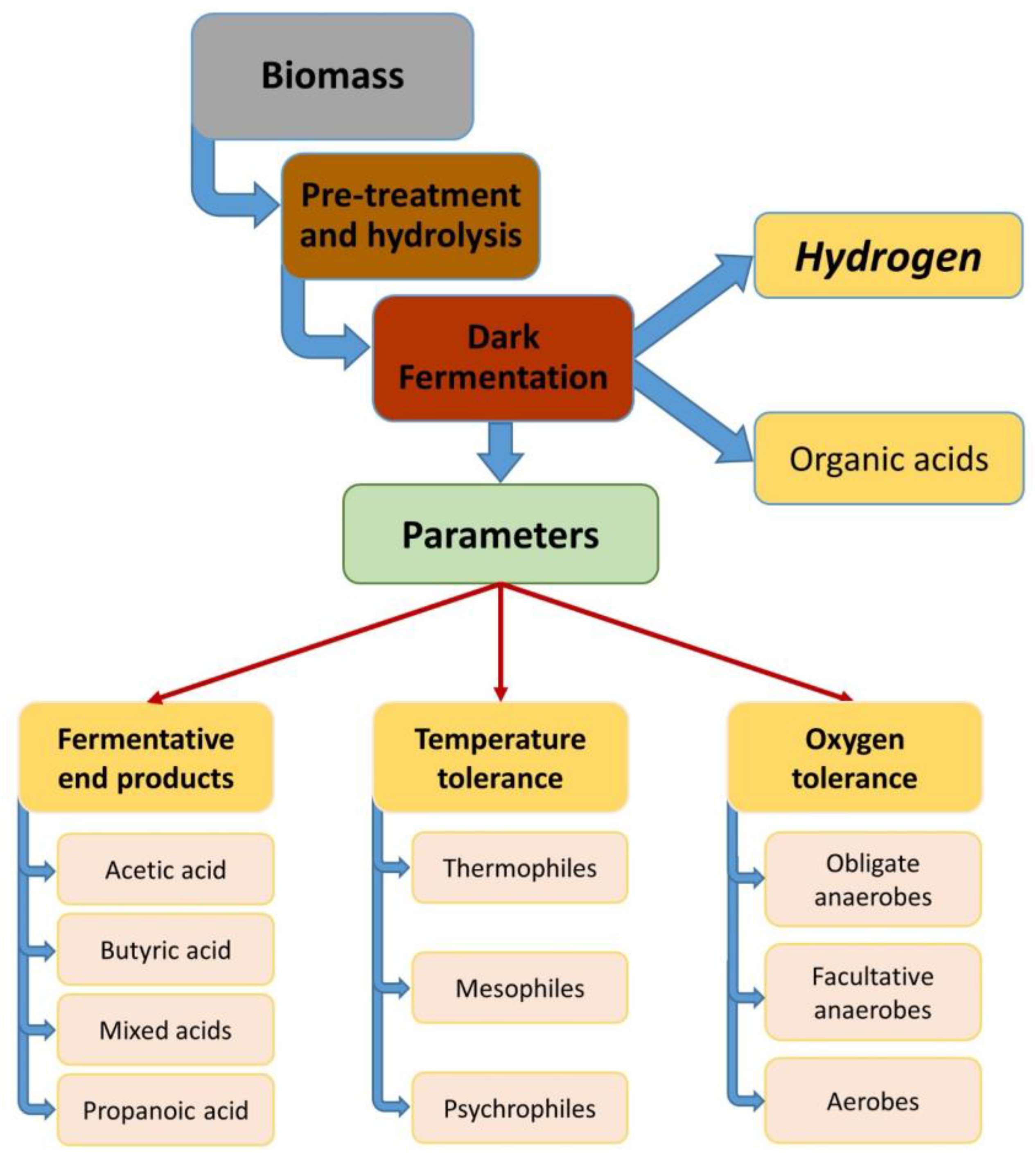
1. Introduction
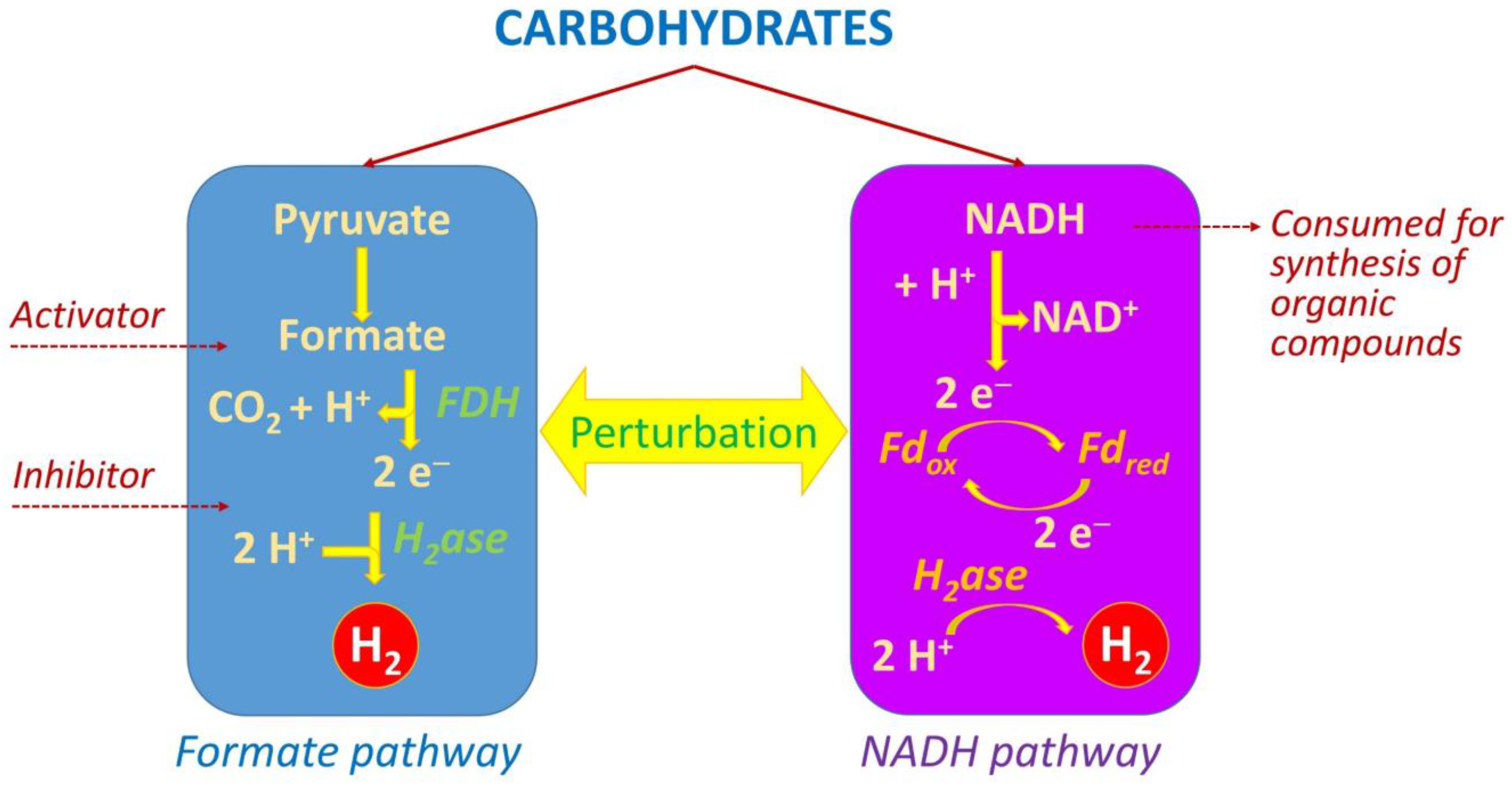
2. Hydrogen Production Pathways Utilized by Dark Fermentation Bacteria
3. Factors Influencing Dark Fermentation Reactions
3.1. Effect of pH
3.2. Effect of Temperature
3.3. Substrates Used by the Dark Fermentation Bacteria
3.4. Hydraulic Retention Time (HRT)
4. Bacteria Used for Hydrogen Production During Dark Fermentation
4.1. Clostridium
4.1.1. Clostridium butyricum
4.1.2. Clostridium beijerinckii
4.1.3. Clostridium pasteurianum
4.1.4. Clostridium thermocellum
4.2. Enterobacter
4.2.1. Enterobacter aerogenes
4.2.2. Klebsiella pneumoniae
4.3. Escherichia Coli
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen production through dark fermentation: Recent trends and advances in transition to a circular bioeconomy. Int. J. Hydrogen Energy 2024, 52, 335–357. [Google Scholar] [CrossRef]
- Sharmila, V.G.; Tamilarasan, K.; Kumar, M.D.; Kumar, G.; Varjani, S.; Kumar, S.A.; Banu, J.R. Trends in dark biohydrogen production strategy and linkages with transition towards low carbon economy: An outlook, cost-effectiveness, bottlenecks and future scope. Int. J. Hydrogen Energy 2022, 47, 15309–15332. [Google Scholar] [CrossRef]
- Liguori, S.; Kian, K.; Buggy, N.; Anzelmo, B.H.; Wilcox, J. Opportunities and challenges of low-carbon hydrogen via metallic membranes. PECS 2020, 80, 100851. [Google Scholar] [CrossRef]
- Hydrogen Supply Outlook 2024: A Reality Check. Available online: https://about.bnef.com/blog/hydrogen-supply-outlook-2024-a-reality-check/ (accessed on 12 November 2024).
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Rossi, F.; Nicolini, A. An experimental investigation to improve the hydrogen production by water photoelectrolysis when cyanin-chloride is used as sensibilizer. Appl. Energy 2012, 97, 763–770. [Google Scholar] [CrossRef]
- Qu, X.; Zeng, H.; Gao, Y.; Mo, T.; Li, Y. Bio-hydrogen production by dark anaerobic fermentation of organic wastewater. Front. Chem. 2022, 10, 978907. [Google Scholar] [CrossRef]
- 4 Startups Providing Clean Hydrogen Production with Microbes. Available online: https://www.greyb.com/blog/microbes-hydrogen-production-startups/# (accessed on 12 November 2024).
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. [Google Scholar] [CrossRef]
- Tiang, M.F.; Hanipa, M.A.F.; Abdul, P.M.; Jahim, J.M.; Mahmod, S.S.; Takriff, M.S.; Lay, C.H.; Reungsang, A.; Wu, S.Y. Recent advanced biotechnological strategies to enhance photo-fermentative biohydrogen production by purple non-sulphur bacteria: An overview. Int. J. Hydrogen Energy 2020, 45, 13211–13230. [Google Scholar] [CrossRef]
- Javed, M.A.; Zafar, A.M.; Hassan, A.A.; Zaidi, A.A.; Farooq, M.; El Badawy, A.; Lundquist, T.; Mohamed, M.M.A.; Al-Zuhair, S. The role of oxygen regulation and algal growth parameters in hydrogen production via biophotolysis. J. Environ. Chem. Eng. 2022, 10, 107003. [Google Scholar] [CrossRef]
- Rochaix, J.D. Regulation of photosynthetic electron transport. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 375–383. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial electrohydrogenesis cell and dark fermentation integrated system enhances biohydrogen production from lignocellulosic agricultural wastes: Substrate pretreatment towards optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Rousseau, R.; Ketep, S.F.; Etcheverry, L.; Délia, M.L.; Bergel, A. Microbial electrolysis cell (MEC): A step ahead towards hydrogen-evolving cathode operated at high current density. Bioresour. Technol. Rep. 2020, 9, 100399. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Wiktorowska-Sowa, E.; Piotrowski, J.; Salamon, A.; Kaźmierczak, W.; Błaszczyk, M.K.; Barberan, A.; Chen, Y.; et al. Dynamics and complexity of dark fermentation microbial communities producing hydrogen from sugar beet molasses in continuously operating packed bed reactors. Front. Microbiol. 2021, 11, 612344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.T.; Ding, J.; Wang, B.Y.; Bao, M.Y.; Liu, B.F.; Pang, J.W.; Ren, N.Q.; Yang, S.S. Advances in the biomass valorization in dark fermentation systems: A sustainable approach for biohydrogen production. J. Chem. Eng. 2024, 2024, 148444. [Google Scholar] [CrossRef]
- UK First in ‘Clean Hydrogen’ Production and Carbon Capture. Available online: https://www.york.ac.uk/news-and-events/news/2024/research/clean-hydrogen-production-carbon-capture/ (accessed on 12 November 2024).
- Yang, Y.; Bu, J.; Tiong, Y.W.; Xu, S.; Zhang, J.; He, Y.; Zhu, M.; Tong, Y.W. Enhanced thermophilic dark fermentation of hydrogen production from food waste by Fe-modified biochar. Environ. Res. 2024, 244, 117946. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Zhu, L.; Du, L.; Ma, Y.; Ma, Y.; Yu, J.; Meng, A. Micro ribonucleic acid 27a aggravates ferroptosis during early ischemic stroke of rats through nuclear factor erythroid-2-related factor 2. Neuroscience 2022, 504, 10–20. [Google Scholar] [CrossRef]
- Zhao, B.; Dong, Z.; Sha, H.; Cao, S.; Duan, J.; Yuan, A.; Song, Z. Thermally modified tourmaline enhances hydrogen production by influencing hydrolysis acidification in two stages during dark fermentation of corn stover. Bioresour. Technol. 2023, 386, 129568. [Google Scholar] [CrossRef]
- Kamran, M. Bioenergy. In Renewable Energy Conversion Systems; Academic Press: Cambridge, MA, USA, 2021; Volume 243. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Fact. 2022, 21, 166. [Google Scholar] [CrossRef]
- Seppälä, J.J.; Puhakka, J.A.; Yli-Harja, O.; Karp, M.T.; Santala, V. Fermentative hydrogen production by Clostridium butyricum and Escherichia coli in pure and cocultures. Int. J. Hydrogen Energy 2011, 36, 10701–10708. [Google Scholar] [CrossRef]
- Nasr, N.; Gupta, M.; Hafez, H.; El Naggar, M.H.; Nakhla, G. Mono-and co-substrate utilization kinetics using mono-and coculture of Clostridium beijerinckii and Clostridium saccharoperbutylacetonicum. Bioresour. Technol. 2017, 241, 152–160. [Google Scholar] [CrossRef]
- Rafieenia, R.; Pivato, A.; Schievano, A.; Lavagnolo, M.C. Dark fermentation metabolic models to study strategies for hydrogen consumers inhibition. Bioresour. Technol. 2018, 267, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Hidalgo, A.M.; Smoliński, A.; Sanchez, A. A meta-analysis of research trends on hydrogen production via dark fermentation. Int. J. Hydrogen Energy 2022, 47, 13300–13339. [Google Scholar] [CrossRef]
- Liu, D.; Sun, Y.; Li, Y.; Lu, Y. Perturbation of formate pathway and NADH pathway acting on the biohydrogen production. Sci. Rep. 2017, 7, 9587. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, D.T.; Ogunbiyi, O.D.; Akamo, D.O.; Otun, K.O.; Akinpelu, D.A.; Adegoke, J.A.; Fapojuwo, D.P.; Oladoye, P.O. Factors affecting biohydrogen production: Overview and perspectives. Int. J. Hydrogen Energy 2023, 48, 27513–27539. [Google Scholar] [CrossRef]
- Sanchez-Ledesma, L.M.; Rodríguez-Victoria, J.A.; Ramírez-Malule, H. Effect of fermentation time, pH, and their interaction on the production of volatile fatty acids from Cassava Wastewater. Water 2024, 16, 1514. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Effect of temperature on biohydrogen and biomethane production using a biochemical potential test with different mixtures of sewage sludge, vinasse and poultry manure. J. Clean. Prod. 2023, 382, 135237. [Google Scholar] [CrossRef]
- Yin, Y.; Song, W.; Wang, J. Inhibitory effect of acetic acid on dark-fermentative hydrogen production. Bioresour. Technol. 2022, 364, 128074. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, Z.; Lu, X.; Song, Y.; Yuan, Z.; Hu, S. Significant in situ sludge yield reduction in an acidic activated sludge system. Water Res. 2024, 261, 122042. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Man, H.C.; Yusoff, M.Z.M.; Hassan, M.A. Microbial characterization of hydrogen-producing bacteria in fermented food waste at different pH values. Int. J. Hydrogen Energy 2011, 36, 9571–9580. [Google Scholar] [CrossRef]
- Tang, T.; Chen, Y.; Liu, M.; Du, Y.; Tan, Y. Effect of pH on the performance of hydrogen production by dark fermentation coupled denitrification. Environ. Res. 2022, 208, 112663. [Google Scholar] [CrossRef]
- Pandey, A.; Sinha, P.; Pandey, A. Hydrogen production by sequential dark and photofermentation using wet biomass hydrolysate of Spirulina platensis: Response surface methodological approach. Int. J. Hydrogen Energy 2021, 46, 7137–7146. [Google Scholar] [CrossRef]
- Mangayil, R.; Santala, V.; Karp, M. Fermentative hydrogen production from different sugars by Citrobacter sp. CMC-1 in batch culture. Int. J. Hydrogen Energy 2011, 36, 15187–15194. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Fuentes, K.L.; Arias, C.A.; Chaparro, T.R. Production of hydrogen from beverage wastewater by dark fermentation in an internal circulation reactor: Effect on pH and hydraulic retention time. Energy Convers. Manag. X 2022, 15, 100232. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Jung, K.W.; Kim, M.S.; Shin, H.S. Effect of initial pH independent of operational pH on hydrogen fermentation of food waste. Bioresour. Technol. 2011, 102, 8646–8652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Zhang, Q.; Li, G.; Xia, C. Comparison of bio-hydrogen and bio-methane production performance in continuous two-phase anaerobic fermentation system between co-digestion and digestate recirculation. Bioresour. Technol. 2020, 318, 124269. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; An, D.; Ren, N.; Zhang, Y.; Chen, Y. Effects of pH and ORP on microbial ecology and kinetics for hydrogen production in continuously dark fermentation. Bioresour. Technol. 2011, 102, 10875–10880. [Google Scholar] [CrossRef]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste biomass valorization for the production of biofuels and value-added products: A comprehensive review of thermochemical, biological and integrated processes. PSEP 2022, 159, 323–344. [Google Scholar] [CrossRef]
- Avargani, V.M.; Zendehboudi, S.; Saady, N.M.C.; Dusseault, M.B. A comprehensive review on hydrogen production and utilization in North America: Prospects and challenges. Energy Conv. Manag. 2022, 269, 115927. [Google Scholar] [CrossRef]
- Ziara, R.M.; Miller, D.N.; Subbiah, J.; Dvorak, B.I. Lactate wastewater dark fermentation: The effect of temperature and initial pH on biohydrogen production and microbial community. Int. J. Hydrogen Energy 2019, 44, 661–673. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tseng, Y.T.; Leu, H.J. Thermophilic biohydrogen fermentation of kitchen waste. Waste Biomass Valorization 2020, 11, 1041–1047. [Google Scholar] [CrossRef]
- Azbar, N.; Dokgöz, F.T.; Keskin, T.; Eltem, R.; Korkmaz, K.S.; Gezgin, Y.; Akbal, Z.; Oncel, S.; Dalay, M.C.; Gonen, C.; et al. Comparative evaluation of bio-hydrogen production from cheese whey wastewater under thermophilic and mesophilic anaerobic conditions. Int. J. Green Energy 2009, 6, 192–200. [Google Scholar] [CrossRef]
- Li, X.H.; Liang, D.W.; Bai, Y.X.; Fan, Y.T.; Hou, H.W. Enhanced H2 production from corn stalk by integrating dark fermentation and single chamber microbial electrolysis cells with double anode arrangement. Int. J. Hydrogen Energy 2014, 39, 8977–8982. [Google Scholar] [CrossRef]
- Wang, A.; Sun, D.; Cao, G.; Wang, H.; Ren, N.; Wu, W.M.; Logan, B.E. Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour. Technol. 2011, 102, 4137–4143. [Google Scholar] [CrossRef]
- Xue, S.; Chen, H.; Wang, F.; Lv, G.; Tan, L.; Liu, G. The effect of substrate acidification on the biohydrogen production by dark fermentation. Int. J. Hydrogen Energy 2024, 49, 177–188. [Google Scholar] [CrossRef]
- Mugnai, G.; Borruso, L.; Mimmo, T.; Cesco, S.; Luongo, V.; Frunzo, L.; Fabbricino, M.; Pirozzi, F.; Cappitelli, F.; Villa, F. Dynamics of bacterial communities and substrate conversion during olive-mill waste dark fermentation: Prediction of the metabolic routes for hydrogen production. Bioresour. Technol. 2021, 319, 124157. [Google Scholar] [CrossRef]
- Valentín-Reyes, J.; García-Reyes, R.B.; García-González, A.; Álvarez-Valencia, L.H.; Rivas-García, P.; de Jesús Cerino-Córdova, F. Mathematical modelling for biohydrogen production by Clostridium beijerinckii. Int. J. Hydrogen Energy 2018, 43, 17602–17610. [Google Scholar] [CrossRef]
- Yin, T.; Wang, W.; Zhuo, S.; Cao, G.; Ren, H.; Li, J.; Xing, D.; Xie, G.; Liu, B. Thermophilic dark fermentation fast start-up of hydrogen production with substrate concentration regulation and moderate pretreatment inoculum. Fuel 2023, 334, 126748. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhu, M.J. Influences of bisphenol A on hydrogen production from food waste by thermophilic dark fermentation, Environ. Res. 2024, 260, 119625. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Krisna, R.; Shukor, H.; Abdul, P.M.; AL-Rajabi, M.M.; Atabani, A.E.; Luthfi, A.A.I.; Gunny, A.A.N. Evaluation of biohydrogen production from rice straw hydrolysate via Clostridium sp. YM1: In-lab fermentation and techno-economic study. Int. J. Hydrogen Energy, 2024; in press. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Santiago, S.G.; Trably, E.; Latrille, E.; Buitrón, G.; Moreno-Andrade, I. The hydraulic retention time influences the abundance of Enterobacter, Clostridium and Lactobacillus during the hydrogen production from food waste. Lett. Appl. Microbiol. 2019, 69, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, W.; Zhang, X.; Zhang, G. Digestion of thermally hydrolyzed sewage sludge by anaerobic sequencing batch reactor. J. Hazard Mater. 2009, 162, 799–803. [Google Scholar] [CrossRef]
- Ri, P.C.; Kim, J.S.; Kim, T.R.; Pang, C.H.; Mun, H.G.; Pak, G.C.; Ren, N.Q. Effect of hydraulic retention time on the hydrogen production in a horizontal and vertical continuous stirred-tank reactor. Int. J. Hydrogen Energy 2019, 44, 17742–17749. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Anburajan, P.; Park, J.H.; Kumar, G.; Sivagurunathan, P.; Kim, S.H. Process performance of biohydrogen production using glucose at various HRTs and assessment of microbial dynamics variation via q-PCR. Int. J. Hydrogen Energy 2017, 42, 27550–27557. [Google Scholar] [CrossRef]
- Kumar, G.; Park, J.H.; Kim, M.S.; Kim, D.H.; Kim, S.H. Hydrogen fermentation of different galactose–glucose compositions during various hydraulic retention times (HRTs). Int. J. Hydrogen Energy 2014, 39, 20625–20631. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Duber, A.; Seifert, K. Dark-fermentative hydrogen production from synthetic lignocellulose hydrolysate by a mixed bacterial culture: The relationship between hydraulic retention time and pH conditions. Bioresour. Technol. 2022, 358, 127309. [Google Scholar] [CrossRef]
- Baik, J.H.; Jung, J.H.; Sim, Y.B.; Park, J.H.; Yang, J.; Kim, S.H. High-rate biohydrogen production from xylose using a dynamic membrane bioreactor. Bioresour. Technol. 2022, 344, 126205. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Principle and application of different pretreatment methods for enriching hydrogen-producing bacteria from mixed cultures. Int. J. Hydrogen Energy 2017, 42, 4804–4823. [Google Scholar] [CrossRef]
- Debabov, V.G. Acetogens: Biochemistry, bioenergetics, genetics, and biotechnological potential. Microbiology 2021, 90, 273–297. [Google Scholar] [CrossRef]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A. Genus, I. Clostridium Prazmowski, 1880. Bergey’s Manual of Systematic Bacteriol. In The Firmicutes, 9th ed.; Springer: New York, NY, USA, 2009; Volume 3, pp. 739–740. [Google Scholar]
- Junghare, M.; Subudhi, S.; Lal, B. Improvement of hydrogen production under decreased partial pressure by newly isolated alkaline tolerant anaerobe, Clostridium butyricum TM-9A: Optimization of process parameters. Int. J. Hydrogen Energy 2012, 37, 3160–3168. [Google Scholar] [CrossRef]
- Tai, J.; Adav, S.S.; Su, A.; Lee, D.J. Biological hydrogen production from phenol-containing wastewater using Clostridium butyricum. Int. J. Hydrogen Energy 2010, 35, 13345–13349. [Google Scholar] [CrossRef]
- Aly, S.S.; Imai, T.; Hassouna, M.S.; Nguyen, D.M.K.; Higuchi, T.; Kanno, A.; Yamamoto, K.; Akada, R.; Sekine, M. Identification of factors that accelerate hydrogen production by Clostridium butyricum RAK25832 using casamino acids as a nitrogen source. Int. J. Hydrogen Energy 2018, 43, 5300–5313. [Google Scholar] [CrossRef]
- Litti, Y.V.; Khuraseva, N.D.; Vishnyakova, A.V.; Zhuravleva, E.A.; Kovalev, A.A.; Kovalev, D.A.; Panchenko, V.A.; Parshina, S.N. Comparative study on biohydrogen production by newly isolated Clostridium butyricum SP4 and Clostridium beijerinckii SP6. Int. J. Hydrogen Energy 2023, 48, 27540–27556. [Google Scholar] [CrossRef]
- RamKumar, N.; Anupama, P.D.; Nayak, T.; Subudhi, S. Scale up of biohydrogen production by a pure strain; Clostridium butyricum TM-9A at regulated pH under decreased partial pressure. Renew. Energy 2021, 170, 1178–1185. [Google Scholar] [CrossRef]
- Kanchanasuta, S.; Prommeenate, P.; Boonapatcharone, N.; Pisutpaisal, N. Stability of Clostridium butyricum in biohydrogen production from non-sterile food waste. Int. J. Hydrogen Energy 2023, 42, 3454–3465. [Google Scholar] [CrossRef]
- Mahato, R.K.; Kumar, D.; Rajagopalan, G. Biohydrogen production from fruit waste by Clostridium strain BOH3. Renew. Energy 2020, 153, 1368–1377. [Google Scholar] [CrossRef]
- Moura, A.G.L.D.; Rabelo, C.A.B.D.S.; Okino, C.H.; Maintinguer, S.I.; Silva, E.L.; Varesche, M.B.A. Enhancement of Clostridium butyricum hydrogen production by iron and nickel nanoparticles: Effects on hydA expression. Int. J. Hydrogen Energy 2020, 45, 28447–28461. [Google Scholar] [CrossRef]
- Braga, J.K.; Stancari, R.A.; Motteran, F.; Malavazi, I.; Varesche, M.B.A. Metals addition for enhanced hydrogen, acetic and butyric acids production from cellulosic substrates by Clostridium butyricum. Biomass Bioenergy 2021, 150, 105679. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Isolation and characterization of a novel strain Clostridium butyricum INET1 for fermentative hydrogen production. Int. J. Hydrogen Energy 2017, 42, 12173–12180. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Zhang, Z.; Zhao, X.; Qi, N.; Han, T. Efficiency enhancement of H2 production by a newly isolated maltose-preferring fermentative bio-hydrogen producer of Clostridium butyricum NH-02. J. Energy Storage 2020, 30, 101426. [Google Scholar] [CrossRef]
- Beckers, L.; Masset, J.; Hamilton, C.; Delvigne, F.; Toye, D.; Crine, M.; Thonart, F.; Hiligsmann, S. Investigation of the links between mass transfer conditions, dissolved hydrogen concentration and biohydrogen production by the pure strain Clostridium butyricum CWBI1009. Biochem. Eng. J. 2015, 98, 18–28. [Google Scholar] [CrossRef]
- Hamilton, C.; Calusinska, M.; Baptiste, S.; Masset, J.; Beckers, L.; Thonart, P.; Hiligsmann, S. Effect of the nitrogen source on the hydrogen production metabolism and hydrogenases of Clostridium butyricum CWBI1009. Int. J. Hydrogen Energy 2018, 43, 5451–5462. [Google Scholar] [CrossRef]
- Subudhi, S.; Lal, B. Fermentative hydrogen production in recombinant Escherichia coli harboring a [FeFe]-hydrogenase gene isolated from Clostridium butyricum. Int. J. Hydrogen Energy 2011, 36, 14024–14030. [Google Scholar] [CrossRef]
- Plangklang, P.; Reungsang, A.; Pattra, S. Enhanced bio-hydrogen production from sugarcane juice by immobilized Clostridium butyricum on sugarcane bagasse. Int. J. Hydrogen Energy 2012, 37, 15525–15532. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, C.Y.; Cheng, C.L.; Lee, D.J.; Chang, J.S. Fermentative hydrogen production by Clostridium butyricum CGS5 using carbohydrate-rich microalgal biomass as feedstock. Int. J. Hydrogen Energy 2012, 37, 15458–15464. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Sarma, S.J.; Brar, S.K.; Le Bihan, Y.; Buelna, G.; Soccol, C.R. Evidence of metabolic shift on hydrogen, ethanol and 1,3-propanediol production from crude glycerol by nitrogen sparging under micro-aerobic conditions using coculture of Enterobacter aerogenes and Clostridium butyricum. Int. J. Hydrogen Energy 2015, 40, 8669–8676. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Sarma, S.J.; Brar, S.K.; Le Bihan, Y.; Buelna, G.; Verma, M. Surfactant mediated enhanced glycerol uptake and hydrogen production from biodiesel waste using coculture of Enterobacter aerogenes and Clostridium butyricum. Renew. Energy 2016, 95, 542–551. [Google Scholar] [CrossRef]
- Fangkum, A.; Reungsang, A. Biohydrogen production from sugarcane bagasse hydrolysate by elephant dung: Effects of initial pH and substrate concentration. Int. J. Hydrogen Energy 2011, 36, 8687–8696. [Google Scholar] [CrossRef]
- Sim, Y.B.; Yang, J.; Joo, H.H.; Jung, J.H.; Kim, D.H.; Kim, S.H. Effect of bioaugmentation using Clostridium butyricum on the start-up and the performance of continuous biohydrogen production. Bioresour. Technol. 2022, 366, 128181. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.H.; Baik, J.H.; Park, J.H.; Yoon, J.J.; Lee, C.Y.; Kim, S.H. Improvement in H2 production from Clostridium butyricum by coculture with Sporolactobacillus vineae. Fuel 2021, 285, 119051. [Google Scholar] [CrossRef]
- Yang, Y.; Nie, X.; Jiang, Y.; Yang, C.; Gu, Y.; Jiang, W. Metabolic regulation in solventogenic clostridia: Regulators, mechanisms and engineering. Biotechnol. Adv. 2018, 36, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Simons, A.D.; van der Wal, H.; Collas, F.; Houweling-Tan, B.; Kengen, S.W.; López-Contreras, A.M. L-Rhamnose metabolism in Clostridium beijerinckii strain DSM 6423. Appl. Environ. Microbiol. 2019, 85, e02656-18. [Google Scholar] [CrossRef] [PubMed]
- Trchounian, K.; Müller, N.; Schink, B.; Trchounian, A. Glycerol and mixture of carbon sources conversion to hydrogen by Clostridium beijerinckii DSM791 and effects of various heavy metals on hydrogenase activity. Int. J. Hydrogen Energy 2017, 42, 7875–7882. [Google Scholar] [CrossRef]
- Khalil, M.E.; Jain, A.; Yang, K.L.; Rajagopalan, G. A remarkable enhancement in hydrogen production from Clostridium beijerinckii G117 by the co-fermentation of crude glycerol and rice bran hydrolysates. Int. J. Hydrogen Energy 2023, 48, 33810–33826. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Meng, J.; Sun, K.; Yan, H. A neutral red mediated electro-fermentation system of Clostridium beijerinckii for effective co-production of butanol and hydrogen. Bioresour. Technol. 2021, 332, 125097. [Google Scholar] [CrossRef]
- Rosa, D.; Medeiros, A.B.P.; Martinez-Burgos, W.J.; do Nascimento Junior, J.R.; de Carvalho, J.C.; Sydney, E.B.; Soccol, C.R. Biological hydrogen production from palm oil mill effluent (POME) by anaerobic consortia and Clostridium beijerinckii. J. Biotech. 2020, 323, 17–23. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Laniecki, M. An unexpected negative influence of light intensity on hydrogen production by dark fermentative bacteria Clostridium beijerinckii. Bioresour. Technol. 2016, 200, 1039–1043. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, X.; Guo, B.; Finneran, K.T.; Zilles, J.L.; Morgenroth, E. Lignocellulosic hydrolysates and extracellular electron shuttles for H2 production using coculture fermentation with Clostridium beijerinckii and Geobacter metallireducens. Bioresour. Technol. 2013, 147, 89–95. [Google Scholar] [CrossRef]
- Zhao, X.; Xing, D.; Fu, N.; Liu, B.; Ren, N. Hydrogen production by the newly isolated Clostridium beijerinckii RZF-1108. Bioresour. Technol. 2011, 102, 8432–8436. [Google Scholar] [CrossRef]
- Skonieczny, M.T.; Yargeau, V. Biohydrogen production from wastewater by Clostridium beijerinckii: Effect of pH and substrate concentration. Int. J. Hydrogen Energy 2009, 34, 3288–3294. [Google Scholar] [CrossRef]
- Schwarz, K.M.; Grosse-Honebrink, A.; Derecka, K.; Rotta, C.; Zhang, Y.; Minton, N.P. Towards improved butanol production through targeted genetic modification of Clostridium pasteurianum. Metab. Eng. 2017, 40, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pradhan, N. A synergistic association between iron reduction and enhanced hydrogen production in Clostridium pasteurianum. Biochem. Eng. J. 2024, 2024, 109216. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Progress in microbiology for fermentative hydrogen production from organic wastes. Crit. Rev. Environ. Sci. Technol. 2019, 49, 825–865. [Google Scholar] [CrossRef]
- Yang, F.; Shi, H.; Mao, X.; Qi, L.; Zhu, M.; Tan, W.; Zhang, X. Mechanism of ZnFe2O4 NPs on biohydrogen production by Clostridium pasteurianum. Int. J. Hydrogen Energy 2024, 70, 557–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, S.; Hao, Q.; Wang, O.; Liu, F. Respiratory electrogen Geobacter boosts hydrogen production efficiency of fermentative electrotroph Clostridium pasteurianum. J. Chem. Eng. 2023, 456, 141069. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.; Wang, M.; Zhang, Z.; Feng, Y. Enhancement of magnetic field on fermentative hydrogen production by Clostridium pasteurianum. Bioresour. Technol. 2021, 341, 125764. [Google Scholar] [CrossRef]
- Wannapokin, A.; Huang, H.T.; Chang, P.H.; Chien, Y.W.; Hung, C.H. Improving production of biohydrogen from COOH-functionalized multiwalled carbon nanotubes through Co-immobilization with Clostridium pasteurianum. Int. J. Hydrogen Energy 2022, 47, 40704–40713. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Kushwaha, D.; Gupta, V.K.; Manikanta, A.; Ramteke, P.W.; Mishra, P.K. Efficient dark fermentative hydrogen production from enzyme hydrolyzed rice straw by Clostridium pasteurianum (MTCC116). Bioresour. Technol. 2017, 238, 552–558. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Wang, S.; Liu, F. Stimulation of ferrihydrite nanorods on fermentative hydrogen production by Clostridium pasteurianum. Bioresour. Technol. 2019, 283, 308–315. [Google Scholar] [CrossRef]
- Sarma, S.; Dubey, V.K.; Moholkar, V.S. Kinetic and thermodynamic analysis (with statistical optimization) of hydrogen production from crude glycerol using Clostridium pasteurianum. Int. J. Hydrogen Energy 2016, 41, 19972–19989. [Google Scholar] [CrossRef]
- Sarma, S.; Anand, A.; Dubey, V.K.; Moholkar, V.S. Metabolic flux network analysis of hydrogen production from crude glycerol by Clostridium pasteurianum. Bioresour. Technol. 2017, 242, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wannapokin, A.; Cheng, Y.T.; Wu, S.Z.; Hsieh, P.H.; Hung, C.H. Potential of bio-hydrogen production by C. pasteurianum co-immobilized with selected nano-metal particles. Int. J. Hydrogen Energy 2021, 46, 11337–11344. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Lai, Y.C.; Chen, K.Y.; Hung, C.H. Explore the possible effect of TiO2 and magnetic hematite nanoparticle addition on biohydrogen production by Clostridium pasteurianum based on gene expression measurements. Int. J. Hydrogen Energy 2016, 41, 21685–21691. [Google Scholar] [CrossRef]
- An, Q.; Cheng, J.R.; Wang, Y.T.; Zhu, M.J. Performance and energy recovery of single and two stage biogas production from paper sludge: Clostridium thermocellum augmentation and microbial community analysis. Renew. Energy 2020, 148, 214–222. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Islam, R.; Cicek, N.; Sparling, R.; Levin, D. Effect of substrate loading on hydrogen production during anaerobic fermentation by Clostridium thermocellum 27405. Appl. Microbiol. Biotechnol. 2006, 72, 576–583. [Google Scholar] [CrossRef]
- Islam, R.; Sparling, R.; Cicek, N.; Levin, D.B. Optimization of influential nutrients during direct cellulose fermentation into hydrogen by Clostridium thermocellum. Int. J. Mol. Sci. 2015, 16, 3116–3132. [Google Scholar] [CrossRef]
- Singer, S.; Magnusson, L.; Hou, D.; Lo, J.; Maness, P.C.; Ren, Z.J. Anaerobic membrane gas extraction facilitates thermophilic hydrogen production from Clostridium thermocellum. Environ. Sci. Water Res. Technol. 2018, 4, 1771–1782. [Google Scholar] [CrossRef]
- Cheng, J.R.; Zhu, M.J. Biohydrogen production from pretreated lignocellulose by Clostridium thermocellum. Biotechnol. Bioprocess Eng. 2016, 21, 87–94. [Google Scholar] [CrossRef]
- Kim, C.; Wolf, I.; Dou, C.; Magnusson, L.; Maness, P.C.; Chou, K.J.; Singer, S.; Sundstrom, E. Coupling gas purging with inorganic carbon supply to enhance biohydrogen production with Clostridium thermocellum. J. Chem. Eng. 2023, 456, 141028. [Google Scholar] [CrossRef]
- An, Q.; Wang, J.L.; Wang, Y.T.; Lin, Z.L.; Zhu, M.J. Investigation on hydrogen production from paper sludge without inoculation and its enhancement by Clostridium thermocellum. Bioresour. Technol. 2018, 263, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.Q.; Liang, L.; Zhu, M.J. Enhanced biohydrogen production from sugarcane bagasse by Clostridium thermocellum supplemented with CaCO3. Bioresour. Technol. 2015, 197, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, F.X.; Xing, X.H. Bioengineering of the Enterobacter aerogenes strain for biohydrogen production. Bioresour. Technol. 2011, 102, 8344–8349. [Google Scholar] [CrossRef]
- Elsamadony, M.; Elreedy, A.; Mostafa, A.; Fujii, M.; Gescher, J.; Shakeri Yekta, S.; Schnürer, A.; Gaillard, J.F.; Pant, D. Perspectives on potential applications of nanometal derivatives in gaseous bioenergy pathways: Mechanisms, life cycle, and toxicity. ACS Sustain. Chem. Eng 2021, 9, 9563–9589. [Google Scholar] [CrossRef]
- Ramprakash, B.; Sivaramakrishnan, R.; Subramani, K.; Incharoensakdi, A. Iron oxide treated Chlorella sp. for enhanced biomass and lipid content coupled to fermentative hydrogen production by Enterobacter aerogenes using hydrolyzate from pretreated biomass. Int. J. Hydrogen Energy 2024, 73, 43–53. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Liu, M.; Zhou, J.; Cen, K. Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using Enterobacter aerogenes. Bioresour. Technol. 2016, 207, 213–219. [Google Scholar] [CrossRef]
- Praptyana, I.R. Biohydrogen production from wood dust mahogany (Swietenia mahagony) by dark fermentation using Enterobacter aerogenes: Effect of ozone pretreatment time and pH. Mater. Today Proc. 2022, 63, S203–S209. [Google Scholar] [CrossRef]
- Jiang, K.; Bai, R.; Gao, T.; Lu, P.; Zhang, J.; Zhang, S.; Xu, F.; Wang, S.; Zhao, H. Optimization of hydrogen production in Enterobacter aerogenes by Complex I peripheral fragments destruction and maeA overexpression. Microb. Cell Fact. 2023, 22, 137. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by Enterobacter aerogenes 2822. Int. J. Hydrogen Energy 2021, 46, 1777–1800. [Google Scholar] [CrossRef]
- Liu, F.; Fang, B. Optimization of bio-hydrogen production from biodiesel wastes by Klebsiella pneumoniae. Biotechnol. J. 2007, 2, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Zhang, X.; Tan, W.S.; Zhu, M.L. Characteristics of fermentative hydrogen production with Klebsiella pneumoniae ECU-15 isolated from anaerobic sewage sludge. Int. J. Hydrogen Energy 2010, 35, 71–80. [Google Scholar] [CrossRef]
- Estevam, A.; Arantes, M.K.; Andrigheto, C.; Fiorini, A.; da Silva, E.A.; Alves, H.J. Production of biohydrogen from brewery wastewater using Klebsiella pneumoniae isolated from the environment. Int. J. Hydrogen Energy 2018, 43, 4276–4283. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Zhu, M.; Tan, W. Effect of the culture media optimization, pH and temperature on the biohydrogen production and the hydrogenase activities by Klebsiella pneumoniae ECU-15. Bioresour. Technol. 2013, 137, 9–17. [Google Scholar] [CrossRef]
- Wu, K.J.; Lin, Y.H.; Lo, Y.C.; Chen, C.Y.; Chen, W.M.; Chang, J.S. Converting glycerol into hydrogen, ethanol, and diols with a Klebsiella sp. HE1 strain via anaerobic fermentation. J. Taiwan Inst. Chem. Eng. 2011, 42, 20–25. [Google Scholar] [CrossRef]
- Chookaew, T.; Sompong, O.; Prasertsan, P. Fermentative production of hydrogen and soluble metabolites from crude glycerol of biodiesel plant by the newly isolated thermotolerant Klebsiella pneumoniae TR17. Int. J. Hydrogen Energy 2012, 37, 13314–13322. [Google Scholar] [CrossRef]
- Maintinguer, S.I.; Fernandes, B.S.; Duarte, I.C.; Saavedra, N.K.; Adorno, M.A.T.; Varesche, M.B.A. Fermentative hydrogen production with xylose by Clostridium and Klebsiella species in anaerobic batch reactors. Int. J. Hydrogen Energy 2011, 36, 13508–13517. [Google Scholar] [CrossRef]
- Chookaew, T.; Sompong, O.; Prasertsan, P. Statistical optimization of medium components affecting simultaneous fermentative hydrogen and ethanol production from crude glycerol by thermotolerant Klebsiella sp. TR17. Int. J. Hydrogen Energy 2014, 39, 751–760. [Google Scholar] [CrossRef]
- Chookaew, T.; Sompong, O.; Prasertsan, P. Biohydrogen production from crude glycerol by immobilized Klebsiella sp. TR17 in a UASB reactor and bacterial quantification under non-sterile conditions. Int. J. Hydrogen Energy 2014, 39, 9580–9587. [Google Scholar] [CrossRef]
- Liu, W.; Pang, J.; Wu, D.; Zhang, L.; Xing, D.; Hu, J.; Li, Y.; Liu, Z. Hydrogen production by a novel Klebsiella pneumoniae strain from sheep rumen uses corn straw as substrate. Energy 2023, 282, 128210. [Google Scholar] [CrossRef]
- Mohammed, A.; Firdaus, A.M.; Zaharah, I.; Mohammed, I.L.; Mohammed, J.N.; Balogun, T.V. Investigation and Scaling of Hydrogen Production by Klebsiella sp. ABZ11 for Optimal Yield and the Kinetics of Batch Fermentation Process. JASEM 2022, 26, 2113–2124. [Google Scholar] [CrossRef]
- Tuttle, A.R.; Trahan, N.D.; Son, M.S. Growth and maintenance of Escherichia coli laboratory strains. Curr. Protoc. 2021, 1, e20. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, B.; Khanna, N.; Das, D. Dark-fermentative biohydrogen production. In Biomass, Biofuels and Biochemical: Biohydrogen, 2nd ed.; Pandey, A., Venkata Mohan, S., Chang, J.S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–122. [Google Scholar] [CrossRef]
- Trchounian, K.; Sawers, R.G.; Trchounian, A. Improving biohydrogen productivity by microbial dark-and photo-fermentations: Novel data and future approaches. Renew. Sustain. Energy Rev. 2017, 80, 1201–1216. [Google Scholar] [CrossRef]
- Soo, C.S.; Yap, W.S.; Hon, W.M.; Phang, L.Y. Mini review: Hydrogen and ethanol co-production from waste materials via microbial fermentation. WJMB 2015, 31, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Sundara Sekar, B.; Seol, E.; Park, S. Co-production of hydrogen and ethanol from glucose in Escherichia coli by activation of pentose-phosphate pathway through deletion of phosphoglucose isomerase (PGI) and overexpression of glucose-6-phosphate dehydrogenase (ZWF) and 6-phosphogluconate dehydrogenase (GND). Biotechnol. Biofuels 2017, 10, 85. [Google Scholar] [CrossRef]
- Ramprakash, B.; Incharoensakdi, A. Dark fermentative hydrogen production from pretreated garden wastes by Escherichia coli. Fuel 2022, 310, 122217. [Google Scholar] [CrossRef]
- Teramoto, H.; Suda, M.; Inui, M. Effects of potential inhibitors present in dilute acid-pretreated corn stover on fermentative hydrogen production by Escherichia coli. Int. J. Hydrogen Energy 2022, 47, 29219–29229. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Magaña, G.; Rodriguez, F.; De Leon-Rodriguez, A.; Sanchez, A. Co-production of ethanol-hydrogen by genetically engineered Escherichia coli in sustainable biorefineries for lignocellulosic ethanol production. J. Chem. Eng. 2021, 406, 126829. [Google Scholar] [CrossRef]
- Rosales-Colunga, L.M.; Razo-Flores, E.; Rodríguez, A.D.L. Fermentation of lactose and its constituent sugars by Escherichia coli WDHL: Impact on hydrogen production. Bioresour. Technol. 2012, 111, 180–184. [Google Scholar] [CrossRef]
- Poladyan, A.; Margaryan, L.; Trchounian, K.; Trchounian, A. Biomass and biohydrogen production during dark fermentation of Escherichia coli using office paper waste and cardboard. Int. J. Hydrogen Energy 2020, 45, 286–293. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Hernandez, V.E.B.; De Leon-Rodriguez, A. Scale-up of hydrogen and ethanol co-production by an engineered Escherichia coli. Fuel 2021, 300, 121002. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Lin, C.Y. Hydrogen and ethanol fermentation of various carbon sources by immobilized Escherichia coli (XL1-Blue). Int. J. Hydrogen Energy 2014, 39, 6881–6888. [Google Scholar] [CrossRef]
- Maru, B.T.; López, F.; Kengen, S.W.M.; Constantí, M.; Medina, F. Dark fermentative hydrogen and ethanol production from biodiesel waste glycerol using a coculture of Escherichia coli and Enterobacter sp. Fuel 2016, 186, 375–384. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Y.; Gao, R.; Tong, J.; Yang, Z. Transferring [NiFe] hydrogenase gene from Rhodopeseudomonas palustris into E. coli BL21 (DE3) for improving hydrogen production. Int. J. Hydrogen Energy 2015, 40, 4329–4336. [Google Scholar] [CrossRef]
- Sharma, P.; Melkania, U. Biochar-enhanced hydrogen production from organic fraction of municipal solid waste using coculture of Enterobacter aerogenes and E. coli. Int. J. Hydrogen Energy 2017, 42, 18865–18874. [Google Scholar] [CrossRef]
- Sharma, P.; Melkania, U. Impact of heavy metals on hydrogen production from organic fraction of municipal solid waste using coculture of Enterobacter aerogenes and E. coli. Waste Manag. 2018, 75, 289–296. [Google Scholar] [CrossRef]
- Rosales-Colunga, L.M.; García, R.G.; Rodríguez, A.D.L. Estimation of hydrogen production in genetically modified E. coli fermentations using an artificial neural network. Int. J. Hydrogen Energy 2010, 35, 13186–13192. [Google Scholar] [CrossRef]
- Hassan, S.H.; Morsy, F.M. Feasibility of installing and maintaining anaerobiosis using Escherichia coli HD701 as a facultative anaerobe for hydrogen production by Clostridium acetobutylicum ATCC 824 from various carbohydrates. Enzyme Microb. Technol. 2015, 81, 56–62. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Li, Y.; Tao, L.; Li, T. Optimization of pH conditions to improve the spore production of Clostridium butyricum NN-2 during fermentation process. Arch. Microbiol. 2020, 202, 1251–1256. [Google Scholar] [CrossRef]
- Seelert, T.; Ghosh, D.; Yargeau, V. Improving biohydrogen production using Clostridium beijerinckii immobilized with magnetite nanoparticles. Appl. Microbiol. Biotechnol. 2015, 99, 4107–4116. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, Y.; Sun, H.; Fu, B.; Zhang, Q.; Wu, C.; Shen, J.; Liu, D.; Wang, Y. Epidemiology and genetic characterization of tet (X4)-positive Klebsiella pneumoniae and Klebsiella quasipneumoniae isolated from raw meat in Chengdu City, China. Biosaf. Health 2024, 6, 116–124. [Google Scholar] [CrossRef]
- Wang, R.Y.; Shi, Z.Y.; Chen, J.C.; Wu, Q.; Chen, G.Q. Enhanced co-production of hydrogen and poly-(R)-3-hydroxybutyrate by recombinant PHB producing E. coli over-expressing hydrogenase 3 and acetyl-CoA synthetase. Metab. Eng. 2012, 14, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.N.; Hu, B.B.; Zhu, M.J. Enhanced hydrogen production and sugar accumulation from spent mushroom compost by Clostridium thermocellum supplemented with PEG8000 and JFC-E. Int. J. Hydrogen Energy 2016, 41, 2383–2390. [Google Scholar] [CrossRef]


| Strain | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 Yield as Mol H2/mol Substrate * | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| DSM2478, NCIMB8082 | Glucose (3) | 37 | 6.5 | Anaerobic jars purged with N2 | Batch (250) | 2.09 | [23] | |
| TM-9A | Glucose (10) | 37 | 8.0 | 24 | 10.1 kPa | Batch (67) | 3.10 | [67] |
| RAK25832 | Glucose (10) | 30 | 8.0 | Serum bottles flushed with N2 | Batch (75) | 1.81 | [69] | |
| SP4 | Hexose and pentose (5) | 30 | 110 rpm, glass vials in N2 flow | Batch (60) | 0.93–1.52 | [70] | ||
| TM-9A | Sugarcane molasses | 37 | 7.5 | 24 | Serum bottles | Batch (120) | 3.34 (synthetic analytical grade glucose) | [71] |
| TISTR 1032 | Synthetic food waste with volatile solids (28) | 37 | 6.0 | 100 rpm | Semi-batch (5000) | 0.02 (volatile solids) | [72] | |
| BOH3 | Fruit waste with sugar (10) | 6.8 | 24 | Purged with N2 | Batch (250) | 2.30 | [73] | |
| CGS2 | Glucose (5) | 37 | 6.8 | 130 rpm | Batch (250) | 0.95 | [74] | |
| CCT 7470 | Cellulose and glucose (5) | 37 | Cellulose medium | Batch (250) | 0.19 (cellulose), 0.58 (glucose) | [75] | ||
| INET1 | Glucose (10) | 35 | 7.0 | 10% v/v inoculum, purged with N2, 100 rpm | Batch (150) | 2.07 | [76] | |
| NH-02 (MT229351) | Maltose (10) | 30 | 7.0 | 23 | Purged with argon, 4% v/v inoculum | Batch (100) | 1.90 | [77] |
| CWBI1009 | Glucose (5) | 7.3 | 400 rpm, N2 sparging | Batch (2500) | 3.10 | [78] | ||
| CWBI1009 | Glucose (5) | 30 | 7.3 | N2 flushing | Batch (270) | 1.43 | [79] | |
| TERI BH05-2 in recombinant E. coli | Glucose (10) | 37 | 7.0 | 48 | N2 flushing, 100 rpm | Batch (67) | 3.20 | [80] |
| TISTR 1032 | Sucrose (25) | 6.5 | Sugarcane bagasse for immobilization | Batch | 1.34 | [81] | ||
| CGS5 | Microalgal biomass hydrolysate (9) | 37 | 5.5 | 30 | Batch (20) | 1.15 | [82] | |
| NRRL B-4112 | Glycerol (10) | 168 | N2 sparging | 1.02 | [83] | |||
| NRRL B-41122 with Enterobacter aerogenes NRRL B-407 | Glycerol (10) | 36 | 6.5 | 150 rpm | Batch (47) | 1.25 | [84] | |
| TISTR 1032 | Sugarcane juice | 37 | 6 | 36 | 10% v/v inoculum, 150 rpm | Continuous (1000) | 1.0 | [85] |
| DSM 10702 | Glucose (5) | 5.5 | 12 | N2 purging | Batch + continuous (4500) | 2.61 | [86] | |
| KCCM 35433 with Sporolactobacillus vineae KCCM 11493BP | Glucose (5) | 35 | 32 | 200 rpm | Batch (160) | 1.84 | [87] | |
| Strain | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 yield as Mol H2/mol Substrate | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| DSM 1820 | Glucose (2) | 37 | 10% v/v, flushed with N2, 100 rpm | Batch (180) | 2.70 ± 0.20 | [24] | ||
| Not specified | Dextrose (3) | 37 | 7.5 | 96 | Flushed with N2, 120 rpm | Batch | 3.58 | [52] |
| DSM 791 | Glycerol (11) | 37 | 7.5 | 96 | Batch (120) | 1.21 | [90] | |
| G117 | Glycerol (20–80) | 39 | 6.8 | 12–72 | Purged with N2, 150 rpm | Batch (120) | 1.18–1.45 | [91] |
| NCIMB 8052 | Glucose (20) | 37 | 6.5 | Cathodic electrofermentation, graphite felt electrode, 130 rpm, sparged with N2 | Batch (150) | 1.51 | [92] | |
| ATCC 8260 | Sucrose (7.5) | 37 | 6.0–7.0 | 1 mL inoculum | Batch (15) | 9.39 | [93] | |
| NCIMB-8052 | Glucose (3) | 32 | 6.5 | 120 | Sparged with N2, 10% v/v inoculum, 120 rpm, light illumination | Batch (60) | 2.47 | [94] |
| NCIMB 8052 | Glucose:xylose 1:5 w/w (3) | 30 | Flushed with N2, 3% v/v inoculum | Batch (26) | 0.09 | [95] | ||
| RZF-118 | Glucose (9) | 35 | 7.0 | 20 | Flushed with N2, 8% v/v inoculum, 140 rpm | Batch (100) | 1.97 | [96] |
| ATCC 8260 | Glucose (3) | 30 | 6.3 | Flushed with argon, 3% v/v inoculum, 180 rpm | Batch (100) | 0.191 | [97] | |
| Strain | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 Yield as Mol H2/mol Substrate | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| Glucose (0.12) | 30 | 7.0 | 60 | Batch (100) | 2.34 ± 0.02 | [103] | ||
| CH5 | Glucose | 35 | 7.0 | 120 rpm | Batch (120) | 1.61 | [104] | |
| MTCC116 | Glucose (54.18) | 37 | 6.6 | 96 | 10% v/v inoculum, 192 rpm | Batch (150) | 3.60·10−3 | [105] |
| DSM525 | Glucose | 37 | Flushing with N2, 5% v/v inoculum, ferrihydrite nanorods | Batch (25) | 3.55 | [106] | ||
| Glycerol (7.4) | 36 | 6.7 | 10% v/v inoculum, 150 rpm | Batch | 0.63 | [107] | ||
| MTCC 116 | Glycerol (7.4) | 36 | 6.7 | 10% v/v inoculum, flushing with N2, 150 rpm | Batch (100) | 2.00–3.00 | [108] | |
| CH5 | Xylose (40) | 35 | 6.8 | Flushed with argon, 120 rpm, 400 mg/L nanometal particles for bacteria immobilization | Batch (120) | 0.16 | [109] | |
| CH5 | Xylose (40) | 35 | 10 mL inoculum, nanometal and soluble iron, 120 rpm, flushed with argon | Batch (120) | 1.46 | [110] | ||
| Strain | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 Yield as Mol H2/mol Substrate * | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| DSM 1237 | Cellulose (25) | 60 | 7.3–7.4 | Batch (20) | 1.30 (hexose) | [114] | ||
| DSM 1313 | Cellulose (5) | 60 | 7.0 | Argon flushing, 60 rpm | Continuous (2000) | 0.76–1.21 (hexose) | [115] | |
| 27405 | Cellulose (5) | 55 | 7.0 | 168 | Purged with N2, 150 rpm | Batch (120) | 1.92 (hexose) | [116] |
| KJC315 | Cellobiose (5) | 60 | 7.0 | 24 | Purged with N2, 100 rpm, 33% CO2 | Batch (130) | 3.26 (hexose) | [117] |
| DSM1313 | Cellulose (10) | 55 | 192 | Purged with N2, 10% v/v inoculum, 150 rpm | Batch (130) | 1.15 (hexose) | [118] | |
| ATCC 27405 | Cellulose (3) | 55 | 168 | 20 mM CaCO3, 10% (v/v) inoculum, 150 rpm | Batch (120) | 5.87 (hexose) | [119] | |
| Strain | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 Yield as Mol H2/mol Substrate * | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| Fermentable sugar | 37 | 72 | Anaerobic, Fe2O3 nanoparticles | Batch (100) | 2.60 | [122] | ||
| ATCC13408 | Glucose (3) | 37 | 6 | Aerobic, 220 rpm, purging with N2, Fe2O3 nanoparticles 200 mg/L | Batch (200) | 1.32–1.55 | [123] | |
| ATCC 13048 | Mahogany wood hydrolysate and preculture medium (45) | 37 | 48 | 120 rpm, 15% v/v inoculum | Batch (250) | 0.03 (glucose) | [124] | |
| IAM1183 | Glucose (15) | 37 | 20 | 200 rpm, purging with N2 | Batch (100) | 1.34 ± 0.21 | [125] | |
| 2822 | Cheese whey (32.5) | 31 | 6.5 | 104 | 250 rpm, 10% v/v inoculum, flushing with argon | Batch (2000) | 0.26 (lactose) | [126] |
| Strain | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 Yield as Mol H2/mol Substrate * | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| DSM 2026 | Glycerol (20.4) | 37 | 6.5 | 24 | 10% v/v inoculum | Batch (5000) | 0.80 | [127] |
| ECU-15 | Glucose (10), Xylose (2), and Cellobiose (1.5) | 150 rpm, N2 flushing | Batch (1000) | 2.07 (glucose) | [128] | |||
| MGH 78578 | Brewery wastewater (3–4) | 35 | 5.5 | 72 | 10% v/v inoculum, 90 rpm | Batch (250) | 0.80–1.67 (glucose) | [129] |
| ECU-15 | Glucose (35.62) | 37 | 6.0 | 150 rpm, flushed with N2 | Batch (1000) | 1.22 | [130] | |
| HE1 | Glycerol (50) | 35 | 6.0 | 200 rpm | Batch (2500) | 0.34 | [131] | |
| TR17 | Glycerol (20) | 40 | 8.0 | 24 | Batch (60) | 0.25 | [132] | |
| Sucrose (3.588) | 37 | 5.5 | 48 | 20% v/v inoculum, 8500 rpm | Batch (100) | 0.80 (xylose) | [133] | |
| TR17 | Glycerol (11.4) | 40 | 8.0 | 10% v/v inoculum, flushed with N2 | Batch (60) | 0.26 | [134] | |
| TR17 | Glycerol (10) | 40 | 8.0 | 12 | Up-flow anaerobic sludge blanket reactor | Batch (1000) | 4.08 | [135] |
| Y7-3 | Corn straw (50) | 37 | 24 | 5% v/v inoculum, 220 rpm | Batch (100) | 0.18 | [136] | |
| ABZ11 | Glucose (9.15) | 34 | 6.8 | 48 | 150 rpm | Batch (2000) | 2.71 | [137] |
| Strain | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 Yield as Mol H2/mol Substrate * | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| K-12 | Garden waste (cellulose, 13) | 33 | Purged with argon | Batch (100) | 2.73 (cellulose) | [143] | ||
| W3110 | Acetate (10) | 33 | 6.3 | Purged with 95% N2 | Batch (69) | 0.21 | [144] | |
| W3110 | Hemi cellulosic hydrolysates (10–15) | 31 | 8.2 | 200 rpm | Batch (10) | 1.15–1.73 mol H2/mol substrate | [145] | |
| WDHL | Glucose (15) Lactose (15) Galactose (15) | 37 | 6.0 | 175 rpm | Batch (1000) | 0.30 1.02 (hexose) 1.12 | [146] | |
| BW25113 | Glucose | 37 | 7.5 | Batch (100) | 0.05 | [147] | ||
| WDH-LF | Glucose | 31 | 8.2 | 400 rpm | Batch (10,000) | 2.82 | [148] | |
| XL1-Blue | Fructose (5) Glucose (5) Xylose (5) | 35 | 6.5 | Purged with argon, 150 rpm | Batch (130) | 1.17 (fructose) 0.96 (glucose) 0.69 (xylose) | [149] | |
| CECT432, CECT434 and E. cloacae MCM2/1 | Glycerol (20) | 37 | 6.3 | 72 | 10% v/v inoculum, purged with argon | Batch (1200) | 4.40·10−3 | [150] |
| BH20 | Glucose (4) | 37 | 16 | 120 rpm | Batch (200) | 0.32 ± 0.01 | [151] | |
| E. coli and Enterobacter aerogenes | Acetate (0.563), butyrate (0.537), propionate (0.059), and lactate (0.214) | 37 | 5.5 | 12.5 g/L biochar, purged with N2 | Batch (500) | 0.33 | [152] | |
| E. coli and Enterobacter aerogenes | Acetate (0.608), butyrate (0.516), propionate (0.051), and lactate (0.191) | 37 | 5.5 | Purged with N2, 50 mL inoculum, gas sampling every 24 h, 10 mg/L copper | Batch (500) | 0.15 | [153] | |
| WDHL | Cheese whey powder | 37 | 175 rpm | Batch (1000) | 1.50·10−3 | [154] | ||
| HD701 | Mixture of glucose, sucrose, starch, acid-hydrolyzed sucrose, and starch | 35 | 7.0 | 10% v/v inoculum, sparged with N2 | Batch (1000) | 2.00 (glucose) | [155] | |
| Bacteria | Advantages | Disadvantages |
|---|---|---|
| C. butyricum |
|
|
| C. beijerinckii |
|
|
| C. pasteurianum |
| |
| C. thermocellum |
|
|
| E. aerogenes |
|
|
| K. pneumoniae |
|
|
| E. coli |
|
| Bacteria | Substrate (Concentration, g/L) | Conditions | Operation Mode (Reactor Volume, mL) | H2 Yield as Mol H2/mol Substrate | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | pH | Time (h) | More Details | |||||
| C. butyricum TM-9A | Sugarcane molasses | 37 | 7.5 | 24 | Serum bottles | Batch (120) | 3.34 (synthetic analytical grade glucose) | [71] |
| C. beijerinckii | Sucrose (7) | 37 | 6.0−7.0 | 1 mL inoculum | Batch (15) | 9.39 | [93] | |
| C. pasteurianum DSM525 | Glucose | 37 | Flushing with N2, 5% inoculum, ferrihydrite nanorods | Batch (25) | 3.55 | [106] | ||
| C. thermocellum ATCC 27405 | Cellulose (3) | 55 | 168 | 10% (v/v) inoculum, 150 rpm, 20 mM CaCO3 | Batch (120) | 5.87 (hexose) | [119] | |
| E. aerogenes | Fermentable sugar | 37 | 72 | Anaerobic, Fe2O3 nanoparticles | Batch (100) | 2.60 | [122] | |
| Klebsiella species TR17 | Glycerol (10) | 40 | 8.0 | 12 | Up-flow anaerobic sludge blanket reactor | Batch (1000) | 4.08 | [135] |
| E. coli WDH-LF | Glucose (16) | 31 | 8.2 | 400 rpm | Batch (10,000) | 2.82 | [148] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production. Appl. Sci. 2024, 14, 10789. https://doi.org/10.3390/app142310789
Gupta S, Fernandes A, Lopes A, Grasa L, Salafranca J. Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production. Applied Sciences. 2024; 14(23):10789. https://doi.org/10.3390/app142310789
Chicago/Turabian StyleGupta, Soumya, Annabel Fernandes, Ana Lopes, Laura Grasa, and Jesús Salafranca. 2024. "Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production" Applied Sciences 14, no. 23: 10789. https://doi.org/10.3390/app142310789
APA StyleGupta, S., Fernandes, A., Lopes, A., Grasa, L., & Salafranca, J. (2024). Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production. Applied Sciences, 14(23), 10789. https://doi.org/10.3390/app142310789









