Effects of CO2 Aeration and Light Supply on the Growth and Lipid Production of a Locally Isolated Microalga, Chlorella variabilis RSM09
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Isolation of Microalgae
2.2. Genetic Identification of Isolated Microalgae
2.3. Effect of CO2 Concentration on Growth of Microalgae
2.4. Effect of Light Intensity and Photoperiod on Growth of Microalgae
2.5. Microalgal Growth Determination
2.6. Microalgal Lipid Extraction
2.7. Fatty Acid Composition Analysis
2.8. Statistical Analysis
3. Results and Discussion
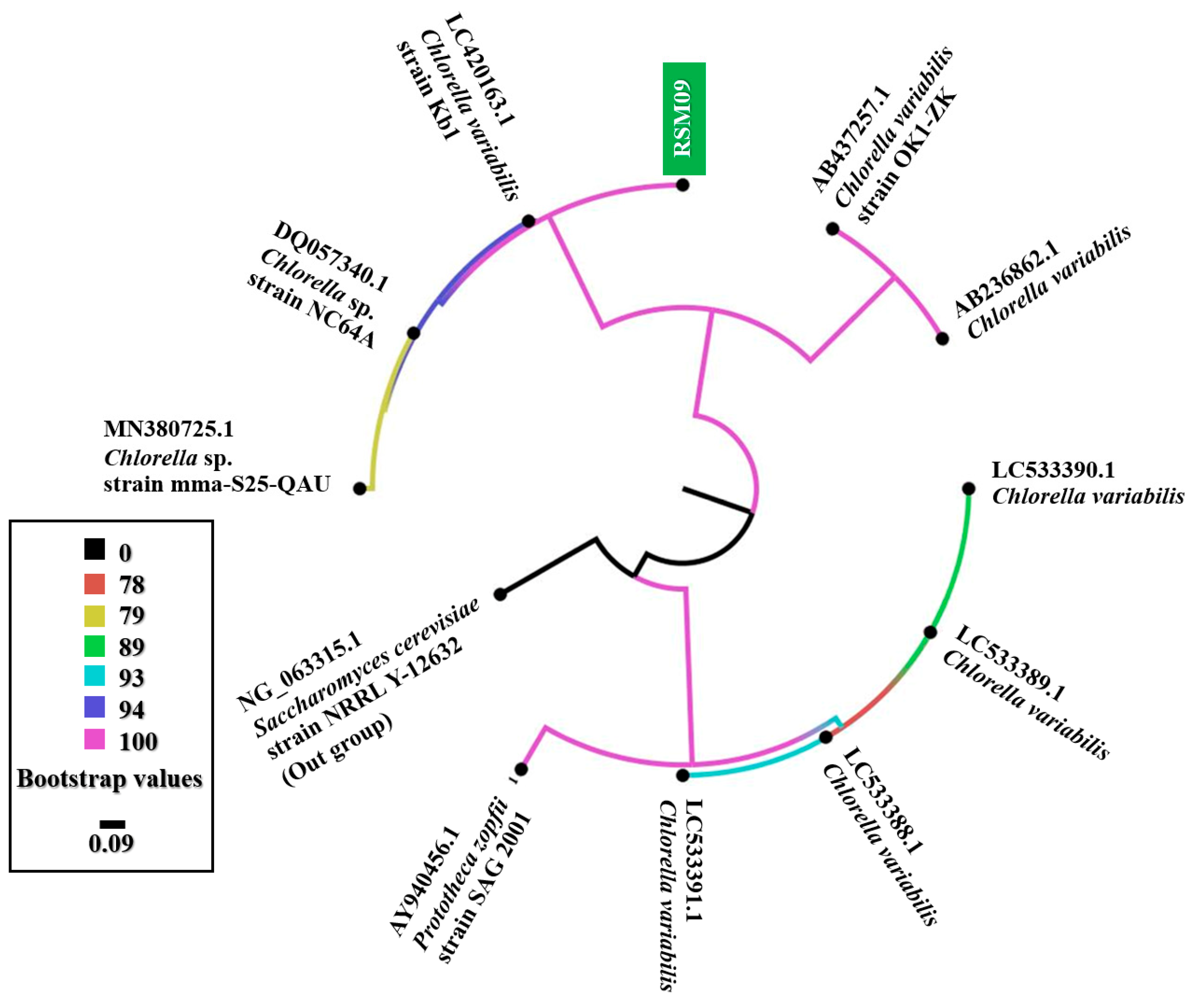
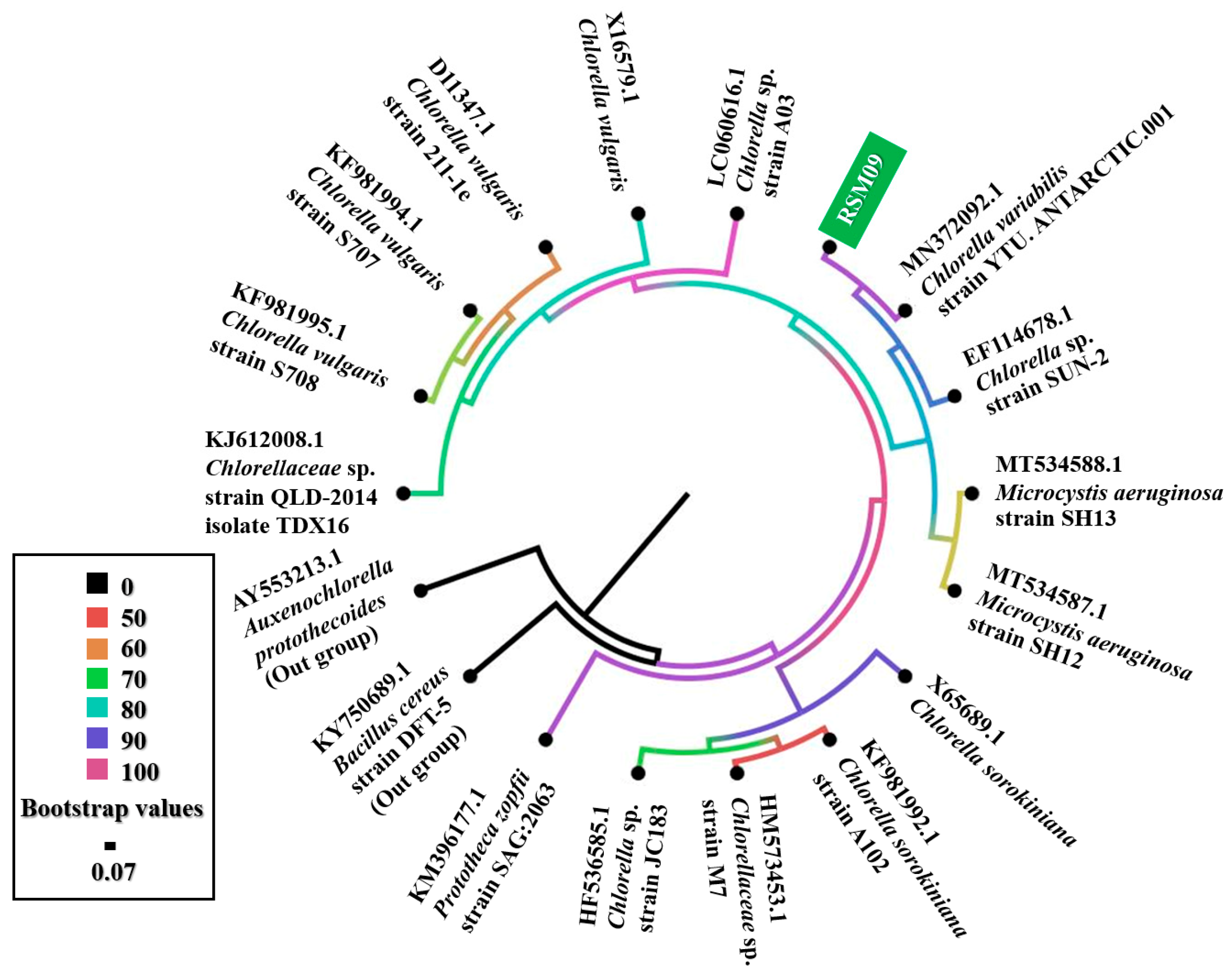
3.1. Genetic Identification of Isolated Microalgae
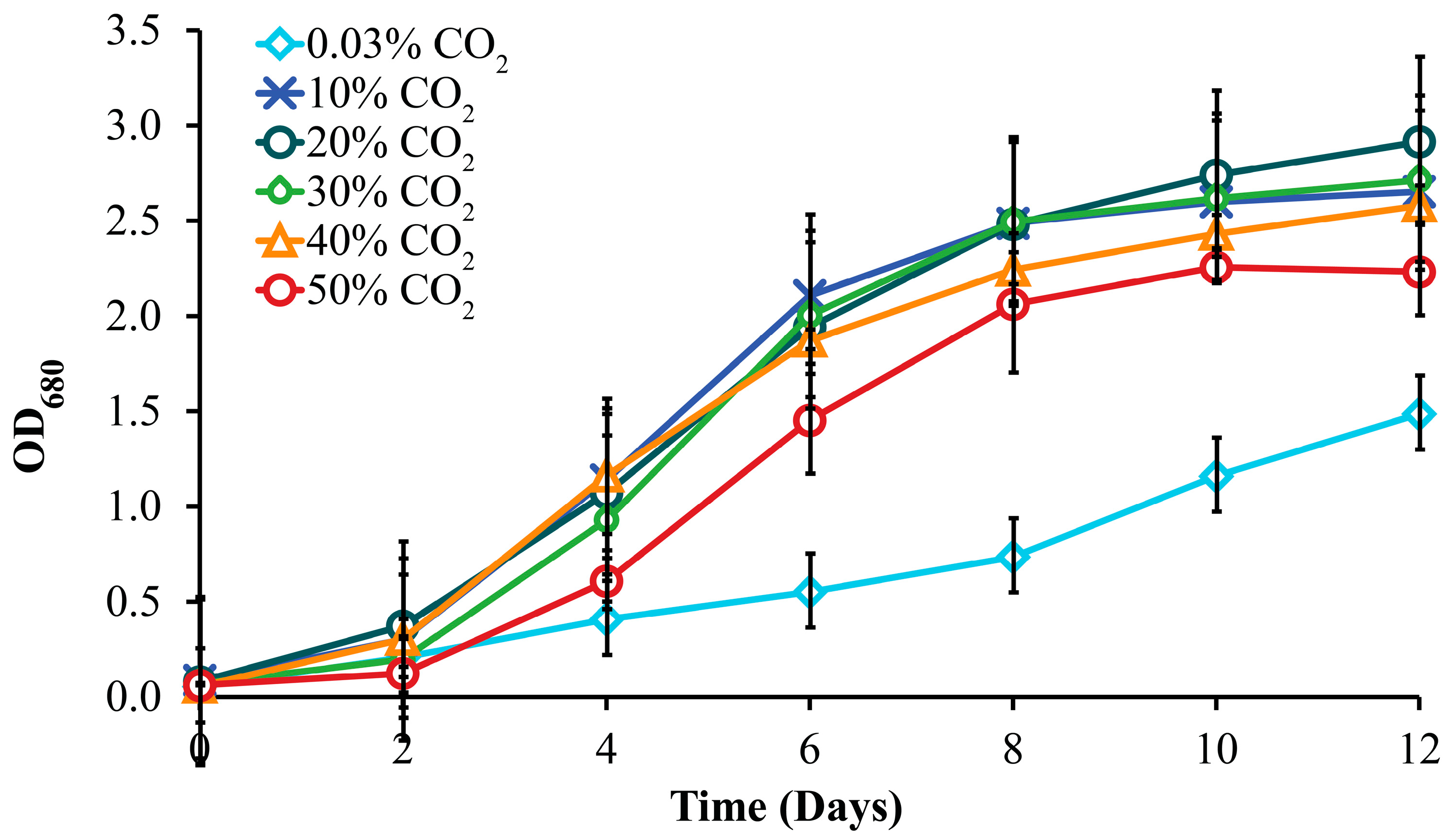
3.2. Effect of CO2 Concentration
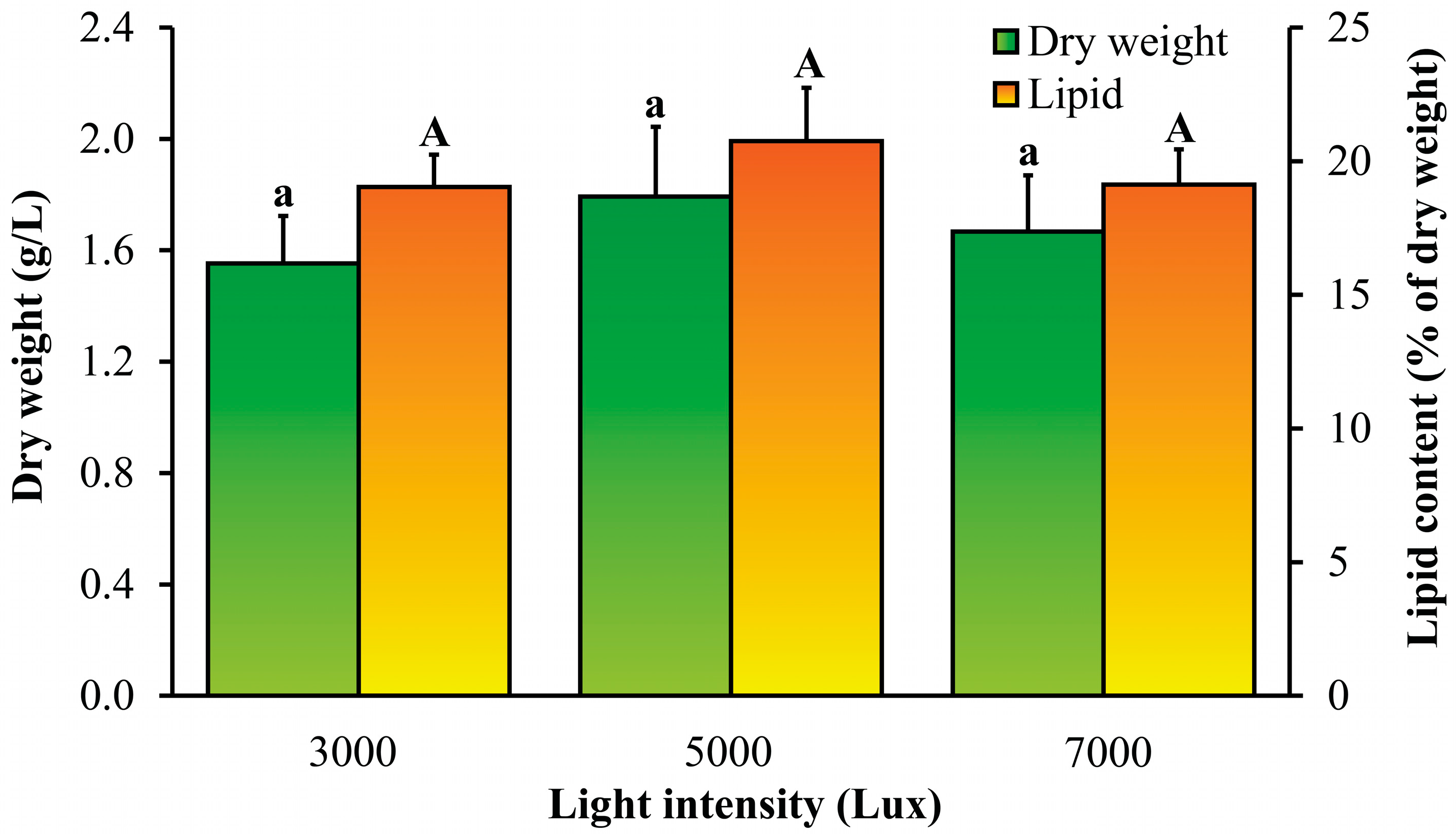
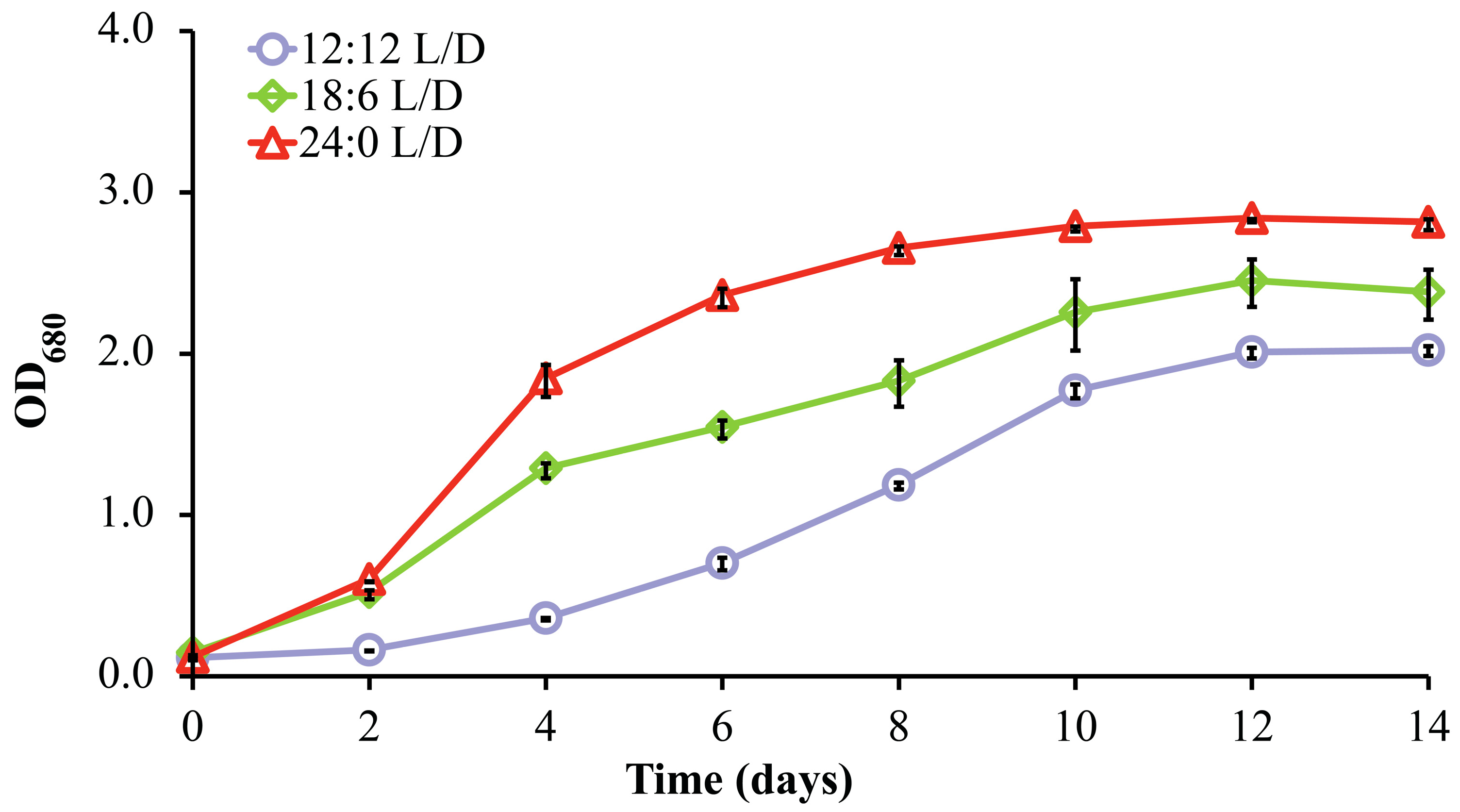
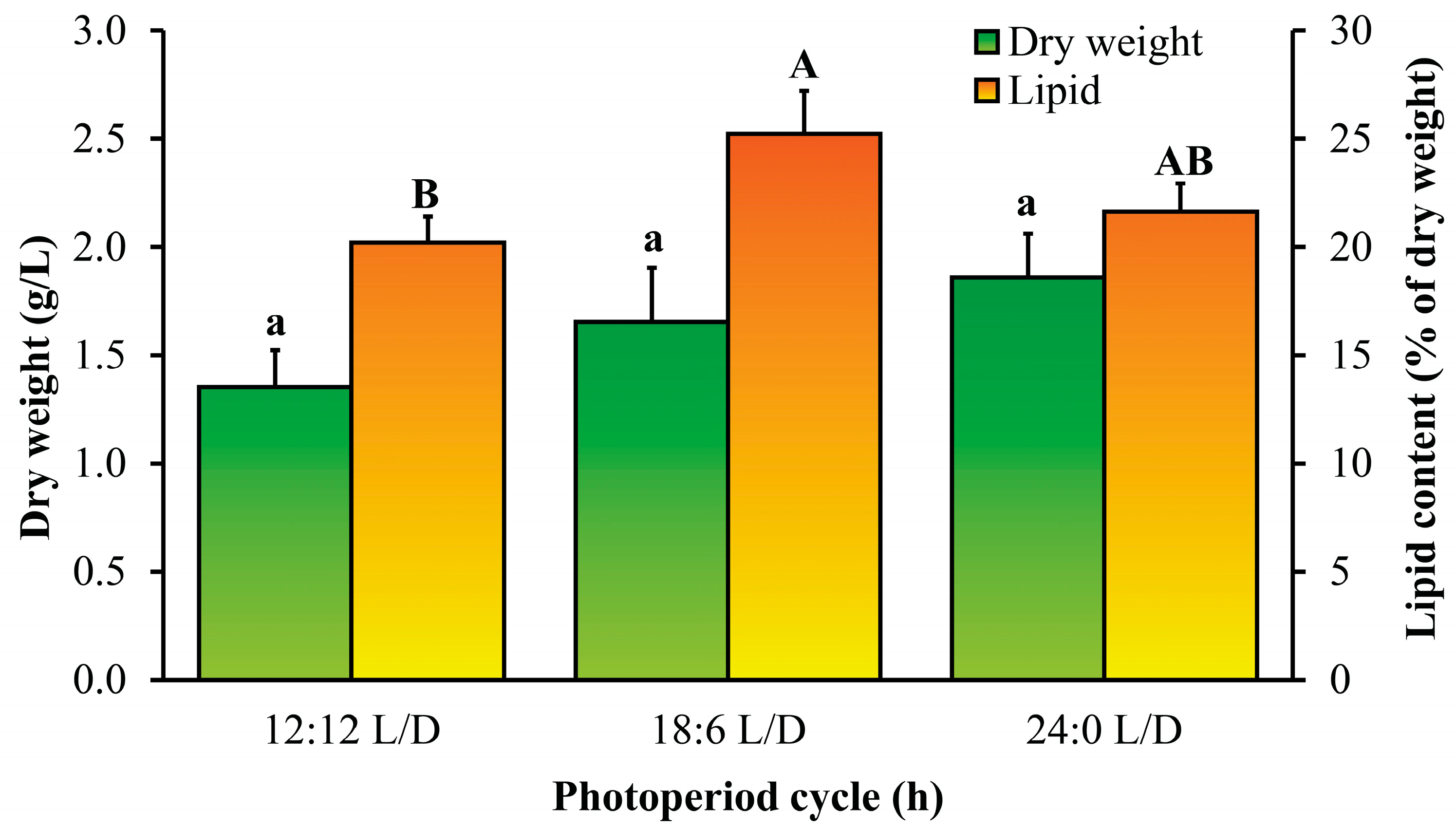
3.3. Effects of Light Intensity and Photoperiod
3.4. Fatty Acid Composition of Lipids from C. variabilis RSM09
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medipally, S.R.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Microalgae as Sustainable Renewable Energy Feedstock for Biofuel Production. Biomed. Res. Int. 2015, 2015, 519513. [Google Scholar] [CrossRef] [PubMed]
- Shuba, E.S.; Kifle, D. Microalgae to Biofuels: ‘Promising’ Alternative and Renewable Energy, Review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Chhandama, M.V.L.; Satyan, K.B.; Changmai, B.; Vanlalveni, C.; Rokhum, S.L. Microalgae as a Feedstock for the Production of Biodiesel: A Review. Bioresour. Technol. Rep. 2021, 15, 100771. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Bonifacio, S.; Clowes, J.; Foulds, A.; Holland, R.; Matthews, J.C.; Percival, C.J.; Shallcross, D.E. Investigation of Biofuel as a Potential Renewable Energy Source. Atmosphere 2021, 12, 1289. [Google Scholar] [CrossRef]
- Chowdhury, H.; Loganathan, B. Third-Generation Biofuels from Microalgae: A Review. Curr. Opin. Green. Sustain. Chem. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Hossain, A.B.M.S.; Salleh, A.; Boyce, A.N.; chowdhury, P.; Naqiuddin, M. Biodiesel Fuel Production from Algae as Renewable Energy. Am. J. Biochem. Biotechnol. 2008, 4, 250–254. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel Production by Microalgal Biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; de Souza, C.O.; Vich, D.V.; de Carvalho, G.C.; Nascimento, M.A. Screening Microalgae Strains for Biodiesel Production: Lipid Productivity and Estimation of Fuel Quality Based on Fatty Acids Profiles as Selective Criteria. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Chu, W.-L. Strategies to Enhance Production of Microalgal Biomass and Lipids for Biofuel Feedstock. Eur. J. Phycol. 2017, 52, 419–437. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, J.N.; Lahiri, S.; Pittman, J.K.; Jana, B.B. Carbon Dioxide Sequestration in Wastewater by a Consortium of Elevated Carbon Dioxide-Tolerant Microalgae. J. CO2 Util. 2015, 10, 105–112. [Google Scholar] [CrossRef]
- Boonma, S.; Chaiklangmuang, S.; Chaiwongsar, S.; Pekkoh, J.; Pumas, C.; Ungsethaphand, T.; Tongsiri, S.; Peerapornpisal, Y. Enhanced Carbon Dioxide Fixation and Bio-oil Production of a Microalgal Consortium. Clean 2015, 43, 761–766. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a High-Density Culture of Chlorella sp. in a Semicontinuous Photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, Y.; Feng, J.; Sun, J.; Zhou, J.; Cen, K. Improving CO2 Fixation Efficiency by Optimizing Chlorella PY-ZU1 Culture Conditions in Sequential Bioreactors. Bioresour. Technol. 2013, 144, 321–327. [Google Scholar] [CrossRef]
- Li, S.; Luo, S.; Guo, R. Efficiency of CO2 Fixation by Microalgae in a Closed Raceway Pond. Bioresour. Technol. 2013, 136, 267–272. [Google Scholar] [CrossRef]
- Dragone, G. Challenges and Opportunities to Increase Economic Feasibility and Sustainability of Mixotrophic Cultivation of Green Microalgae of the Genus Chlorella. Renew. Sustain. Energy Rev. 2022, 160, 112284. [Google Scholar] [CrossRef]
- Chankhong, K.; Chotigeat, W.; Iewkittayakorn, J. Effects of Culture Medium on Growth Kinetics and Fatty Acid Composition of Chlorella sp. T12. Songklanakarin J. Sci. Technol. 2018, 40, 1098–1104. [Google Scholar]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella Vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef]
- Indrayani, I.; Haslianti, H.; Asmariani, A.; Muskita, W.; Balubi, A.M. Growth, Biomass and Lipid Productivity of a Newly Isolated Tropical Marine Diatom, Skeletonema sp. UHO29, under Different Light Intensities. Biodiversitas 2020, 21, 1498–1503. [Google Scholar] [CrossRef]
- Kumaran, K.; Lam, M.K.; Tan, X.B.; Uemura, Y.; Lim, J.W.; Khoo, C.G.; Lee, K.T. Cultivation of Chlorella Vulgaris Using Plant-Based and Animal Waste-Based Compost: A Comparison Study. Procedia Eng. 2016, 148, 679–686. [Google Scholar] [CrossRef]
- Odeh, W.; Sweiss, M.; Ahmad, F.H.; Arabeyyat, Z.; Alnsour, W.; Aldabbas, M.; Hasan, M. Isolation and Identification of Green Microalgae from Northern Jordan. J. Pure Appl. Microbiol. 2023, 17, 2205–2214. [Google Scholar] [CrossRef]
- Burja, A.M.; Tamagnini, P.; Bustard, M.T.; Wright, P.C. Identification of the Green Alga, Chlorella vulgaris (SDC1) Using Cyanobacteria Derived 16S RDNA Primers: Targeting the Chloroplast. FEMS Microbiol. Lett. 2001, 202, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Jun, S.-Y.; Lee, J.-Y.; Ahn, C.-Y.; Oh, H.-M. Selection of Microalgae for Lipid Production under High Levels Carbon Dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method for Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Slover, H.T.; Lanza, E. Quantitative Analysis of Food Fatty Acids by Capillary Gas Chromatography. J. Am. Oil Chem. Soc. 1979, 56, 933–943. [Google Scholar] [CrossRef]
- Kumar, K.; Dasgupta, C.N.; Nayak, B.; Lindblad, P.; Das, D. Development of Suitable Photobioreactors for CO2 Sequestration Addressing Global Warming Using Green Algae and Cyanobacteria. Bioresour. Technol. 2011, 102, 4945–4953. [Google Scholar] [CrossRef]
- Wawrzyńczak, D. Industrial Carbon Dioxide Capture and Utilization Technology. In The Carbon Chain in Carbon Dioxide Industrial Utilization Technologies; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–15. [Google Scholar]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 Biofixation and Fatty Acid Composition of Scenedesmus Obliquus and Chlorella Pyrenoidosa in Response to Different CO2 Levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I. High-CO2 Tolerance in Microalgae: Possible Mechanisms and Implications for Biotechnology and Bioremediation. Biotechnol. Lett. 2013, 35, 1745–1752. [Google Scholar] [CrossRef]
- Wong, Y.-K.; Ho, K.C.; Lai, P.-K.; Leung, C.-C.; Ho, Y.-M.; Lee, O.-K.; Yung, K.-L.; Leung, H.-M. A Study on Algal Growth Behaviour under Different Sparing Period of CO2 Supplementation. In Proceedings of the 1st International Conference on Beneficial Uses of Algal Biomass, ICBUAB, Hong Kong, China, 5–8 November 2013; pp. 19–28. [Google Scholar]
- Al-Qasm, M.; Raut, N.; Talebi, S.; Al-Rajhi, S.; Al-Barwani, T. A Review of Effect of Light on Microalgae Growth. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012. [Google Scholar]
- Sorokin, C.; Krauss, R.W. The Effects of Light Intensity on the Growth Rates of Green Algae. Plant Physiol. 1958, 33, 109–113. [Google Scholar] [CrossRef]
- Shu, C.-H.; Tsai, C.-C.; Chen, K.-Y.; Liao, W.-H.; Huang, H.-C. Enhancing High Quality Oil Accumulation and Carbon Dioxide Fixation by a Mixed Culture of Chlorella sp. and Saccharomyces Cerevisiae. J. Taiwan. Inst. Chem. Eng. 2013, 44, 936–942. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of Light Intensity and Nitrogen Starvation on CO2 Fixation and Lipid/Carbohydrate Production of an Indigenous Microalga Scenedesmus Obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Mandotra, S.K.; Kumar, P.; Suseela, M.R.; Nayaka, S.; Ramteke, P.W. Evaluation of Fatty Acid Profile and Biodiesel Properties of Microalga Scenedesmus Abundans under the Influence of Phosphorus, PH and Light Intensities. Bioresour. Technol. 2016, 201, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ropero, J.E.; Lidueñez-Ballesteros, V.S.; Rodríguez-Bohórquez, A.D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; López-Barrera, G.L.; Barajas-Solano, A.F.; Bryan, S.J.; Zuorro, A. The Effect of LEDs on Biomass and Phycobiliproteins Production in Thermotolerant Oscillatoria sp. Appl. Sci. 2022, 12, 11664. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. The Influence of Light Intensity and Photoperiod on the Growth and Lipid Content of Microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of Light Intensity and Photoperiod on Biomass and Fatty Acid Composition of the Microalgae, Chlorella Vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Lim, K.C.; Zaleha, K. Effect of Photoperiod on the Cellular Fatty Acid Composition of Three Tropical Marine Microalgae. Malays. Appl. Biol. J. 2013, 42, 41–49. [Google Scholar]
- Pekkoh, J.; Duangjan, K.; Phinyo, K.; Kaewkod, T.; Ruangrit, K.; Thurakit, T.; Pumas, C.; Pathom-aree, W.; Cheirsilp, B.; Gu, W.; et al. Turning Waste CO2 into Value-Added Biorefinery Co-Products Using Cyanobacterium Leptolyngbya sp. KC45 as a Highly Efficient Living Photocatalyst. Chem. Eng. J. 2023, 460, 141765. [Google Scholar] [CrossRef]
- Klyachko-Gurvich, G.L.; Tsoglin, L.N.; Doucha, J.; Kopetskii, J.; Shebalina (ryabykh), I.B.; Semenenko, V.E. Desaturation of Fatty Acids as an Adaptive Response to Shifts in Light Intensity 1. Physiol. Plant 1999, 107, 240–249. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Insights into the Physiology of Chlorella Vulgaris Cultivated in Sweet Sorghum Bagasse Hydrolysate for Sustainable Algal Biomass and Lipid Production. Sci. Rep. 2021, 11, 6779. [Google Scholar] [CrossRef]



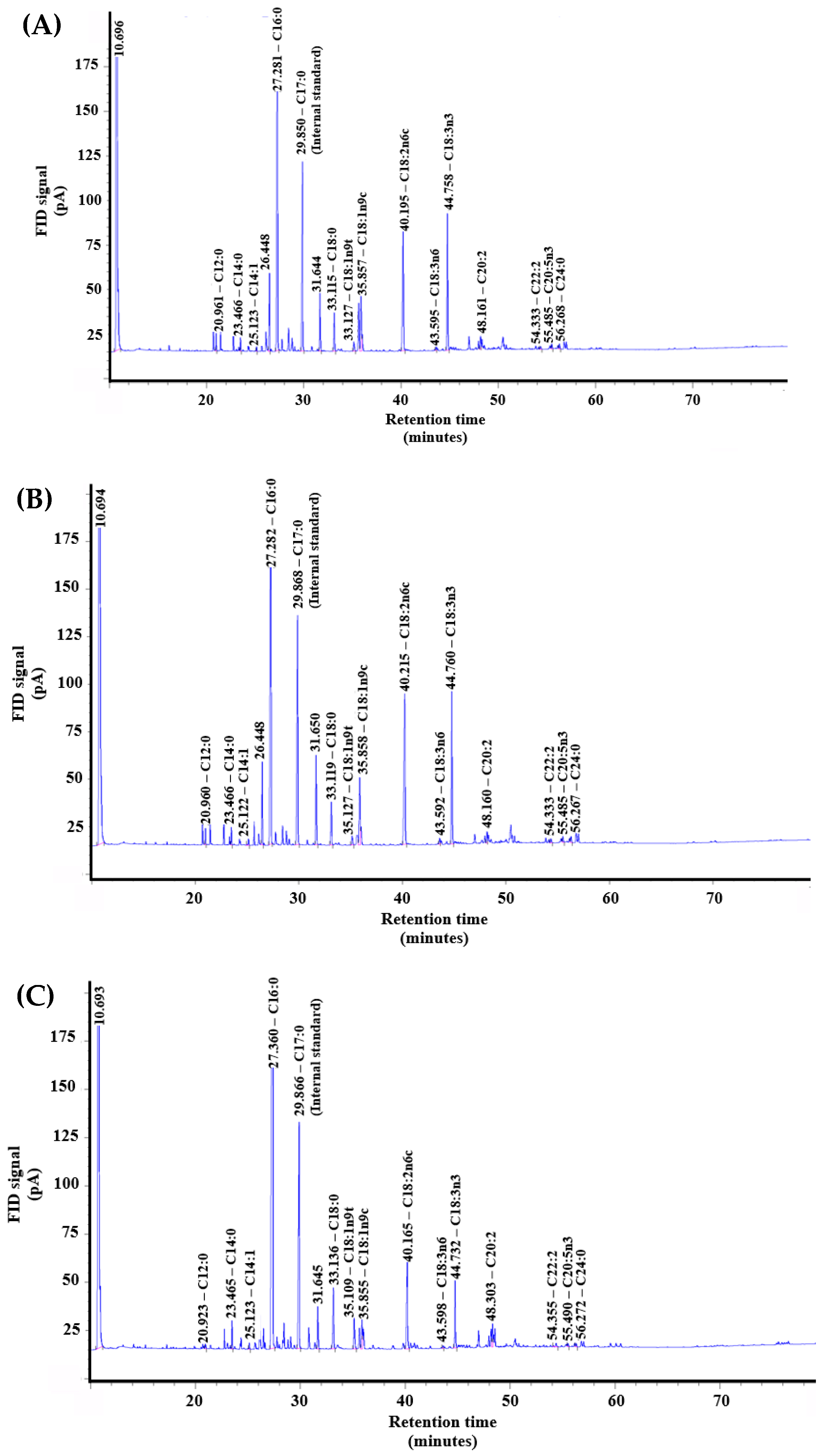
| Fatty Acid | FAME Composition (% of Total FAME) | ||
|---|---|---|---|
| 12:12 L/D | 18:6 L/D | 24:0 L/D | |
| Lauric (C12:0) | 0.86 | 0.70 | 0.24 |
| Myristic (C14:0) | 0.68 | 0.79 | 0.96 |
| Myristoleic (C14:1) | 0.26 | 0.38 | 0.26 |
| Palmitic (C16:0) | 45.09 | 42.27 | 70.50 |
| Stearic (C18:0) | 4.40 | 4.37 | 5.18 |
| Oleic (C18:1) | 7.82 | 8.26 | 5.23 |
| Linoleic (C18:2) | 18.29 | 21.06 | 8.35 |
| Linolenic (C18:3) | 17.76 | 17.47 | 5.71 |
| Eicosadienoic (C20:2) | 1.82 | 1.37 | 1.79 |
| Eicosapentaenoic (C20:5) | 0.70 | 0.75 | 0.33 |
| Docosadienoic (C22:2) | 0.58 | 0.73 | 0.65 |
| Tetracosanoic (C24:0) | 1.74 | 1.85 | 0.80 |
| Saturated fatty acids (SFAs) | 52.77 | 49.98 | 77.68 |
| Monounsaturated fatty acids (MUFAs) | 8.08 | 8.64 | 5.49 |
| Polyunsaturated fatty acids (PUFAs) | 39.15 | 41.38 | 16.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantarasiri, A.; Ungwiwatkul, S. Effects of CO2 Aeration and Light Supply on the Growth and Lipid Production of a Locally Isolated Microalga, Chlorella variabilis RSM09. Appl. Sci. 2024, 14, 10512. https://doi.org/10.3390/app142210512
Chantarasiri A, Ungwiwatkul S. Effects of CO2 Aeration and Light Supply on the Growth and Lipid Production of a Locally Isolated Microalga, Chlorella variabilis RSM09. Applied Sciences. 2024; 14(22):10512. https://doi.org/10.3390/app142210512
Chicago/Turabian StyleChantarasiri, Aiya, and Sunisa Ungwiwatkul. 2024. "Effects of CO2 Aeration and Light Supply on the Growth and Lipid Production of a Locally Isolated Microalga, Chlorella variabilis RSM09" Applied Sciences 14, no. 22: 10512. https://doi.org/10.3390/app142210512
APA StyleChantarasiri, A., & Ungwiwatkul, S. (2024). Effects of CO2 Aeration and Light Supply on the Growth and Lipid Production of a Locally Isolated Microalga, Chlorella variabilis RSM09. Applied Sciences, 14(22), 10512. https://doi.org/10.3390/app142210512







