Myocardial Strain Assessment for Early Duchenne Muscular Dystrophy Diagnosis in Pediatric Patients Using Cardiac MRI
Abstract
1. Introduction
- -
- A dataset was collected for this study that comprised data of 28 DMD patients and 20 healthy controls.
- -
- Exploration of the utility of myocardial strain measurements: This study is among the first to explore the utility of cardiac magnetic resonance-feature tracking (CMR-FT) for early diagnosis of Duchenne muscular dystrophy (DMD) in pediatric patients, a population where early detection is crucial for timely intervention.
- -
- Our research highlights the use of CMR-FT in detecting myocardial strain abnormalities in DMD patients without relying on late gadolinium enhancement (LGE).
- -
- Investigation of the association of LV geometry with strain parameters.
2. Materials and Methods
2.1. Study Population
2.2. MRI Acquisition and Data Analysis
2.3. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. CMR Characteristics
3.2.1. Functional and Geometry
| Variable | DMD (n = 28) | Controls (n = 20) | p-Value |
|---|---|---|---|
| Subject characteristics | |||
| Age (year) | 10.56 ± 1.79 | 11.30 ± 2.71 | 0.057 |
| Weight (kg) | 31.36 ± 6.45 | 42.85 ± 12.73 | 0.001 |
| Height (cm) | 133.29 ± 10.56 | 148.45 ± 14.74 | <0.001 |
| BSA (m2) | 1.06 ± 0.15 | 1.34 ± 0.25 | <0.001 |
| CMR characteristics of LV Functional | |||
| LVEDV/BSA (mL/m2) | 73.07 ± 10.74 | 80.09 ± 6.80 | 0.887 |
| LVESV/BSA (mL/m2) | 33.07 ± 5.58 | 30.87 ± 4.69 | 0.150 |
| SV/BSA (mL/m2) | 41.96 ± 7.65 | 48.48 ± 7.06 | 0.005 |
| Myocardial mass/BSA (g/m2) | 40.54 ± 4.80 | 40.20 ± 5.25 | 0.820 |
| LVEF (%) | 56.14 ± 3.51 | 61.60 ± 4.66 | 0.003 |
| Geometry | |||
| ED wall thickness (mm) | 6.72 ± 2.71 | 8. 18 ± 1.76 | <0.001 |
| ES wall thickness (mm) | 7.38 ± 2.75 | 8.89 ± 2.52 | <0.001 |
| Wall thickening (%) | 55.85 ± 8.98 | 72.83 ± 7.45 | <0.001 |
3.2.2. Strain Measurements
3.3. Correlation Between Myocardial Strain and LGE Assessment
3.4. Correlation Between Global Strain Parameters and LV Geometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Yang, D.; Wan, K.; Luo, Y.; Sun, J.Y.; Zhang, T.J.; Li, W.H.; Greiser, A.; Jolly, M.P.; Zhang, Q.; et al. Distribution pattern of left-ventricular myocardial strain analyzed by a cine MRI based deformation registration algorithm in healthy Chinese volunteers. Sci. Rep. 2017, 7, 45314. [Google Scholar] [CrossRef]
- Scatteia, A.; Baritussio, A.; Bucciarelli, D.C. Strain imaging using cardiac magnetic resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef]
- Swoboda, P.P.; Erhayiem, B.; Mcdiarmid, A.K.; Lancaster, R.E.; Lyall, G.K.; Dobson, L.E.; Ripley, D.P.; Musa, T.A.; Garg, P.; Ferguson, C.; et al. Relationship between cardiac deformation parameters measured by cardiovascular magnetic resonance and aerobic fitness in endurance athletes. J. Cardiovasc. Magn. Reson. 2016, 18, 48. [Google Scholar] [CrossRef]
- Buss, S.J.; Breuninger, K.; Lehrk, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef]
- Rajiah, P.S.; Kalisz, K.; Broncano, J.; Goerne, H.; Collins, J.D.; François, C.J.; Ibrahim, E.S.; Agarwal, P.P. Myocardial Strain Evaluation with Cardiovascular MRI: Physics, Principles, and Clinical Applications. Radiographics 2022, 42, 968–990. [Google Scholar] [CrossRef]
- Obokata, M.; Nagata, Y.; Wu, V.C.; Kado, Y.; Kurabayashi, M.; Otsuji, Y.; Takeuchi, M. Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 525–532. [Google Scholar] [CrossRef]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Osypiuk, E.; Lehman, B.T.; Stanchev, P.; Aragam, J.; Benjamin, E.J.; Solomon, S.D.; Vasan, R.S. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: The Framingham Heart Study. Circ. Cardiovasc. Imaging 2013, 6, 692–699. [Google Scholar] [CrossRef]
- Dandel, M.; Lehmkuhl, H.; Knosalla, C.; Suramelashvili, N.; Hetzer, R. Strain and Strain Rate Imaging by Echocardiography—Basic Concepts and Clinical Applicability. Curr. Cardiol. Rev. 2009, 5, 133–148. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef]
- Götte, M.J.; Germans, T.; Rüssel, I.K.; Zwanenburg, J.J.; Marcus, J.T.; van-Rossum, A.C.; van-Veldhuisen, D.J. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: Studies in normal and impaired left ventricular function. J. Am. Coll. Cardiol. 2006, 48, 2002–2011. [Google Scholar] [CrossRef]
- Awadi, R.; Benameur, N.; Kraiem, T.; Labidi, S. A quasi-static biomechanical model of the human myocardium based on Cardiac Magnetic Resonance images. Procedia Computer Sci. 2023, 2019, 1177–1184. [Google Scholar] [CrossRef]
- Benameur, N.; Mahmoudi, R.; Caiani, E.G.; Arous, Y.; Saâdaoui, F.; Mahjoubi, H. Assessment of the relationship between regional wall motion abnormality score revealed by parametric imaging and the extent of LGE with CMR. Clin. Imaging 2022, 89, 68–77. [Google Scholar] [CrossRef]
- Cao, J.J.; Ngai, N.; Duncanson, L.; Cheng, J.; Gliganic, K.; Chen, Q. A comparison of both DENSE and feature tracking techniques with tagging for the cardiovascular magnetic resonance assessment of myocardial strain. J. Cardiovasc. Magn. Reson. 2018, 20, 1097–6647. [Google Scholar] [CrossRef]
- Brady, B.; King, G.; Murphy, R.T.; Walsh, D. Myocardial strain: A clinical review. Ir. J. Med. Sci. 2023, 192, 1649–1656. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Ma, Y.; Wang, P.; Li, Y.; Tian, J.; Yue, X.; Su, G.; Li, B. Predictive value of myocardial strain on myocardial infarction size by cardiac magnetic resonance imaging in ST segment elevation myocardial infarction with preserved left ventricular ejection fraction. Front. Pharmacol. 2022, 13, 1015390. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Paul, J.; Li, H.; Ma, G.S.; Buckert, D.; Rasche, V. Left ventricular myocardial strain quantification with two- and three-dimensional cardiovascular magnetic resonance based tissue tracking. Quant. Imaging Med. Surg. 2021, 11, 1421–1436. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Q.; Cao, L.; Zhao, Z.; Zhao, J.; Lu, Q.; Zeng, L.; Zhang, M.; Pohost, G.M.; Li, K. Correlation between left ventricular myocardial strain and left ventricular geometry in healthy adults: A cardiovascular magnetic resonance-feature tracking study. Int. J. Cardiovasc. Imaging 2019, 35, 2057–2065. [Google Scholar] [CrossRef]
- Polacin, M.; Karolyi, M.; Eberhard, M.; Alkadhi, H.; Kozerke, S.; Manka, R. Segmental strain for scar detection in acute myocardial infarcts and in follow-up exams using non-contrast CMR cine sequences. BMC Cardiovasc. Disord. 2022, 22, 226. [Google Scholar] [CrossRef]
- Hor, K.N.; Wansapura, J.; Markham, L.W.; Mazur, W.; Cripe, L.H.; Fleck, R.; Benson, D.W.; Gottliebson, W.M. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: A cardiac magnetic resonance tagging study. J. Am. Coll. Cardiol. 2009, 53, 1204–1210. [Google Scholar] [CrossRef]
- Taqatqa, A.; Bokowski, J.; Al-Kubaisi, M.; Khalil, A.; Miranda, C.; Alaksham, H.; Fughhi, I.; Kenny, D.; Diab, K.A. The Use of Speckle Tracking Echocardiography for Early Detection of Myocardial Dysfunction in Patients with Duchenne Muscular Dystrophy. Pediatr. Cardiol. 2016, 37, 1422–1428. [Google Scholar] [CrossRef]
- Siegel, B.; Olivieri, L.; Gordish, D.H. Spurney CF Myocardial Strain Using Cardiac MR Feature Tracking and Speckle Tracking Echocardiography in Duchenne Muscular Dystrophy Patients. Pediatr. Cardiol. 2018, 39, 478–483. [Google Scholar] [CrossRef]
- Oreto, L.; Vita, G.L.; Mandraffino, G.; Carerj, S.; Calabrò, M.P.; Manganaro, R.; Cusmà-Piccione, M.; Todaro, M.C.; Sframeli, M.; Cinquegrani, M.; et al. Impaired myocardial strain in early stage of Duchenne muscular dystrophy: Its relation with age and motor performance. Acta Myol. 2020, 39, 191–199. [Google Scholar]
- Panovský, R.; Pešl, M.; Máchal, J.; Holeček, T.; Feitová, V.; Juříková, L.; Masárová, L.; Pešlová, E.; Opatřil, L.; Mojica-Pisciotti, M.L.; et al. Quantitative assessment of left ventricular longitudinal function and myocardial deformation in Duchenne muscular dystrophy patients. Orphanet J. Rare Dis. 2021, 16, 57. [Google Scholar] [CrossRef]
- Prakash, N.; Suthar, R.; Sihag, B.K.; Debi, U.; Kumar, R.M.; Sankhyan, N. Cardiac MRI and Echocardiography for Early Diagnosis of Cardiomyopathy Among Boys with Duchenne Muscular Dystrophy: A Cross-Sectional Study. Front. Pediatr. 2022, 10, 818608. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alsaied, T.; Henson, S.E.; Gandhi, J.; Patel, P.; Khoury, P.; Villa, C.; Ryan, T.D.; Wittekind, S.G.; Lang, S.M.; et al. Left Ventricular Magnetic Resonance Imaging Strain Predicts the Onset of Duchenne Muscular Dystrophy-Associated Cardiomyopathy. Circ. Cardiovasc. Imaging 2020, 13, e011526. [Google Scholar] [CrossRef]
- Earl, C.C.; Jauregui, A.M.; Lin, G.; Hor, K.N.; Markham, L.W.; Soslow, J.H.; Goergen, C.J. Regional 4D Cardiac Magnetic Resonance Strain Predicts Cardiomyopathy Progression in Duchenne Muscular Dystrophy. medRxiv 2023, 7, 23298238. [Google Scholar] [CrossRef]
- Florian, A.; Ludwig, A.; Engelen, M.; Waltenberger, J.; Rösch, S.; Sechtem, U.; Yilmaz, A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J. Cardiovasc. Magn. Reson. 2014, 16, 81. [Google Scholar] [CrossRef]
- Xu, K.; Xu, H.Y.; Xu, R.; Xie, L.J.; Yang, Z.G.; Yu, L.; Zhou, B.; Fu, H.; Liu, H.; Cai, X.T.; et al. Global segmental and layer specific analysis of myocardial involvement in Duchenne muscular dystrophy by cardiovascular magnetic resonance native T1 mapping. J. Cardiovasc. Magn. Reson. 2021, 23, 110. [Google Scholar] [CrossRef]
- Wren, T.A.; Bluml, S.; Tseng, O.L.; Gilsanz, V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: Preliminary study. AJR Am. J. Roentgenol. 2008, 190, W8–W12. [Google Scholar] [CrossRef]
- Ward, L.M.; Weber, D.R. Growth, pubertal development, and skeletal health in boys with Duchenne Muscular Dystrophy. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 39–48. [Google Scholar] [CrossRef] [PubMed]
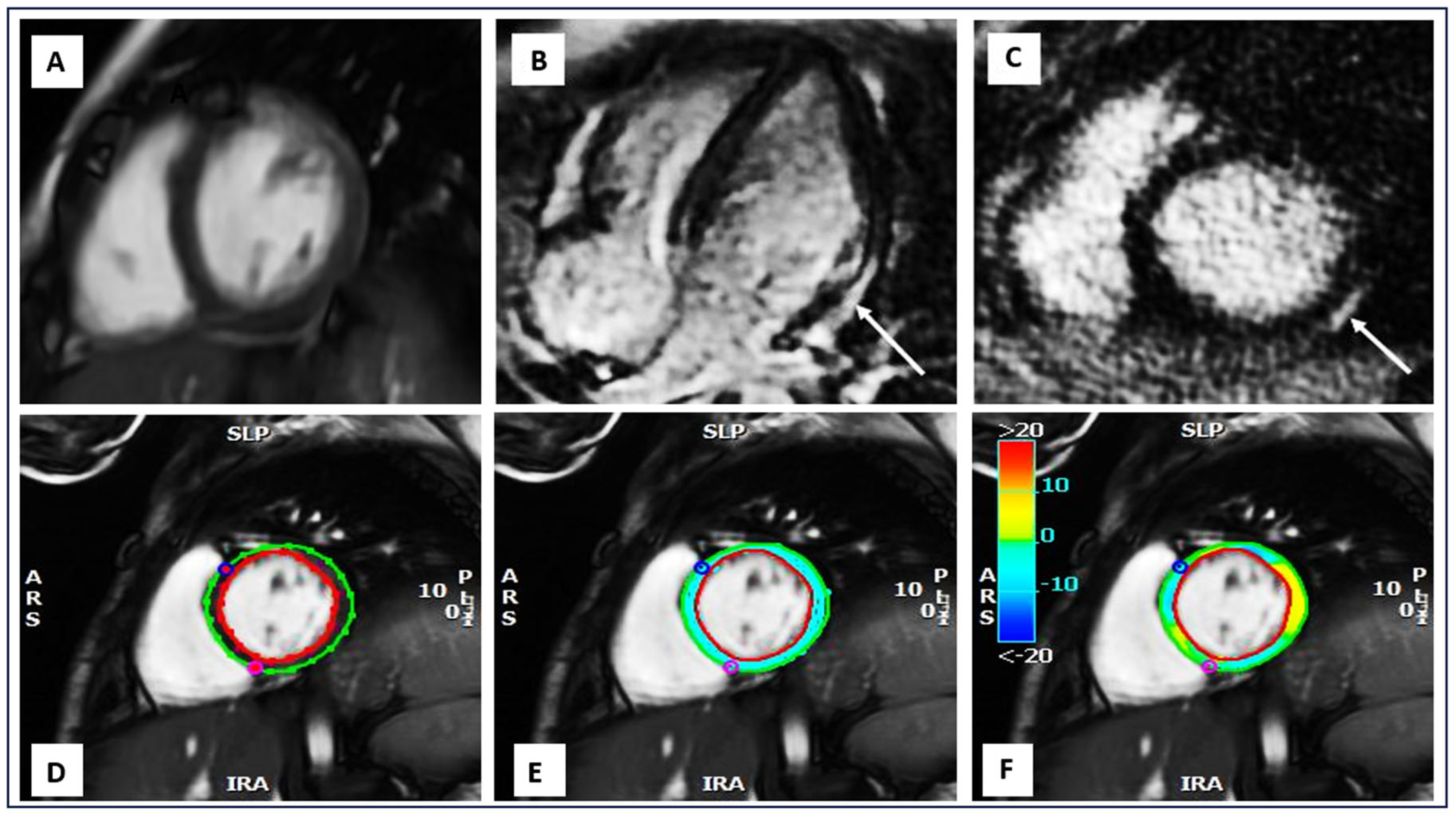

| Study | Number of Subjects (n) | Modality | Technique | Strain Measurements |
|---|---|---|---|---|
| Hor et al. (2009) [19] | 70 DMD/16 controls | MRI | HARmonic Phase | |
| Taqatqa et al. (2016) [20] | 19 DMD/16 controls | Echocardiography | STE | GCS/GLS |
| Siegel et al. (2017) [21] | 24 DMD/8 controls | MRI/Echocardiography | FT/STE | GCS |
| Oreto et al. (2020) [22] | 32 DMD/24 controls | Echocardiography | STE | GCS/CLS |
| Panovsky et al. (2021) [23] | 51 DMD/18 controls | MRI/Echocardiography | FT/MAPSE | GCS/GLS/GRS |
| Prakash et al. (2022) [24] | 38 DMD | MRI/Echocardiography | TTE/STE/TDI | GS |
| Our study (2024) | 28 DMD/20 controls | MRI | FT | GCS/GLS/GRS |
| Variable | DMD (n = 28) | Controls (n = 20) | p-Value |
|---|---|---|---|
| GCS (%) | −16.12 ± 3.55 | −19.34 ± 4.48 | <0.001 |
| GLS (%) | −13.79 ± 5.28 | −17.13 ± 5.94 | <0.001 |
| GRS (%) | 27.49 ± 2.44 | 31.69 ± 2.53 | <0.001 |
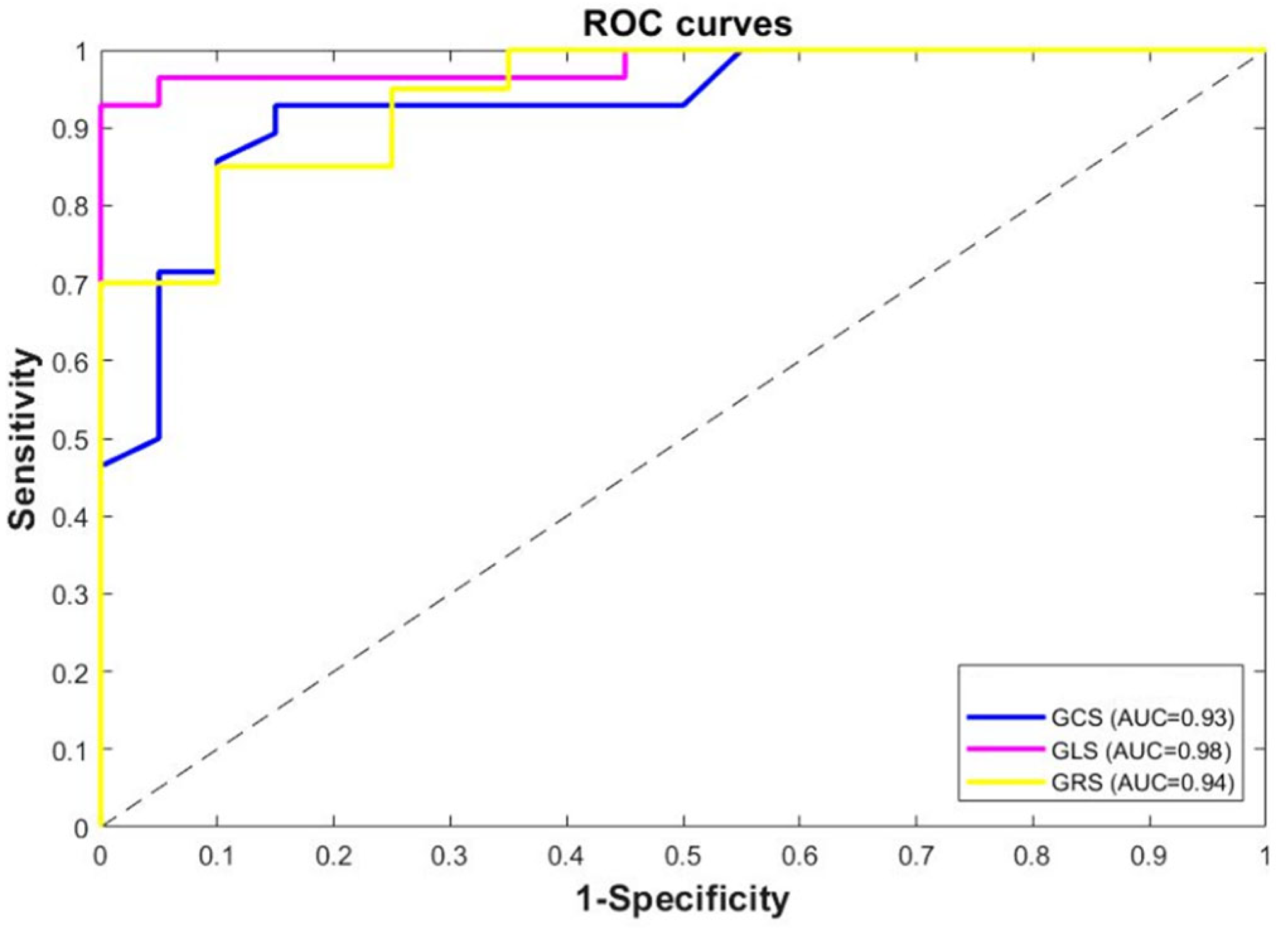
| Variable | AUC (95% CI) | p-Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| GCS (%) | 0.93 | 0.003 | 86 | 90 |
| GLS (%) | 0.98 | 0.001 | 96 | 95 |
| GRS (%) | 0.94 | 0.046 | 90 | 93 |
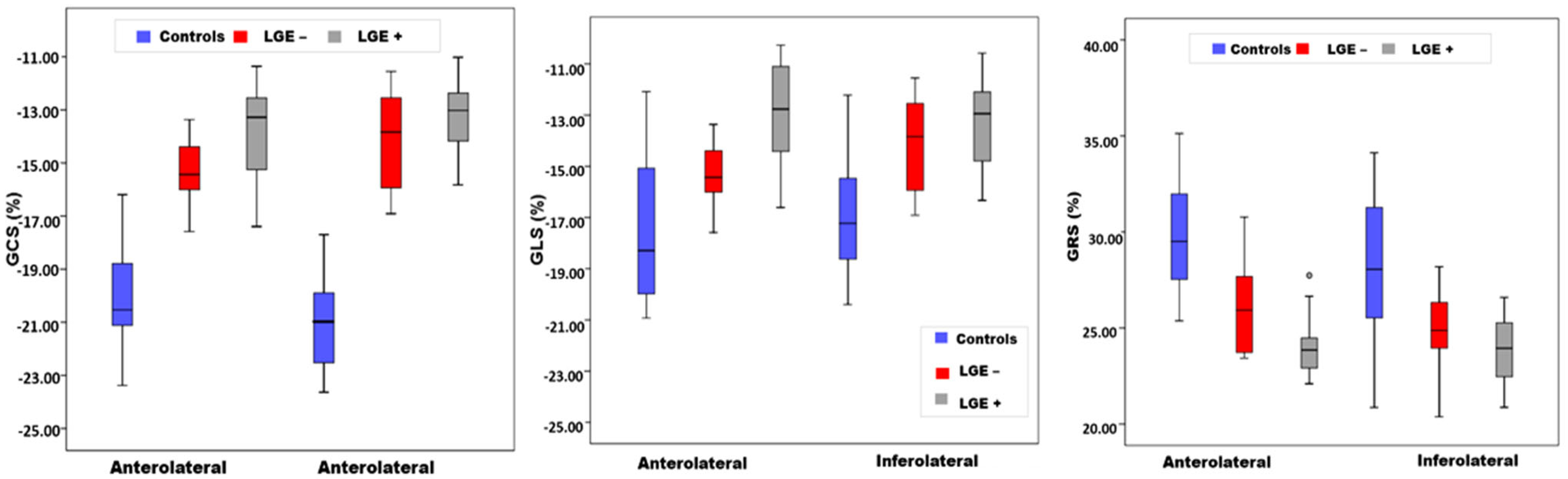
| Segment | Controls (n = 20) | LGE− (n = 12) | LGE+ (n = 16) | p-Value |
|---|---|---|---|---|
| Circumferential strain (%) | ||||
| Anterolateral | −20.23 ± 1.85 | −15.32 ± 1.32 | −13.80 ± 1.78 | 0.033 |
| Inferolateral | −20.78 ± 1.70 | −14.05 ±1.79 | −13.49 ± 1.65 | 0.042 |
| Longitudinal strain (%) | ||||
| Anterolateral | −18.58 ± 2.79 | −14.86 ± 1.41 | −12.89 ± 1.85 | 0.046 |
| Inferolateral | −16.94 ± 2.37 | −13.93 ± 1.80 | −13.42 ± 1.62 | 0.040 |
| Radial strain (%) | ||||
| Anterolateral | 29.72 ± 2.58 | 26.18 ± 2.45 | 24.32 ± 1.55 | 0.043 |
| Inferolateral | 28.10 ± 3.53 | 24.89 ± 1.93 | 23.74 ± 2.21 | 0.030 |
| Variable | GCS (%) | GLS (%) | GRS (%) | |||
|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | |
| ES wall thickness (mm) | −0.26 | 0.002 | −0.09 | 0.003 | 0.06 | 0.82 |
| ED wall thickness (mm) | 0.20 | 0.319 | −0.06 | 0.730 | 0.03 | 0.92 |
| Wall thickening (%) | −0.30 | 0.010 | −0.29 | 0.040 | 0.08 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awadi, R.; Benameur, N.; Hafsi, H.; Younes, T.B.; Arous, Y.; Labidi, S.; Tavares, J.M.R.S. Myocardial Strain Assessment for Early Duchenne Muscular Dystrophy Diagnosis in Pediatric Patients Using Cardiac MRI. Appl. Sci. 2024, 14, 10341. https://doi.org/10.3390/app142210341
Awadi R, Benameur N, Hafsi H, Younes TB, Arous Y, Labidi S, Tavares JMRS. Myocardial Strain Assessment for Early Duchenne Muscular Dystrophy Diagnosis in Pediatric Patients Using Cardiac MRI. Applied Sciences. 2024; 14(22):10341. https://doi.org/10.3390/app142210341
Chicago/Turabian StyleAwadi, Rania, Narjes Benameur, Hassen Hafsi, Thouraya Ben Younes, Younes Arous, Salam Labidi, and João Manuel R. S. Tavares. 2024. "Myocardial Strain Assessment for Early Duchenne Muscular Dystrophy Diagnosis in Pediatric Patients Using Cardiac MRI" Applied Sciences 14, no. 22: 10341. https://doi.org/10.3390/app142210341
APA StyleAwadi, R., Benameur, N., Hafsi, H., Younes, T. B., Arous, Y., Labidi, S., & Tavares, J. M. R. S. (2024). Myocardial Strain Assessment for Early Duchenne Muscular Dystrophy Diagnosis in Pediatric Patients Using Cardiac MRI. Applied Sciences, 14(22), 10341. https://doi.org/10.3390/app142210341










