Chemical and Thermal Changes in Mg3Si2O5 (OH)4 Polymorph Minerals and Importance as an Industrial Material
Abstract
1. Introduction
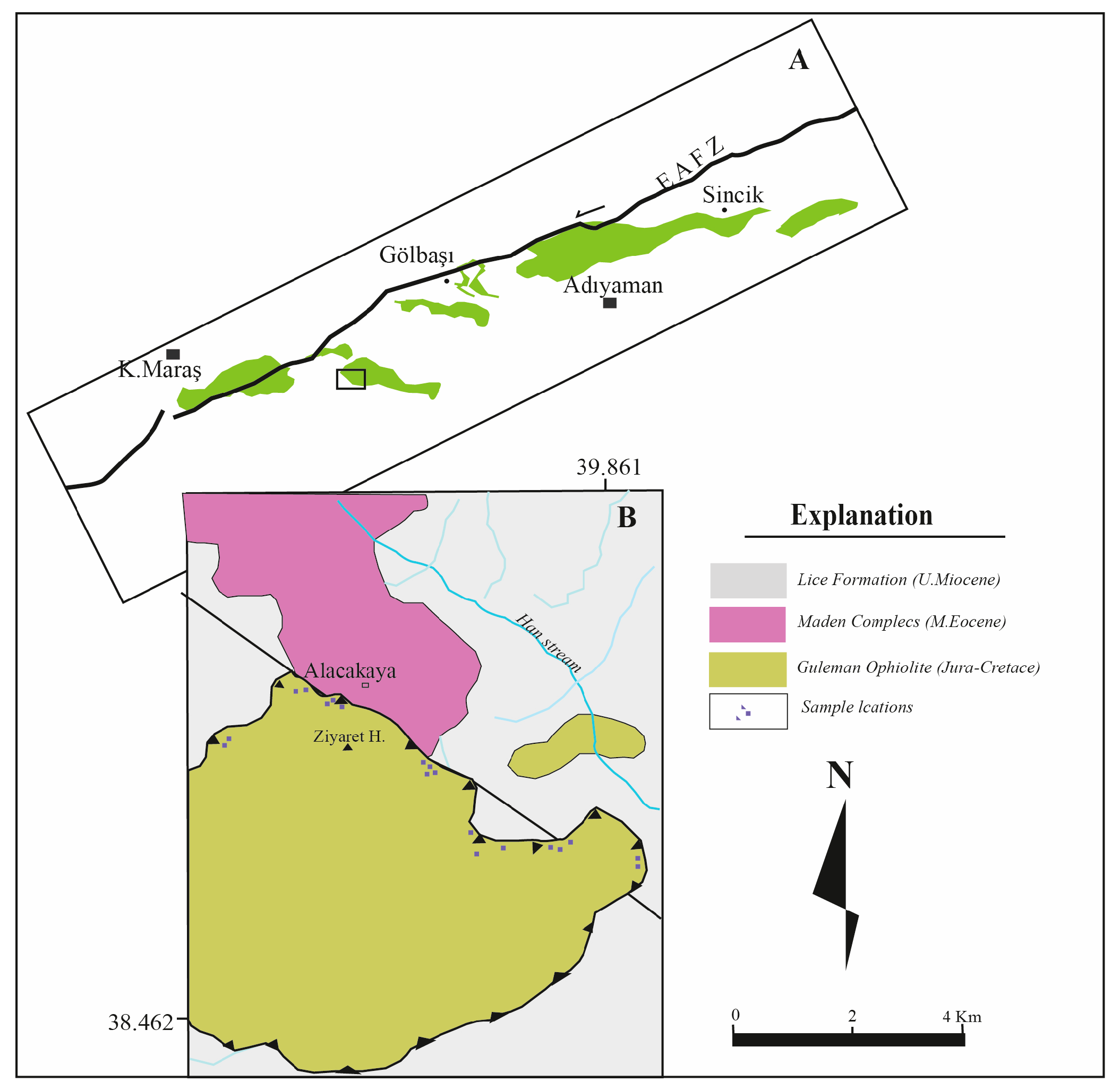
2. Materials and Methods
3. Findings
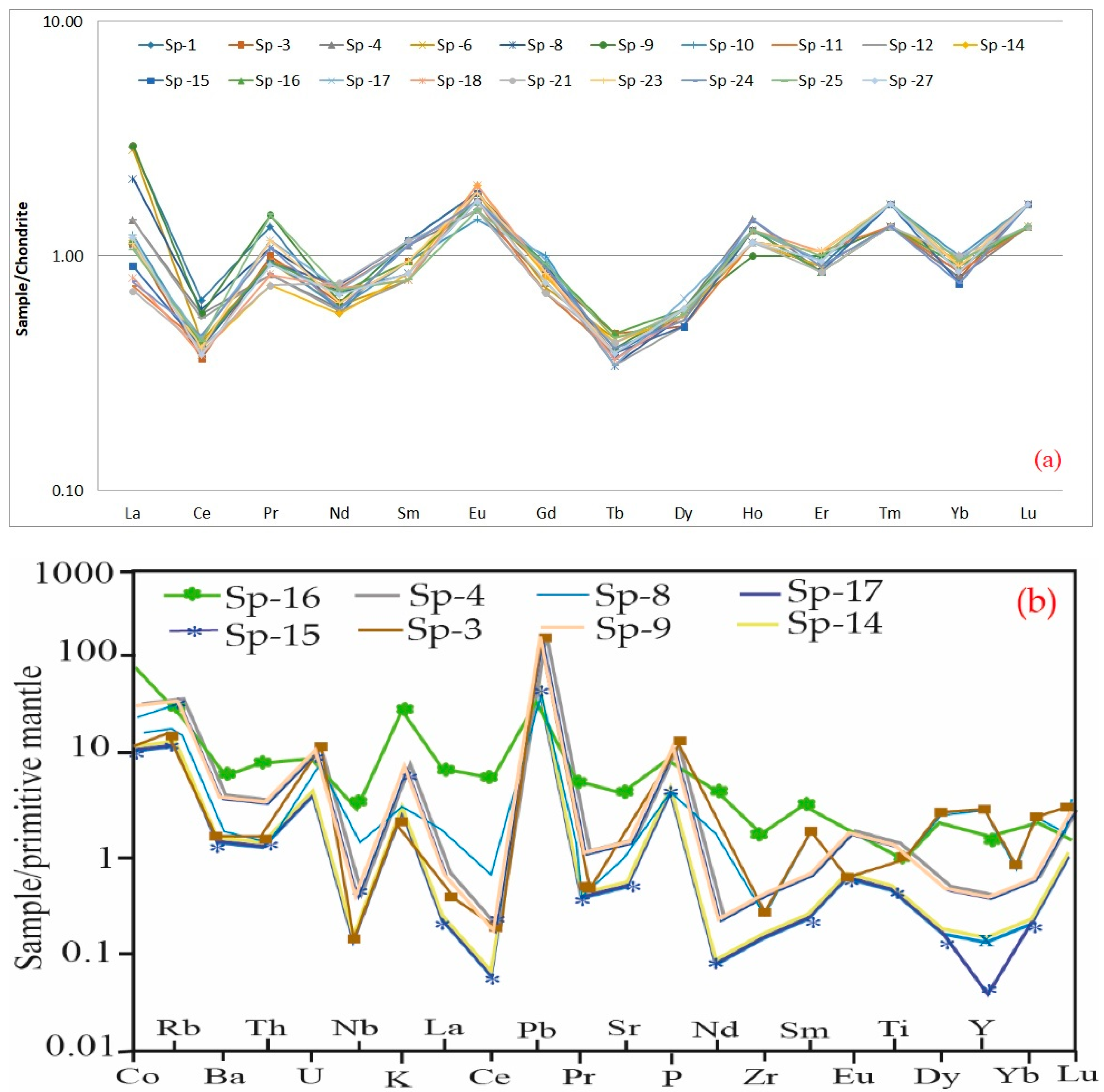
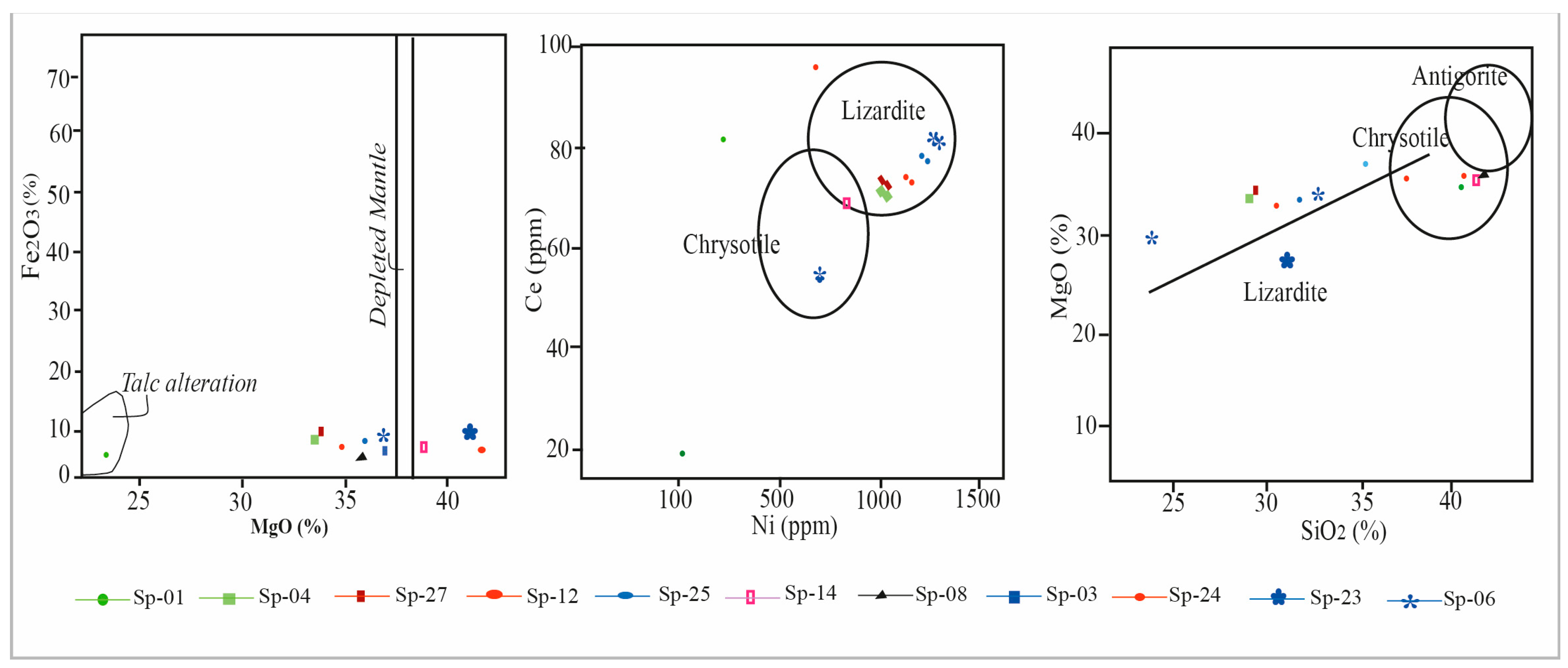
3.1. Geochemistry
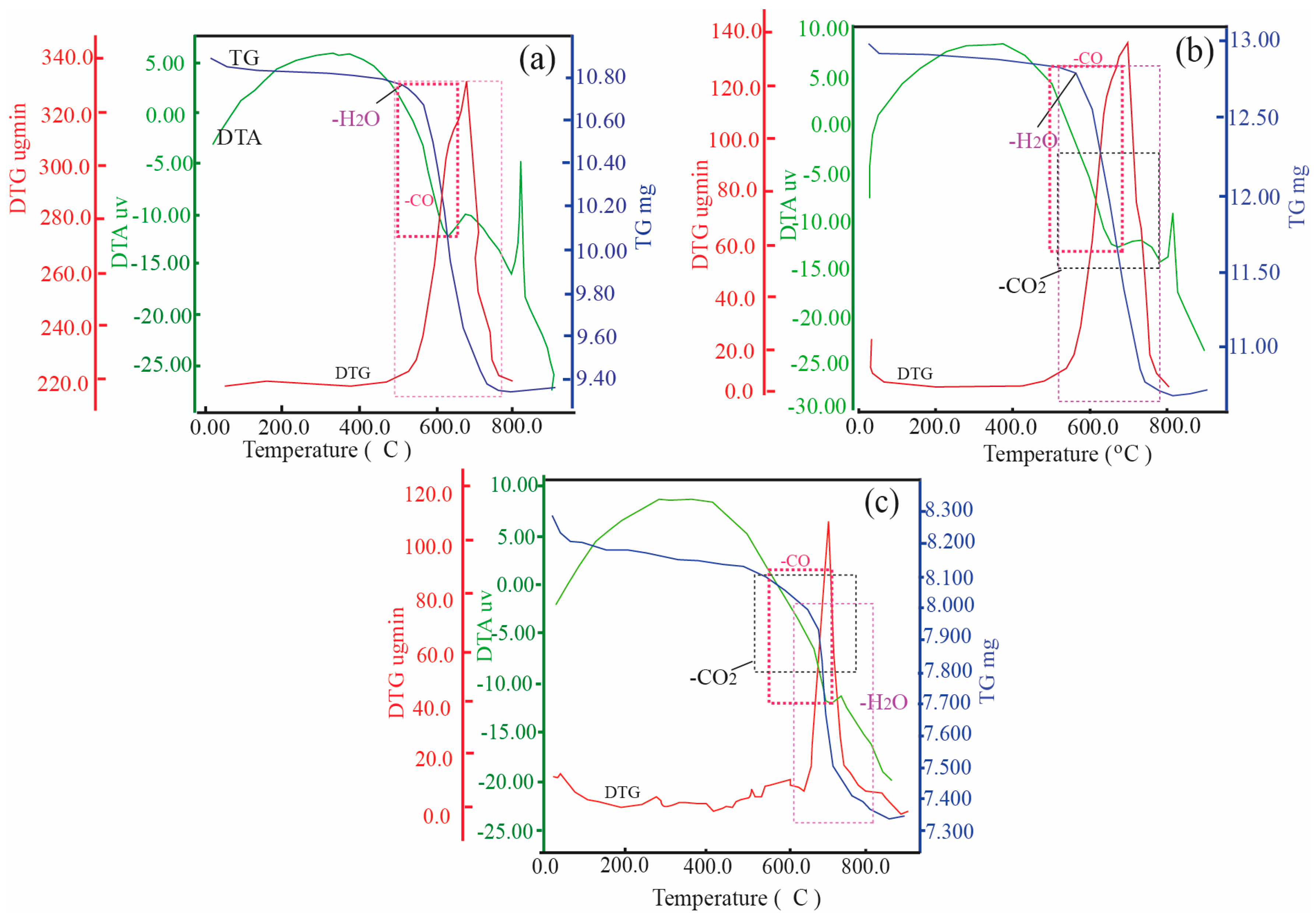
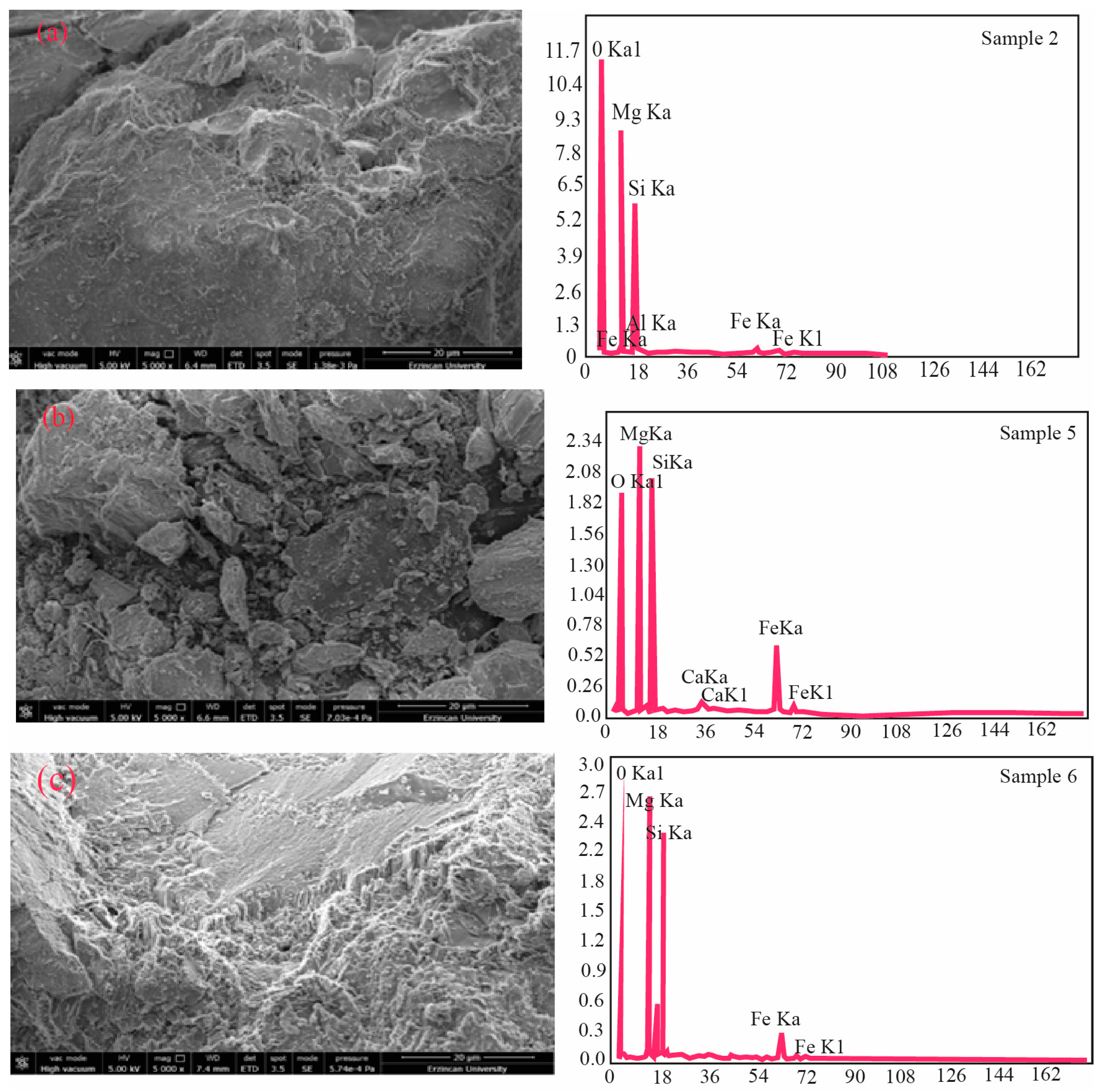
3.2. Microstructure Characteristics (SEM, TG, DTA)
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kılıç, A.D.; İnceöz, M. Mineralogical, geochemical and isotopic effect of silica in ultramaphic systems, eastern Anatolian Turkey. Geochem. Int. 2015, 53, 369–382. [Google Scholar] [CrossRef]
- Dilek, Y.; Flower, M.F.J. Arc-trench rollback and forearc accretion: 2. Model template for Albania, Cyprus, and Oman. In Ophiolites in Earth History; Dilek, Y., Robinson, P.T., Eds.; Geological Society of London: London, UK, 2003; Volume 218, pp. 43–68. [Google Scholar]
- Rakovan, J. Word to the Wise: Serpentine, California’s State Rock. Rocks Miner. 2011, 86, 63–68. [Google Scholar] [CrossRef]
- Evans, B.W.; Hattori, K.; Baronnet, A. Serpentinite: What, why, where? Elements 2013, 9, 99–106. [Google Scholar] [CrossRef]
- Wicks, F.J.; Whittaker, E.J.W. A reappraisal of the structures of the serpentine minerals. Can. Mineral. 1975, 13, 227–243. [Google Scholar]
- Evans, B.W. Lizardite versus antigorite serpentinite: Magnetite, hydrogen, and life. Geology 2010, 38, 879–882. [Google Scholar] [CrossRef]
- Morgan, A. Acid Leaching Studies of Chrysotile Asbestos from Mines in the Coalinga Region of California and From Quebec and British Columbia. Ann. Occup. Hyg. 1997, 41, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- O’Hanley, D.S.; Wicks, F.J. Conditions of formations of lizardite, chrysotile and antigorite, Cassiar, British Columbia. Can. Mineral. 1995, 33, 753–773. [Google Scholar]
- Sanna, A.; Wang, X.; Lacinska, A.; Styles, M.; Paulson, T.; Maroto-Valer, M. Enhancing Mg extraction from lizardite-rich serpentine for CO2 mineral sequestration. Miner. Eng. 2013, 49, 135–144. [Google Scholar] [CrossRef]
- Johannes, W. An experimental investigation of the system MgO-SiO2-H2O-CO2. Am. J. Sci. 1969, 267, 1083–1104. [Google Scholar] [CrossRef]
- Moody, J.B. Serpentinisation a review. Lithos 1976, 9, 125–138. [Google Scholar] [CrossRef]
- Früh-Green, G.L.; Connolly, J.A.D.; Plas, A. Serpentinization of oceanic peridotites: Implications for geochemical cycles and biological activity. Subseafloor Biosph. Mid-Ocean. Ridges 2004, 144, 119–136. [Google Scholar]
- Page, N.J. Chemical differences among the serpentine B polymorphs. Am. Mineral. 1968, 53, 201–215. [Google Scholar]
- Kamegaki, S.; Smith, D.; Ryu, M.; Ng, S.H.; Huang, H.-H.; Maasoumi, P.; Vongsvivut, J.; Moraru, D.; Katkus, T.; Juodkazis, S.; et al. Four-Polarisation Camera for Anisotropy Mapping at Three Orientations: Micro-Grain of Olivine. Coatings 2023, 13, 1640. [Google Scholar] [CrossRef]
- Brady, J.B. Chrysotile and other asbestos minerals: A comprehensive study. Environ. Geol. 2000, 39, 303–311. [Google Scholar]
- Barnes, L.; Le Marche, V.C., Jr.; Himmelberg, G.R. Geochemical evidence of present day serpentinization. Science 1967, 156, 830–832. [Google Scholar] [CrossRef]
- Sleep, N.H.; Bird, D.K.; Pope, E.C. Serpentinite and the dawn of life. Philos. Trans. R. Soc. B 2011, 366, 2857–2869. [Google Scholar] [CrossRef] [PubMed]
- Barnes, I.; O’Neil, J.R. The relationship between fluids in some fresh Alpine-type ultramafics and possible modern serpentinization, Westem United States. Geol. Soc. Am. Bull. 2019, 80, 1947–1960. [Google Scholar] [CrossRef]
- Ulmer, P.; Trommsdorff, V. Serpentine Stability to Mantle Depths and Subduction-Related Magmatism. Science 1995, 268, 858–861. [Google Scholar] [CrossRef]
- Rouméjon, S.; Cannat, M. Serpentinization of mantle-derived peridotites at mid-ocean ridges: Mesh texture development in the context of tectonic exhumation. Geochem. Geophys. Geosyst. 2019, 15, 2354–2379. [Google Scholar] [CrossRef]
- Chittenden, E.T.; Stanton, D.J.; Watson, J.; Dodson, K.J. Serpentine and dunite as magnesium fertilisers. N. Z. J. Agric. Res. 1967, 10, 160–171. [Google Scholar] [CrossRef]
- Mcnaught, K.J.; Dorofaeff, F.D.; Karlovsky, J. Effect of magnesium fertilisers and season on levels of inorganic nutrients in a pasture on Hamilton clay loam. N. Z. J. Agric. Res. 1968, 11, 533–550. [Google Scholar] [CrossRef][Green Version]
- Błońska, E.; Januszek, K.; Małek, S.; Wanıc, T. Effects of serpentinite fertilizer on the chemical properties and enzyme activity of young spruce soils. Int. Agrophys. 2016, 30, 401–414. [Google Scholar] [CrossRef]
- Luz, A.B.; Lapido-Loureiro, F.E.; Sampaıo, J.A.; Castılhos, Z.C.; Bezerra, M.S. Rochas, minerais e rotas tecnológicas para a produção de fertilizantes alternativos. In Agrominerais para o Brasil; CETEM/MCT: Rio de Janeiro, Brazil, 2010; pp. 61–88. [Google Scholar]
- Carmignano, O.; Vieira, S.S.; Brandão, P.R.; Bertoli, A.; Lago, R.M. Serpentinites: Mineral Structure, Properties and Technological Applications. J. Braz. Chem. Soc. 2020, 31, 2–14. [Google Scholar] [CrossRef]
- Blaskowskı, A.E.; Bergmann, M.; Sılveıra, C.A.P.; Garnıer, J.; Camargo, M.A.; Cavalcante, O.A. Potencial das rochas das pilhas de rejeitos da mineração Ferbasa-Cia de Ferroligas da Bahia como corretivos e remineralizadores de solo. In Anais do III Congresso Brasileiro de Rochagem, 8 a 11 de novembro de 2016; Bamberg, A.L., Silveira, C.A.P., de Souza Martins, É., Bergmann, M., Martinazzo, R., Theodoro, S.H., Eds.; Embrapa Clima Temperado: Pelotas, Brazil; Embrapa Cerrados: Brasília, Brazil; Triunfal Gráfica e Editora: Assis, Brazil, 2016; Volume 455, pp. 121–127. [Google Scholar]
- Şaşmaz, A.; Kılıç, A.D.; Akgül, B.; Sasmaz, B. A spectral approach on mineralogy and geochemistry of garnet skarns in Arc-Type 447 Granitoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 122037. [Google Scholar] [CrossRef]
- Hoskins, J.A. Chrysotile asbestos cement and the Grenfell Tower fire. Toxicol. Appl. Pharmacol. 2018, 361, 171. [Google Scholar] [CrossRef]
- Nguyen, H.; Kaas, A.; Kinnunen, P.; Carvelli, V.; Monticelli, C.; Yliniemi, J.; Illikainen, M. Fiber reinforced alkali-activated stone wool composites fabricated by hot-pressing technique. Mater. Des. 2020, 186, 108–315. [Google Scholar] [CrossRef]
- Grozeva, N.G. Carbon and Mineral Transformations in Seafloor Serpentinization Systems. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2018. [Google Scholar]
- Kakooei, H.; Marioryad, H. Evaluation of exposure to the airborne asbestos in an automobile brake and clutch manufacturing industry in Iran. Regul. Toxicol. Pharmacol. 2010, 56, 143–147. [Google Scholar] [CrossRef]
- Sakakibara, M.; Uehara, S. What is asbestos? Rock Miner. Sci. 2006, 35, 3–10. [Google Scholar] [CrossRef]
- Znamenáčková, I.; Dolinská, S.; Lovás, M.; Hredzák, S.; Matik, M.; Tomčová, J.; Čablík, V. Application of microwave energy at treatment of asbestos cement (eternit). IOP Conf. Ser. Earth Environ. Sci. 2016, 44, 052023. [Google Scholar] [CrossRef]
- Iwaszko, J. Making asbestos-cement products safe using heat treatment. Case Stud. Constr. Mater. 2019, 10, e00221. [Google Scholar] [CrossRef]
- Priest, G.; Horner, J.A. Fibrous ceramic aluminum silicate as an alternative to asbestos liners. J. Prosthet. Dent. 1980, 44, 51–56. [Google Scholar] [CrossRef]
- Abrajano, T.A. Geochemistry of reduced gas related to serpentinization of the Zambales ophiolite, Philippines. Appl. Geochem. 1990, 5, 625–630. [Google Scholar] [CrossRef]
- McCollom, T.M. Geochemical constraints on primary productivity in submarine hydrothermal vent plumes. Deep-Sea Res. Lett. 2000, 47, 85–101. [Google Scholar] [CrossRef]
- Carmingnano, O. Employment of serpentinite rock in architecture. J. Mater. Sci. Eng. 2023, 301, 495–498. [Google Scholar]
- Whittaker, E.J.W.; Zussman, J. The characterization of serpentine minerais by X-Ray diffraction. Mineral. Mag. 1956, 31, 107–126. [Google Scholar]
- McDonough, W.F. The composition of the Earth. Chem. Geol. 1989, 120, 3–4. [Google Scholar]
- Kılıç, A.D. (Fırat University, Elazığ, Türkiye). A New Perspective on U-Pb Geochronology in Zoned Garnets and Identification of Inclusion Minerals through Raman Spectroscopy, as well as Stable Isotopes and Metallic Enrichments in Serpentinites. Fırat University Project Number MF 24.49. 2024; (Unpublished work). [Google Scholar]
- Me’vel, C. Serpentinization of abyssal peridotites at mid-ocean ridges. Comptes Rendus Geosci. 2003, 335, 825–852. [Google Scholar] [CrossRef]
- Cavallo, A. Resour. Policy 2018, 59, 17. [Google Scholar]
- Özkan, Y.Z. Guleman ofiyolitinde metamorfizma izleri. TJK Symp. 1989, 11, 29–32. (In Turkish) [Google Scholar]
- Kılıç, A.D.; Yıldırım, Ö. Redox Reactions in Antigorite Serpantinites. Int. Conf. Innov. Acad. Stud. 2023, 3, 276–279. [Google Scholar] [CrossRef]
- Deschamps, F.; Godard, M.; Guillot, S.; Hattori, K. Geochemistry of subduction zone serpentinites: A review. Lithos 2013, 178, 96–127. [Google Scholar] [CrossRef]
- Yada, K.; Iishi, K. Serpentine minerals hydrothermally synthesized and their microstructures. J. Cryst. Growth 1974, 24, 627–630. [Google Scholar] [CrossRef]
- Niu, Y. Bulk-rock major and trace element compositions of abyssal peridotites: Implications for mantle melting, melt extraction and post-melting processes beneath mid-ocean ridges. J. Petrol. 2004, 45, 2423–2458. [Google Scholar] [CrossRef]
- Ball, M.C.; Taylor, H.F.W. The dehydration of chrysotile in air and under hydrothermal conditions. Mineral. Mag. 1963, 33, 467–482. [Google Scholar] [CrossRef]
- Viti, C. Serpentine minerals discrimination by thermal analysis. Am. Mineral. 2010, 95, 631–638. [Google Scholar] [CrossRef]
- Alt, J.C.; Shanks, W.C. Stable isotope compositions of serpentine seamounts in the Mariana forearc: Serpentinization processes, fluid sources and sulphur metasomatism. Earth Planet. Sci. Lett. 2006, 242, 272–285. [Google Scholar] [CrossRef]
- Poirier, J.-P. Light elements in the Earth’s outer core: A critical review. Phys. Earth Planet. Inter. 1994, 85, 319–337. [Google Scholar] [CrossRef]
- Surour, A.A. Chemistry of serpentine “polymorphs” in the Pan-African serpentinites from the Eastern Desert of Egypt, with an emphasis on the effect of superimposed thermal metamorphism. Mineral. Petrol. 2017, 111, 99–119. [Google Scholar] [CrossRef]
- Miyashiro, A.; Shido, F.; Ewing, M. Composition and origin of serpentinites from the Mid-Atlantic Ridge near 24 and 30°N. Contrib. Mineral. Petrol. 1969, 23, 117–127. [Google Scholar] [CrossRef]
- Hattori, K.H.; Wallis, S.; Enami, T.; Mizukami, T. Subduction of mantle wedge peridotites: Evidence from the Higashi-akaishi ultramafic body in the Sanbagawa metamorphic belt. Island Arc. 2010, 19, 192–207. [Google Scholar] [CrossRef]
- Hellebrand, E.; Snow, J.E.; Dick, H.J.B.; Hofmann, A.E. Coupled major and trace elements as indicators of the extent of melting in mid-ocean-ridge peridotites. Nature 2001, 410, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Fantini, R.; Sisti, M.; Arletti, R.; Malferrari, D.; Gamberini, M.C.; Zapparoli, M.; Gualtieri, A.F. Identification and quantification protocol of hazardous-metal bearing minerals: Ni in serpentinite rocks from Valmalenco (Sondrio, Central Alps, Northern Italy). J. Hazard. Mater. 2024, 476, 134–928. [Google Scholar] [CrossRef]



| Major Oxides | Na2O (%) | MgO (%) | Al2O3 (%) | SiO2 (%) | P2O5 (%) | K2O (%) | CaO (%) | TiO2 (%) | MnO (%) | Fe2O3 (%) | LOI | TOTAL | Sc | V | Co | Ni | Cu | Zn | Ga | Rb | Sr | Y | Zr | Nb | Mo | Cd | Ba | Ti | Cr | Sn | Mn | Cs | Ta |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sp-01 | 0.3 | 22.8 | 6.9 | 37.3 | 0.1 | 1.1 | 4.5 | 0.2 | 0.3 | 5 | 11.3 | 99.6 | 20 | 72 | 18 | 100 | 14 | 35 | 9.9 | 10 | 102 | 7.3 | 19 | 2.4 | 5 | 0.1 | 33 | 22 | 226 | 10 | 814 | 1.1 | 0.2 |
| Sp-03 | 0.1 | 38.8 | 0.5 | 40.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 7.4 | 11.8 | 100.4 | 4 | 12 | 87 | 965 | 3 | 26 | 0.9 | 10 | 10 | 0.2 | 2 | 0.1 | 5 | 2 | 49 | 32 | 233 | 11 | 803 | 0.1 | 0.1 |
| Sp-04 | 0.1 | 34.9 | 0.6 | 35.1 | 0.1 | 0.1 | 4.9 | 0.1 | 0.1 | 7.2 | 11.4 | 100.4 | 3 | 12 | 91 | 972 | 19 | 446 | 1.1 | 10 | 32 | 0.4 | 2.9 | 0.2 | 5 | 2 | <10 | 32 | 432 | 10 | 619 | 0.1 | <0.1 |
| Sp-06 | 0.1 | 38.2 | 0.3 | 41.9 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 6.6 | 11.6 | 100.6 | 6 | 16 | 75 | 1545 | 3 | 42 | 1.7 | 10 | 10 | 0.1 | 1.6 | 0.1 | 5 | 0.6 | <10 | 36 | 625 | 10 | 934 | 1.2 | <0.1 |
| Sp-08 | 0.1 | 37.7 | 0.8 | 41.5 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 6.6 | 11.3 | 100.2 | 8 | 27 | 73 | 1819 | 4 | 26 | 2 | 10 | 10 | 0.2 | 1.9 | 0.1 | 5 | 1.1 | <10 | 32 | 112 | 11 | 722 | 0.1 | <0.1 |
| Sp-09 | 0.1 | 37.2 | 0.8 | 42.1 | 0.1 | 0.1 | 0.4 | 0.1 | 0.2 | 6.6 | 9.1 | 100.1 | 5 | 15 | 54 | 1197 | 4 | 32 | 2.2 | 10 | 10 | 14.4 | 2.6 | 1 | 5 | 0.8 | <10 | 30 | 201 | <10 | 274 | 0.1 | <0.1 |
| Sp-10 | 0.1 | 37.3 | 2.6 | 38.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 9 | 8.0 | 99.9 | 9 | 24 | 81 | 727 | 5 | 35 | 1.1 | 10 | 10 | 1.5 | 2.7 | 0.1 | 5 | 1.6 | <10 | 66 | 772 | <10 | 565 | 0.1 | <0.1 |
| Sp-11 | 0.1 | 33.5 | 1.9 | 37.8 | 0.1 | 0.1 | 4.23 | 0.1 | 0.1 | 8.3 | 1.6 | 99.8 | 7 | 33 | 96 | 1184 | 15 | 34 | 1 | 10 | 29 | 1.2 | 3.4 | 0.1 | 5 | 1.1 | <10 | 33 | 381 | <10 | 415 | 0.1 | <0.1 |
| Sp-12 | 0.1 | 36.7 | 0.5 | 37.1 | 0.1 | 0.1 | 2.6 | 0.1 | 0.1 | 6.7 | 3.4 | 99.8 | 20 | 44 | 82 | 1757 | 24 | 44 | 0.8 | 10 | 140 | 0.4 | 2.5 | 0.1 | 5 | 0.8 | <10 | 34 | 206 | <10 | 324 | 0.1 | <0.1 |
| Sp-14 | 0.1 | 38.5 | 0.6 | 39.3 | 0.1 | 0.1 | 1 | 0.1 | 0.1 | 74 | 1.3 | 99.9 | 6 | 36 | 74 | 1503 | 17 | 33 | 1.1 | 10 | 20 | 0.3 | 2 | 0.1 | 5 | 1.1 | <10 | 55 | 189 | <10 | 217 | 0.1 | 0.1 |
| Sp-15 | 0.1 | 38.4 | 0.8 | 41.8 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 10 | 1.3 | 99.9 | 7 | 19 | 96 | 2033 | 19 | 34 | 0.8 | 10 | 10 | 0.4 | 2.3 | 0.1 | 5 | 1.1 | <10 | 58 | 204 | <10 | 219 | 0.1 | 0.1 |
| Sp-16 | 0.1 | 37.6 | 0.8 | 40 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 7.9 | 1.9 | 99.9 | 5 | 31 | 74 | 1513 | 12 | 31 | 1.5 | 10 | 10 | 0.3 | 1.9 | 0.1 | 5 | 1.3 | <10 | 37 | 311 | <10 | 812 | 0.1 | <0.1 |
| Sp-17 | 0.1 | 37.9 | 0.6 | 40.1 | 0.1 | 0.1 | 0.8 | 0.1 | 0.1 | 10.6 | 1.4 | 99.9 | 12 | 28 | 75 | 1739 | 13 | 29 | 0.7 | 10 | 10 | 0.1 | 1.9 | 0.1 | 5 | 0.7 | <10 | 44 | 197 | <10 | 613 | 0.1 | 0.1 |
| Sp-18 | 0.1 | 36.2 | 0.8 | 38.4 | 0.1 | 0.1 | 2.4 | 0.1 | 0.1 | 7.9 | 1.2 | 99.8 | 8 | 43 | 89 | 1019 | 113 | 47 | 0.9 | 10 | 10 | 2.8 | 2.6 | 0.1 | 5 | 1.5 | <10 | 31 | 312 | <10 | 913 | 0.1 | <0.1 |
| Sp-21 | 0.1 | 38.1 | 0.4 | 37.7 | 0.1 | 0.1 | 0.5 | 0.1 | 0.2 | 10.0 | 1.7 | 99.9 | 9 | 26 | 80 | 1740 | 7 | 41 | 0.7 | 10 | 10 | 0.2 | 1.3 | 0.1 | 5 | 0.7 | <10 | 52 | 423 | <10 | 577 | 0.1 | <0.1 |
| Sp-23 | 0.1 | 41.1 | 0.7 | 43.1 | 0.1 | 0.1 | 0.8 | 0.1 | 0.1 | 9.67 | 1.7 | 99.9 | 0 | 15 | 73 | 1687 | 11 | 36 | 0.9 | 10 | 10 | 0.1 | 1.6 | 0.1 | 5 | 1.1 | <10 | 35 | 178 | <10 | 311 | 0.1 | <0.1 |
| Sp-24 | 0.1 | 42.1 | 0.6 | 41.7 | 0.1 | 0.1 | 3.42 | 0.1 | 0.1 | 7.1 | 1.4 | 99.8 | 0 | 27 | 91 | 1860 | 23 | 41 | 0.9 | 10 | 10 | 0.6 | 1.5 | 0.1 | 5 | 0.9 | <10 | 37 | 120 | 10 | 293 | 0.1 | <0.1 |
| Sp-25 | 0.1 | 36.7 | 3.3 | 37.4 | 0.1 | 0.1 | 5.1 | 0.1 | 0.1 | 7.8 | 2.0 | 99.8 | 0 | 37 | 79 | 1175 | 4 | 28 | 2.9 | 10 | 75.4 | 2.7 | 4.5 | 0.3 | 5 | 0.8 | <10 | 33 | 344 | <10 | 435 | 0.1 | <0.1 |
| Sp-27 | 0.1 | 33.8 | 3.3 | 40.0 | 0.1 | 0.1 | 1 | 0.1 | 0.1 | 9.1 | 1.4 | 99.8 | 0 | 34 | 68 | 1323 | 20 | 37 | 2.1 | 18 | 10 | 2.8 | 4.6 | 0.3 | 5 | 0.7 | <10 | 34 | 221 | <10 | 209 | 0.1 | <0.1 |
| Trace Elements (ppm) | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ∑REE | ∑LREE | ∑HREE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sp-01 | 0.90 | 0.48 | 0.16 | 0.34 | 0.22 | 0.11 | 0.18 | 0.18 | 0.18 | 0.08 | 0.19 | 0.05 | 0.18 | 0.04 | 3.29 | 2.39 | 0.90 |
| Sp-03 | 0.35 | 0.27 | 0.12 | 0.38 | 0.18 | 0.12 | 0.22 | 0.22 | 0.16 | 0.09 | 0.18 | 0.04 | 0.17 | 0.05 | 2.55 | 1.64 | 0.91 |
| Sp-04 | 0.44 | 0.42 | 0.10 | 0.36 | 0.21 | 0.11 | 0.20 | 0.18 | 0.19 | 0.10 | 0.18 | 0.04 | 0.17 | 0.04 | 2.74 | 1.84 | 0.90 |
| Sp-06 | 0.88 | 0.32 | 0.11 | 0.37 | 0.15 | 0.13 | 0.19 | 0.21 | 0.17 | 0.09 | 0.20 | 0.05 | 0.19 | 0.04 | 3.10 | 2.15 | 0.95 |
| Sp-08 | 0.66 | 0.44 | 0.13 | 0.42 | 0.16 | 0.12 | 0.24 | 0.16 | 0.18 | 0.08 | 0.19 | 0.04 | 0.18 | 0.05 | 3.05 | 2.17 | 0.88 |
| Sp-09 | 0.92 | 0.42 | 0.18 | 0.38 | 0.22 | 0.13 | 0.23 | 0.19 | 0.19 | 0.07 | 0.21 | 0.04 | 0.20 | 0.04 | 3.42 | 2.67 | 0.94 |
| Sp-10 | 0.38 | 0.30 | 0.12 | 0.36 | 0.18 | 0.10 | 0.26 | 0.17 | 0.19 | 0.08 | 0.22 | 0.05 | 0.21 | 0.05 | 2.67 | 1.70 | 0.97 |
| Sp-11 | 0.23 | 0.31 | 0.12 | 0.44 | 0.21 | 0.12 | 0.18 | 0.18 | 0.16 | 0.08 | 0.22 | 0.04 | 0.18 | 0.04 | 2.51 | 1.61 | 0.90 |
| Sp-12 | 0.44 | 0.40 | 0.10 | 0.35 | 0.15 | 0.13 | 0.22 | 0.16 | 0.16 | 0.09 | 0.18 | 0.05 | 0.19 | 0.04 | 2.66 | 1.79 | 0.87 |
| Sp-14 | 0.36 | 0.29 | 0.09 | 0.34 | 0.16 | 0.14 | 0.21 | 0.20 | 0.18 | 0.08 | 0.19 | 0.04 | 0.20 | 0.05 | 2.53 | 1.59 | 0.94 |
| Sp-15 | 0.28 | 0.30 | 0.11 | 0.45 | 0.22 | 0.13 | 0.23 | 0.18 | 0.16 | 0.09 | 0.18 | 0.05 | 0.16 | 0.05 | 2.59 | 1.72 | 0.87 |
| Sp-16 | 0.36 | 0.31 | 0.11 | 0.42 | 0.18 | 0.12 | 0.24 | 0.22 | 0.19 | 0.09 | 0.18 | 0.04 | 0.19 | 0.04 | 2.69 | 1.74 | 0.95 |
| Sp-17 | 0.34 | 0.33 | 0.14 | 0.43 | 0.21 | 0.12 | 0.25 | 0.16 | 0.21 | 0.09 | 0.20 | 0.05 | 0.20 | 0.05 | 2.78 | 1.82 | 0.96 |
| Sp-18 | 0.25 | 0.28 | 0.10 | 0.44 | 0.15 | 0.14 | 0.22 | 0.17 | 0.18 | 0.09 | 0.22 | 0.05 | 0.19 | 0.05 | 2.53 | 1.58 | 0.95 |
| Sp-21 | 0.22 | 0.30 | 0.09 | 0.46 | 0.22 | 0.11 | 0.18 | 0.20 | 0.19 | 0.08 | 0.18 | 0.04 | 0.21 | 0.04 | 2.52 | 1.58 | 0.94 |
| Sp-23 | 0.35 | 0.30 | 0.14 | 0.38 | 0.18 | 0.13 | 0.22 | 0.18 | 0.19 | 0.08 | 0.22 | 0.05 | 0.19 | 0.05 | 2.66 | 1.70 | 0.96 |
| Sp-24 | 0.24 | 0.34 | 0.13 | 0.36 | 0.21 | 0.12 | 0.23 | 0.19 | 0.17 | 0.10 | 0.19 | 0.04 | 0.16 | 0.05 | 2.53 | 1.63 | 0.90 |
| Sp-25 | 0.33 | 0.32 | 0.18 | 0.43 | 0.15 | 0.11 | 0.24 | 0.21 | 0.18 | 0.09 | 0.21 | 0.05 | 0.20 | 0.04 | 2.74 | 1.76 | 0.98 |
| Sp-27 | 0.37 | 0.28 | 0.11 | 0.41 | 0.16 | 0.12 | 0.20 | 0.18 | 0.19 | 0.08 | 0.20 | 0.05 | 0.18 | 0.05 | 2.58 | 1.65 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şaşmaz, A.; Kılıç, A.D.; Konakçı, N. Chemical and Thermal Changes in Mg3Si2O5 (OH)4 Polymorph Minerals and Importance as an Industrial Material. Appl. Sci. 2024, 14, 10298. https://doi.org/10.3390/app142210298
Şaşmaz A, Kılıç AD, Konakçı N. Chemical and Thermal Changes in Mg3Si2O5 (OH)4 Polymorph Minerals and Importance as an Industrial Material. Applied Sciences. 2024; 14(22):10298. https://doi.org/10.3390/app142210298
Chicago/Turabian StyleŞaşmaz, Ahmet, Ayşe Didem Kılıç, and Nevin Konakçı. 2024. "Chemical and Thermal Changes in Mg3Si2O5 (OH)4 Polymorph Minerals and Importance as an Industrial Material" Applied Sciences 14, no. 22: 10298. https://doi.org/10.3390/app142210298
APA StyleŞaşmaz, A., Kılıç, A. D., & Konakçı, N. (2024). Chemical and Thermal Changes in Mg3Si2O5 (OH)4 Polymorph Minerals and Importance as an Industrial Material. Applied Sciences, 14(22), 10298. https://doi.org/10.3390/app142210298








