Effect of the Compounding Method on the Development of High-Performance Binary and Ternary Blends Based on PPE
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Blends Preparation with a Twin-Screw Extruder
2.2. Blends Preparation with Internal Mixing
2.3. Formulations
2.4. Characterization
3. Results and Discussion
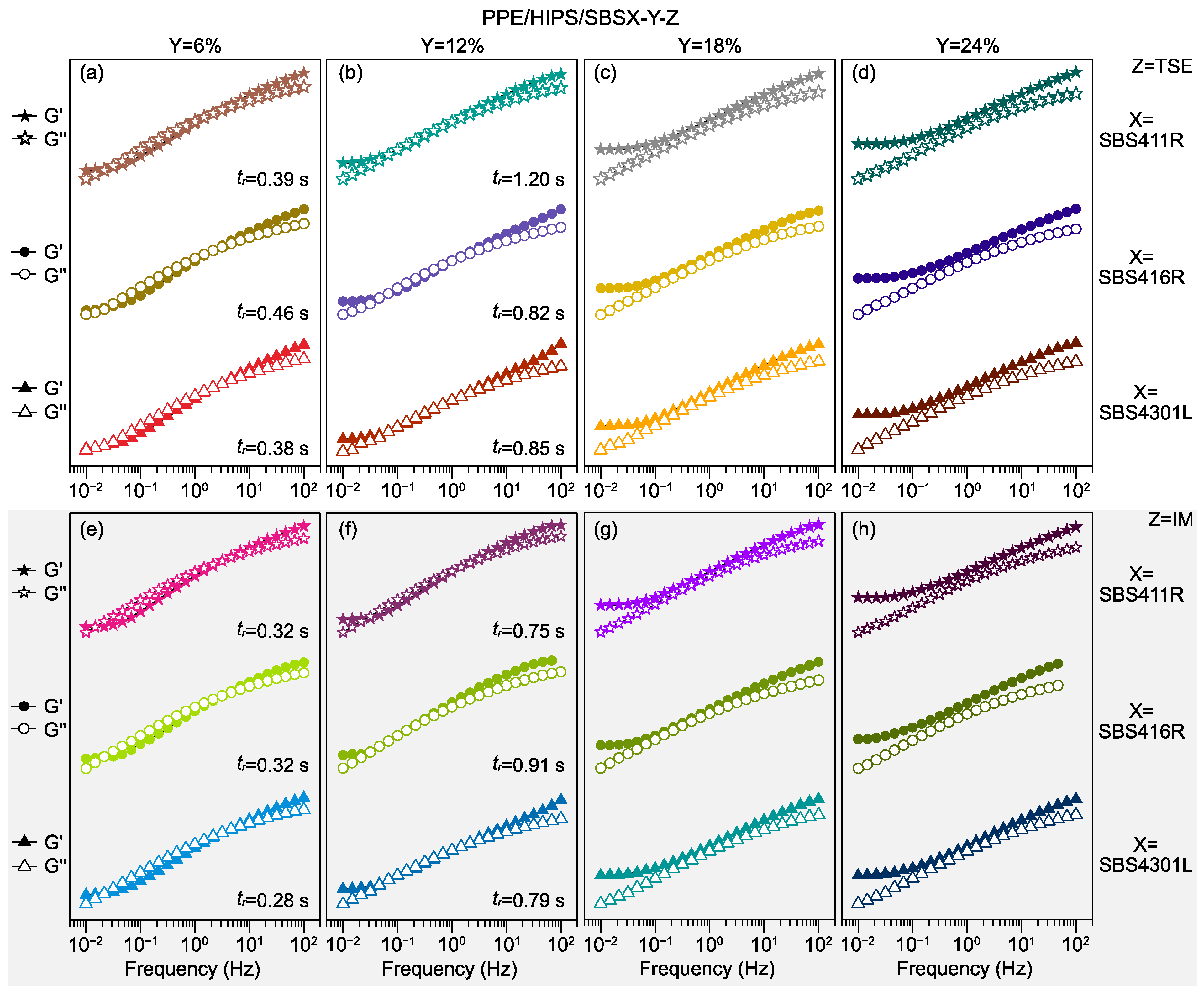
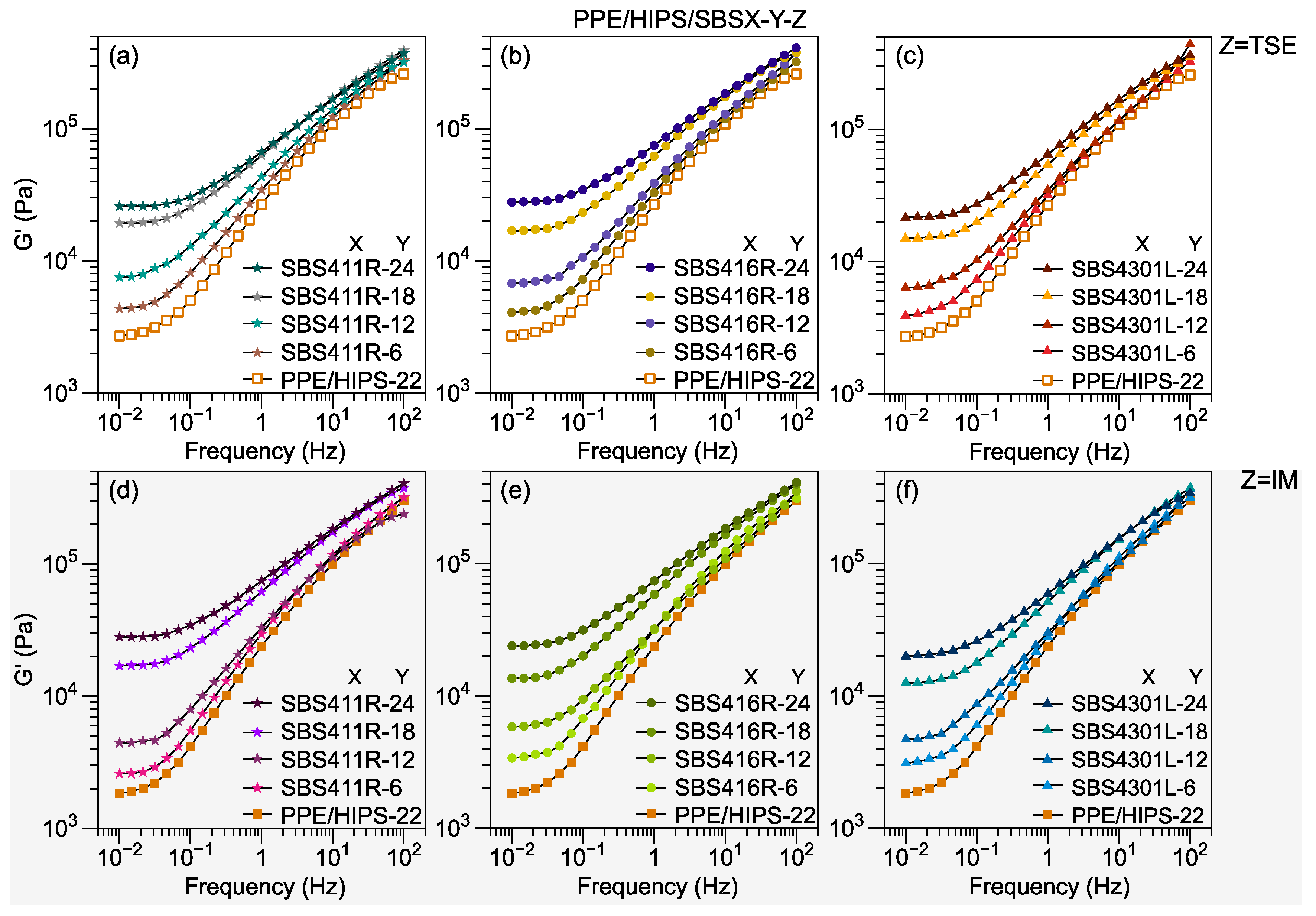
3.1. Effect of SBS Architecture, Content, and Molecular Weight
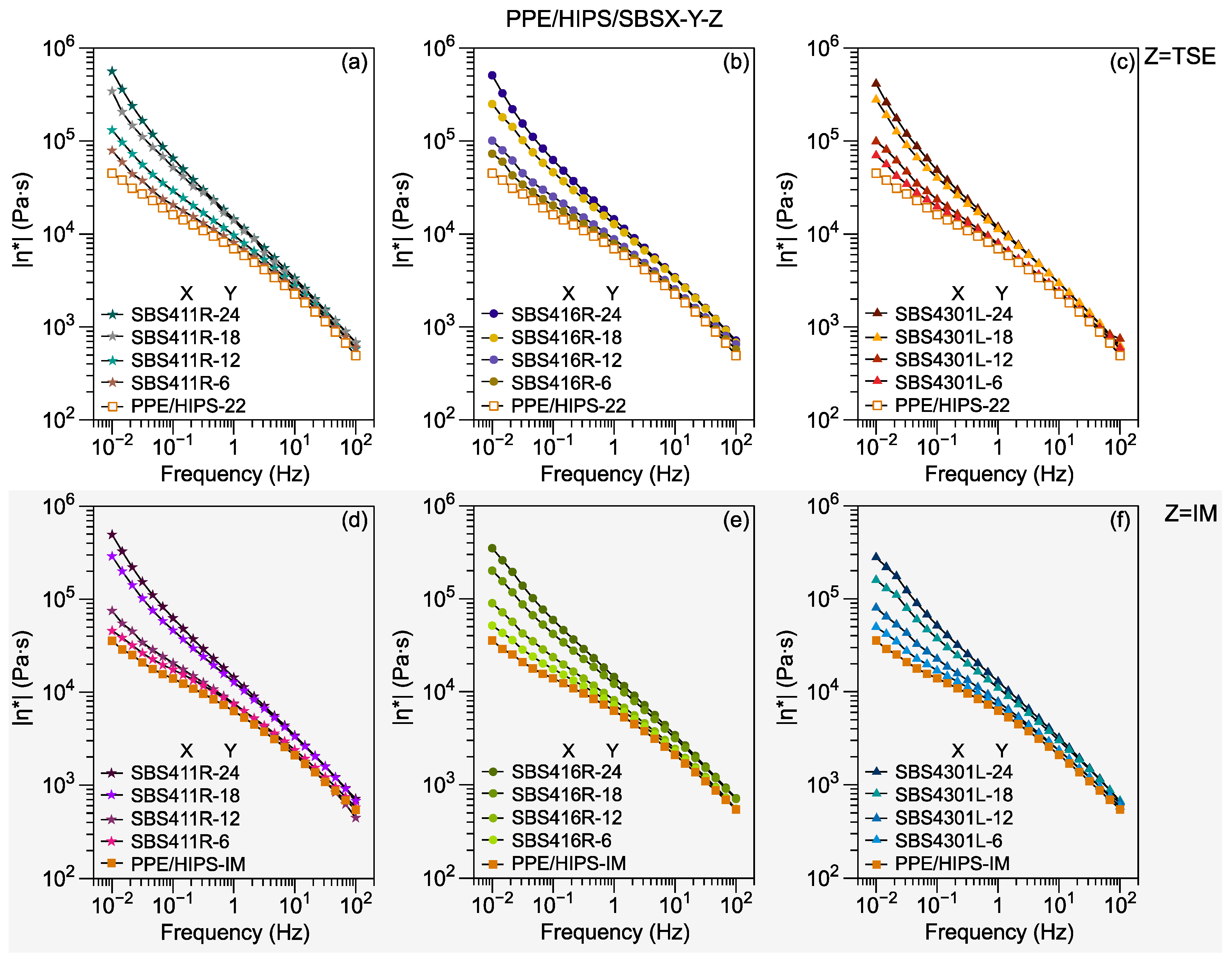
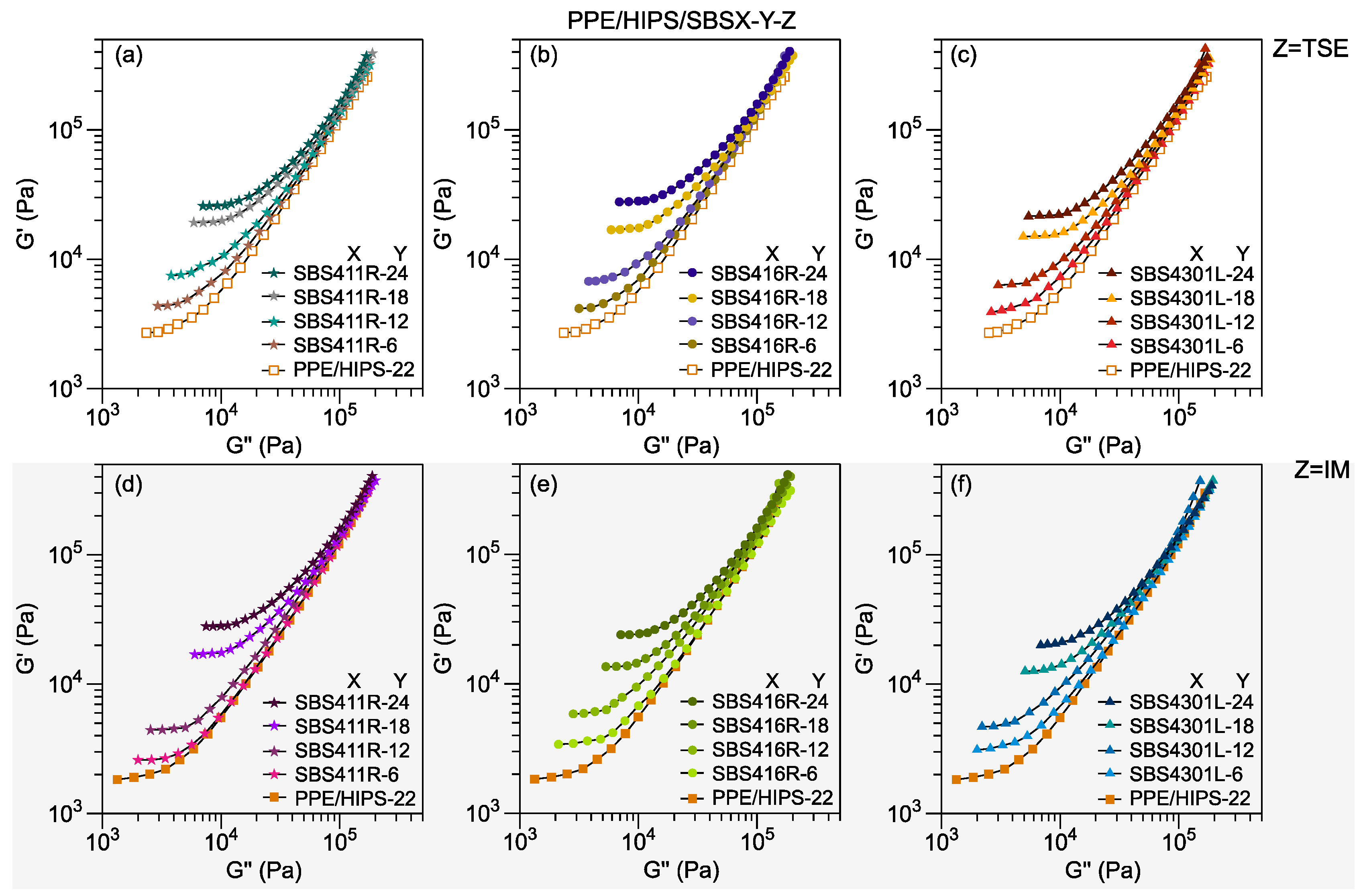
3.2. Viscosity
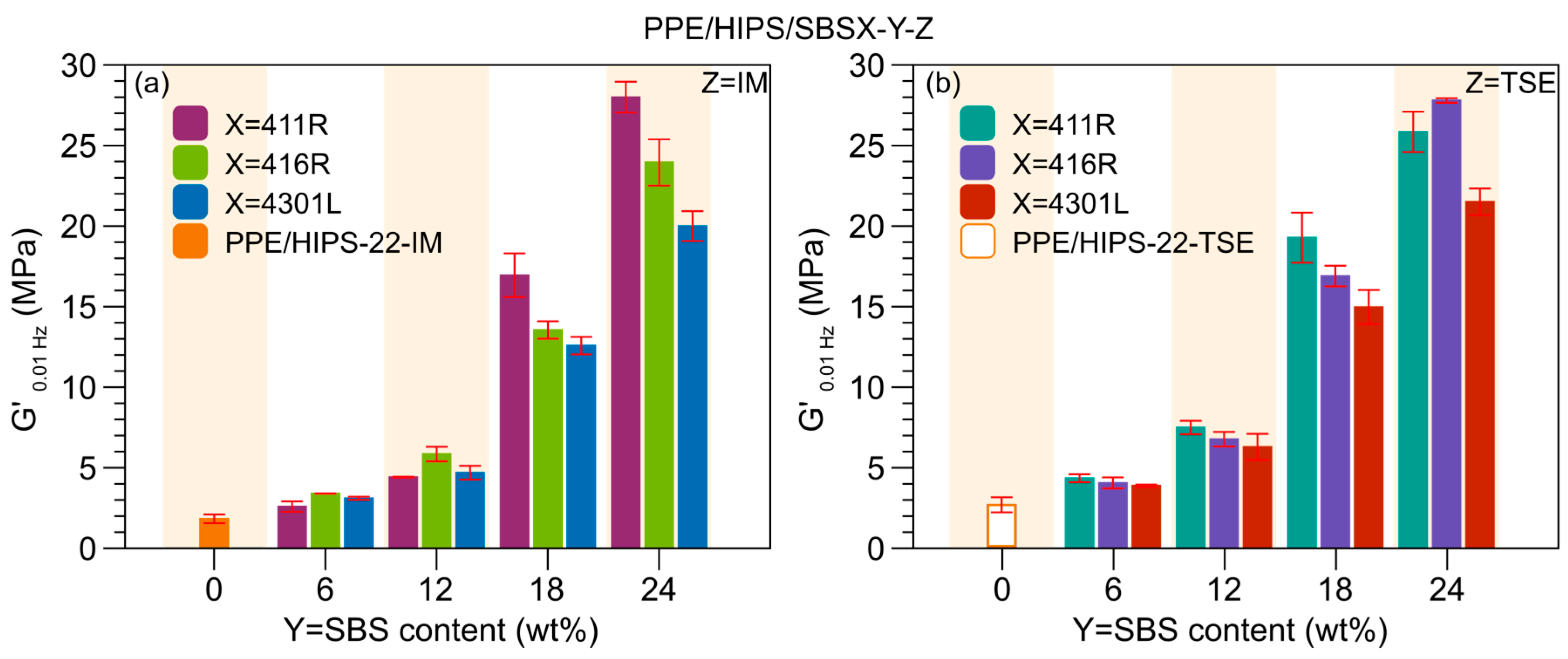
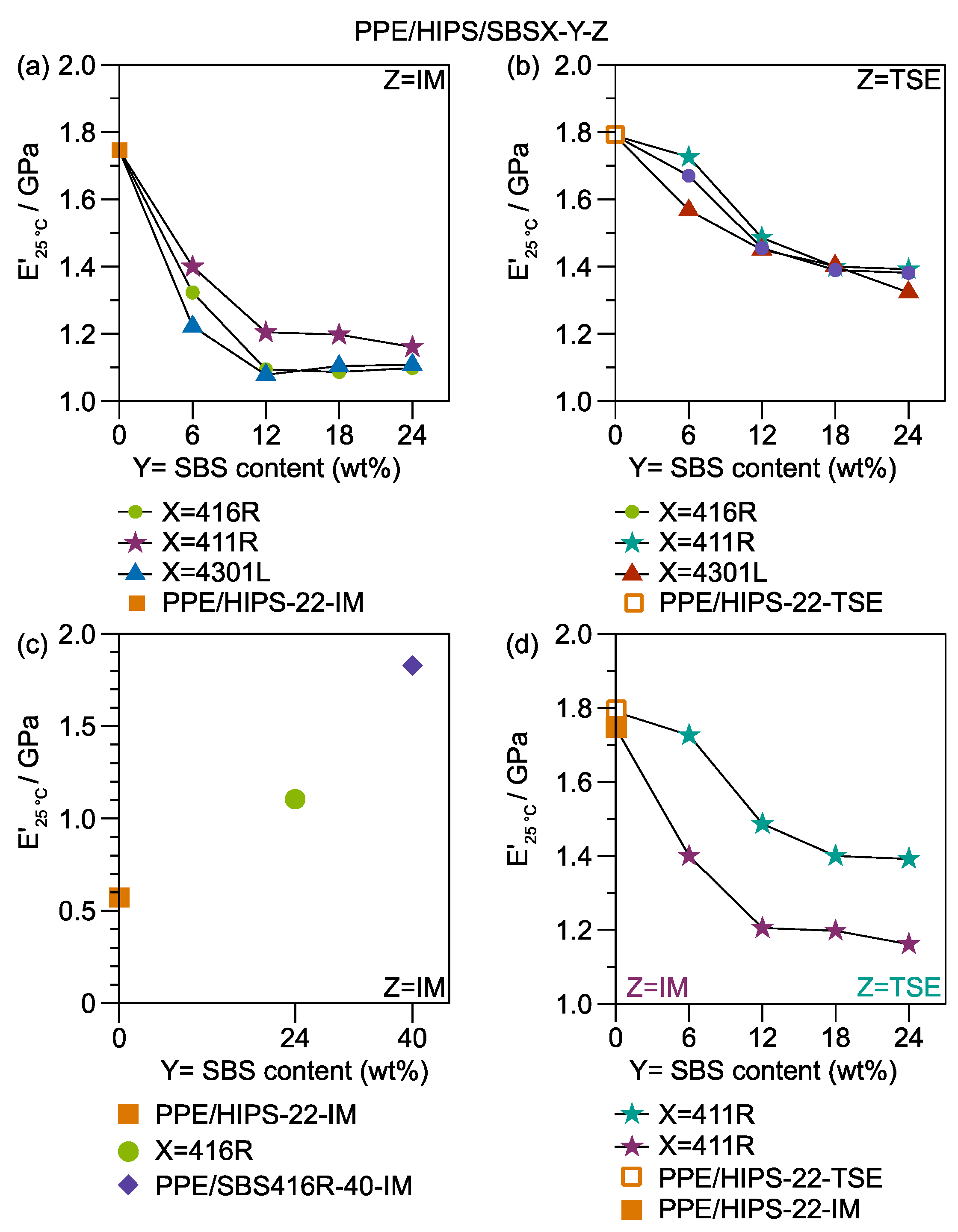
3.3. Thermomechanical Stability
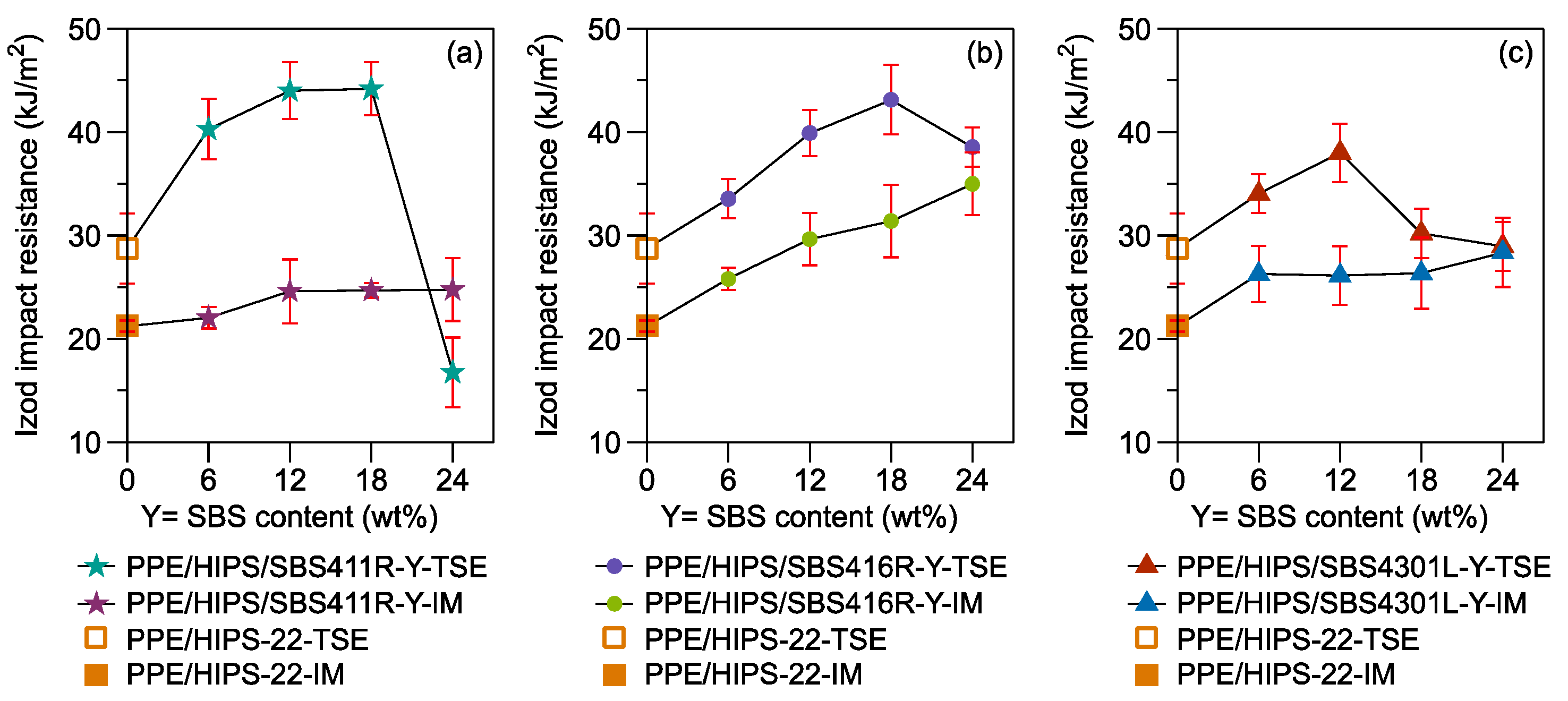
3.4. Toughening
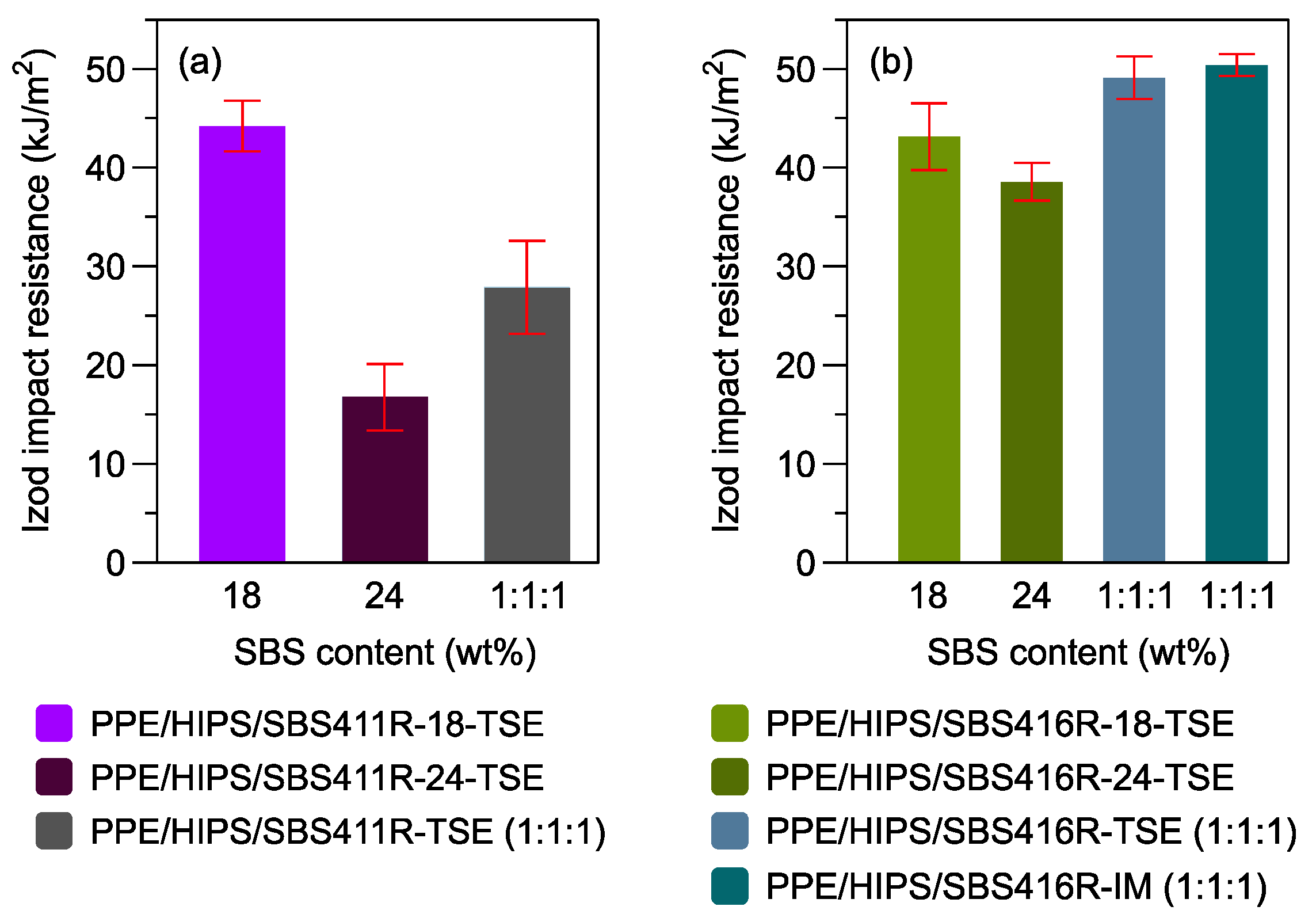
3.5. Impact Resistance in PPE/HIPS/SBS Blends at 1:1:1 Ratio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hay, A.S. Polymerization by Oxidative Coupling: Discovery and Commercialization of PPO® and Noryl® Resins. J. Polym. Sci. Part Polym. Chem. 1998, 36, 505–517. [Google Scholar] [CrossRef]
- L’Abee, R.M.A.; Duin, M.V.; Spoelstra, A.B.; Goossens, J.G.P. The Rubber Particle Size to Control the Properties-Processing Balance of Thermoplastic/Cross-Linked Elastomer Blends. Soft Matter 2010, 6, 1758–1768. [Google Scholar] [CrossRef]
- Ribeiro, V.F.; Domingues, N.S.; Riegel, I.C. Estudo Da Recuperação Das Propriedades de Poliestireno de Alto Impacto (HIPS) Através Da Incorporação de Borracha Termoplástica Tipo Estireno-Butadieno-Estireno (SBS). Polimeros 2012, 22, 186–192. [Google Scholar] [CrossRef]
- Wang, X.; Feng, W.; Li, H.; Ruckenstein, E. Optimum Toughening via a Bicontinuous Blending: Toughening of PPO with SEBS and SEBS-g-Maleic Anhydride. Polymer 2002, 43, 37–43. [Google Scholar] [CrossRef]
- Salehiyan, R.; Ray, S.S.; Stadler, F.J.; Ojijo, V. Rheology-Microstructure Relationships in Melt-Processed Polylactide/Poly(Vinylidene Fluoride) Blends. Materials 2018, 11, 2450. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility Improvements of PLA-Based Binary and Ternary Blends with Controlled Morphology Using PBAT, PBSA, and Nanoclay. Compos. Part B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Ajitha, A.R.; Thomas, S. Chapter 1—Introduction: Polymer Blends, Thermodynamics, Miscibility, Phase Separation, and Compatibilization. In Compatibilization of Polymer Blends; Ajitha, A.R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–29. ISBN 978-0-12-816006-0. [Google Scholar]
- Bahrami, R.; Löbling, T.I.; Schmalz, H.; Müller, A.H.E.; Altstädt, V. Micromechanics of “Raspberry” Morphology in PPE/SAN Polymer Blends Compatibilized with Linear ABC Triblock Terpolymers. Polymer 2015, 80, 52–63. [Google Scholar] [CrossRef]
- Gupta, S.; Krishnamurthy, R.; Preschilla, N.; Biswas, A.; Bhowmick, A.K. Development and Properties of Novel Thermoplastic Elastomer Based on Poly (Phenylene Ether). Rubber Chem. Technol. 2007, 80, 642–660. [Google Scholar] [CrossRef]
- Puskas, J.E.; Kwon, Y.; Altstädt, V.; Kontopoulou, M. Blends of Poly(2,6-Dimethyl-1,4-Phenylene Oxide) (PPO) with Polystyrene-Based Thermoplastic Rubbers: A Comparative Study. Polymer 2007, 48, 590–597. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Hwung, D.-S. Miscibility Study on Polyphenylene Oxide and SBS Triblock Copolymer Blends. Eur. Polym. J. 1994, 30, 1191–1195. [Google Scholar] [CrossRef]
- Shokoohi, S.; Arefazar, A.; Naderi, G. Compatibilized Polypropylene/Ethylene–Propylene–Diene-Monomer/Polyamide6 Ternary Blends: Effect of Twin Screw Extruder Processing Parameters. Mater. Des. 2011, 32, 1697–1703. [Google Scholar] [CrossRef]
- Canto, L.B. On the Coarsening of the Phase Morphology of PP/EVA Blends during Compounding in a Twin Screw Extruder. Polym. Test. 2014, 34, 175–182. [Google Scholar] [CrossRef]
- Gallego, R.; García-López, D.; López-Quintana, S.; Gobernado-Mitre, I.; Merino, J.C.; Pastor, J.M. Toughening of PA6/mEPDM Blends by Two Methods of Compounding, Extruder and Internal Mixer: Rheological, Morphological and Mechanical Characterization. Polym. Bull. 2008, 60, 665–675. [Google Scholar] [CrossRef]
- Goharpey, F.; Foudazi, R.; Nazockdast, H.; Katbab, A.A. Determination of Twin-Screw Extruder Operational Conditions for the Preparation of Thermoplastic Vulcanizates on the Basis of Batch-Mixer Results. J. Appl. Polym. Sci. 2008, 107, 3840–3847. [Google Scholar] [CrossRef]
- Shahbikian, S.; Carreau, P.J.; Heuzey, M.-C.; Ellul, M.D.; Cheng, J.; Shirodkar, P.; Nadella, H.P. Morphology Development of EPDM/PP Uncross-linked/Dynamically Cross-linked Blends. Polym. Eng. Sci. 2012, 52, 309–322. [Google Scholar] [CrossRef]
- ASTM 256; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA. [CrossRef]
- Zou, Z.M.; Sun, Z.Y.; An, L.J. Effect of Fumed Silica Nanoparticles on the Morphology and Rheology of Immiscible Polymer Blends. Rheol. Acta 2014, 53, 43–53. [Google Scholar] [CrossRef]
- Nakayama, D.; Wu, F.; Mohanty, A.K.; Hirai, S.; Misra, M. Biodegradable Composites Developed from PBAT/PLA Binary Blends and Silk Powder: Compatibilization and Performance Evaluation. ACS Omega 2018, 3, 12412–12421. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, J.; Dhakate, S.R.; Singh, B.P. Phase Transition and Anomalous Rheological Properties of Graphene Oxide-Carbon Nanotube Acrylonitrile Butadiene Styrene Hybrid Composites. Compos. Part B Eng. 2018, 154, 337–350. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook: 4th Edition; Vincentz Network: Hanover, Germany, 2019; ISBN 978-3-7486-0036-7. [Google Scholar]
- Arrigo, R.; Mascia, L.; Clarke, J.; Malucelli, G. Structure Evolution of Epoxidized Natural Rubber (Enr) in the Melt State by Time-Resolved Mechanical Spectroscopy. Materials 2020, 13, 946. [Google Scholar] [CrossRef]
- Liew, C.W.; Durairaj, R.; Ramesh, S. Rheological Studies of PMMA-PVC Based Polymer Blend Electrolytes with LiTFSI as Doping Salt. PLoS ONE 2014, 9, e102815. [Google Scholar] [CrossRef]
- Hernández-Alamilla, M.; Valadez-Gonzalez, A. The Effect of Two Commercial Melt Strength Enhancer Additives on the Thermal, Rheological and Morphological Properties of Polylactide. J. Polym. Eng. 2016, 36, 31–41. [Google Scholar] [CrossRef]
- Rinawa, K.; Maiti, S.N.; Sonnier, R.; Cuesta, J.-M.L. Non-Isothermal Crystallization Kinetics and Thermal Behaviour of PA12/SEBS-g-MA Blends. Bull. Mater. Sci. 2015, 38, 1315–1327. [Google Scholar] [CrossRef]
- Lafranche, E.; Krawczak, P. Toughness Improvement of Novel Biobased Aromatic Polyamide/SEBS/Organoclay Ternary Hybrids. J. Appl. Polym. Sci. 2020, 137, 48888. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Li, M.; Kang, H.; Zhang, L. Renewable and Supertoughened Polylactide-Based Composites: Morphology, Interfacial Compatibilization, and Toughening Mechanism. Ind. Eng. Chem. Res. 2016, 55, 9195–9204. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, J.; Fei, M.; Qiu, R.; Sakai, E. Manufacturing of Thermally Remoldable Blends from Epoxidized Soybean Oil and Poly(Lactic Acid) via Dynamic Cross-Linking in a Twin-Screw Extruder. Ind. Eng. Chem. Res. 2018, 57, 7516–7524. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Zhang, J.; Zhu, W.; Tian, T. Remarkably Improved Toughness and Thermal Stability of Poly (Vinyl Chloride) (PVC)/Poly (α-Methylstyrene-Acrylonitrile) (α-MSAN) Blend with the Assistance of Two Impact Modifiers. Polym. Test. 2016, 51, 1–5. [Google Scholar] [CrossRef]
- Adhikari, R.; Michler, G.H. Influence of Molecular Architecture on Morphology and Micromechanical Behavior of Styrene/Butadiene Block Copolymer Systems. Prog. Polym. Sci. 2004, 29, 949–986. [Google Scholar] [CrossRef]
- Weidisch, R.; Laatsch, J.; Michler, G.H.; Arnold, M.; Schade, B.; Fischer, H. Transition from Crazing to Shear Deformation in Star Block Copolymers. Macromolecules 2002, 35, 6585–6591. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, Z.; Zhang, J. Study of the Phase Morphology and Toughness in Poly (Vinyl Chloride)/Acrylonitrile–Styrene-acrylic/Styrene–Butadiene–Styrene Ternary Blends Influenced by Interfacial/Surface Tension. J. Appl. Polym. Sci. 2019, 136, 47721. [Google Scholar] [CrossRef]
- Meng, X.; Liu, X.; Li, Z.; Zhou, Q. Positron Annihilation Lifetime Spectroscopy for Miscibility Investigations of Styrene–Butadiene–Styrene Copolymer/Polystyrene Blends. Polym. Eng. Sci. 2014, 54, 785–793. [Google Scholar] [CrossRef]
- Adhikari, R. Influence of Uncoupled Diblock Molecules on Mechanical Properties of Styerene/Butadiene/Styrene Triblock Copolymers. Nepal J. Sci. Technol. 2012, 12, 149–156. [Google Scholar] [CrossRef]
- Xie, L.; Xu, H.; Niu, B.; Ji, X.; Chen, J.; Li, Z.-M.; Hsiao, B.S.; Zhong, G.-J. Unprecedented Access to Strong and Ductile Poly(Lactic Acid) by Introducing In Situ Nanofibrillar Poly(Butylene Succinate) for Green Packaging. Biomacromolecules 2014, 15, 4054–4064. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, W.; Zhou, C. Rheological Characterization of Droplet-Matrix versus Co-Continuous Morphology. J. Macromol. Sci. Part B 2006, 45, 889–898. [Google Scholar] [CrossRef]
- Martin, C. Twin Screw Extruders as Continuous Mixers for Thermal Processing: A Technical and Historical Perspective. AAPS PharmSciTech 2016, 17, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Hylton, D.; Mintz, E.A. Rheological and Thermo-Mechanical Properties of Poly(Lactic Acid)/Lignin-Coated Cellulose Nanocrystal Composites. ACS Sustain. Chem. Eng. 2017, 5, 1711–1720. [Google Scholar] [CrossRef]
- Bair, H.E. Quantitative Thermal Analysis of Polyblends. Polym. Eng. Sci. 1970, 10, 247–250. [Google Scholar] [CrossRef]
- Asthana, S.; Kennedy, J.P. Novel Polyisobutylene Stars. XXIII. Thermal, Mechanical, and Processing Characteristics of Poly(Phenylene Ether)/Polydivinylbenzene(Polyisobutylene-b-Polystyrene)37 Blends. J. Appl. Polym. Sci. 2002, 86, 2866–2872. [Google Scholar] [CrossRef]
- Saron, C.; Sanchez, E.M.S.; Isabel Felisberti, M. Thermal and Photochemical Degradation of PPO/HIPS Blends. J. Appl. Polym. Sci. 2007, 104, 3269–3276. [Google Scholar] [CrossRef]
- Dae Han, C.; Kim, J.K. On the Use of Time-Temperature Superposition in Multicomponent/Multiphase Polymer Systems. Polymer 1993, 34, 2533–2539. [Google Scholar] [CrossRef]
- Shen, H.; Luan, T.; Xie, B.; Yang, W.; Yang, M. Rheological Behaviors and Molecular Weight Distribution Characteristics of Bimodal High-density Polyethylene. J. Appl. Polym. Sci. 2011, 121, 1543–1549. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Rheological, Morphological and Mechanical Studies of Sustainably Sourced Polymer Blends Based on Poly(Lactic Acid) and Polyamide 11. Polymers 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, M.; Haghtalab, A.; Famili, N. Influence on Compounding Methods on Rheology and Morphology of Linear Low Density Polyethylene/Poly(Lactic Acid). Appl. Rheol. 2016, 26, 64746. [Google Scholar] [CrossRef]
- Maroufkhani, M.; Katbab, A.; Liu, W.; Zhang, J. Polylactide (PLA) and Acrylonitrile Butadiene Rubber (NBR) Blends: The Effect of ACN Content on Morphology, Compatibility and Mechanical Properties. Polymer 2017, 115, 37–44. [Google Scholar] [CrossRef]
- Thirtha, V.; Lehman, R.; Nosker, T. Morphological Effects on Glass Transition Behavior in Selected Immiscible Blends of Amorphous and Semicrystalline Polymers. Polymer 2006, 47, 5392–5401. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M.; Lei, L.; Wu, X. Improvement of Tensile and Thermal Properties of Poly(Lactic Acid) Composites with Admicellar-Treated Rice Straw Fiber. Chem. Eng. J. 2011, 173, 659–666. [Google Scholar] [CrossRef]
- Adhikari, R.; Huy, T.A.; Buschnakowski, M.; Michler, G.H.; Knoll, K. Asymmetric PS- Block -(PS-Co-PB)- Block -PS Block Copolymers: Morphology Formation and Deformation Behaviour. New J. Phys. 2004, 6, 28. [Google Scholar] [CrossRef][Green Version]
- Masson, J.F.; Bundalo-Perc, S.; Delgado, A. Glass Transitions and Mixed Phases in Block SBS. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 276–279. [Google Scholar] [CrossRef]
- Picchioni, F.; Casentini, E.; Passaglia, E.; Ruggeri, G. Blends of SBS Triblock Copolymer with Poly(2,6-dimethyl-1,4-phenylene Oxide)/Polystyrene Mixture. J. Appl. Polym. Sci. 2003, 88, 2698–2705. [Google Scholar] [CrossRef]
- Boudenne, A.; Ibos, L.; Candau, Y.; Thomas, S. Handbook of Multiphase Polymer Systems, Volume 1, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; ISBN 978-0-470-71420-1. [Google Scholar]
- Saengow, C.; Giacomin, A.J. Review of Nonlinear Oscillatory Shear Flow Notations and Presentations: Polymeric Liquids. Curr. Opin. Colloid Interface Sci. 2019, 43, 26–38. [Google Scholar] [CrossRef]
- Abramova, A.V.; Abramov, V.O.; Bayazitov, V.M.; Voitov, Y.; Straumal, E.A.; Lermontov, S.A.; Cherdyntseva, T.A.; Braeutigam, P.; Weiße, M.; Günther, K. A Sol-Gel Method for Applying Nanosized Antibacterial Particles to the Surface of Textile Materials in an Ultrasonic Field. Ultrason. Sonochem. 2020, 60, 104788. [Google Scholar] [CrossRef]
- Choi, S.H.; Cho, I.; Kim, K.U. Toughening of Syndiotactic Polystyrene and Poly(2,6-Dimethyl-1,4-Diphenylene Oxide) Blends. I. Influence of Mixing Protocol and Blend Conditions. Polym. J. 1999, 31, 828–835. [Google Scholar] [CrossRef][Green Version]
- Ruckdäschel, H.; Sandler, J.K.W.; Altstädt, V.; Schmalz, H.; Abetz, V.; Müller, A.H.E. Toughening of Immiscible PPE/SAN Blends by Triblock Terpolymers. Polymer 2007, 48, 2700–2719. [Google Scholar] [CrossRef]
- Li, R.; Wu, L.; Li, B.-G. Poly(l-Lactide) Materials with Balanced Mechanical Properties Prepared by Blending with PEG-Mb-PPA Multiblock Copolymers. Ind. Eng. Chem. Res. 2017, 56, 2773–2782. [Google Scholar] [CrossRef]
- Lee, J.K.; Han, C.D. Evolution of Polymer Blend Morphology during Compounding in an Internal Mixer. Polymer 1999, 40, 6277–6296. [Google Scholar] [CrossRef]
- Shang, M.; Wu, Y.; Shentu, B.; Weng, Z. Toughening of PBT by POE/POE- g -GMA Elastomer through Regulating Interfacial Adhesion and Toughening Mechanism. Ind. Eng. Chem. Res. 2019, 58, 12650–12663. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Tjong, S.C.; Xu, S.A.; Mai, Y.W. Impact-Specific Essential Work of Fracture of Maleic Anhydride-Compatibilized Polypropylene/Elastomer Blends and Their Composites. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1881–1892. [Google Scholar] [CrossRef]
- Jelcic, Z.; Holjevac-Grguric, T.; Rek, V. Mechanical Properties and Fractal Morphology of High-Impact Polystyrene/Poly(Styrene-b-Butadiene-b-Styrene) Blends. Polym. Degrad. Stab. 2005, 90, 295–302. [Google Scholar] [CrossRef]
- Liu, H.; Song, W.; Chen, F.; Guo, L.; Zhang, J. Interaction of Microstructure and Interfacial Adhesion on Impact Performance of Polylactide (PLA) Ternary Blends. Macromolecules 2011, 44, 1513–1522. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Paul, D.R. Notched Impact Behavior of Polymer Blends: Part 1: New Model for Particle Size Dependence. Polymer 2009, 50, 5539–5548. [Google Scholar] [CrossRef]
- Martínez-barrera, G.; Menchaca, C.; Pietkiewicz, D.; Brostow, W. Polystyrene + Styrene-Butadiene Blends: Mechanical and Morphological Properties. Mater. Sci. Medžiagotyra 2004, 10, 166–173. [Google Scholar]
- Mehrabi Mazidi, M.; Edalat, A.; Berahman, R.; Hosseini, F.S. Highly-Toughened Polylactide-(PLA-) Based Ternary Blends with Significantly Enhanced Glass Transition and Melt Strength: Tailoring the Interfacial Interactions, Phase Morphology, and Performance. Macromolecules 2018, 51, 4298–4314. [Google Scholar] [CrossRef]
- Ge, T.; Grest, G.S.; Robbins, M.O. Structure and Strength at Immiscible Polymer Interfaces. ACS Macro Lett. 2013, 2, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zeng, Q.; Niu, D.; Yang, W.; Dong, W.; Chen, M.; Ma, P. Design of Supertoughened and Heat-Resistant PLLA/Elastomer Blends by Controlling the Distribution of Stereocomplex Crystallites and the Morphology. Macromolecules 2019, 52, 1092–1103. [Google Scholar] [CrossRef]

| Sample | PPE (wt%) | HIPS (wt%) | SBS (wt%) |
|---|---|---|---|
| PPE/HIPS-22-IM | 77.8 | 22.2 | 0 |
| PPE/HIPS/SBSX-6-IM | 73.1 | 20.9 | 6 |
| PPE/HIPS/SBSX-12-IM | 68.5 | 19.5 | 12 |
| PPE/HIPS/SBSX-18-IM | 63.8 | 18.2 | 18 |
| PPE/HIPS/SBSX-24-IM | 60 | 16 | 24 |
| PPE/HIPS-40-IM | 60 | 40 | 0 |
| PPE/SBS416-40-IM | 60 | 0 | 40 |
| PPE/HIPS-22-TSE | 77.8 | 22.2 | 0 |
| PPE/HIPS/SBSX-6-TSE | 73.1 | 20.9 | 6 |
| PPE/HIPS/SBSX-12-TSE | 68.5 | 19.5 | 12 |
| PPE/HIPS/SBSX-18-TSE | 63.8 | 18.2 | 18 |
| PPE/HIPS/SBSX-24-TSE | 60 | 16 | 24 |
| PPE/HIPS/SBS411R-TSE (1:1:1) | 33.33 | 33.33 | 33.33 |
| PPE/HIPS/SBS416R-TSE (1:1:1) | 33.33 | 33.33 | 33.33 |
| PPE/HIPS/SBS416R-IM (1:1:1) | 33.33 | 33.33 | 33.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Martínez, E.I.; Zaragoza-Contreras, E.A.; Vega-Rios, A.; Flores-Gallardo, S.G. Effect of the Compounding Method on the Development of High-Performance Binary and Ternary Blends Based on PPE. Appl. Sci. 2024, 14, 10264. https://doi.org/10.3390/app142210264
López-Martínez EI, Zaragoza-Contreras EA, Vega-Rios A, Flores-Gallardo SG. Effect of the Compounding Method on the Development of High-Performance Binary and Ternary Blends Based on PPE. Applied Sciences. 2024; 14(22):10264. https://doi.org/10.3390/app142210264
Chicago/Turabian StyleLópez-Martínez, Erika Ivonne, Erasto Armando Zaragoza-Contreras, Alejandro Vega-Rios, and Sergio Gabriel Flores-Gallardo. 2024. "Effect of the Compounding Method on the Development of High-Performance Binary and Ternary Blends Based on PPE" Applied Sciences 14, no. 22: 10264. https://doi.org/10.3390/app142210264
APA StyleLópez-Martínez, E. I., Zaragoza-Contreras, E. A., Vega-Rios, A., & Flores-Gallardo, S. G. (2024). Effect of the Compounding Method on the Development of High-Performance Binary and Ternary Blends Based on PPE. Applied Sciences, 14(22), 10264. https://doi.org/10.3390/app142210264






