Development of Micro-Nano Structured Electrodes for Enhanced Reactivity: Improving Efficiency Through Nano-Bubble Generation
Abstract
1. Introduction
2. Experimental Section
2.1. Material Selection
2.2. Electrode Modification
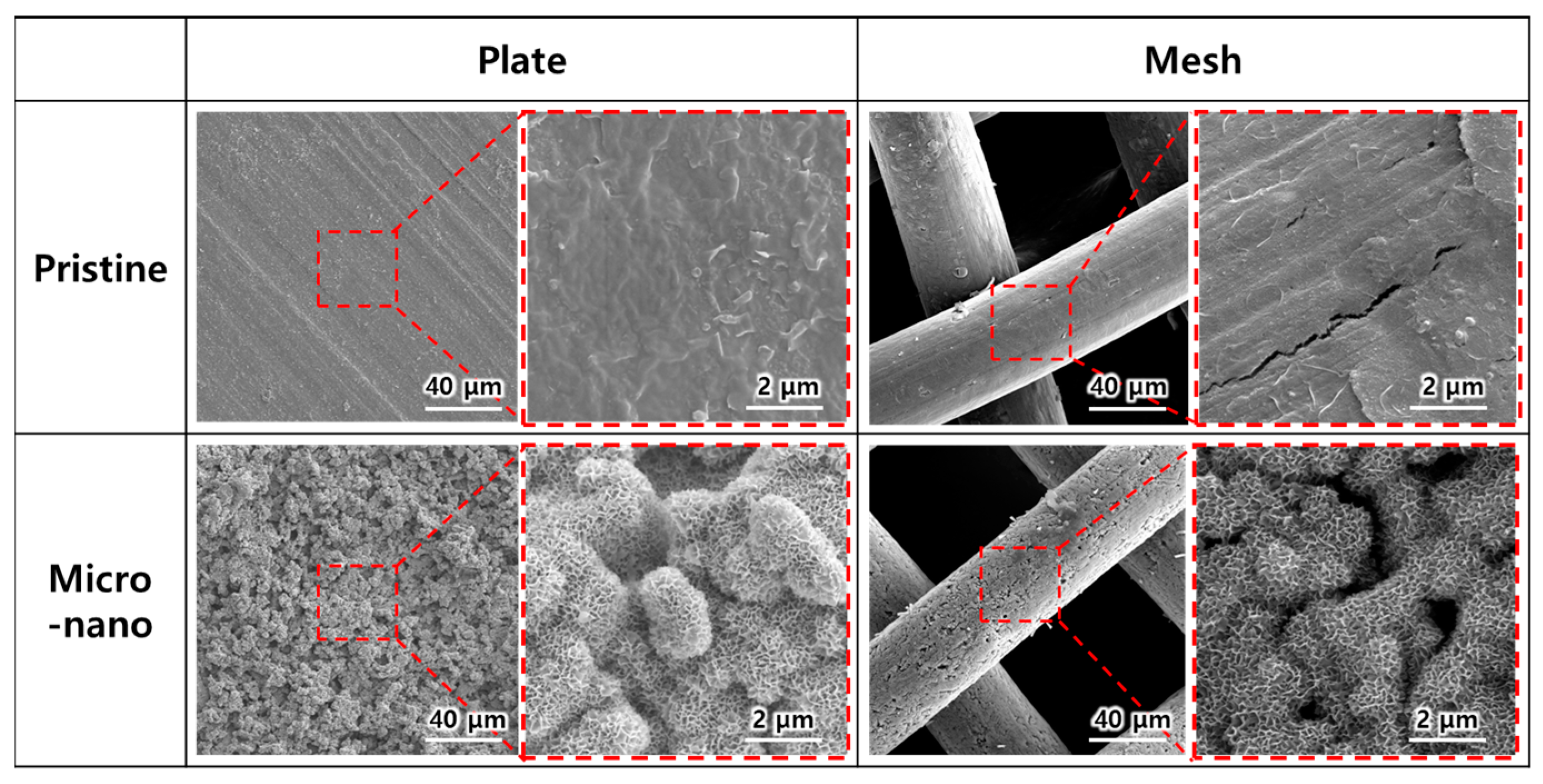
2.2.1. Surface Treatment
2.2.2. Characterization of Electrode Surface Morphology and Bubble Behavior
2.3. Evaluation of EF Performance
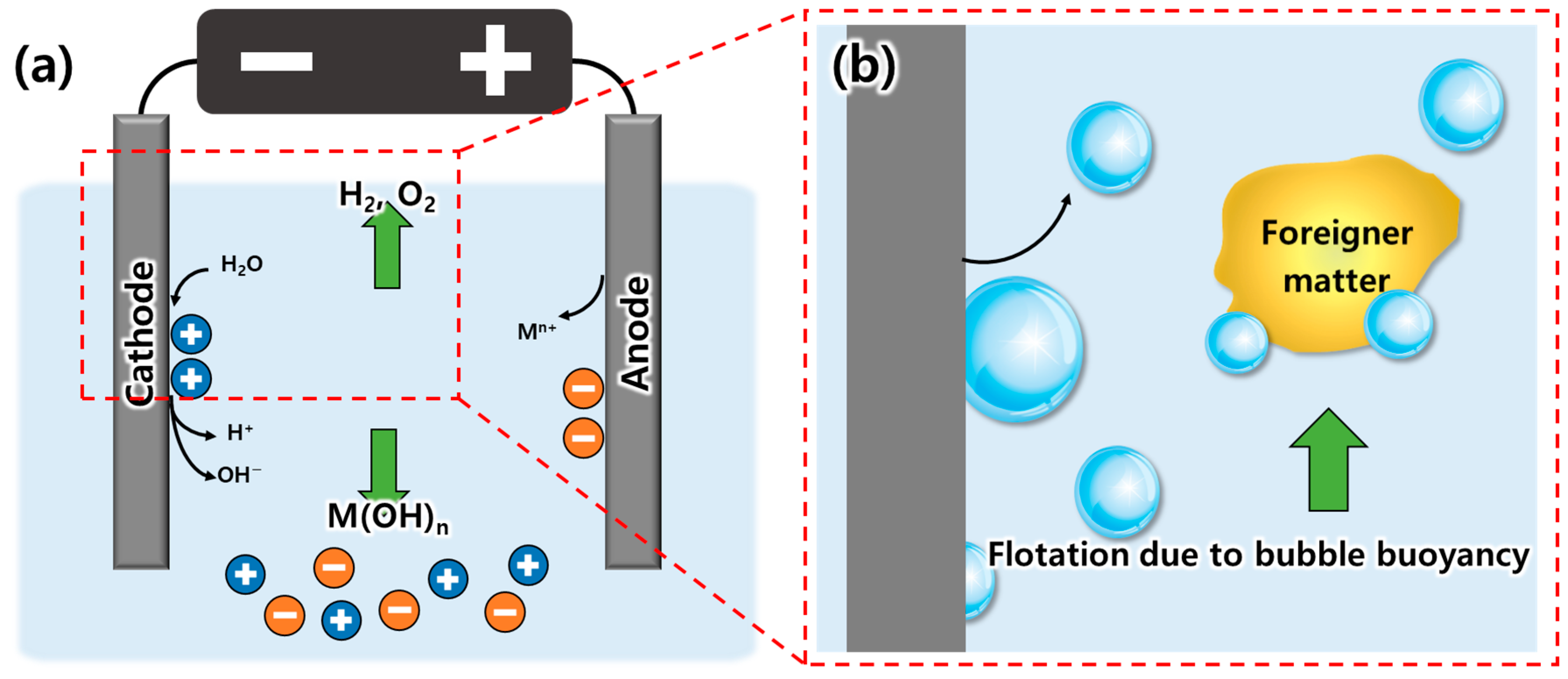
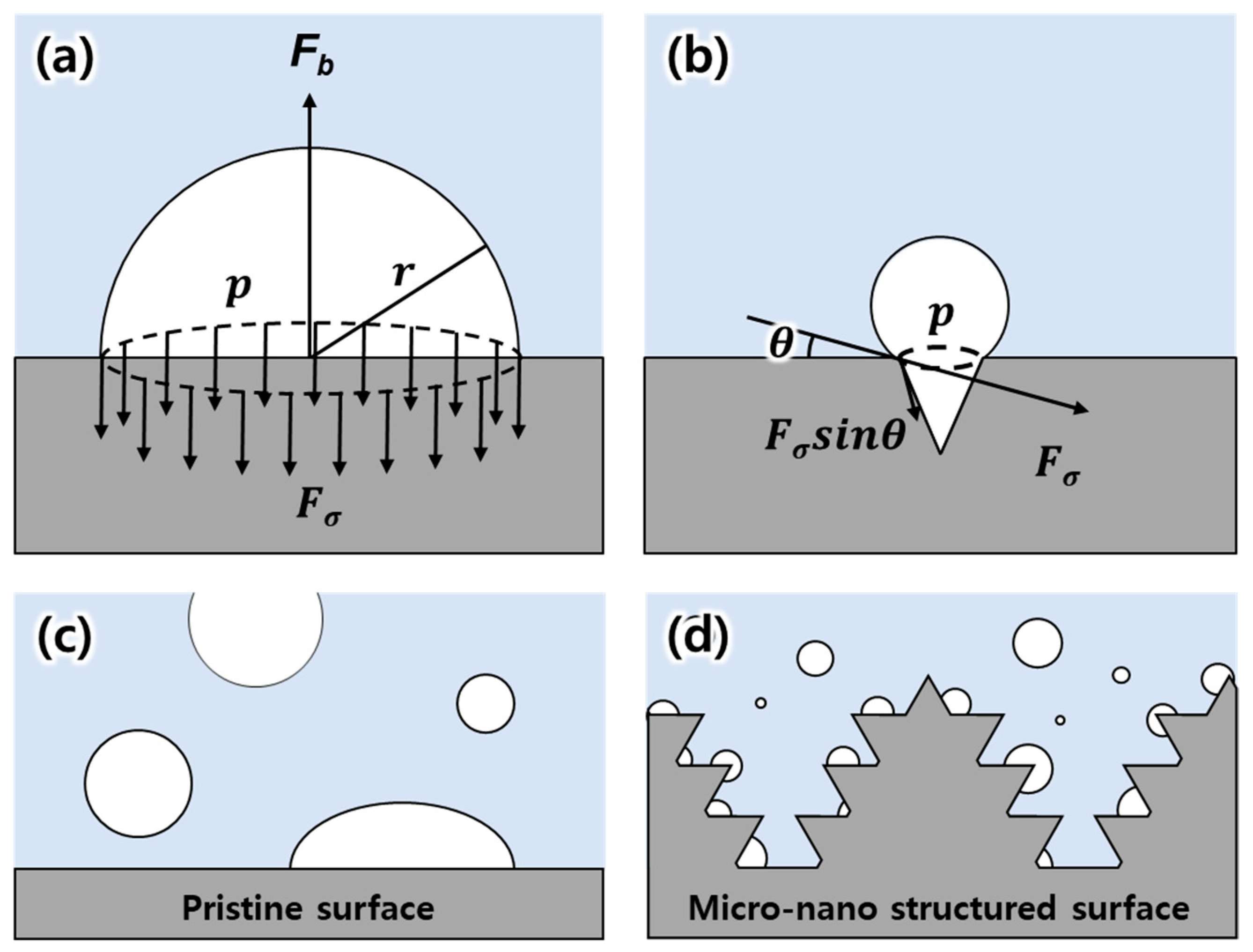
3. Results and Discussion
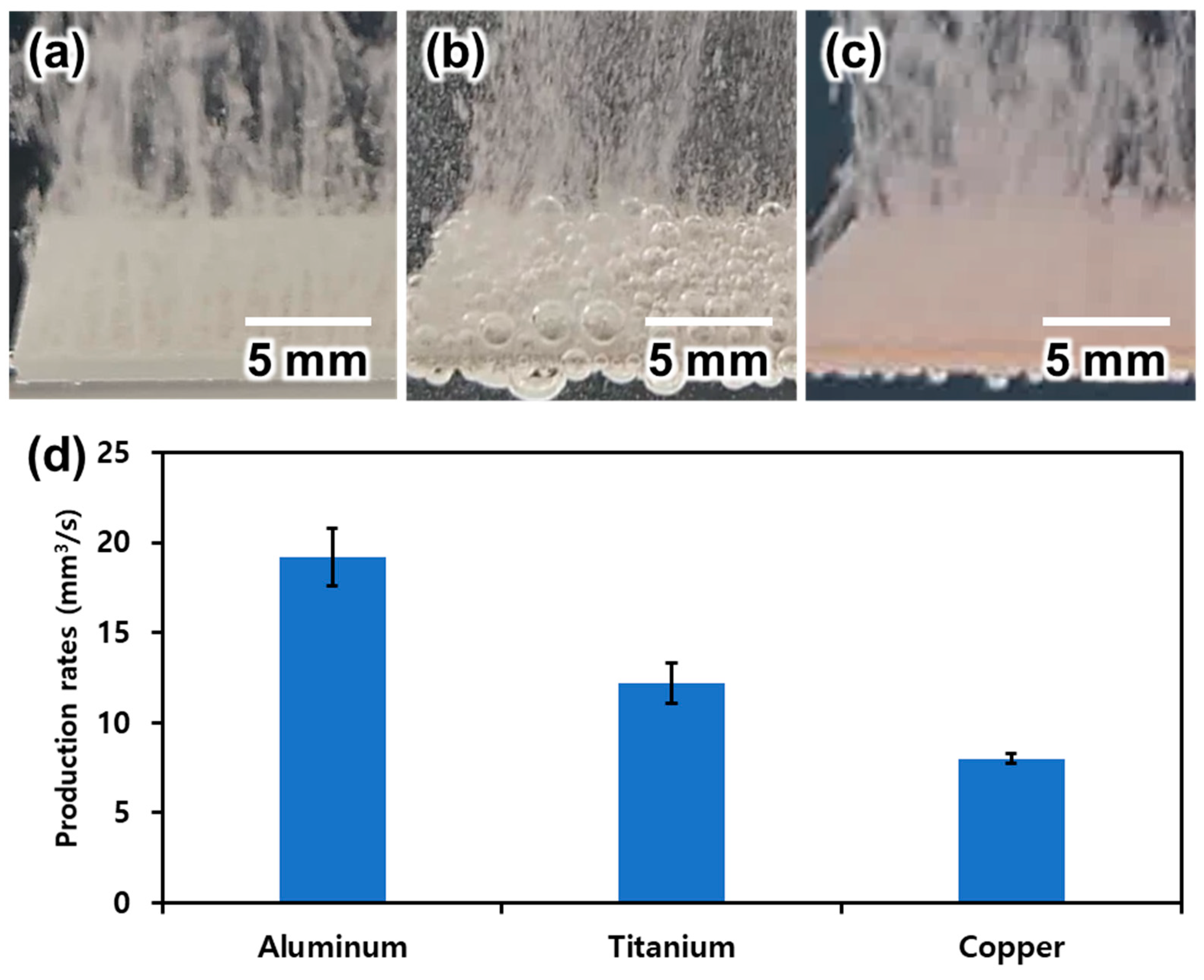
3.1. Material Selection
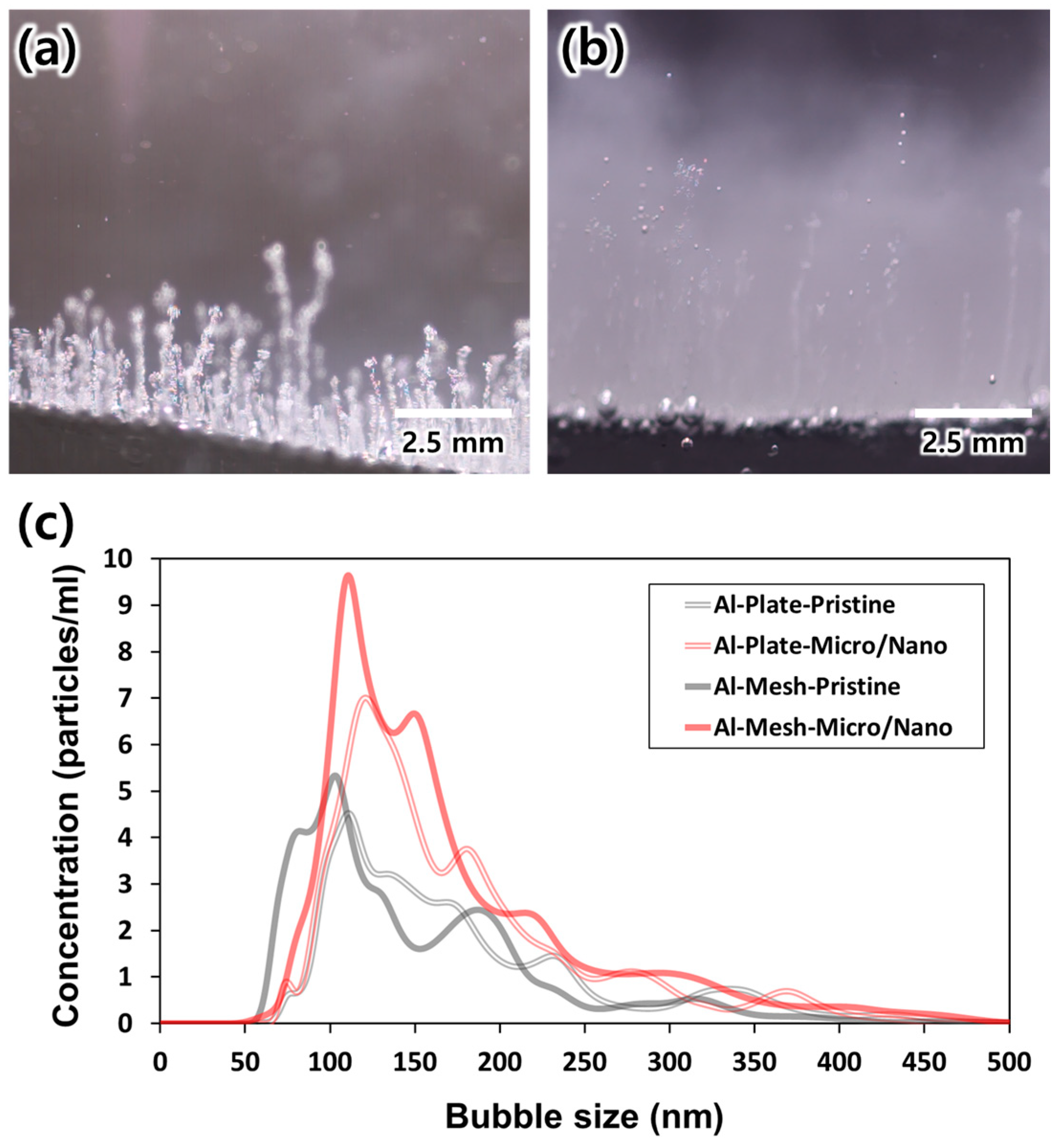
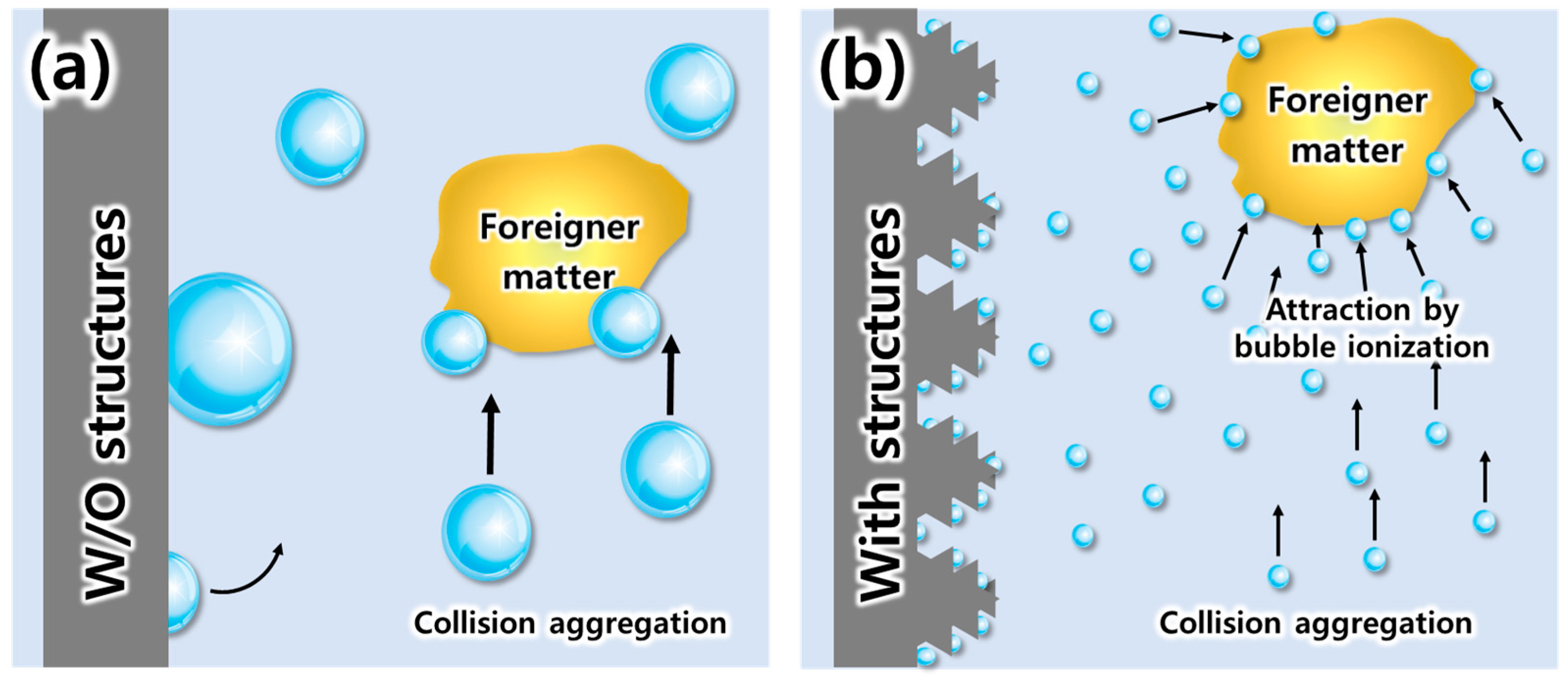
3.2. Evaluation of Bubble Generation for Various Types of Electrodes
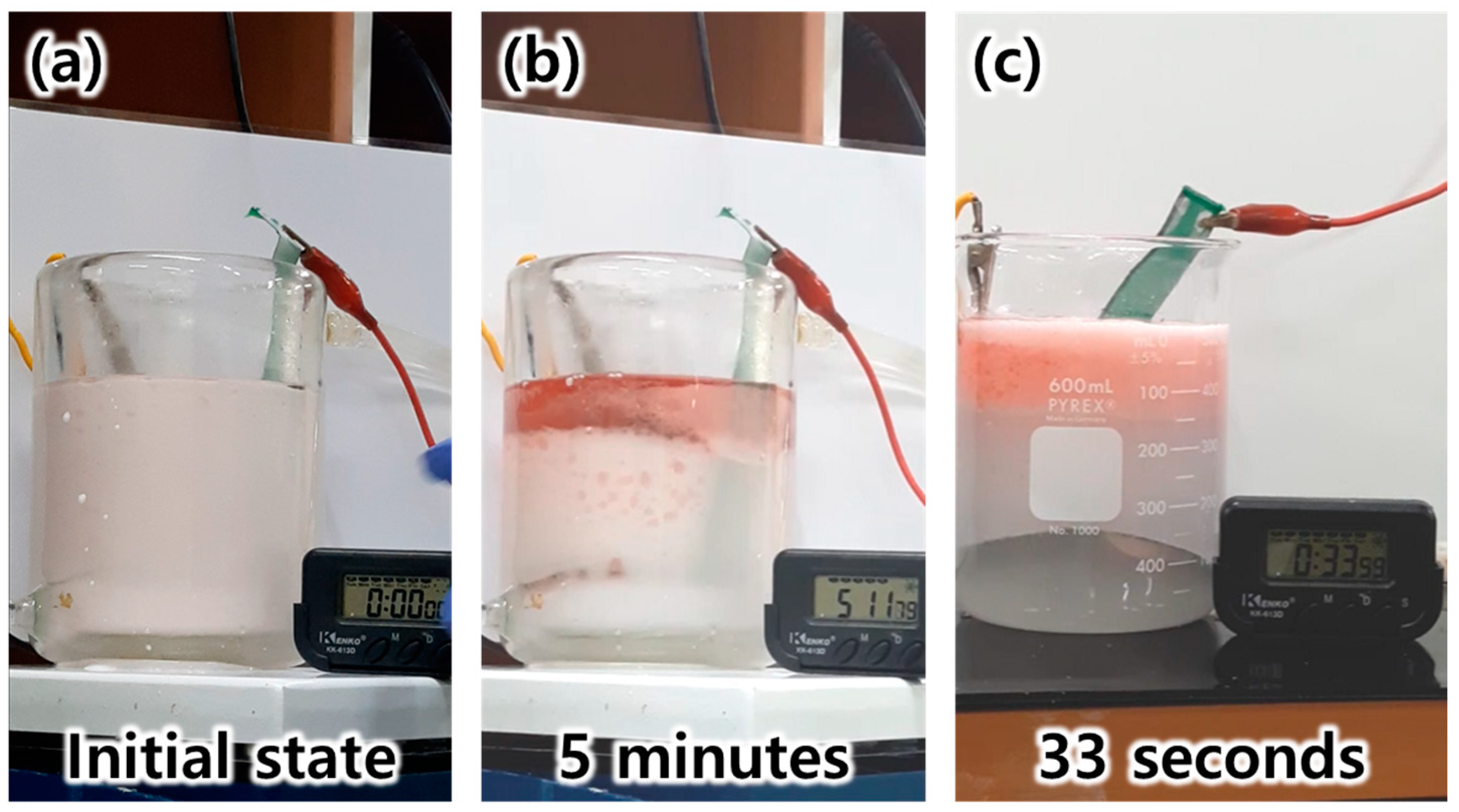
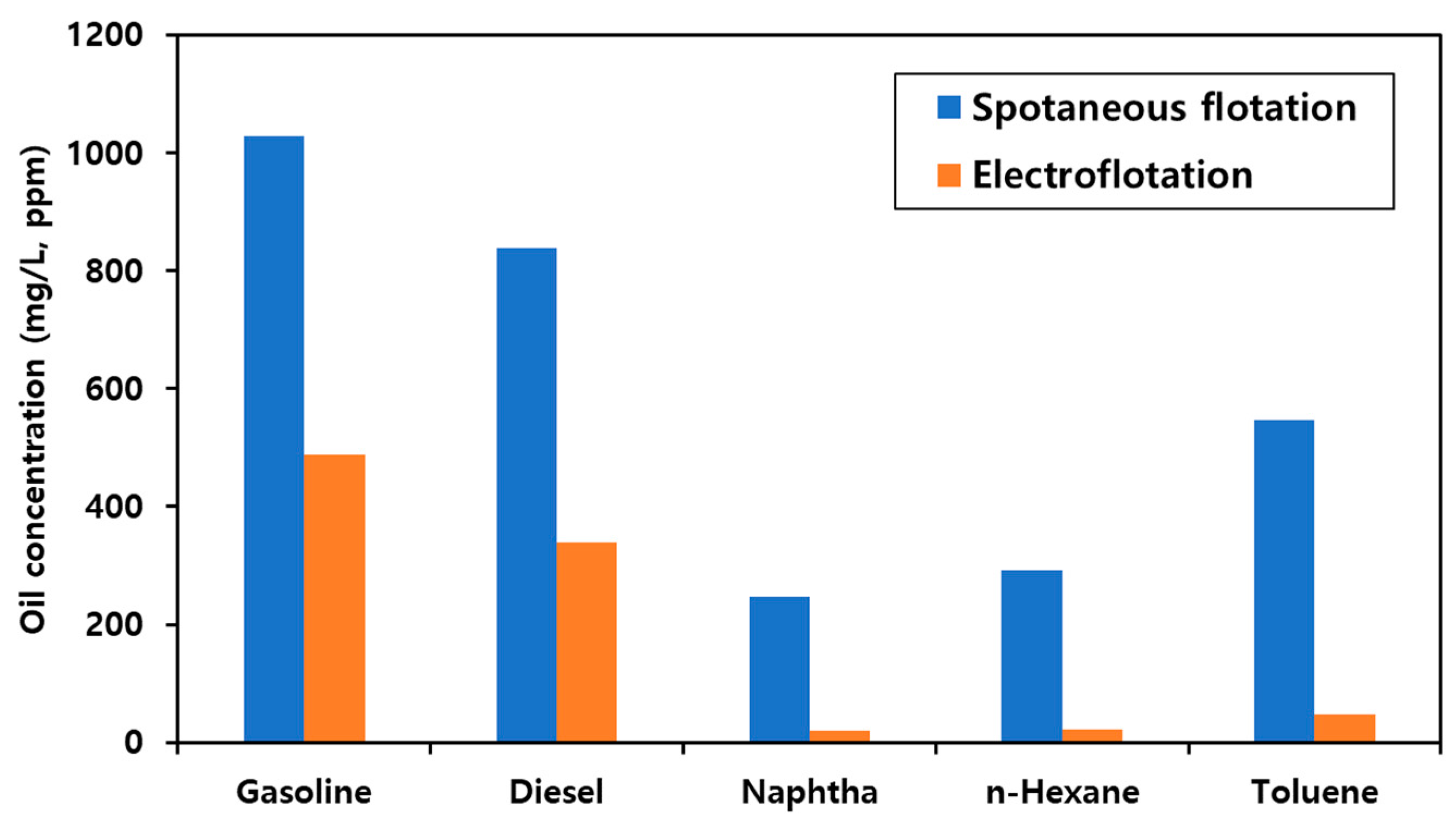
3.3. Evaluation of EF Performance for Micro-Nano Structured Mesh Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cecchini, R.; Pelosi, G. Alessandro Volta and his battery. IEEE Antennas Propag. Mag. 1992, 34, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Guo, H.; Niu, C.; Zhao, Y.; Xu, L.; Li, Q.; Mai, L. Advances in structure and property optimizations of battery electrode materials. Joule 2017, 1, 522–547. [Google Scholar] [CrossRef]
- Arthur, T.S.; Bates, D.J.; Cirigliano, N.; Johnson, D.C.; Malati, P.; Mosby, J.M.; Perre, E.; Rawls, M.T.; Prieto, A.L.; Dunn, B. Three-dimensional electrodes and battery architectures. MRS Bull. 2011, 36, 523–531. [Google Scholar] [CrossRef]
- Yang, R.; Yao, W.; Tang, B.; Zhang, F.; Lei, X.; Lee, C.; Tang, Y. Development and challenges of electrode materials for rechargeable Mg batteries. Energy Storage Mater. 2021, 42, 687–704. [Google Scholar] [CrossRef]
- Lee, Y.K. The effect of active material conductive additives and binder in a cathode composite electrode on battery performance. Energies 2019, 12, 658. [Google Scholar] [CrossRef]
- Park, S.; Shi, B.; Shang, Y.; Deng, K.; Fu, K. Structured electrode additive manufacturing for lithium-ion batteries. Nano Lett. 2022, 22, 9462–9469. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsh, D.; Hu, L. Thick electrode batteries: Principles, opportunities, and challenge. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Su, Y.; Fu, Y.; Wei, Y.; Yan, J.; Mao, B. The electrode/ionic liquid interface: Electric double layer and metal electrodeposition. ChemPhysChem 2010, 11, 2764–2778. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, H.; Hao, Z.; Yu, M.; Chen, X.; Chen, J. Electrodeposition of (hydro)oxides for an oxygen evolution electrode. Chem. Sci. 2020, 11, 10614–10625. [Google Scholar] [CrossRef]
- Hao, F.; Verma, A.; Mukherjee, P.P. Electrodeposition stability of metal electrodes. Energy Storage Mater. 2019, 20, 1–6. [Google Scholar] [CrossRef]
- Litser, S.; McLean, G. PEM fuel cell electrodes. J. Power Sources 2004, 130, 61–76. [Google Scholar] [CrossRef]
- Kundu, S.; Fowler, M.W.; Simon, L.C.; Grot, S. Morphological features (defects) in fuel cell membrane electrode assemblies. J. Power Sources 2006, 157, 650–656. [Google Scholar] [CrossRef]
- Chen, D.; Pei, P.; Li, Y.; Ren, P.; Meng, Y.; Song, X.; Wu, Z. Proton exchange membrane fuel cell stack consistency: Evaluation methods, influencing factors, membrane electrode assembly parameters and improvement measures. Energy Convers. Manag. 2022, 261, 115651. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent progress in metal-organic framework-based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Yue, T.; Shen, B.; Gao, P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: A review. Renew. Sustain. Energy Rev. 2022, 158, 112131. [Google Scholar] [CrossRef]
- Wan, L.; Xu, Z.; Xu, Q.; Pang, M.; Lin, D.; Liu, J.; Wang, B. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis. Energy Environ. Sci. 2023, 16, 1384. [Google Scholar] [CrossRef]
- Mayerhofer, B.; McLaughlin, D.; Bohm, T.; Hegelheimer, M.; Seeberger, D.; Thiele, S. Bipolar membrane electrode assemblies for water electrolysis. ACS Appl. Energy Mater. 2020, 3, 9635–9644. [Google Scholar] [CrossRef]
- Liang, S.; Jiang, M.; Lun, H.; Ma, Y.; Yang, J. A high-rate electrode with grotthuss topochemistry for membrane-free decoupled acid water electrolysis. Adv. Energy Mater. 2021, 11, 2102057. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Habibzadeh, F.; Salvatore, D.A.; Ren, S.; Simpson, G.L.; Wheeler, D.G.; Liu, A.; Berlinguette, C.P. Porous metal electrodes enable efficient electrolysis of carbon capture solutions. Enegry Environ. Sci. 2022, 15, 705–713. [Google Scholar] [CrossRef]
- Mockl, M.; Ernst, M.F.; Kornherr, M.; Allebrod, F.; Bernt, M.; Byrknes, J.; Eickes, C.; Gebauer, C.; Moskovteseva, A.; Gasteiger, H.A. Durability testing of low-iridium PEM water electrolysis membrane electrode assemblies. J. Electrochem. Soc. 2022, 169, 064505. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Yang, P.; Wang, D.; Xu, H.; Wang, B.; Meng, F.; Zhang, J.; An, M. Electrodeposition: Synthesis of advanced transition metal-based catalyst for hydrogen production via electrolysis of water. J. Energy Chem. 2021, 57, 547–566. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, R.; Zou, J.; Yang, H. Surface design strategy of catalysts for water electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A. Electrode wear and material removal rate during EDM of aluminum and mild steel using copper and brass electrodes. Int. J. Adv. Manuf. Technol. 2008, 39, 482–487. [Google Scholar] [CrossRef]
- Ran, Q.; Shi, H.; Meng, H.; Zeng, S.P.; Wan, W.B.; Zhang, W.; Wen, Z.; Lang, X.Y.; Jiang, Q. Aluminum-copper alloy anode materials for high-energy aqueous aluminum batteries. Nat. Commun. 2022, 13, 576. [Google Scholar] [CrossRef]
- Dhanabalan, S.; Sivakumar, K.; Narayanan, C.S. Experimental investigation on electrical discharge machining of titanium alloy using copper, brass and aluminum electrodes. J. Eng. Sci. Technol. 2015, 10, 72–80. [Google Scholar]
- Su, H.; Wu, D.; Li, C.; Li, C.; Zhang, C. Research advances on electrode materials for solid oxide electrolysis cells. Prog. Nat. Sci. Mater. Int. 2023, 33, 309–319. [Google Scholar] [CrossRef]
- Hughes, J.P.; Clipsham, J.; Chavushoglu, H.; Rowley-Neale, S.J.; Banks, C.E. Polymer electrolyte electrolysis: A review of the activity and stability of non-precious metal hydrogen evolution reaction and oxygen evolution reaction catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110709. [Google Scholar] [CrossRef]
- Khouya, A. Hydrogen production costs of a polymer electrolyte membrane electrolysis powered by a renewable hybrid system. Int. J. Hydrogen Energy 2021, 46, 14005–14023. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Salla, M.; Qin, N.; Gao, M.; Ji, Y.; Huang, S.; Wu, S.; Zhang, R.; Lu, Z.; et al. Decoupled redox catalytic hydrogen production with a robust electrolyte-borne electron and proton carrier. J. Am. Chem. Soc. 2020, 143, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ulleberg, Ø. Modeling of advanced alkaline electrolyzers: A system simulation approach. Int. J. Hydrogen Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Chen, M.; Chou, S.F.; Blaabjerg, F.; Davari, P. Overview of power electronic converter topologies enabling large-scale hydrogen production via water electrolysis. Appl. Sci. 2022, 12, 1906. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Shen, J.; Li, J.; Li, B.; Zheng, Y.; Bao, X.; Guo, J.; Guo, Y.; Lai, C.; Lei, W.; Wang, S.; et al. Ambient fast synthesis of superaerophobic/superhydrophilic electrode for superior electrocatalytic water oxidation. Energy Environ. Mater. 2023, 6, e12462. [Google Scholar] [CrossRef]
- Andaveh, R.; Darband, G.B.; Maleki, M.; Rouhaghdam, A.S. Superaerophobic/superhydrophilic surfaces as advanced electrocatalysts for the hydrogen evolution reaction: A comprehensive review. J. Mater. Chem. A 2022, 10, 5147–5173. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Cho, H.; Park, B.; Kim, D.; Hwang, W. Robust superhydrophilic/hydrophobic surface based on self-aggregated Al2O3 nanowires by single-step anodization and self-assembly method. ACS Appl. Mater. Interfaces 2012, 4, 5074–5078. [Google Scholar] [CrossRef]
- Cho, H.; Kim, D.; Lee, C.; Hwang, W. A simple fabrication method for mechanically robust superhydrophobic surface by hierarchical aluminum hydroxide structures. Curr. Appl. Phys. 2013, 13, 762–767. [Google Scholar] [CrossRef]
- Kwak, W.; Hwang, W. Facile method for preparing superoleophobic surfaces with hierarchical microcubic/nanowire structures. Nanotechnology 2015, 27, 055301. [Google Scholar] [CrossRef]
- Tiringer, U.; Van Dam, J.P.B.; Abrahami, S.T.; Terryn, H.; Kovač, J.; Milošev, I.; Mol, J.M.C. Scrutinizing the importance of surface chemistry versus surface roughness for aluminium/sol-gel film adhesion. Surf. Interfaces 2021, 26, 101417. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, W.; Cho, H. Development of a versatile coating based on hydrolysis-assisted self-bonding and structure evolution of aluminum nitride nanopowder: Application toward repairing severe damages on superhydrophobic surfaces. Surf. Coat. Technol. 2023, 460, 129431. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Almeida, C.C.D.; Costa, P.R.F.D.; Melo, M.J.D.M.; Santos, E.V.D.; Martínez-Huitle, C.A. Application of electrochemical technology for water treatment of Brazilian industry effluents. J. Mex. Chem. Soc. 2014, 58, 276–286. [Google Scholar]
- Hacha, R.R.; Merma, A.G.; Couto, H.J.B.; Torem, M.L. Measurement and analysis of H2 and O2 bubbles diameter produced by electroflotation processes in a modified Partridge-Smith cell. Powder Technol. 2019, 342, 308–320. [Google Scholar] [CrossRef]
- Santos, G.D.O.S.; de Salles Pupo, M.M.; Vasconcelos, V.M.; Eguiluz, K.I.B.; Banda, G.R.S. Electroflotation. In Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2018; pp. 77–118. [Google Scholar]
- Kyzas, G.Z.; Matis, K.A. Electroflotation process: A review. J. Mol. Liq. 2016, 220, 657–664. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Nass, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potential and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Vespsalainen, M.; Sillanpaa, M. Electrocoagulation in the treatment of industrial waters and wastewaters. In Advanced Water Treatment; Sillanpaa, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–78. [Google Scholar]
- Mouedhen, G.; Feki, M.; Wery, M.D.P.; Ayedi, H.F. Behavior of aluminum electrodes in electrocoagulation process. J. Hazard. Mater. 2008, 150, 124–135. [Google Scholar] [CrossRef]
- Gurney, R.W. The quantum mechanics of electrolysis. Contain. Pap. Math. Phys. Character 1931, 134, 137–154. [Google Scholar]
- Liger-Belair, G.; Marchal, R.; Robillard, B.; Vignes-Adler, M.; Maujean, A.; Jeandet, P. Study of effervescence in a glass of champagne: Frequencies of bubble formation, growth rates, and velocities of rising bubbles. Am. J. Enol. Vitic. 1999, 50, 317–323. [Google Scholar] [CrossRef]
- Aune, R.; Battezzati, L.; Brooks, R.; Egry, I.; Fecht, H.J.; Garandet, J.P.; Mills, K.C.; Passerone, A.; Quested, P.N.; Ricci, E.; et al. Surface tension and viscosity of industrial alloys from parabolic flight experiments—Results of the ThermoLab project. Microgravity-Sci. Technol. 2005, 16, 11–14. [Google Scholar] [CrossRef]
- Brillo, J.; Egry, I. Surface tension of nickel, copper, iron and their binary alloys. J. Mater. Sci. 2005, 40, 2213–2216. [Google Scholar] [CrossRef]
- Bainbridge, I.F.; Taylor, J.A. The surface tension of pure aluminum and aluminum alloys. Metall. Mater. Trans. A 2013, 44, 3901–3909. [Google Scholar] [CrossRef]
- Bian, H.; Kurwitz, C.; Sun, Z.; Cheng, K.; Chen, K. Enhanced nucleate boiling on 3D-printed micro-porous structured surface. Appl. Therm. Eng. 2018, 141, 422–434. [Google Scholar] [CrossRef]
- Landau, L.D.; Akhierzer, A.I.; Lifshitz, E.M. Heat. In General Physics: Mechanics and Molecular Physics, 1st ed.; Pergamon Press: Oxford, UK, 1967; pp. 144–149. [Google Scholar]
- Yoon, R.H.; Yordan, J.L. Zeta-potential measurements on microbubbles generated using various surfactants. J. Colloid Interface Sci. 1986, 113, 430–438. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Kim, M.; Park, J.-H.; Choi, B.; Hwang, W. Development of Micro-Nano Structured Electrodes for Enhanced Reactivity: Improving Efficiency Through Nano-Bubble Generation. Appl. Sci. 2024, 14, 9952. https://doi.org/10.3390/app14219952
Lee K, Kim M, Park J-H, Choi B, Hwang W. Development of Micro-Nano Structured Electrodes for Enhanced Reactivity: Improving Efficiency Through Nano-Bubble Generation. Applied Sciences. 2024; 14(21):9952. https://doi.org/10.3390/app14219952
Chicago/Turabian StyleLee, Kwangseok, Moonsu Kim, Jung-Hyung Park, Bonggi Choi, and Woonbong Hwang. 2024. "Development of Micro-Nano Structured Electrodes for Enhanced Reactivity: Improving Efficiency Through Nano-Bubble Generation" Applied Sciences 14, no. 21: 9952. https://doi.org/10.3390/app14219952
APA StyleLee, K., Kim, M., Park, J.-H., Choi, B., & Hwang, W. (2024). Development of Micro-Nano Structured Electrodes for Enhanced Reactivity: Improving Efficiency Through Nano-Bubble Generation. Applied Sciences, 14(21), 9952. https://doi.org/10.3390/app14219952





