Enhancing Biogas Production Through the Co-Digestion of Fish Waste (FW) and Water Hyacinth (WH) Using Cow Dung as an Inoculum: Effect of FW/WH Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Substrate and Inoculum
2.2. Analytical Methods
2.3. Measurement of Volatile Fatty Acids and Methane Content
2.4. Model for Biogas Production and Statistical Analysis
3. Preparation of Reactors
4. Results and Discussion
4.1. Initial Characterization of FW and WH
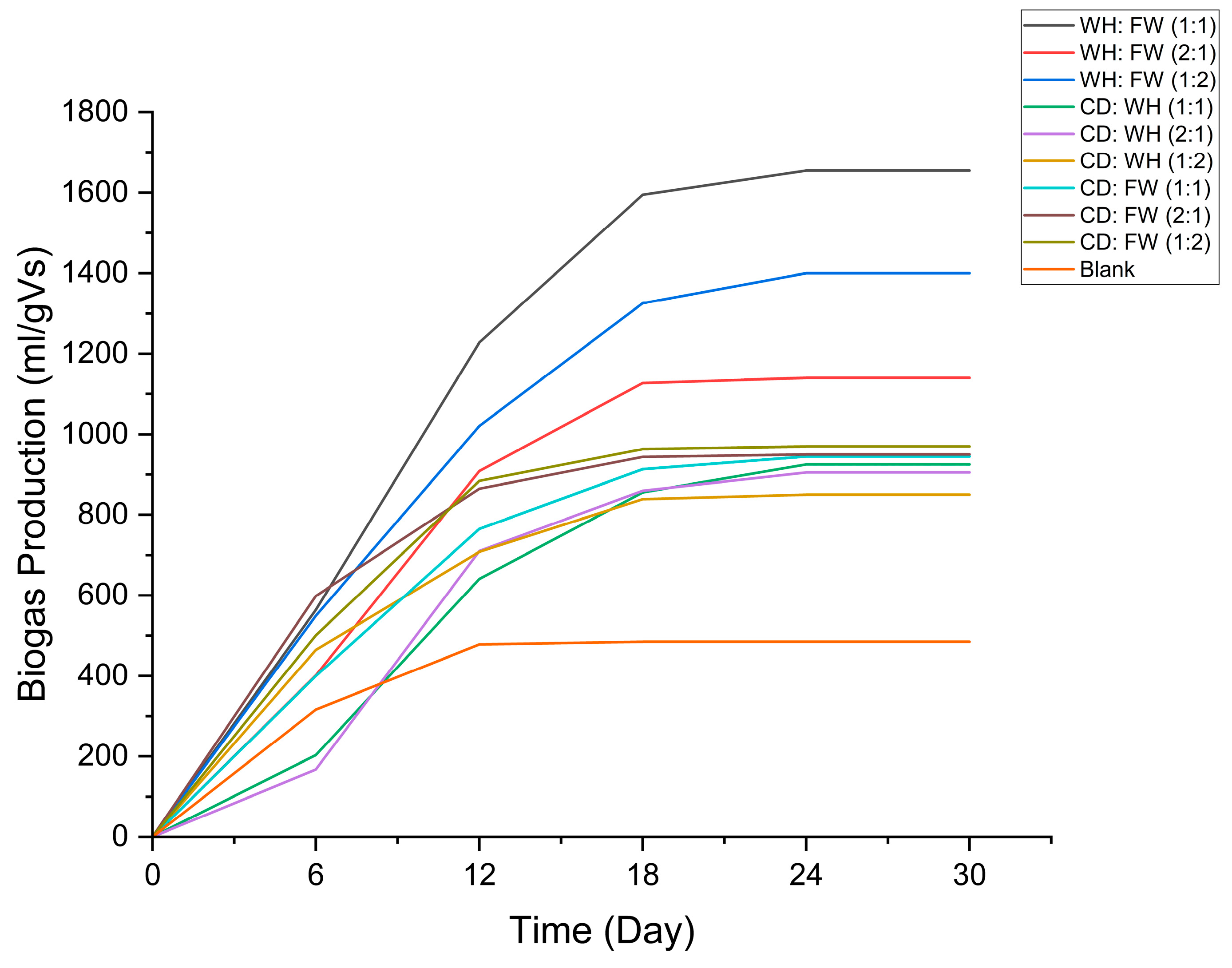
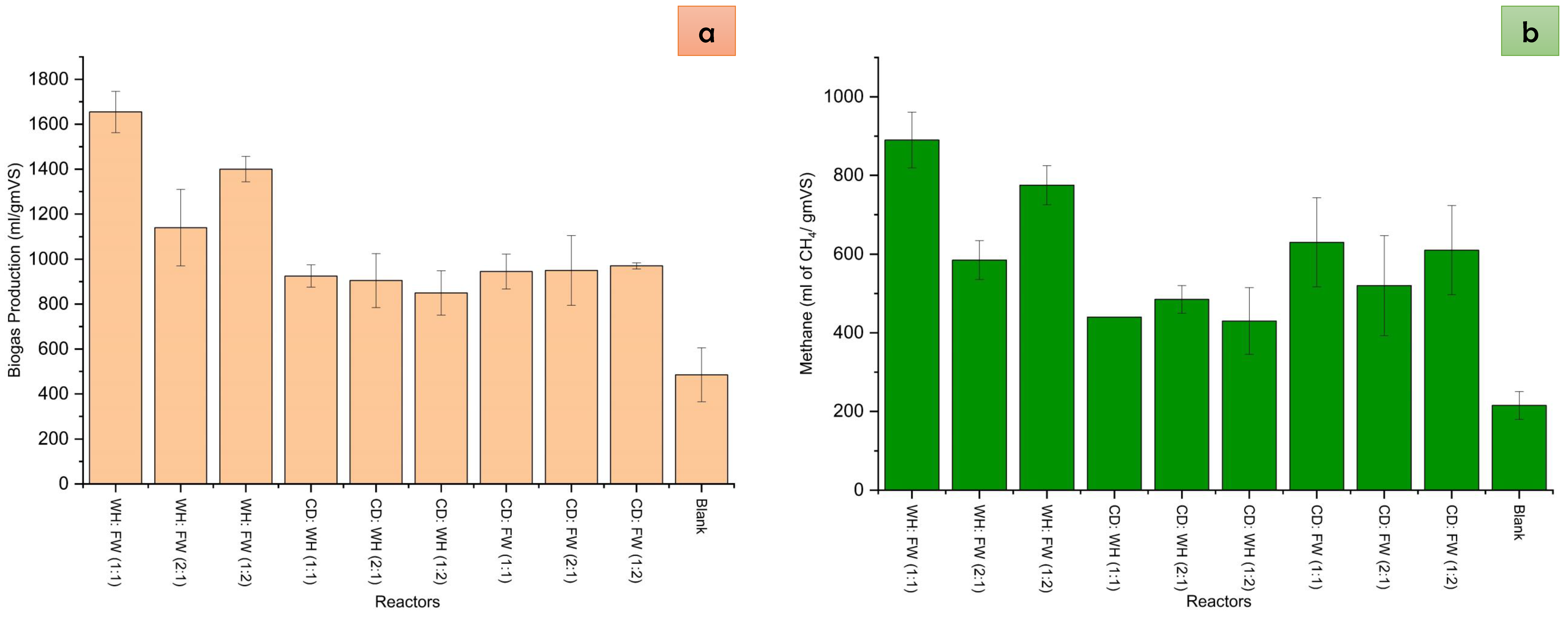
4.2. Cumulative Biogas Production and Methane Content
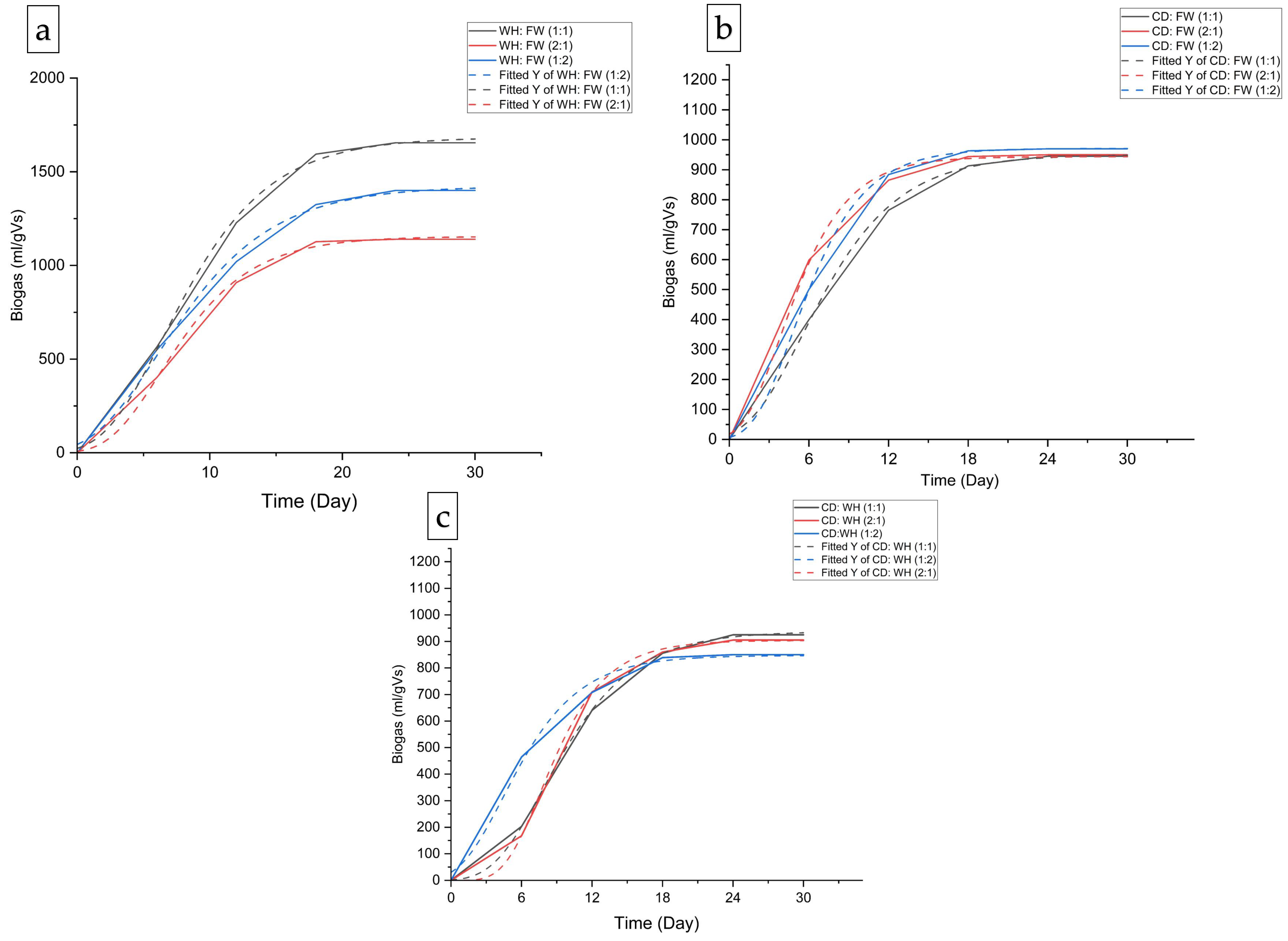
4.3. Modified Gompertz Model
4.4. Removal of Chemical Oxygen Demand
4.5. Biogas Production and COD Reduction
4.6. Reduction in Volatile Fatty Acids
4.7. Propionic Acid and Acetic Acid Ratio
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saha, C.K.; Nandi, R.; Akter, S.; Hossain, S.; Kabir, K.B.; Kirtania, K.; Islam, M.T.; Guidugli, L.; Reza, M.T.; Alam, M.M. Technical prospects and challenges of anaerobic co-digestion in Bangladesh: A review. Renew. Sustain. Energy Rev. 2024, 197, 114412. [Google Scholar] [CrossRef]
- Ibro, M.K.; Ancha, V.R.; Lemma, D.B. Impacts of anaerobic co-digestion on different influencing parameters: A critical review. Nat. Sustain. 2022, 14, 9387. [Google Scholar] [CrossRef]
- Ibro, M.K.; Ancha, V.R.; Lemma, D.B.; Pohl, M. Enhancing Biodegradability of Coffee Husk and Water Hyacinth Using Food Waste: Synergistic and Kinetic Evaluation Under Co-digestion. Bioenergy Res. 2024, 20, 1953–1970. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ammar, M.; Zou, D.; Korai, R.M.; Li, X. An insight into the anaerobic co-digestion of municipal solid waste and food waste: Influence of co-substrate mixture ratio and substrate to inoculum ratio on biogas production. Appl. Biochem. Biotech. 2019, 187, 1356–1370. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Owamah, H.I.; Ikpeseni, S.C.; Alfa, M.I.; Oyebisi, S.O.; Gopikumar, S.; Samuel, O.D.; Ilabor, S.C. Influence of inoculum/substrate ratio on biogas yield and kinetics from the anaerobic co-digestion of food waste and maize husk. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100558. [Google Scholar] [CrossRef]
- Al-Iraqi, A.R.; Gandhi, B.P.; Folkard, A.M.; Barker, P.A.; Semple, K.T. Influence of inoculum to substrate ratio and substrates mixing ratio on biogas production from the anaerobic co-digestion of Phragmites australis and food waste. Bioenergy Res. 2024, 17, 1277–1287. [Google Scholar] [CrossRef]
- Agrawal, A.; Chaudhari, P.K.; Ghosh, P. Effect of inoculums type and optimization of inoculum to substrate ratio on the kinetics of biogas production of fruit and vegetable waste. Environ. Eng. Res. 2024, 29, 220518. [Google Scholar] [CrossRef]
- e Silva, A.D.; dos Santos, A.L.; Malveira, I.C.; Girão, B.H.; dos Santos, A.B. Effect of thermo-alkaline pretreatment and substrate inoculum ratio on methane production from dry and semi-dry anaerobic digestion of swine manure. Renew. Energy 2024, 231, 121015. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Singh, S.P.; Atkinson, J.D.; Lam, S.S.; Iqbal, H.M.; Tong, Y.W. Biotransformation of food waste into biogas and hydrogen fuel–a review. Int. J. Hydrog. Energy 2024, 52, 46–60. [Google Scholar] [CrossRef]
- Venslauskas, K.; Navickas, K.; Rubežius, M.; Žalys, B.; Gegeckas, A. Processing of Agricultural Residues with a High Concentration of Structural Carbohydrates into Biogas Using Selective Biological Products. Sustainability 2024, 16, 1553. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; De Blasio, C. Process simulation of co-HTC of sewage sludge and food waste digestates and supercritical water gasification of aqueous effluent integrated with biogas plants. Energy 2024, 291, 130221. [Google Scholar] [CrossRef]
- Ward, A.J.; Løes, A.K. The potential of fish and fish oil waste for bioenergy generation: Norway and beyond. Biofuels 2011, 2, 375–387. [Google Scholar] [CrossRef]
- MFAHD. Inland Fisheries, Department of Fisheries, Government of India. 2024. Available online: https://dof.gov.in/inland-fisheries#:~:text=India%20has%20around%202.36%20million%20Ha%20of%20Tanks,from%20tanks%20and%20pond%20is%208.5%20million%20MT (accessed on 26 June 2024).
- Rajendiran, N.; Ganesan, S.; Velmurugan, N.; Venkatachalam, S.S. Synergistic effect of biogas production from co-digestion of fish and vegetable market wastes and kinetic modelling. Biomass Convers. Biorefin. 2024, 14, 12329–12341. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Bharathiraja, B.; Rithika, J.; Dhanasree, S.; Ezhilarasi, V.; Lavanya, A.; Praveenkumar, R. Production of biofuels from fish wastes: An overview. Biofpr 2019, 10, 301–307. [Google Scholar] [CrossRef]
- MFAHD. Pradhan Mantri Matsya Sampada Yojana—A Scheme to Bring About Blue Revolution Through Sustainable and Responsible Development of Fisheries Sector in India. Ministry of Fisheries, Animal Husbandry & Dairying, Government of India. 2020. Available online: https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1625535 (accessed on 26 June 2024).
- Ayyappan, S.; Jena, J.K. Environmental Issues in Indian Freshwater Aquaculture. Asian Fish. Sci. 1999, 1, 13–31. [Google Scholar]
- Chowdhury, P.; Viraraghavan, T.; Srinivasan, A. Biological treatment processes for fish processing wastewater–A review. Bioresour. Technol. 2010, 101, 439–449. [Google Scholar] [CrossRef]
- Ahumada, R.; Rudolph, A.; Contreras, S. Evaluation of coastal waters receiving fish processing waste: Lota Bay as a case study. Environ. Monit. Assess. 2004, 90, 89–99. [Google Scholar] [CrossRef]
- Gildberg, A.R. Enzymes and bioactive peptides from fish waste related to fish silage, fish feed and fish sauce production. J. Aquat. Food Prod. Technol. 2004, 13, 3–11. [Google Scholar] [CrossRef]
- Brod, E.; Øgaard, A.F. Closing global P cycles: The effect of dewatered fish sludge and manure solids as P fertiliser. Waste Manag. 2021, 135, 190–198. [Google Scholar] [CrossRef]
- Kébé, N.N.; Rieker, C.; Fall, P.A.; Diouf, D.; Ndiaye, D.; Mockenhaupt, T.; Beuel, P.; Bursche, J. Anaerobic Co-digestion of fish processing waste with cow manure and waste of market (rests of fruits and vegetables): A lab scale batch test. Sustain. Bioenergy Syst. 2021, 11, 45. [Google Scholar] [CrossRef]
- Midlen, A.; Redding, T.A. Environmental Management for Aquaculture; Springer: Dordrecht, The Netherlands, 1998; ISBN 978-0-412-59500-4. [Google Scholar]
- Netshivhumbe, R.; Faloye, F.; Tolessa, A.; Görgens, J.; Goosen, N. Anaerobic co-digestion of fish sludge originating from a recirculating aquaculture system. Waste Biomass Valori. 2024, 17, 1–17. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Kamal, M.; Mahmoud, N.S. Phytoremediation of aquaculture wastewater for water recycling and production of fish feed. Environ. Int. 2005, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.H.; Hizar, N.H.; Lim, S.R.; Amin, M.F.M.; Hassin, N.H.; Rasat, M.S.M.; Ahmad, M.I.; Razab, M.K.A.A.; Abdullah, N.H. Phytoremediation of aquaculture wastewater by Colocasia esculenta, Pistia stratiotes, and Limnocharis flava. JTRSS 2017, 5, 93–97. [Google Scholar] [CrossRef]
- Koley, A.; Bray, D.; Banerjee, S.; Sarhar, S.; Thahur, R.G.; Hazra, A.K.; Mandol, N.C.; Chaudhury, S.; Ross, A.B.; Camargo-Valero, M.A.; et al. Water hyacinth (Eichhornia crassipes) a sustainable strategy for heavy metals removal from contaminated waterbodies. In Bioremediation of Toxic Metal (loid)s; CRC Press: Boca Raton, FL, USA, 2022; pp. 95–114. [Google Scholar]
- Koley, A.; Thahur, R.G.; Das, K.; Gupta, N.; Banerjee, A.; Show, B.K.; Ghosh, A.; Chaudhury, S.; Hazra, A.M.; Nahar, G.; et al. Growth Dynamics and Nutrient Removal from Biogas Slurry Using Water Hyacinth. Sustainability 2024, 16, 4450. [Google Scholar] [CrossRef]
- Koley, A.; Ghosh, A.; Banerjee, S.; Gupta, N.; Thakur, R.G.; Show, B.K.; Chaudhury, S.; Ross, A.B.; Nahar, G.; Balachandran, S. Phytoremediation of wastewater discharged from paper and pulp, textile and dairy industries using water hyacinth (Eichhornia crassipes). In Bioremediation for Sustainable Environmental Cleanup; CRC Press: Boca Raton, FL, USA, 2024; pp. 238–261. [Google Scholar]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Din, M.F.M.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. Environ. Manage. 2015, 163, 125–133. [Google Scholar] [CrossRef]
- Center, T.D.; Spencer, N.R. The phenology and growth of water hyacinth (Eichhornia crassipes (Mart.) Solms) in a eutrophic north-central Florida lake. Aquat. Bot. 1981, 10, 1–32. [Google Scholar] [CrossRef]
- Bote, M.A.; Naik, V.R.; Jagadeeshgouda, K.B. Review on water hyacinth weed as a potential bio fuel crop to meet collective energy needs. Mater. Sci. Energy Technol. 2020, 3, 397–406. [Google Scholar] [CrossRef]
- Najmudeen, T.M.; ArakkalFebna, M.A.; Rojith, G.; Zacharia, P.U. Characterisation of biochar from water hyacinth (Eichhornia crassipes) and the effects of biochar on the growth of fish and paddy in integrated culture systems. JCR 2019, 86, 225–234. [Google Scholar] [CrossRef]
- Manivannan, A.; Narendhirakannan, R.T. Bioethanol production from aquatic weed water hyacinth (Eichhornia crassipes) by yeast fermentation. Waste Biomass Valori. 2015, 6, 209–216. [Google Scholar] [CrossRef]
- Nugraha, W.D.; Syafrudin; Pradita, L.L.; Matin, H.H.A.; Budiyono. Biogas production from water hyacinth (Eichhornia crassipes): The effect of F/M ratio. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 150, p. 012019. [Google Scholar]
- Show, B.K.; Shivakumaran, G.; Koley, A.; Ghosh, A.; Chaudhury, S.; Hazra, A.K.; Balachandran, S. Effect of thermal and NaOH pretreatment on water hyacinth to enhance the biogas production. Environ. Sci. Pollut. Res. 2023, 30, 120984–120993. [Google Scholar] [CrossRef] [PubMed]
- Koley, A.; Mukhopadhyay, P.; Show, B.K.; Ghosh, A.; Balachandran, S. OP30: Biogas production potentiality of water hyacinth, Pistia and duckweed: A comparative analysis. In Proceedings of the National Symposium: “Recent Trends in Sustainable Technology-Techno-Commercial Developments”, Kolkata, India, 9–10 September 2022; pp. 143–148. [Google Scholar]
- Koley, A.; Mukhopadhyay, P.; Gupta, N.; Singh, A.; Ghosh, A.; Show, B.K.; Thahur, R.G.; Chadhury, S.; Hazra, A.K.; Balachandran, S. Biogas production potential of aquatic weeds as the next-generation feedstock for bioenergy production: A review. Environ. Sci. Pollut. Res. 2023, 30, 111802–111832. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.K.; Bhui, I.; Banerjee, S.N.; Goswami, R.; Chakraborty, A.K.; Shome, A.; Balachandran, S.; Chaudhury, S. Biogas production from locally available aquatic weeds of Santiniketan through anaerobic digestion. Clean Technol. Environ. Policy 2015, 17, 1681–1688. [Google Scholar] [CrossRef]
- Gbiete, D.; Sprafke, J.; Kongnine, D.M.; Narra, S.; Kpelou, P.; Mouzou, E.; Agboka, K. Potential for Biogas Production from Water Hyacinth and Banana Peels: A Case Study of Substrates Harvested from Lomé, Togo. Fuels 2024, 5, 494–507. [Google Scholar] [CrossRef]
- Chai, A.; Wong, Y.S.; Ong, S.A.; Lutpi, N.A.; Sam, S.T.; Wirach, T.; Kee, W.C.; Khoo, H.C. Exploring the potential of thermophilic anaerobic co-digestion between agro-industrial waste and water hyacinth: Operational performance, kinetic study and degradation pathway. Bioprocess Biosyst. 2023, 46, 995–1009. [Google Scholar] [CrossRef]
- Kinattinkara, S.; Arumugam, T.; Samiappan, N.; Sivakumar, V.; Velusamy, S.; Murugesan, M.; Shanmugamoorthy, M. Deriving an alternative energy using anaerobic co-digestion of water hyacinth, food waste, and cow manure. JREE 2023, 10, 19–25. [Google Scholar]
- Agori, J.E.; Iwemah, E.R.; Etuke, J.O.; Umukoro, L.O. Utilization of digestate from anaerobic co-digestion of water hyacinth and poultry waste as a sustainable source of organic fertilizer. WJAETS 2023, 10, 074–081. [Google Scholar]
- Nugraha, W.D.; Pradita, L.L. Biogas Production from Water Hyacinth. In Biogas-Recent Advances and Integrated Approaches; IntechOpen: London, UK, 2020. [Google Scholar]
- Hudakorn, T.; Sritrakul, N. Biogas and biomass pellet production from water hyacinth. Energy Rep. 2020, 6, 532–538. [Google Scholar] [CrossRef]
- Sarto, S.; Hildayati, R.; Syaichurrozi, I. Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew. Energy 2019, 132, 335–350. [Google Scholar] [CrossRef]
- Patil, J.H.; AntonyRaj, M.; Gavimath, C.C. Study on effect of pretreatment methods on biomethanation of water hyacinth. Int. J. Adv. Biotechnol. Res. 2011, 2, 143–147. [Google Scholar]
- Vaidyanathan, S.; Kavadia, K.M.; Shroff, K.C.; Mahajan, S.P. Biogas production in batch and semicontinuous digesters using water hyacinth. Biotechnol. Bioeng. 1985, 27, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Structure of Lignocellulosic Biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer Briefs in Molecular Science; Springer: Singapore, 2016. [Google Scholar]
- Auma, E.O. Anaerobic Co-Digestion of Water Hyacinth (Eichhornia crassipes) with Ruminal Slaughterhouse Waste Under Mesophilic Conditions. Ph.D. Dissertation, University of Nairobi, Nairobi, Kenya, 2020. [Google Scholar]
- Patil, J.H.; Raj, M.A.; Gavimath, C.C.; Hooli, V.R. A comparative study on anaerobic co-digestion of water hyacinth with poultry litter and cow dung. Int. J. Chem. Sci. Appl. 2011, 2, 148–155. [Google Scholar]
- Nges, I.A.; Mbatia, B.; Björnsson, L. Improved utilization of fish waste by anaerobic digestion following omega-3 fatty acids extraction. J. Environ. Manag. 2012, 110, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Solli, L.; Bergersen, O.; Sørheim, R.; Briseid, T. Effects of a gradually increased load of fish waste silage in co-digestion with cow manure on methane production. Waste Manag. 2014, 34, 1553–1559. [Google Scholar] [CrossRef]
- Ingabire, H.; Ntambara, B.; Mazimpaka, E. Characterization and analysis of fish waste as feedstock for biogas production. Int. J. Low-Carbon Technol. 2023, 18, 212–217. [Google Scholar] [CrossRef]
- Hortence, I.; Maurice, T.; Arimi, M. Effect of co-digestion with water hyacinth, inoculum concentration and dilution on biogas production of fish waste. Energy Rep. 2023, 10, 4819–4823. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; American Public Health Association: Washington, DC, USA, 2017; Volume 10, ISBN 978-0-87553-287-5. [Google Scholar]
- ASTM D 1252-00, TEST METHOD A; Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. ASTM International: West Conshohocken, PA, USA, 2009.
- APHA. American Water Works Association. Water Environment Federation. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Print Office: Washington, DC, USA, 1954.
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Strömberg, S.; Nistor, M.; Liu, J. Towards eliminating systematic errors caused by the experimental conditions in Biochemical Methane Potential (BMP) tests. Waste Manag. 2014, 34, 1939–1948. [Google Scholar] [CrossRef]
- Tjørve, K.M.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, 0178691. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modelling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Moharir, S.; Bondre, A.; Vaidya, S.; Patankar, P.; Kanaskar, Y.; Karne, H. Comparative analysis of the amount of biogas produced by different cultures using the modified Gompertz model and Logistic model. Eur. J. Sustain. Dev. Res. 2020, 4, 0141. [Google Scholar] [CrossRef] [PubMed]
- Budiyono, I.S.; Sumardiono, S. Kinetic model of biogas yield production from vinasse at various initial pH: Comparison between modified Gompertz model and first order kinetic model. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 2798–2805. [Google Scholar]
- Nazurally, N. Anaerobic digestion of fish waste and seagrass/macroalgae: Potential sustainable waste management for tropical Small Island Developing States. J. Mater. Cycles Waste Manag. 2018, 20, 1724–1735. [Google Scholar] [CrossRef]
- Cadavid-Rodríguez, L.S.; Vargas-Muñoz, M.A.; Plácido, J. Biomethane from fish waste as a source of renewable energy for artisanal fishing communities. Sustain. Energy Technol. Assess. 2019, 34, 110–115. [Google Scholar] [CrossRef]
- Sarker, S. By-products of fish-oil refinery as potential substrates for biogas production in Norway: A preliminary study. Results Eng. 2020, 6, 100137. [Google Scholar] [CrossRef]
- Choe, U.; Mustafa, A.M.; Lin, H.; Xu, J.; Sheng, K. Effect of bamboo hydrochar on anaerobic digestion of fish processing waste for biogas production. Bioresour. Technol. 2019, 283, 340–349. [Google Scholar] [CrossRef]
- Xu, J.; Mustafa, A.M.; Sheng, K. Effects of inoculum to substrate ratio and co-digestion with bagasse on biogas production of fish waste. Environ. Technol. 2017, 38, 2517–2522. [Google Scholar] [CrossRef]
- OjikutuAbimbola, O.; Osokoya Olumide, O. Evaluation of biogas production from food waste. IJES 2014, 3, 1–7. [Google Scholar]
- Kafle, G.K.; Kim, S.H. Evaluation of the biogas productivity potential of fish waste: A lab scale batch study. Biosyst. Eng. 2012, 37, 302–313. [Google Scholar] [CrossRef]
- Bhui, I.; Banerjee, S.N.; Chaudhury, S.; Balachandran, S. Biogas production by co-digestion of locally available aquatic weeds (Eichornia crassipes and Salvinia cucullata) with kitchen waste. In Proceedings of the International Conference on Renewable Energy and Sustainable Environment, Pollachi, India, 3–5 August 2015; Dr. Mahalingam College of Engineering and Technology: Pollachi, India, 2015. [Google Scholar]
- Suthar, S.; Sharma, B.; Kumar, K.; Banu, J.R.; Tyagi, V.K. Enhanced biogas production in dilute acid-thermal pretreatment and cattle dung biochar mediated biomethanation of water hyacinth. Fuel 2022, 307, 121897. [Google Scholar] [CrossRef]
- Nalinga, Y.; Legonda, I. Experimental investigation on biogas production from anaerobic co-digestion of water hyacinth and fish waste. Int. J. Innov. Sci. Res. Technol. 2016, 4, 1–8. [Google Scholar]
- Mshandete, A.; Kivaisi, A.; Rubindamayugi, M.; Mattiasson, B.O. Anaerobic batch co-digestion of sisal pulp and fish wastes. Bioresour. Technol. 2004, 95, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bücker, F.; Marder, M.; Peiter, M.R.; Lehn, D.N.; Esquerdo, V.M.; de Almeida Pinto, L.A.; Konrad, O. Fish waste: An efficient alternative to biogas and methane production in an anaerobic mono-digestion system. Renew. Energy 2020, 147, 798–805. [Google Scholar] [CrossRef]
- Salam, B.; Islam, M.; Rahman, M.T. Biogas from anaerobic digestion of fish waste. In Proceedings of the International Conference on Mechanical Engineering, Dhaka, Bangladesh, 26–28 December 2009; Volume 26, pp. 1–3. [Google Scholar]
- Hanafiah, M.M.; Ali, M.Y.M.; Aziz, N.I.H.A.; John, A. Biogas production from agrowaste and effluents. Acta Chem. Malays. 2017, 1, 13–15. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef]
- Tasnim, F.; Iqbal, S.A.; Chowdhury, A.R. Biogas production from anaerobic co-digestion of cow manure with kitchen waste and Water Hyacinth. Renew. Energy 2017, 109, 434–439. [Google Scholar] [CrossRef]
- Choi, H.J. Assessment of sludge reduction and biogas potential from anaerobic co-digestion using an acidogenically fermented fishery byproduct with various agricultural wastes. Water Air Soil Pollut. 2020, 231, 336. [Google Scholar] [CrossRef]
- Harnadek, C.M.W.; Guilford, N.G.H.; Edwards, E.A. Chemical oxygen demand analysis of anaerobic digester contents. STEM Fellow. J. 2015, 1, 2–5. [Google Scholar] [CrossRef]
- Mudhoo, A.; Moorateeah, P.R.; Mohee, R. Effects of Microwave Heating on Biogas Production, Chemical Oxygen Demand and Volatile Solids. Int. J. Environ. Chem. Ecol. Geol. Geophy. Eng. 2012, 6, 609–614. [Google Scholar]
- Hill, D.T.; Cobb, S.A.; Bolte, J.P. Using volatile fatty acid relationships to predict anaerobic digester failure. Trans. ASAE 1987, 30, 496–0501. [Google Scholar] [CrossRef]



| (a) | ||||||
| Inoculum (g) | Substrate (g) | Ratio | Total (g) | |||
| CD:WH (2:1) | 226.74 | 173.25 | 2 | 400 | ||
| CD:FW (2:1) | 354.39 | 45.60 | 2 | 400 | ||
| CD:WH (1:2) | 98.61 | 301.38 | 0.5 | 400 | ||
| CD:FW (1:2) | 264.08 | 135.91 | 0.5 | 400 | ||
| CD:WH (1:1) | 158.21 | 241.78 | 1 | 400 | ||
| CD:FW (1:1) | 318.13 | 81.86 | 1 | 400 | ||
| Blank | 400 | - | - | 400 | ||
| (b) | ||||||
| WH (g) | FW(g) | Ratio | Total (g) | CD (g) | Total (g) | |
| WH:FW (1:1) | 256.76 | 43.23 | 1 | 300 | 100 | 400 |
| WH:FW (2:1) | 276.70 | 23.29 | 2 | 300 | 100 | 400 |
| WH:FW (1:2) | 224.41 | 75.58 | 0.5 | 300 | 100 | 400 |
| Moisture Content (%) | Volatile Solids (%) | Total Solids (%) | Ash Content (%) | pH | C/N Ratio | Reference |
|---|---|---|---|---|---|---|
| FW | ||||||
| 48.6 ± 0.3 | 96.2 ± 0.5 | 51.4 ± 0.1 | 3.8 ± 0.4 | 7.2 ± 0.001 | [67] | |
| 74.8 | 88.9 | 25.2 | - | 7.4 | 5.7 | [68] |
| 28.95 | 30.63 | 3.90 | [69] | |||
| 67.1–81.43 | 27.50–55.5 | 31.30–32.2 | 2.14–5.7 | 3–10.1 | [55] | |
| 39.20 ± 0.27 | 43.54 ± 0.21 | 2.14 ± 0.05 | 10.72 ± 0.14 | [70] | ||
| 61.58 ± 2.1 | 93.84 ± 1.0 | 38.11 ± 1.9 | 0.51 ± 0.05 | 6.42 ± 0.1 | 5.79 ± 0.12 | [56] |
| 93.74 | 38.08 | 8.0 | [71] | |||
| 81.43 | - | - | 2.14 | - | 5.01 | [72] |
| 68.7 | 27.50 | 31.30 | 5.7 | - | 4.1 | [73] |
| 20.72 | 25.93 | 1.96 | 6.5 | 8.69 | Present Study | |
| WH | ||||||
| 94.22 ± 2.20 | 99.48 ± 1.11 | 5.58 ± 0.06 | 16.65 ± 0.35 | 7.19 ± 0.12 | 21.25 ± 0.25 | [56] |
| 95.2 | 67.61 | - | - | 5.82 | 25.7 ± 1.3 | [37] |
| - | 87.4 ± 1.1 | 12.4 ± 1.3 | - | - | 28.8 ± 1.5 | [74] |
| - | 86.2 ± 0.9 | 11.4 ± 1.4 | - | - | 29.0 ± 1.3 | [40] |
| 86.5 ± 1.52 | 78.95 ± 1.44 | 102.56 ± 4.32 | 4.52 ± 0.07 | - | 18.12 ± 0.23 | [75] |
| - | 3.49 | 4.09 | 0.121 | 6.98 | 22.93 | Present Study |
| Inoculum | Substrate | Operating Condition | Biogas Yield/BMP (mL/g VS−1) | Methane (%) | Reference |
|---|---|---|---|---|---|
| Sludge | FW, seagrass, microalgae | ISR: 1:3 HTR: 26 days | 8410 *a | [67] | |
| CD | FW | ISR: 1:1.2 HTR: 15 days Temp: Ambient | 2 *b | [79] | |
| Industrial inoculum | FW | HTR: 20 days | 1546 *a | 17.7 | [80] |
| Anaerobic sludge from wastewater treatment reactor | FW | HTR: 28 days Temp: Mesophilic | 464.5 | 75.5 *c | [68] |
| Anaerobic digestion sludge | Ensilaged FW Soap stock, alkaline fish glycerin, light ethyl monoester, dark ethyl monoester | HTR: 65 Days Temp: 39 ± 1 | FE: 985 *a FE + SS: 1738 *a FE + AFG: 1818 *a FE + LME: 2089 *a FE + DME: 1948 *a | FE: 73 FE + SS: 69 FE + AFG: 72 FE + LME: 72 FE + DME: 71 | [69] |
| Fish intestines | 50.12 | [55] | |||
| Sludge from the anaerobic fermentation tank | Fish processing waste, bamboo hydrochar | ISR: 1:2 HRT: 36 days Temp: 37 ± 1 °C | 292 *b | 74.9 | [70] |
| Digestate from biogas plant | FW, WH | ISR: 25:75 HRT: 20 days Temp: 37 °C | 700 *a (approx.) | 68.15 | [56] |
| Sludge of municipal anaerobic digester | FW | HRT: 21 days Temp: 37 ± 1 °C | 433.4 | 73.34 | [71] |
| Sludge of municipal anaerobic digester | FW, bagasse | ISR: 2.19 HRT: 21 days Temp: 37 ± 1 °C | 409.5 | 67.8 | [71] |
| Swine manure | FW, bread waste silage | ISR: 80:20 HRT: 96 days | 763 | 63.1 | [81] |
| Swine manure | FW, brewery grain waste | ISR: 40:60 HRT: 96 days | 671 | 65.8 | [81] |
| Swine Manure | FW | HRT: 60 days Temp: 36.5 °C | 757 | 73 | [73] |
| CD and blood with a ratio of 1:1 | FW WH | ISR: 1:1, 1:2 and 2:1 Temp: 25.3 °C to 33.4 HRT: 21 days | FW: WH (1:1)–0.30 *d FW: WH (1:2)–0.55 *d FW: WH (2:1)–0.35–0.45 *d (Approx.) | FW: WH (1:1)–63 FW: WH (1:2)–73.3 FW: WH (2:1)–65 | [76] |
| Anaerobic microbiota of a biogas plant sludge mixture | FW | HRT: 20 days Temp: 35 °C | 791.78 | 540.5 *c | [78] |
| Anaerobic microbiota of a biogas plant sludge mixture | Fish crude oil waste | HRT: 20 days Temp: 35 °C | 733.93 | 426.36 *c | [78] |
| WH | |||||
| Cow manure and sewage sludge | WH | ISR: HRT: Temp: 37 °C | 812 *a | 65 | [82] |
| CD | WH (pretreated 5% NaoH) | ISR: 2:1 HRT: 25 days Temp: 37 °C | 142.61 *b | 64.59 | [37] |
| Cow manure | WH | ISR: 2:1 HRT: 30 days Temp: 37 °C | 410 *b | - | [38] |
| Waste activated sludge | WH | F/M: 1.5 HRT: 35 days Temp: 35 °C | 2053 *a | 54.67 ± 0.6 | [75] |
| Present Study (Co-Digestion) | |||||
| CD | WH FW | WH:FW: 1:1 HRT: 24 days Temp: 37 °C | 1655 ± 91.92 | 890 ± 70.71 *c | Present Study |
| CD | WH FW | WH:FW: 1:2 HRT: 24 days Temp: 37 °C | 1400 ± 56.56 | 775 ± 49.49 *c | Present Study |
| CD | WH FW | WH:FW: 2:1 HRT: 24 days Temp: 37 °C | 1140 ±169.70 | 585 ± 49.4 *c | Present Study |
| Reactors | Mean | Grouping | COD | Mean | Grouping | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biogas production | WH:FW (1:1) | 1339.3 | A | Reduction in COD | COD 3 | 6488.6 | A | |||
| WH:FW (1:2) | 1139.0 | A | B | COD 6 | 5013.4 | B | ||||
| WH:FW (2:1) | 943.4 | B | C | COD 9 | 4123.8 | C | ||||
| CD:FW (2:1) | 861.5 | B | C | COD 12 | 3248.5 | D | ||||
| CD:FW (1:2) | 857.5 | B | C | COD 15 | 1936.3 | E | ||||
| CD:FW (1:1) | 793.6 | B | C | COD 18 | 1414.0 | E | F | |||
| CD:WH (1:2) | 742.3 | C | COD 21 | 1001.4 | F | |||||
| CD:WH (1:1) | 709.8 | C | ||||||||
| CD:WH (2:1) | 709.5 | C | ||||||||
| Time (Day) | WH:FW (1:1): CD | WH:FW (2:1): CD | WH:FW (1:2): CD | CD:WH (1:1) | CD:WH (2:1) | CD:WH (1:2) | CD:FW (1:1) | CD:FW (2:1) | CD:FW (1:2) | Blank |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.76 | 1.06 | 0.83 | 1.02 | 0.95 | 0.70 | 0.49 | 1.21 | 1.04 | 0.63 |
| 3 | 0.95 | 1.30 | 1.17 | 0.93 | 1.11 | 0.89 | 1.06 | 0.98 | 1.02 | 0.34 |
| 6 | 0.24 | 0.24 | 0.22 | 0.26 | 0.23 | 0.26 | 0.21 | 0.23 | 0.20 | 0.28 |
| 9 | 0.41 | 0.46 | 0.35 | 0.61 | 0.52 | 0.53 | 0.31 | 0.43 | 0.32 | 0.35 |
| 12 | 2.67 | 5.00 | 2.56 | 6.19 | 4.56 | 3.87 | 2.99 | 7.16 | 2.26 | 1.58 |
| 15 | 4.43 | 3.32 | 3.26 | 2.84 | 2.14 | 3.78 | 5.49 | 2.91 | 2.64 | 4.62 |
| 18 | 0.31 | 0.00 | 0.14 | 0.16 | 0.00 | 0.41 | 3.04 | 0.32 | 0.56 | 1.58 |
| 21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.56 | 0.00 | 0.00 | 0.17 |
| 24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, G.; Koley, A.; Garai, S.; Balachandran, S.; Ross, A.B. Enhancing Biogas Production Through the Co-Digestion of Fish Waste (FW) and Water Hyacinth (WH) Using Cow Dung as an Inoculum: Effect of FW/WH Ratio. Appl. Sci. 2024, 14, 9880. https://doi.org/10.3390/app14219880
Nahar G, Koley A, Garai S, Balachandran S, Ross AB. Enhancing Biogas Production Through the Co-Digestion of Fish Waste (FW) and Water Hyacinth (WH) Using Cow Dung as an Inoculum: Effect of FW/WH Ratio. Applied Sciences. 2024; 14(21):9880. https://doi.org/10.3390/app14219880
Chicago/Turabian StyleNahar, Gaurav, Apurba Koley, Subhadip Garai, Srinivasan Balachandran, and Andrew B. Ross. 2024. "Enhancing Biogas Production Through the Co-Digestion of Fish Waste (FW) and Water Hyacinth (WH) Using Cow Dung as an Inoculum: Effect of FW/WH Ratio" Applied Sciences 14, no. 21: 9880. https://doi.org/10.3390/app14219880
APA StyleNahar, G., Koley, A., Garai, S., Balachandran, S., & Ross, A. B. (2024). Enhancing Biogas Production Through the Co-Digestion of Fish Waste (FW) and Water Hyacinth (WH) Using Cow Dung as an Inoculum: Effect of FW/WH Ratio. Applied Sciences, 14(21), 9880. https://doi.org/10.3390/app14219880







