The Histopathological Examination of the Degeneration of Menisci in Osteoarthritic Knees Using an Adapted Bonar Score: Does Osteoarthritis Equally Influence the Lateral and Medial Menisci?
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Assessment
2.2. Surgical Technique
2.3. Histopathological Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markes, A.R.; Hodax, J.D.; Ma, C.B. Meniscus Form and Function. Clin. Sports Med. 2020, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mameri, E.S.; Dasari, S.P.; Fortier, L.M.; Verdejo, F.G.; Gursoy, S.; Yanke, A.B.; Chahla, J. Review of Meniscus Anatomy and Biomechanics. Curr. Rev. Musculoskelet. Med. 2022, 15, 323–335. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Wanivenhaus, F.; Burge, A.J.; Warren, R.F.; Rodeo, S.A. The Human Meniscus: A Review of Anatomy, Function, Injury, and Advances in Treatment. Clin. Anat. 2015, 28, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Skalski, M.R.; Patel, D.B.; White, E.A.; Tomasian, A.; Gross, J.S.; Vangsness, C.T.; Matcuk, G.R. Illustrative Review of Knee Meniscal Tear Patterns, Repair and Replacement Options, and Imaging Evaluation. Clin. Imaging 2021, 69, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues. Cells 2019, 8, 324. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The Knee Meniscus: Structure–Function, Pathophysiology, Current Repair Techniques, and Prospects for Regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef]
- Hutchinson, I.D.; Moran, C.J.; Potter, H.G.; Warren, R.F.; Rodeo, S.A. Restoration of the Meniscus: Form and Function. Am. J. Sports Med. 2014, 42, 987–998. [Google Scholar] [CrossRef]
- Nguyen, J.C.; De Smet, A.A.; Graf, B.K.; Rosas, H.G. MR Imaging–Based Diagnosis and Classification of Meniscal Tears. RadioGraphics 2014, 34, 981–999. [Google Scholar] [CrossRef]
- Luvsannyam, E.; Jain, M.S.; Leitao, A.R.; Maikawa, N.; Leitao, A.E. Meniscus Tear: Pathology, Incidence, and Management. Cureus 2022, 14, e25121. [Google Scholar] [CrossRef]
- Mordecai, S.C. Treatment of Meniscal Tears: An Evidence Based Approach. World J. Orthop. 2014, 5, 233. [Google Scholar] [CrossRef]
- Mohamadi, A.; Momenzadeh, K.; Masoudi, A.; Walley, K.C.; Ierardi, K.; Ramappa, A.; DeAngelis, J.P.; Nazarian, A. Evolution of Knowledge on Meniscal Biomechanics: A 40 Year Perspective. BMC Musculoskelet. Disord. 2021, 22, 625. [Google Scholar] [CrossRef] [PubMed]
- Pasiński, M.; Zabrzyńska, M.; Adamczyk, M.; Sokołowski, M.; Głos, T.; Ziejka, M.; Augustynowicz, P.; Boguszewski, K.; Piotrowski, W.; Michał, B.; et al. A Current Insight into Human Knee Menisci. Transl. Res. Anat. 2023, 32, 100259. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The Role of Synovitis in Osteoarthritis Pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Olivotto, E.; Trisolino, G.; Belluzzi, E.; Lazzaro, A.; Strazzari, A.; Pozzuoli, A.; Cigolotti, A.; Ruggieri, P.; Evangelista, A.; Ometto, F.; et al. Macroscopic Synovial Inflammation Correlates with Symptoms and Cartilage Lesions in Patients Undergoing Arthroscopic Partial Meniscectomy: A Clinical Study. J. Clin. Med. 2022, 11, 4330. [Google Scholar] [CrossRef]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Koga, H.; Sekiya, I. Degenerative Meniscus in Knee Osteoarthritis: From Pathology to Treatment. Life 2022, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Englund, M.; Guermazi, A.; Lohmander, L.S. The Meniscus in Knee Osteoarthritis. Rheum. Dis. Clin. N. Am. 2009, 35, 579–590. [Google Scholar] [CrossRef]
- Pauli, C.; Grogan, S.P.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.K.; D’Lima, D.D. Macroscopic and Histopathologic Analysis of Human Knee Menisci in Aging and Osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef]
- Ozeki, N.; Seil, R.; Krych, A.J.; Koga, H. Surgical Treatment of Complex Meniscus Tear and Disease: State of the Art. J. ISAKOS 2021, 6, 35–45. [Google Scholar] [CrossRef]
- Zabrzyńska, M.; Grzanka, D.; Zielińska, W.; Jaworski, Ł.; Pękala, P.; Gagat, M. The Bonar Score in the Histopathological Assessment of Tendinopathy and Its Clinical Relevance—A Systematic Review. Medicina 2021, 57, 367. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Franceschi, F.; Rabitti, C.; Denaro, V. Movin and Bonar Scores Assess the Same Characteristics of Tendon Histology. Clin. Orthop. Relat. Res. 2008, 466, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Is the Inflammation Process Absolutely Absent in Tendinopathy of the Long Head of the Biceps Tendon? Histopathologic Study of the Long Head of the Biceps Tendon after Arthroscopic Treatment. Pol. J. Pathol. 2017, 68, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.; Dahlstrom, J.E.; Twin, J.; Cook, J.; Scott, A. The Bonar Score Revisited: Region of Evaluation Significantly Influences the Standardized Assessment of Tendon Degeneration. J. Sci. Med. Sport 2014, 17, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Min, B.-H.; Choi, B.H.; Kim, Y.J.; Kim, M.; Suh-Kim, H.; Kim, J.H. The Degeneration of Meniscus Roots Is Accompanied by Fibrocartilage Formation, Which May Precede Meniscus Root Tears in Osteoarthritic Knees. Am. J. Sports Med. 2015, 43, 3034–3044. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Gagat, M.; Łapaj, Ł.; Paczesny, Ł.; Yataganbaba, A.; Szwedowski, D.; Huri, G. Relationship between Long Head of the Biceps Tendon Histopathology and Long-Term Functional Results in Smokers. A Time to Reevaluate the Bonar Score? Ther. Adv. Chronic Dis. 2021, 12, 204062232199026. [Google Scholar] [CrossRef]
- Weidow, J.; Cederlund, C.-G.; Ranstam, J.; Kärrholm, J. Ahlbäck Grading of Osteoarthritis of the Knee: Poor Reproducibility and Validity Based on Visual Inspection of the Joint. Acta Orthop. 2006, 77, 262–266. [Google Scholar] [CrossRef]
- Zhu, S.; Tong, G.; Xiang, J.; Qiu, S.; Yao, Z.; Zhou, X.; Lin, L. Microstructure Analysis and Reconstruction of a Meniscus. Orthop. Surg. 2021, 13, 306–313. [Google Scholar] [CrossRef]
- Ashraf, S.; Wibberley, H.; Mapp, P.I.; Hill, R.; Wilson, D.; Walsh, D.A. Increased Vascular Penetration and Nerve Growth in the Meniscus: A Potential Source of Pain in Osteoarthritis. Ann. Rheum. Dis. 2011, 70, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.S.; Solon, L.F.; Lake, S.P.; Castile, R.M.; Hill, J.R.; Brophy, R.H. Mechanical and Microstructural Properties of Meniscus Roots Vary by Location. Am. J. Sports Med. 2022, 50, 2733–2739. [Google Scholar] [CrossRef]
- Zabrzyńska, M.; Pasiński, M.; Gagat, M.; Kułakowski, M.; Woźniak, Ł.; Elster, K.; Antosik, P.; Zabrzyński, J. The Association between the Extent of the Osteoarthritic Meniscus Degeneration and Cigarette Smoking—A Pilot Study. Medicina 2024, 60, 323. [Google Scholar] [CrossRef]
- Albano, D.; Martinelli, N.; Bianchi, A.; Romeo, G.; Bulfamante, G.; Galia, M.; Sconfienza, L.M. Posterior Tibial Tendon Dysfunction: Clinical and Magnetic Resonance Imaging Findings Having Histology as Reference Standard. Eur. J. Radiol. 2018, 99, 55–61. [Google Scholar] [CrossRef]
- Kurdziel, M.D.; Moravek, J.E.; Wiater, B.P.; Davidson, A.; Seta, J.; Maerz, T.; Baker, K.C.; Wiater, J.M. The Impact of Rotator Cuff Deficiency on Structure, Mechanical Properties, and Gene Expression Profiles of the Long Head of the Biceps Tendon (LHBT): Implications for Management of the LHBT during Primary Shoulder Arthroplasty. J. Orthop. Res. 2015, 33, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.M.; Twin, J.; Dahlstrom, J.E.; Cook, J.L.; Cormick, W.; Smith, P.N.; Scott, A. Increased Substance P Expression in the Trochanteric Bursa of Patients with Greater Trochanteric Pain Syndrome. Rheumatol. Int. 2014, 34, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Lundgreen, K.; Lian, Ø.; Scott, A.; Engebretsen, L. Increased Levels of Apoptosis and P53 in Partial-Thickness Supraspinatus Tendon Tears. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1636–1641. [Google Scholar] [CrossRef]
- Docking, S.I.; Cook, J.; Chen, S.; Scarvell, J.; Cormick, W.; Smith, P.; Fearon, A. Identification and Differentiation of Gluteus Medius Tendon Pathology Using Ultrasound and Magnetic Resonance Imaging. Musculoskelet. Sci. Pract. 2019, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Furumatsu, T.; Maehara, A.; Miyazawa, S.; Kamatsuki, Y.; Hino, T.; Ozaki, T. Histological Alterations to the Hamstring Tendon Caused by Cleaning during Autograft Preparation. Muscle Ligaments Tendons J. 2019, 09, 217. [Google Scholar] [CrossRef]
- Lundgreen, K.; Lian, O.B.; Scott, A.; Nassab, P.; Fearon, A.; Engebretsen, L. Rotator Cuff Tear Degeneration and Cell Apoptosis in Smokers versus Nonsmokers. Arthroscopy 2014, 30, 936–941. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Process of Neovascularisation Compared with Pain Intensity in Tendinopathy of the Long Head of the Biceps Brachii Tendon Associated with Concomitant Shoulder Disorders, after Arthroscopic Treatment. Microscopic Evaluation Supported by Immunohistochemical. Folia Morphol. 2018, 77, 378–385. [Google Scholar] [CrossRef]
- Fedje-Johnston, W.; Tóth, F.; Albersheim, M.; Carlson, C.S.; Shea, K.G.; Rendahl, A.; Tompkins, M. Changes in Matrix Components in the Developing Human Meniscus. Am J Sports Med 2021, 49, 207–214. [Google Scholar] [CrossRef]
- Lin, K.M.; Gadinsky, N.E.; Klinger, C.E.; Dyke, J.P.; Rodeo, S.A.; Green, D.W.; Fabricant, P.D.; Helfet, D.L.; Shea, K.G.; Lazaro, L.E. Increased Vascularity in the Neonatal versus Adult Meniscus: Evaluation with Magnetic Resonance Imaging. Cartilage 2021, 13, 1562S–1569S. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Human Knee Menisci: Structure, Composition, and Function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, A.; Nakamura, N.; Horibe, S. Age-Related Changes in the Knee Meniscus. Knee 2017, 24, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Snoeker, B.A.M.; Bakker, E.W.P.; Kegel, C.A.T.; Lucas, C. Risk Factors for Meniscal Tears: A Systematic Review Including Meta-Analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 352–367. [Google Scholar] [CrossRef]
- Akkawi, I.; Draghetti, M.; Zmerly, H. Degenerative Meniscal Lesions: Conservative versus Surgical Management. Acta Biomed. Atenei Parm. 2022, 92, e2021354. [Google Scholar] [CrossRef]
- Buchbinder, R.; Harris, I.A.; Sprowson, A. Management of Degenerative Meniscal Tears and the Role of Surgery. Br. J. Sports Med. 2016, 50, 1413–1416. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Gale, D.; Hunter, D.J.; Aliabadi, P.; Clancy, M.; Felson, D.T. Incidental Meniscal Findings on Knee MRI in Middle-Aged and Elderly Persons. N. Engl. J. Med. 2008, 359, 1108–1115. [Google Scholar] [CrossRef]
- Englund, M.; Niu, J.; Guermazi, A.; Roemer, F.W.; Hunter, D.J.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Nevitt, M.C.; Zhang, Y.Q.; et al. Effect of Meniscal Damage on the Development of Frequent Knee Pain, Aching, or Stiffness. Arthritis Rheum. 2007, 56, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Kornaat, P.R.; Bloem, J.L.; Ceulemans, R.Y.T.; Riyazi, N.; Rosendaal, F.R.; Nelissen, R.G.; Carter, W.O.; Hellio Le Graverand, M.-P.; Kloppenburg, M. Osteoarthritis of the Knee: Association between Clinical Features and MR Imaging Findings. Radiology 2006, 239, 811–817. [Google Scholar] [CrossRef]
- Beaufils, P.; Pujol, N. Management of Traumatic Meniscal Tear and Degenerative Meniscal Lesions. Save the Meniscus. Orthop. Traumatol. Surg. Res. 2017, 103, S237–S244. [Google Scholar] [CrossRef]
- Bergkvist, D.; Dahlberg, L.E.; Neuman, P.; Englund, M. Knee Arthroscopies: Who Gets Them, What Does the Radiologist Report, and What Does the Surgeon Find?: An Evaluation from Southern Sweden. Acta Orthop. 2016, 87, 12–16. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Roemer, F.W.; Aliabadi, P.; Yang, M.; Lewis, C.E.; Torner, J.; Nevitt, M.C.; Sack, B.; Felson, D.T. Meniscal Tear in Knees without Surgery and the Development of Radiographic Osteoarthritis among Middle-aged and Elderly Persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009, 60, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.M.; Osthaus, F.; Schwer, J.; Warnecke, D.; Faschingbauer, M.; Sgroi, M.; Ignatius, A.; Dürselen, L. Osteoarthritis-Related Degeneration Alters the Biomechanical Properties of Human Menisci Before the Articular Cartilage. Front. Bioeng. Biotechnol. 2021, 9, 659989. [Google Scholar] [CrossRef] [PubMed]
- Englund, M.; Haugen, I.K.; Guermazi, A.; Roemer, F.W.; Niu, J.; Neogi, T.; Aliabadi, P.; Felson, D.T. Evidence That Meniscus Damage May Be a Component of Osteoarthritis: The Framingham Study. Osteoarthr. Cartil. 2016, 24, 270–273. [Google Scholar] [CrossRef]
- Sharma, L.; Song, J.; Dunlop, D.; Felson, D.; Lewis, C.E.; Segal, N.; Torner, J.; Cooke, T.D.V.; Hietpas, J.; Lynch, J.; et al. Varus and Valgus Alignment and Incident and Progressive Knee Osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1940–1945. [Google Scholar] [CrossRef]
- Hunter, D.J.; Niu, J.; Felson, D.T.; Harvey, W.F.; Gross, K.D.; McCree, P.; Aliabadi, P.; Sack, B.; Zhang, Y. Knee Alignment Does Not Predict Incident Osteoarthritis: The Framingham Osteoarthritis Study. Arthritis Rheum. 2007, 56, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Habata, T.; Ishimura, M.; Ohgushi, H.; Tamai, S.; Fujisawa, Y. Axial Alignment of the Lower Limb in Patients with Isolated Meniscal Tear. J. Orthop. Sci. 1998, 3, 85–89. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Yang, L.; Zhang, K.; Shi, J.; Zhu, L.; Liang, H.; Wang, X.; Jiang, Q. Biomechanical Analysis of the Effect of Medial Meniscus Degenerative and Traumatic Lesions on the Knee Joint. Am. J. Transl. Res. 2019, 11, 542–556. [Google Scholar]
- Nakagawa, Y.; Mukai, S.; Yabumoto, H.; Tarumi, E.; Nakamura, T. Cartilage Degeneration and Alignment in Severe Varus Knee Osteoarthritis. Cartilage 2015, 6, 208–215. [Google Scholar] [CrossRef]








| Characteristics | Total | Meniscus | p-Value | |
|---|---|---|---|---|
| Medial | Lateral | |||
| n | 83 | 41 | 42 | |
| Valgus knees | 18 | 9 | 9 | |
| Varus knees | 65 | 32 | 33 | |
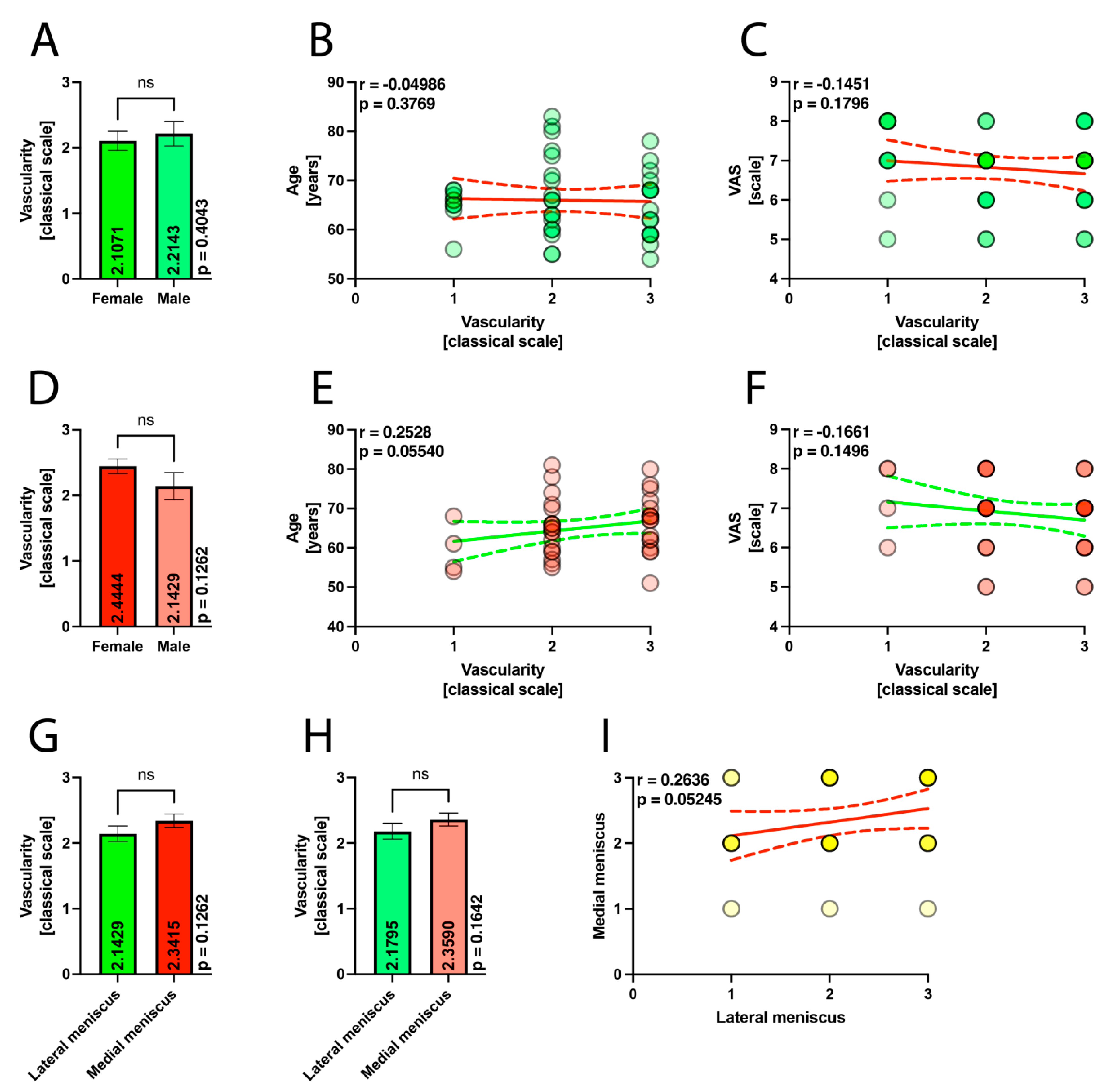
| Female | 29 | 27 | 28 | |
| Male | 15 | 14 | 14 | |
| Age | 65.56 (range 51–83; SD = 7.14) | 65.14 (range 51–81; SD = 7.15) | 65.97 (range 54–83; SD = 7.19) | p = 0.9134 |
| VAS | 6.83 (range 5–8; SD = 0.90) | 6.85 (range 5–8; SD = 0.90) | 6.80 (range 5–8; SD = 0.91) | p = 0.9819 |
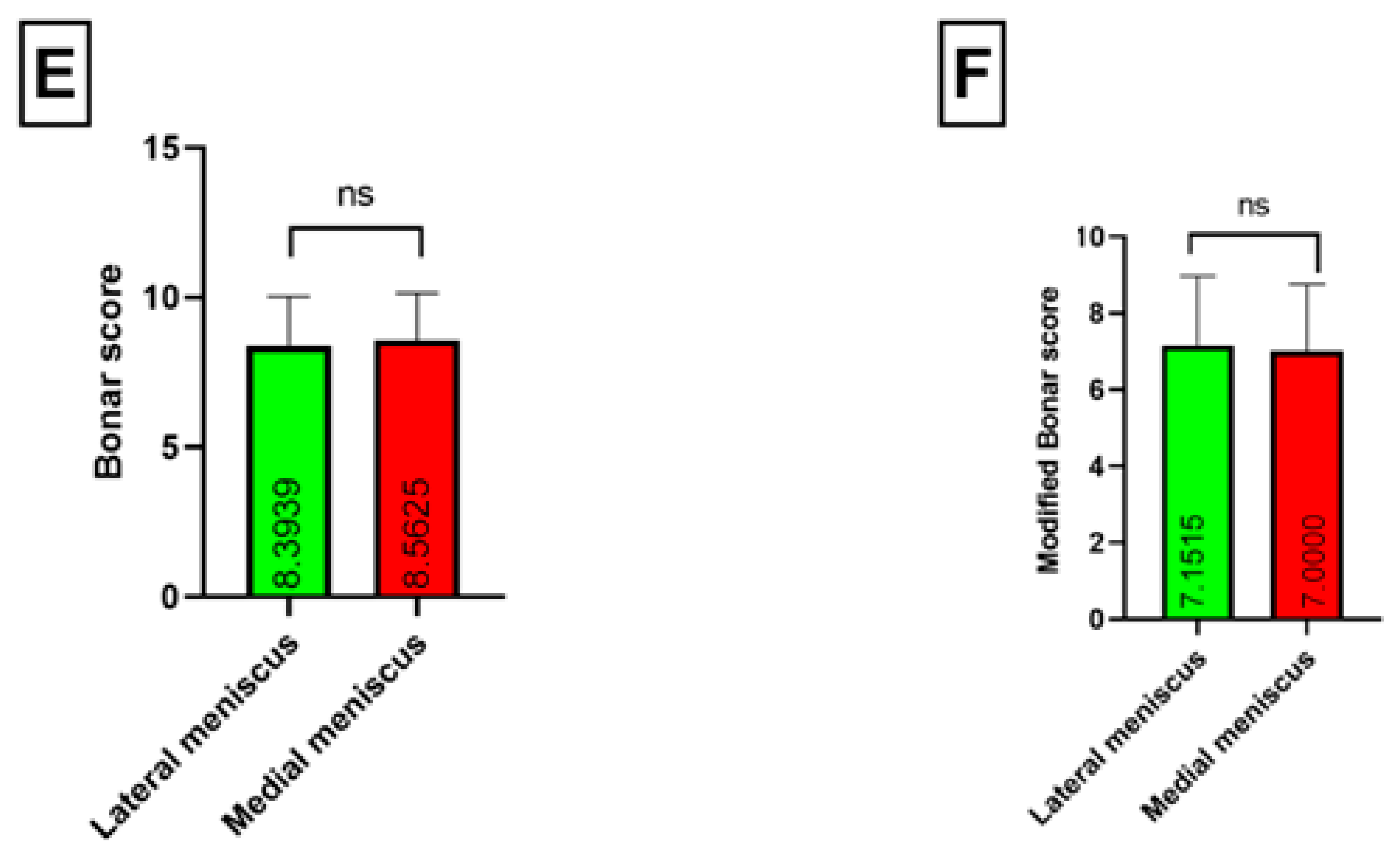
| Classical Bonar score | 8.3976 (range 4–12; SD = 1.5613) | 8.4878 (range 4–11; SD = 1.6751) | 8.3571 (6–12; SD = 1.5113) | p = 0.8657 |
| Modified Bonar score | 6.9398 (3–11; SD = 1.7967) | 6.8049 (3–10; SD = 1.8469) | 7.0714 (3–11; SD = 1.7585) | p = 0.9378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabrzyńska, M.; Gagat, M.; Antosik, P.; Woźniak, Ł.; Kułakowski, M.; Elster, K.; Zabrzyński, J. The Histopathological Examination of the Degeneration of Menisci in Osteoarthritic Knees Using an Adapted Bonar Score: Does Osteoarthritis Equally Influence the Lateral and Medial Menisci? Appl. Sci. 2024, 14, 9659. https://doi.org/10.3390/app14219659
Zabrzyńska M, Gagat M, Antosik P, Woźniak Ł, Kułakowski M, Elster K, Zabrzyński J. The Histopathological Examination of the Degeneration of Menisci in Osteoarthritic Knees Using an Adapted Bonar Score: Does Osteoarthritis Equally Influence the Lateral and Medial Menisci? Applied Sciences. 2024; 14(21):9659. https://doi.org/10.3390/app14219659
Chicago/Turabian StyleZabrzyńska, Maria, Maciej Gagat, Paulina Antosik, Łukasz Woźniak, Michał Kułakowski, Karol Elster, and Jan Zabrzyński. 2024. "The Histopathological Examination of the Degeneration of Menisci in Osteoarthritic Knees Using an Adapted Bonar Score: Does Osteoarthritis Equally Influence the Lateral and Medial Menisci?" Applied Sciences 14, no. 21: 9659. https://doi.org/10.3390/app14219659
APA StyleZabrzyńska, M., Gagat, M., Antosik, P., Woźniak, Ł., Kułakowski, M., Elster, K., & Zabrzyński, J. (2024). The Histopathological Examination of the Degeneration of Menisci in Osteoarthritic Knees Using an Adapted Bonar Score: Does Osteoarthritis Equally Influence the Lateral and Medial Menisci? Applied Sciences, 14(21), 9659. https://doi.org/10.3390/app14219659







