In Silico Investigation of Taurodispacamide A and Strepoxazine A from Agelas oroides S. as Potential Inhibitors of Neuroblastoma Targets Reveals Promising Anticancer Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. PASS Study
2.2. Pharmacokinetic Features-ADMT
- The evaluation of caco-2 cell permeability (nm/s) serves as a means to determine the ability of a potential drug to traverse the intestinal epithelium and enter the bloodstream. Generally, a drug is deemed orally available if it exhibits high permeability [33].
- Human intestinal absorption (HIA %) is a significant parameter used to predict the bioavailability of a drug following oral administration. Higher HIA values signify effective absorption, indicating a greater likelihood of the drug attaining therapeutic concentrations within the systemic circulation [28].
- P-glycoprotein (P-gp) is a protein that functions as an efflux pump present in the cell membranes of the liver, kidneys, and intestines. Its primary role within the cell is to eliminate undesired substances or drugs in a non-specific manner, thereby reducing their accumulation [34].
- Blood-brain barrier penetration involves a complex network of endothelial cells that serve the crucial function of safeguarding the brain against the entry of potentially harmful substances, such as toxins, into the central nervous system [35].
- The MDCK (Madin-Darby Canine Kidney) cell permeability, measured in nm/s, represents an epithelial cell line derived from the kidney of female canines. It serves as a valuable model for studying the transport of drugs across the intestinal epithelium [36].
- Plasma protein binding (%) is a measurement that indicates the extent to which drugs bind to proteins present in the plasma, such as albumin and globulin. This measurement is utilized to estimate drug distribution and assess drug-drug metabolism in relation to protein binding [37].
- Cytochrome P450 enzymes (CYPs) are predominantly located in the liver and are also present in tissues such as the kidneys and lungs. These enzymes play a vital role in metabolizing a wide range of endogenous substances (lipid metabolism) as well as exogenous compounds (drugs, toxins, and carcinogens). In our evaluation, we assessed the activity of key CYPs involved in drug metabolism, including CYP2C19, CYP2C9, CYP3A4, CYP2D6 [38].
- In terms of toxicity assessment, our compounds underwent Ames tests to evaluate their mutagenic potential [39]. Additionally, to ascertain their carcinogenicity, carcinogenicity prediction was performed using mice and rats as model organisms, owing to their genetic and physiological resemblance to humans. In order to assess cardiac safety, we predicted the inhibitory capacity of the human ether-related gene (HERG) channel, which is a voltage-gated potassium channel crucial for regulating the cardiac repolarization process [40].
2.3. Molecular Docking
2.4. Normal Mode Analysis
3. Results and Discussion
3.1. Pass Study
3.2. ADMT
3.3. Molecular Docking
3.3.1. The Focal Adhesion Kinase 1
3.3.2. The Caspase-3
3.3.3. Phosphatidylinositol 4,5-Bisphosphate 3-Kinase Catalytic Subunit Gamma Isoform
3.3.4. Telomerase Reverse Transcriptase
3.3.5. Osm-9-like TRP Channel 1
3.3.6. RAC-Alpha Serine/Threonine-Protein Kinase
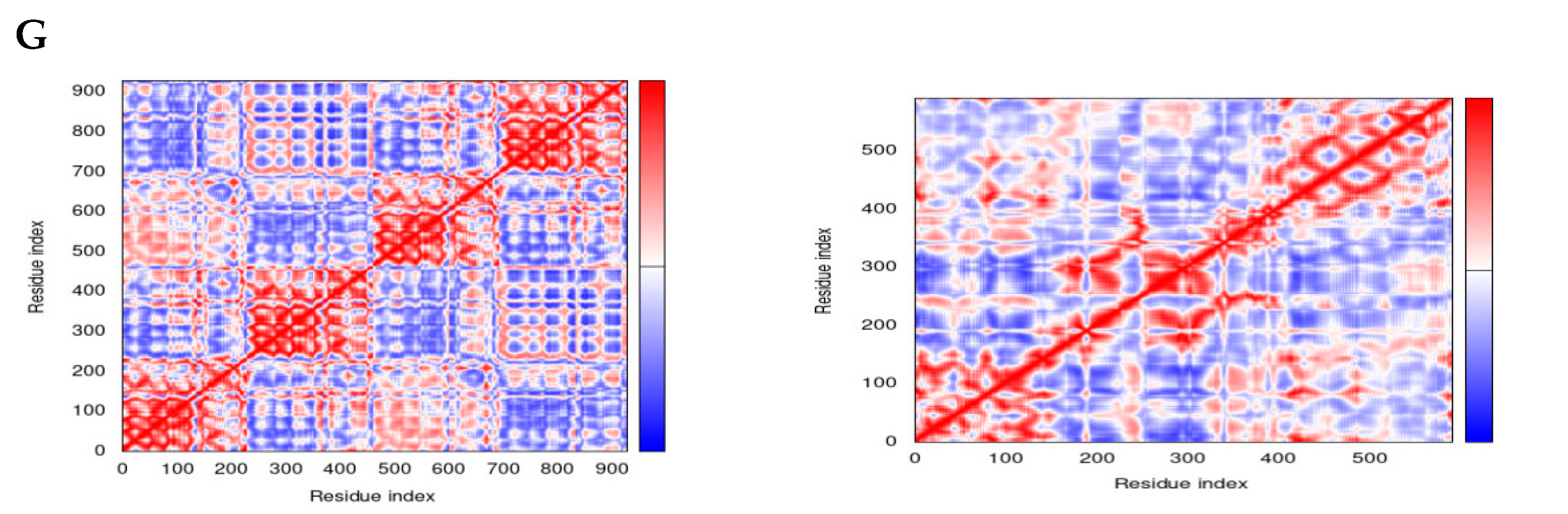
3.4. Normal Mode Analysis
4. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Segura, M.F.; Soriano, A.; Roma, J.; Piskareva, O.; Jiménez, C.; Boloix, A.; Fletcher, J.I.; Haber, M.; Gray, J.C.; Cerdá-Alberich, L. Methodological advances in the discovery of novel neuroblastoma therapeutics. Expert Opin. Drug Discov. 2022, 17, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Capasso, M.; Diskin, S.J. Genetics and genomics of neuroblastoma. In Cancer Genetics; Springer: Boston, MA, USA, 2010; pp. 65–84. [Google Scholar]
- Russo, C.; Cohn, S.L.; Petruzzi, M.J.; de Alarcon, P.A. Long-term neurologic outcome in children with opsoclonus-myoclonus associated with neuroblastoma: A report from the Pediatric Oncology Group. Med. Pediatr. Oncol. Off. J. SIOP-Int. Soc. Pediatr. Oncol. (Soc. Int. D’oncologie Pédiatrique) 1997, 28, 284–288. [Google Scholar] [CrossRef]
- Franks, L.M.; Bollen, A.; Seeger, R.C.; Stram, D.O.; Matthay, K.K. Neuroblastoma in adults and adolescents: An indolent course with poor survival. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997, 79, 2028–2035. [Google Scholar] [CrossRef]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Tomolonis, J.A.; Agarwal, S.; Shohet, J.M. Neuroblastoma pathogenesis: Deregulation of embryonic neural crest development. Cell Tissue Res. 2018, 372, 245–262. [Google Scholar] [CrossRef]
- Fukuta, M.; Nakai, Y.; Kirino, K.; Nakagawa, M.; Sekiguchi, K.; Nagata, S.; Matsumoto, Y.; Yamamoto, T.; Umeda, K.; Heike, T. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE 2014, 9, e112291. [Google Scholar] [CrossRef]
- Lasorella, A.; Noseda, M.; Beyna, M.; Iavarone, A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 2000, 407, 592–598. [Google Scholar] [CrossRef]
- Alborzinia, H.; Flórez, A.F.; Kreth, S.; Brückner, L.M.; Yildiz, U.; Gartlgruber, M.; Odoni, D.I.; Poschet, G.; Garbowicz, K.; Shao, C.; et al. MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat. Cancer 2022, 3, 471–485. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Lindskog, M.; Ponthan, F.; Pettersen, I.; Elfman, L.; Orrego, A.; Sveinbjörnsson, B.; Kogner, P. NSAIDs in neuroblastoma therapy. Cancer Lett. 2005, 228, 195–201. [Google Scholar] [CrossRef]
- Bou-Salah, L.; Benarous, K.; Linani, A.; Rabhi, F.; Chaib, K.; Chine, I.; Bensaidane, H.; Yousfi, M. Anti-inflammatory drugs as new inhibitors to xanthine oxidase: In vitro and in silico approach. Mol. Cell. Probes 2021, 58, 101733. [Google Scholar] [CrossRef]
- Weinstein, J.L.; Katzenstein, H.M.; Cohn, S.L. Advances in the Diagnosis and Treatment of Neuroblastoma. Oncologist 2003, 8, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Strother, D.R.; London, W.B.; Schmidt, M.L.; Brodeur, G.M.; Shimada, H.; Thorner, P.; Collins, M.H.; Tagge, E.; Adkins, S.; Reynolds, C.P. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: Results of Children’s Oncology Group study P9641. J. Clin. Oncol. 2012, 30, 1842. [Google Scholar] [CrossRef]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wang, W.; Liu, G.; Xian, W.; McKeon, F.; Zhou, J.; Zhang, R. Targeting the p53-MDM2 pathway for neuroblastoma therapy: Rays of hope. Cancer Lett. 2021, 496, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Yang, J. Promising Molecular Targets and Novel Therapeutic Approaches in Neuroblastoma. Curr. Pharmacol. Rep. 2023, 9, 43–58. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Y.; Zhao, Z.; Gao, M. Developing targeted therapies for neuroblastoma by dissecting the effects of metabolic reprogramming on tumor microenvironments and progression. Theranostics 2024, 14, 3439–3469. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Batel, R.; Schröder, H.C.; Müller, I.M. Traditional and modern biomedical prospecting: Part I—The history. Evid.-Based Complement. Altern. Med. 2004, 1, 71–82. [Google Scholar] [CrossRef]
- Liang, J.; She, J.; Fu, J.; Wang, J.; Ye, Y.; Yang, B.; Liu, Y.; Zhou, X.; Tao, H. Advances in Natural Products from the Marine-Sponge-Associated Microorganisms with Antimicrobial Activity in the Last Decade. Mar. Drugs 2023, 21, 236. [Google Scholar] [CrossRef]
- Choi, C.; Cho, Y.; Son, A.; Shin, S.-W.; Lee, Y.-J.; Park, H.C. Therapeutic Potential of (−)-Agelamide D, a Diterpene Alkaloid from the Marine Sponge Agelas sp., as a Natural Radiosensitizer in Hepatocellular Carcinoma Models. Mar. Drugs 2020, 18, 500. [Google Scholar] [CrossRef]
- Chu, M.-J.; Li, M.; Ma, H.; Li, P.-L.; Li, G.-Q. Secondary metabolites from marine sponges of the genus Agelas: A comprehensive update insight on structural diversity and bioactivity. RSC Adv. 2022, 12, 7789–7820. [Google Scholar] [CrossRef]
- Lee, S.; Tanaka, N.; Takahashi, S.; Tsuji, D.; Kim, S.-Y.; Kojoma, M.; Itoh, K.; Kobayashi, J.i.; Kashiwada, Y. Agesasines A and B, Bromopyrrole Alkaloids from Marine Sponges Agelas spp. Mar. Drugs 2020, 18, 455. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking11Edited by F. E. Cohen. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Ortí, E.S.; Chacón, P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.K.; Singh, D.; Lagunin, A.; Poroikov, V. PASS-assisted exploration of new therapeutic potential of natural products. Med. Chem. Res. 2011, 20, 1509–1514. [Google Scholar] [CrossRef]
- Linani, A.; Benarous, K.; Bou-salah, L.; Yousfi, M. Hispidin, Harmaline, and Harmine as potent inhibitors of bovine xanthine oxidase: Gout treatment, in vitro, ADMET prediction, and SAR studies. Bioorg. Chem. 2021, 112, 104937. [Google Scholar] [CrossRef]
- Lee, S.; Lee, I.; Kim, H.; Chang, G.; Chung, J.; No, K. The PreADME Approach: Web-based program for rapid prediction of physico-chemical, drug absorption and drug-like properties. In EuroQSAR Designing Drugs and Crop Protectants: Processes, Problems and Solutions; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 418–420. [Google Scholar]
- Garg, S.; Roy, A. In silico analysis of selected alkaloids against main protease (Mpro) of SARS-CoV-2. Chem.-Biol. Interact. 2020, 332, 109309. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Wattamwar, A.S.; Pekamwar, S.S.; Chandak, P.G. Antioxidant, antimicrobial activity and in silico PASS prediction of Annona reticulata Linn. root extract. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 140–148. [Google Scholar] [CrossRef]
- Khurana, N.; Ishar, M.P.S.; Gajbhiye, A.; Goel, R.K. PASS assisted prediction and pharmacological evaluation of novel nicotinic analogs for nootropic activity in mice. Eur. J. Pharmacol. 2011, 662, 22–30. [Google Scholar] [CrossRef]
- Linani, A.; Serseg, T.; Benarous, K.; Bou-salah, L.; Yousfi, M.; Alama, M.N.; Ashraf, G.M. Cupressus sempervirens L. flavonoids as potent inhibitors to xanthine oxidase: In vitro, molecular docking, ADMET and PASS studies. J. Biomol. Struct. Dyn. 2023, 41, 7055–7068. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Z.; Cai, Z. Prediction of human intestinal absorption by GA feature selection and support vector machine regression. Int. J. Mol. Sci. 2008, 9, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Lefauconnier, J.M.; Hauw, J.J. The blood-brain barrier. II. Physiological data (conclusion). Rev. Neurol. 1984, 140, 89–109. [Google Scholar] [PubMed]
- Luo, S.; Kansara, V.S.; Zhu, X.; Mandava, N.K.; Pal, D.; Mitra, A.K. Functional characterization of sodium-dependent multivitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol. Pharm. 2006, 3, 329–339. [Google Scholar] [CrossRef][Green Version]
- Nation, R.L.; Theuretzbacher, U.; Tsuji, B.T. Concentration-dependent plasma protein binding: Expect the unexpected. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 122, 341–346. [Google Scholar] [CrossRef]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar]
- Ames, B.N.; Durston, W.E.; Yamasaki, E.; Lee, F.D. Carcinogens are mutagens: A simple test system combining liver homogenates for activation and bacteria for detection. Proc. Natl. Acad. Sci. USA 1973, 70, 2281–2285. [Google Scholar] [CrossRef]
- Hedley, P.L.; Jørgensen, P.; Schlamowitz, S.; Wangari, R.; Moolman-Smook, J.; Brink, P.A.; Kanters, J.K.; Corfield, V.A.; Christiansen, M. The genetic basis of long QT and short QT syndromes: A mutation update. Hum. Mutat. 2009, 30, 1486–1511. [Google Scholar] [CrossRef]
- Todorov, L.; Saso, L.; Benarous, K.; Traykova, M.; Linani, A.; Kostova, I. Synthesis, Structure and Impact of 5-Aminoorotic Acid and Its Complexes with Lanthanum(III) and Gallium(III) on the Activity of Xanthine Oxidase. Molecules 2021, 26, 4503. [Google Scholar] [CrossRef]
- HyperChem(TM) Professional, version 8.0; Hypercube Inc.: Gainesville, FL, USA, 2009.
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. BIOVIA Workbook, Release 2017; BIOVIA Pipeline Pilot; Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]
- Magoulas, G.E. Ageladine A, a Bromopyrrole Alkaloid from the Marine Sponge Agelas nakamurai. Compounds 2023, 3, 107–121. [Google Scholar] [CrossRef]
- Varijakzhan, D.; Loh, J.-Y.; Yap, W.-S.; Yusoff, K.; Seboussi, R.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Bioactive compounds from marine sponges: Fundamentals and applications. Mar. Drugs 2021, 19, 246. [Google Scholar] [CrossRef] [PubMed]
- Elissawy, A.M.; Soleiman Dehkordi, E.; Mehdinezhad, N.; Ashour, M.L.; Mohammadi Pour, P. Cytotoxic alkaloids derived from marine sponges: A comprehensive review. Biomolecules 2021, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Taglialatela-Scafati, O. Two novel pyrrole-imidazole alkaloids from the Mediterranean sponge Agelas oroides. Tetrahedron Lett. 2000, 41, 9917–9922. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The single global macromolecular structure archive. Methods Mol. Biol. 2017, 1607, 627–641. [Google Scholar]
- Kovacs, J.A.; Chacón, P.; Abagyan, R. Predictions of protein flexibility: First-order measures. Proteins 2004, 56, 661–668. [Google Scholar] [CrossRef]
- Lopéz-Blanco, J.R.; Garzón, J.I.; Chacón, P. iMod: Multipurpose normal mode analysis in internal coordinates. Bioinformatics 2011, 27, 2843–2850. [Google Scholar] [CrossRef]
- Zamir, E.; Geiger, B. Focal Adhesions. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 128–133. [Google Scholar] [CrossRef]
- Tan, H.; Liu, Y.; Gong, C.; Zhang, J.; Huang, J.; Zhang, Q. Synthesis and evaluation of FAK inhibitors with a 5-fluoro-7H-pyrrolo [2,3-d]pyrimidine scaffold as anti-hepatocellular carcinoma agents. Eur. J. Med. Chem. 2021, 223, 113670. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Ponder, K.G.; Boise, L.H. The prodomain of caspase-3 regulates its own removal and caspase activation. Cell Death Discov. 2019, 5, 56. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Y.; He, S.; Cheng, J.; Gong, Y.; Zhang, Z.; Yang, X.; Xu, B.; Liu, X.; Li, C.-Y. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett. 2017, 385, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Kusama, T.; Hamanaka, Y.; Koga, T.; Endo, H.; Tatsuta, M.; Inoue, M. Cross talk between apoptosis and invasion signaling in cancer cells through caspase-3 activation. Cancer Res. 2005, 65, 9121–9125. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Bohn, E.; Kröber, S.M.; Xiao, Y.-j.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Jonges, L.E.; Nagelkerke, J.F.; Ensink, N.G.; van der Velde, E.A.; Tollenaar, R.A.E.M.; Fleuren, G.J.; van de Velde, C.J.H.; Morreau, H.; Kuppen, P.J.K. Caspase-3 activity as a prognostic factor in colorectal carcinoma. Lab. Investig. 2001, 81, 681–688. [Google Scholar] [CrossRef]
- Bernard, A.; Chevrier, S.; Beltjens, F.; Dosset, M.; Viltard, E.; Lagrange, A.; Derangère, V.; Oudot, A.; Ghiringhelli, F.; Collin, B.; et al. Cleaved Caspase-3 Transcriptionally Regulates Angiogenesis-Promoting Chemotherapy Resistance. Cancer Res. 2019, 79, 5958–5970. [Google Scholar] [CrossRef]
- Eckhart, L.; Ballaun, C.; Hermann, M.; VandeBerg, J.L.; Sipos, W.; Uthman, A.; Fischer, H.; Tschachler, E. Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol. Biol. Evol. 2008, 25, 831–841. [Google Scholar] [CrossRef]
- Foster, F.M.; Traer, C.J.; Abraham, S.M.; Fry, M.J. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003, 116, 3037–3040. [Google Scholar] [CrossRef]
- Fry, M.J. Structure, regulation and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1994, 1226, 237–268. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Le, P.T.; Cheng, H.; Ninkovic, S.; Plewe, M.; Huang, X.; Wang, H.; Bagrodia, S.; Sun, S.; Knighton, D.R.; LaFleur Rogers, C.M.; et al. Design and synthesis of a novel pyrrolidinyl pyrido pyrimidinone derivative as a potent inhibitor of PI3Kα and mTOR. Bioorg. Med. Chem. Lett. 2012, 22, 5098–5103. [Google Scholar] [CrossRef]
- Schaich, M.A.; Sanford, S.L.; Welfer, G.A.; Johnson, S.A.; Khoang, T.H.; Opresko, P.L.; Freudenthal, B.D. Mechanisms of nucleotide selection by telomerase. eLife 2020, 9, e55438. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Sub-Cell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef]
- Nadezhdin, K.D.; Neuberger, A.; Nikolaev, Y.A.; Murphy, L.A.; Gracheva, E.O.; Bagriantsev, S.N.; Sobolevsky, A.I. Extracellular cap domain is an essential component of the TRPV1 gating mechanism. Nat. Commun. 2021, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Coffer, P.J.; Jin, J.; Woodgett, J.R. Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 1998, 335, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cron, P.; Thompson, V.; Good, V.M.; Hess, D.; Hemmings, B.A.; Barford, D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell 2002, 9, 1227–1240. [Google Scholar] [CrossRef]
- Lin, K.; Lin, J.; Wu, W.I.; Ballard, J.; Lee, B.B.; Gloor, S.L.; Vigers, G.P.; Morales, T.H.; Friedman, L.S.; Skelton, N.; et al. An ATP-site on-off switch that restricts phosphatase accessibility of Akt. Sci. Signal. 2012, 5, ra37. [Google Scholar] [CrossRef]
- Awan, F.M.; Obaid, A.; Ikram, A.; Janjua, H.A. Mutation-Structure-Function Relationship Based Integrated Strategy Reveals the Potential Impact of Deleterious Missense Mutations in Autophagy Related Proteins on Hepatocellular Carcinoma (HCC): A Comprehensive Informatics Approach. Int. J. Mol. Sci. 2017, 18, 139. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Srivastava, A.; Rao, K.K.; Balaji, P.V. Monomerization alters the dynamics of the lid region in Campylobacter jejuni CstII: An MD simulation study. J. Biomol. Struct. Dyn. 2016, 34, 778–791. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Pandian, J.R.; Drake, E.; Vinayak, A.; Verma, M.; Cascio, D. B-factor Analysis and Conformational Rearrangement of Aldose Reductase. Curr. Proteom. 2014, 11, 151–160. [Google Scholar] [CrossRef]
- Carugo, O. How large B-factors can be in protein crystal structures. BMC Bioinform. 2018, 19, 61. [Google Scholar] [CrossRef]


| Code | 2D Structure | Source |
|---|---|---|
| Mol1 (Ageladine A) PubID 1: 10089677 MW 2: 357.00 g/mol F 3: C10H7Br2N5 |  | Agelas nakamurai H. [45] |
| Mol2 (Oroidin) PubID: 6312649 MW: 389.05 g/mol F: C11H11Br2N5O | 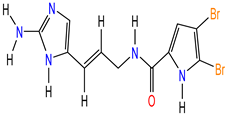 | Agelas oroides S. [46,47] |
| Mol3 (Strepoxazine A) PubID: 132526082 MW: 342.30 g/mol F: C17H14N2O6 | 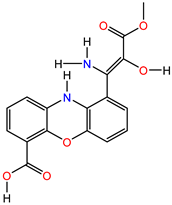 | |
| Mol4 (Cyclooroidin) PubID: 10739060 MW: 389.05 g/mol F: C11H11Br2N5O | 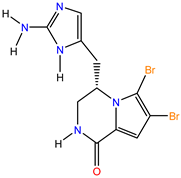 | Agelas oroides S. [48] |
| Mol5 (Taurodispacamide A) PubID: 135501141 MW: 512.18 g/mol F: C13H16Br2N6O4S |  |
| PDB ID | 2IJM | 3DEI | 4FA6 | 6USR | 7LQZ | 4EKK |
| Code | FAK | Ca3 | PI3K | TERT | TRPV1 | AKT1 |
| Target | Focal Adhesion Kinase 1 | Caspase-3 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Telomerase reverse transcriptase | Osm-9-like TRP channel 1 | RAC-alpha serine/threonine-protein kinase |
| Molecules | Pa 1 | Pi 2 | PBA 3 | PBAn 4 |
|---|---|---|---|---|
| Mol1 | 0.964 | 0.001 | MAP kinase 1 inhibitor | 383 |
| 0.903 | 0.002 | Protein kinase inhibitor | ||
| 0.896 | 0.002 | Raf kinase inhibitor | ||
| Mol2 | 0.960 | 0.001 | MAP kinase 1 inhibitor | 68 |
| 0.883 | 0.002 | Protein kinase inhibitor | ||
| 0.878 | 0.002 | Raf kinase inhibitor | ||
| Mol3 | 0.772 | 0.010 | Fusarinine-C ornithinesterase inhibitor | 901 |
| 0.686 | 0.030 | Pro-opiomelanocortin converting enzyme inhibitor | ||
| 0.643 | 0.016 | Preneoplastic conditions treatment | ||
| Mol4 | 0.917 | 0.002 | MAP kinase 1 inhibitor | 109 |
| 0.825 | 0.003 | Mycothiol-S-conjugate amidase inhibitor | ||
| 0.699 | 0.003 | Antineoplastic (brain cancer) | ||
| Mol5 | 0.953 | 0.001 | MAP kinase 1 inhibitor | 68 |
| 0.807 | 0.002 | Coenzyme-B sulfoethylthiotransferase inhibitor | ||
| 0.805 | 0.003 | Mycothiol-S-conjugate amidase inhibitor |
| Pharmacokinetics | Mol1 | Mol2 | Mol3 | Mol4 | Mol5 |
|---|---|---|---|---|---|
| Absorption | |||||
| Caco-2 cell 1 permeability (nm/s) > 20 | 3.93 | 19.77 | 6.45 | 19.23 | 19.67 |
| Human intestinal absorption (HIA %) 70 to 100% | 86.03 | 79.45 | 26.88 | 84.73 | 40.14 |
| Water solubility (g/L) | 55.97 | 0.154 | 51.38 | 27.24 | 4.20 |
| P-glycoprotein inhibition a substrate of it indicates high levels of absorption | Non | Non | Non | Non | Non |
| Distribution | |||||
| Blood-brain barrier penetration (C.brain/C.blood) >2 cross the blood–brain barrier easily | 0.28 | 0.08 | 0.03 | 0.24 | 0.04 |
| MDCK 2 cell permeability (nm/s) Low (<1 nm/s), moderate (1–10 nm/s), and high (>10 nm/s) | 0.55 | 0.46 | 0.57 | 0.57 | 0.49 |
| Plasma protein binding (%) 80 to 100% is considered high, 50 to 80% (moderate), <50% (low) | 49.01 | 60.14 | 32.43 | 38.70 | 56.46 |
| Skin permeability (logKp 3, cm/hour) <−2.5 considered high permeable | −5.19 | −5.02 | −5.12 | −5.20 | −4.82 |
| Metabolism | |||||
| Cytochrome P450 2C19 inhibition | Non | Inhibitor | Non | Non | Inhibitor |
| Cytochrome P450 2C9 inhibition | Non | Non | Non | Non | Non |
| Cytochrome P450 2D6 inhibition | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor |
| Cytochrome P450 2D6 substrate | Substrate | Substrate | Substrate | Substrate | Substrate |
| Cytochrome P450 3A4 inhibition | Non | Inhibitor | Non | Non | Non |
| Cytochrome P450 3A4 substrate | Weakly | Weakly | Weakly | Weakly | Weakly |
| Toxicity | |||||
| Ames test | Mutagen | Mutagen | non-mutagen | Mutagen | Mutagen |
| Carcinogenicity (Mouse) | Negative | Negative | Negative | Negative | Negative |
| Carcinogenicity (Rat) | Positive | Positive | Negative | Positive | Negative |
| HERG 4_inhibition | Medium risk | Medium risk | low_risk | Medium risk | Ambiguous |
| TA100 (+S9) | Positive | Positive | Negative | Positive | Negative |
| TA100 (−S9) | Negative | Negative | Negative | Negative | Negative |
| Ames TA1535 (+S9) | Positive | Negative | Negative | Positive | Negative |
| Ames TA1535 (−S9) | Positive | Positive | Negative | Positive | Positive |
| Ligand | PLPchem Score | Closest Residues | Interactions Type | Length (Å) | Fav/Unfav 1 Bond |
|---|---|---|---|---|---|
| FAK | |||||
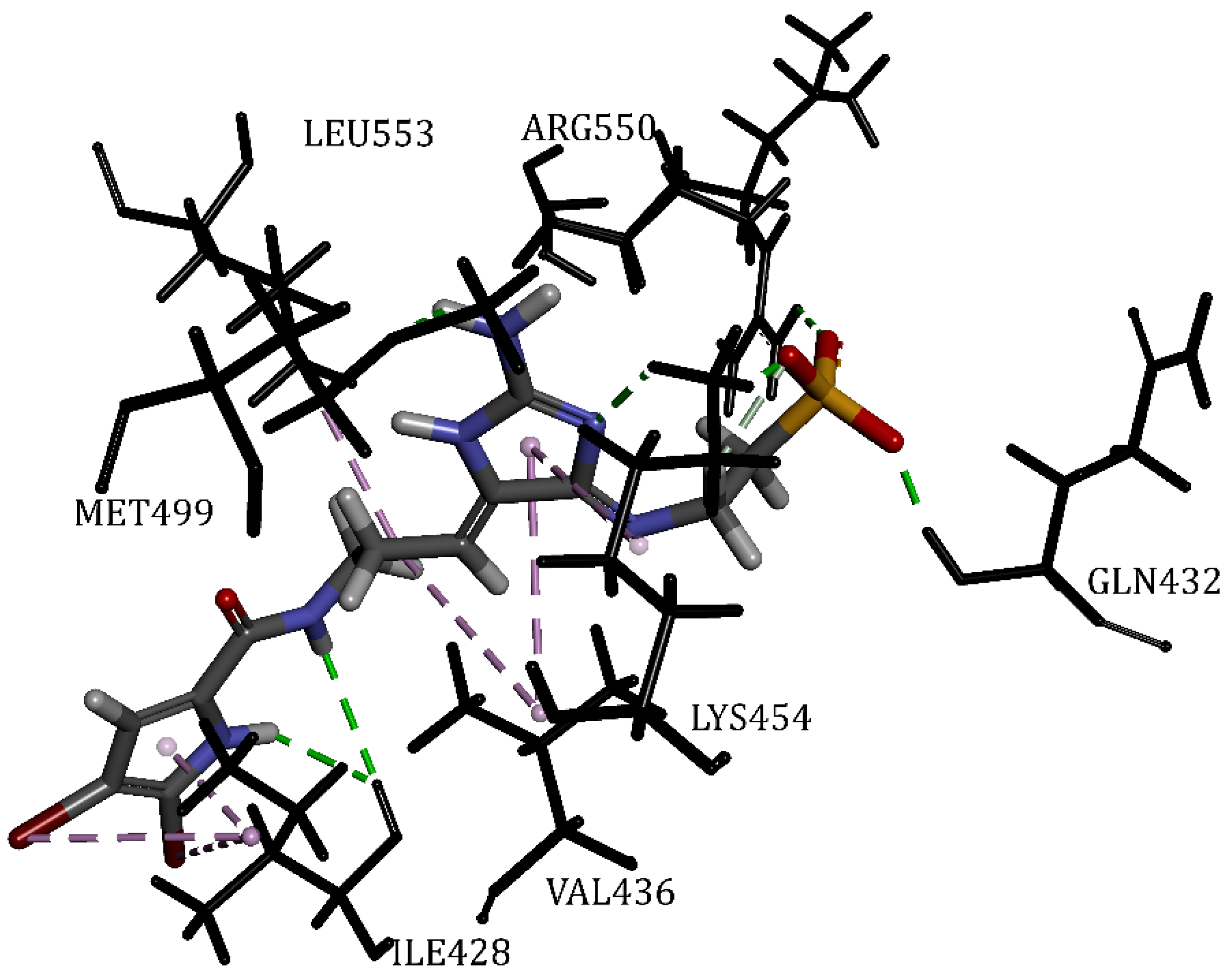
| ATP | 61.64 | Cys502, Leu553, Met499, Ala452, Glu506, Arg550, Gln432, Gly431, Val436, Lys454, Ile428 | Hydrogen Bond | 1.60 | 21/0 |
| Pi-Sigma | 2.64 | ||||
| Pi Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol1 | 38.60 | Cys502, Met499, Leu553, Ile428, Val436, Ala452, | Hydrogen Bond | 2.14 | 10/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol2 | 46.40 | Cys502, Met499, Leu553, Ile428, Val436, Ala452, Leu501 | Hydrogen Bond | 1.95 | 10/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol3 | 44.93 | Cys502, Leu553, Met499, Ala452, Val436, Lys454, Gln432 | Hydrogen Bond | 2.02 | 11/1 |
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol4 | 49.30 | Cys502, Leu553, Val436, Lys454, Ile428 | Hydrogen Bond | 1.54 | 8/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Mol5 | 51.42 | Leu553, Met499, Arg550, Gln432, Val436, Lys454, Ile428 | Hydrogen Bond | 1.67 | 15/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Ca3 | |||||
| RXB | 50.30 | Phe256, Leu168 | Hydrogen Bond | 2.05 | 3/0 |
| Pi-Alkyl | >3.4 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
| Mol1 | 41.04 | Phe256, Leu168, Cys170, Thr166, Tyr204 | Hydrogen Bond | 1.75 | 10/0 |
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol2 | 46.86 | Phe256, Leu168, Thr166, Tyr204 | Hydrogen Bond | 2.62 | 5/0 |
| Pi-Sigma | 2.87 | ||||
| Pi Alkyl | >3.4 | ||||
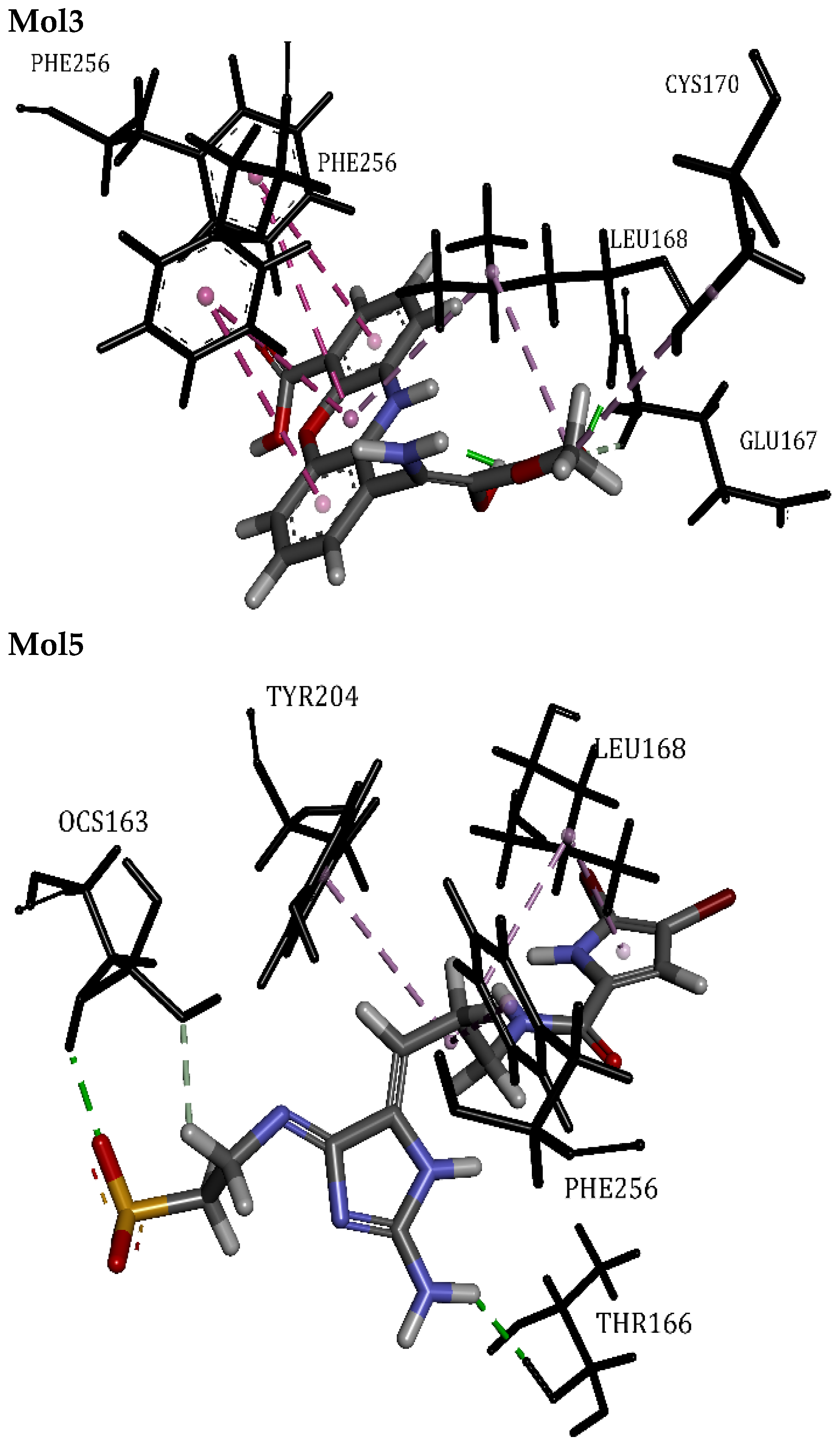
| Mol3 | 60.49 | Phe256, Leu168, Cys170, Glu167 | Hydrogen Bond | 1.60 | 10/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
| Mol4 | 38.22 | Phe256, Leu168, Tyr204, Glu167, His121 | Hydrogen Bond | 1.88 | 7/0 |
| Pi-Alkyl | >3.4 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
| Mol5 | 61.14 | Phe256, Leu168, Thr166, Tyr204, Ocs163 | Hydrogen Bond | 2.04 | 7/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| PI3K | |||||
| OTA | 71.97 | Met804, Met953, Ile831, Ile879, Ile881, Ile963, Lys833, Val882, Tyr867, Phe961 | Hydrogen Bond | 2.08 | 16/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol1 | 44.15 | Met804, Met953, Ile831, Ile881, Ile963, Val882, Pro810 | Hydrogen Bond | 1.63 | 12/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | > 5.0 | ||||
| Mol2 | 49.42 | Met804, Met953, Ile831, Ile879, Ile881, Ile963, Val882, Pro810, Ser806 | Hydrogen Bond | 2.06 | 13/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
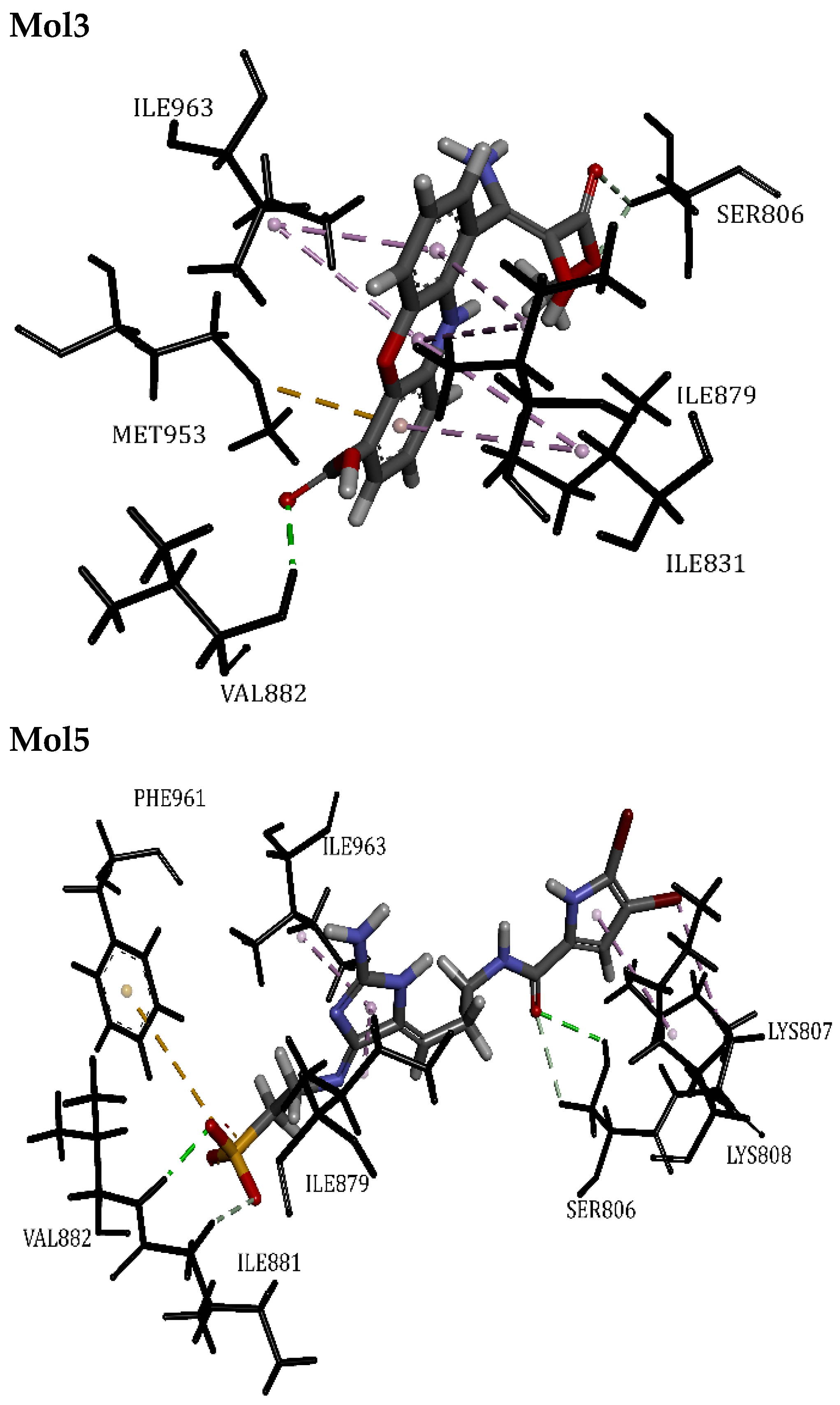
| Mol3 | 56.06 | Ile831, Ile879, Ile963, Met953, Val882, Ser806 | Hydrogen Bond | 2.06 | 10/0 |
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol4 | 53.06 | Ile831, Ile879, Ile963, Met953, Tyr867, Trp812, Asp964 | Hydrogen Bond | 1.83 | 10/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Mol5 | 55.92 | Ile879, Ile881, Ile963, Lys807, Lys808, Val882, Phe961, Ser806 | Hydrogen Bond | 2.05 | 9/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| TERT | |||||
| G2P | 58.02 | Gly309, Gln308, Asp310, Ala195, Arg194, Asp254 | Hydrogen Bond | 1.83 | 9/1 |
| Pi-Sigma | 2.88 | ||||
| Mol1 | 38.59 | Asp251, Ile252, Gln308, Tyr256, Val342, Ala255 | Hydrogen Bond | 1.99 | 9/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Pi Stacked | >3.4 | ||||
| Mol2 | 47.58 | Asp254, Tyr256, Val342, Ala255, Ala195, Phe193 | Hydrogen Bond | 1.76 | 12/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Pi Stacked | >3.4 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
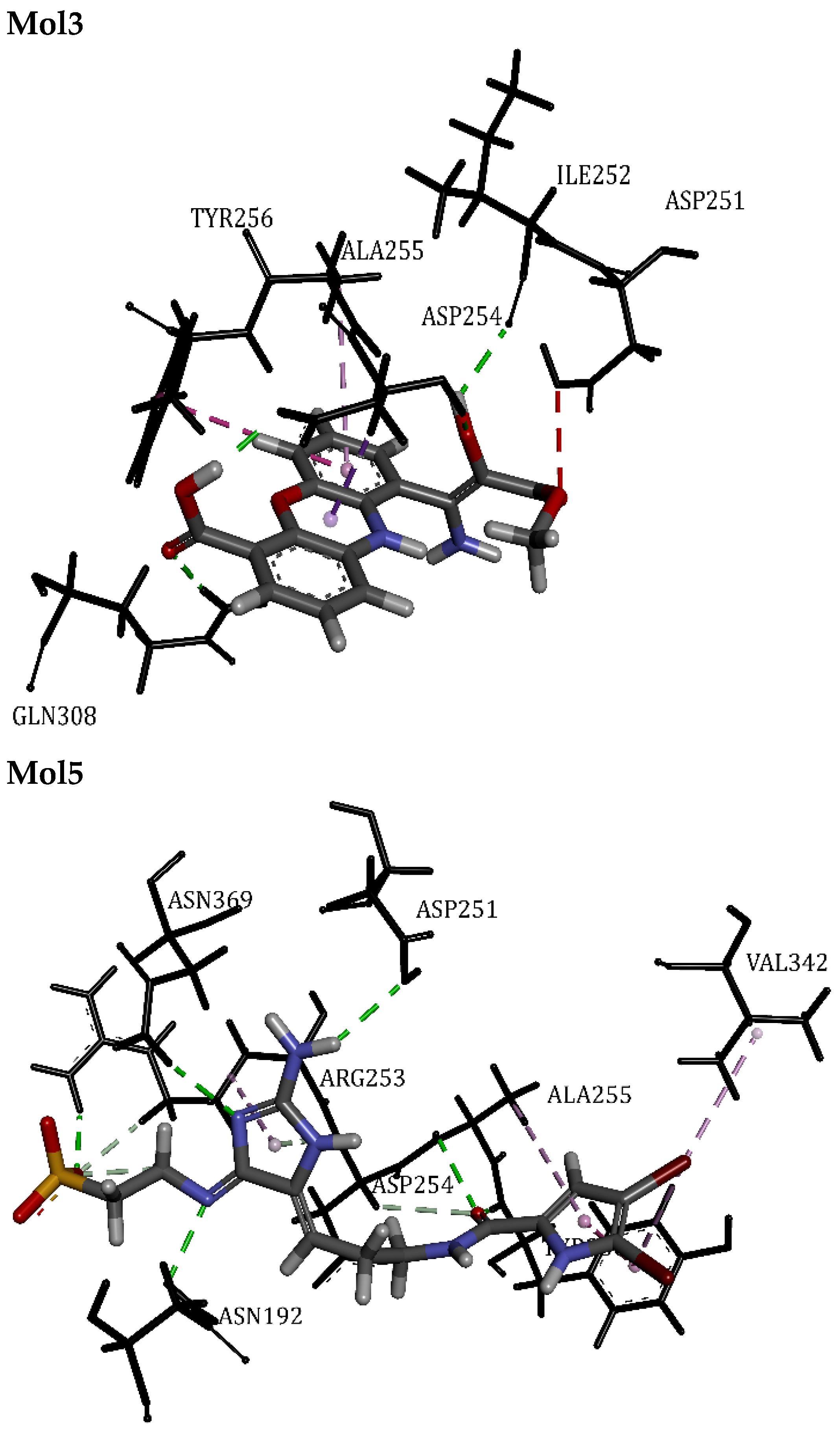
| Mol3 | 53.74 | Asp254, Asp251, Gln308, Tyr256, Ile252 | Hydrogen Bond | 1.74 | 7/1 |
| Pi-Sigma | 2.70 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
| Mol4 | 42.93 | Tyr256, Ala255, Ala195, Arg194 | Hydrogen Bond | 3.03 | 6/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Mol5 | 56.74 | Asp254, Asp251, Tyr256, Asn192, Val342, Ala255, Asn369, Arg253 | Hydrogen Bond | 1.93 | 15/0 |
| Alkyl | >3.4 | ||||
| Pi-Alkyl | >3.4 | ||||
| Pi-Pi Stacked | >3.4 | ||||
| TRPV1 | |||||
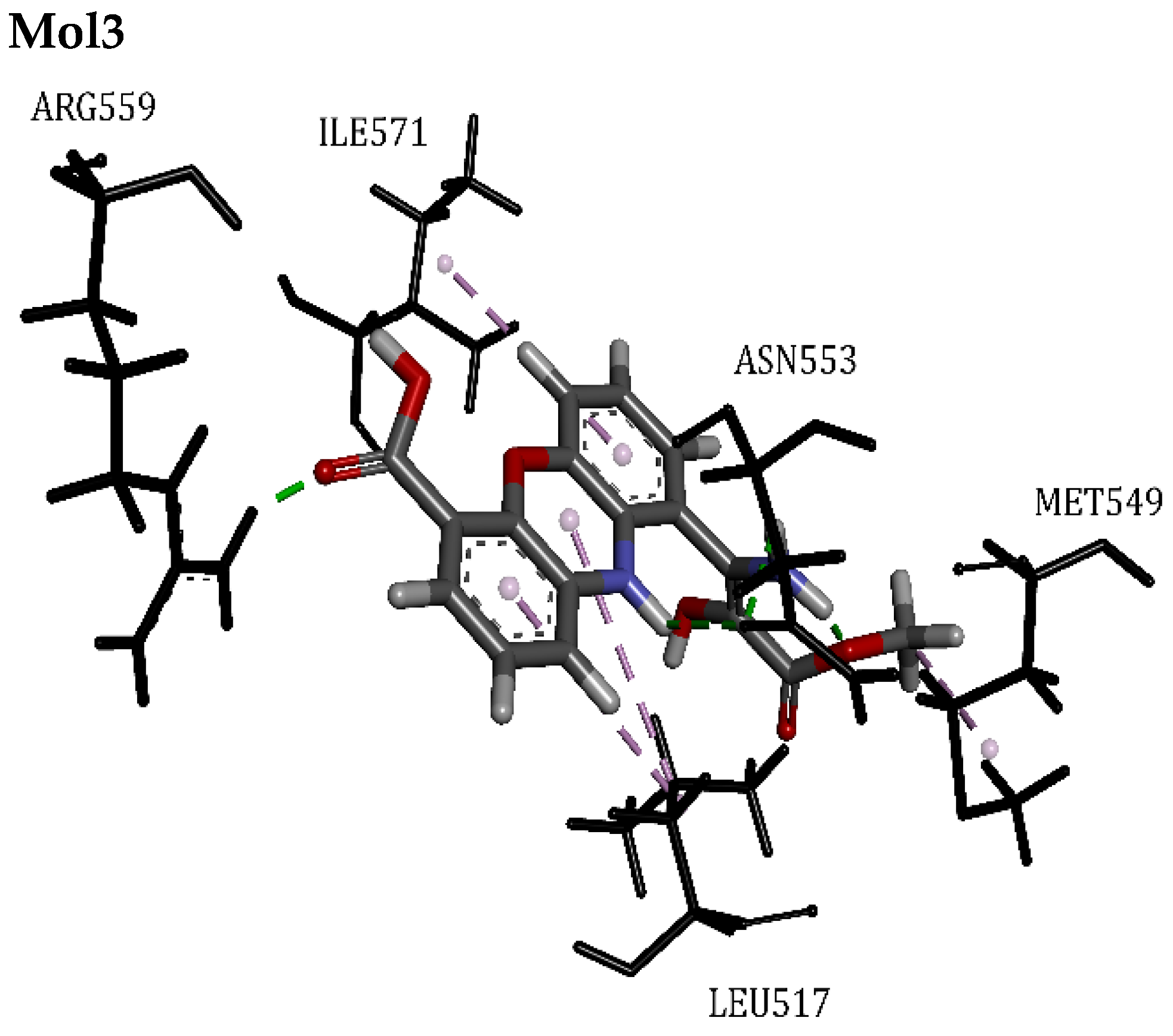
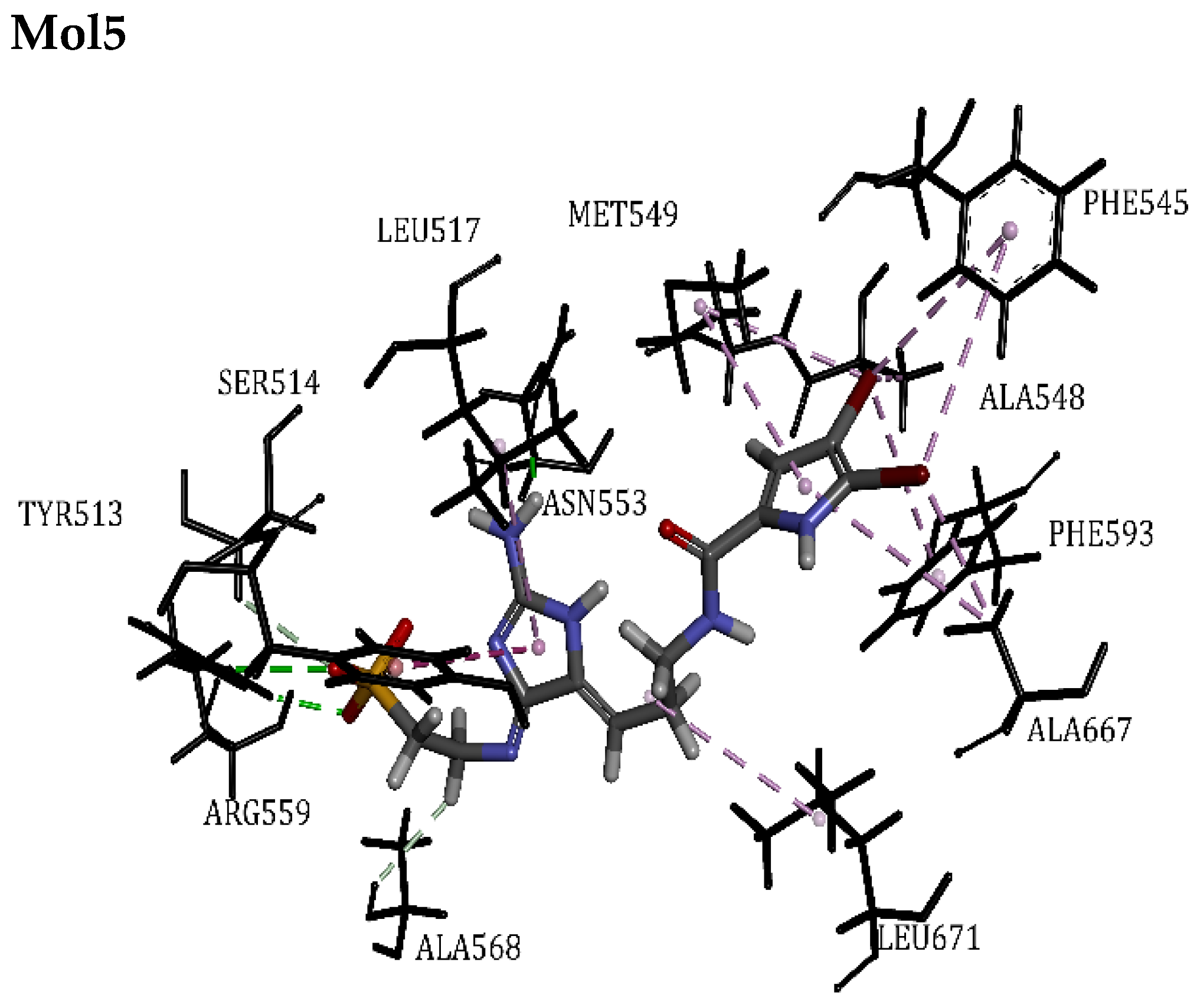
| RTX | 83.61 | Tyr513, Arg559, Ile571, Ile575, Met549, Leu517, Leu555, Asn553, Met516, Phe593, Ala548 | Hydrogen Bond | 2.49 | 14/1 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
| Mol1 | 39.66 | Leu517, Leu555, Leu671, Ala568, Met549, Ile571 | Hydrogen Bond | 2.97 | 8/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Mol2 | 45.95 | Ala548, Tyr513, Tyr556, Met549, Leu517, Phe593 | Hydrogen Bond | 2.46 | 9/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Mol3 | 49.18 | Met549, Leu517, Asn553, Ile571, Arg559 | Hydrogen Bond | 1.60 | 8/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Mol4 | 43.47 | Leu517, Leu555, Ala568, Tyr513, Phe518, Ile571, Arg559 | Hydrogen Bond | 1.72 | 10/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
| Mol5 | 47.68 | Ser514, Ala667, Phe545, Ala568, Asn553, Leu671, Leu517, Tyr513, Phe593, Ala548, Met549, Arg559 | Hydrogen Bond | 1.84 | 17/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Pi-Pi T-shaped | >3.4 | ||||
| AKT1 | |||||
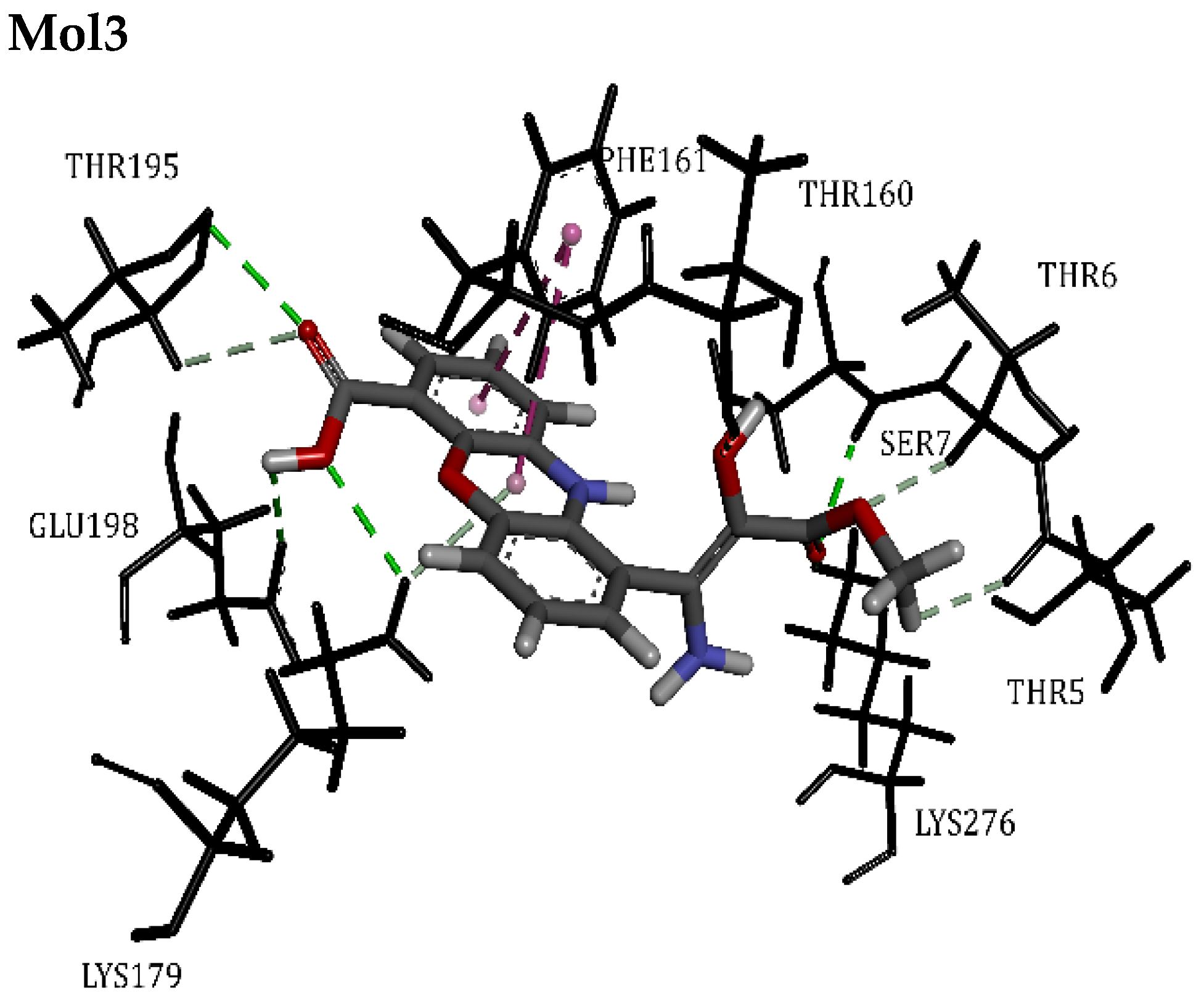
| ANP | 70.98 | Lys276, Lys179, Val164, Met281, Leu156, Ala177, Ala230, Glu228, Tyr229, Ser7, Thr5 | Hydrogen Bond | 1.85 | 14/0 |
| Pi-Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol1 | 37.66 | Phe438, Leu156, Val164, Met281, Met227, Lys179, Ala177, Glu228, Thr291, Tyr229, Ala230, | Hydrogen Bond | 1.84 | 17/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Halogen (Br) | 2.48 | ||||
| Mol2 | 42.74 | Leu156, Val164, Met281, Leu181, Phe161, Asn279 | Hydrogen Bond | 2.73 | 7/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol3 | 57.59 | Lys276, Lys179, Thr160, Phe161, Thr195, Glu198, Thr5, Thr6, Ser7 | Hydrogen Bond | 2.08 | 12/0 |
| Pi-Pi T-shaped | >3.4 | ||||
| Mol4 | 46.66 | Lys276, Lys179, Val164, Met281, Leu156, Ala177, Met227, Asp292, Phe438, | Hydrogen Bond | 2.07 | 12/2 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Mol5 | 51.47 | Thr160, Gly159, Ala177, Val164, Met281, Met227, Phe438, Thr291, Tyr229, Glu228, Leu156, Arg4, Thr5, Thr6, Ser7 | Hydrogen Bond | 2.20 | 19/0 |
| Pi-Alkyl | >3.4 | ||||
| Alkyl | >3.4 | ||||
| Pi-Sulfur | >5.0 | ||||
| Halogen (Br) | 3.03 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linani, A.; Bensenouci, S.; Hafsa, B.l.; Benarous, K.; Serseg, T.; Bou-Salah, L.; Alhatlani, B.Y. In Silico Investigation of Taurodispacamide A and Strepoxazine A from Agelas oroides S. as Potential Inhibitors of Neuroblastoma Targets Reveals Promising Anticancer Activity. Appl. Sci. 2024, 14, 9306. https://doi.org/10.3390/app14209306
Linani A, Bensenouci S, Hafsa Bl, Benarous K, Serseg T, Bou-Salah L, Alhatlani BY. In Silico Investigation of Taurodispacamide A and Strepoxazine A from Agelas oroides S. as Potential Inhibitors of Neuroblastoma Targets Reveals Promising Anticancer Activity. Applied Sciences. 2024; 14(20):9306. https://doi.org/10.3390/app14209306
Chicago/Turabian StyleLinani, Abderahmane, Sabrina Bensenouci, Ben lahbib Hafsa, Khedidja Benarous, Talia Serseg, Leila Bou-Salah, and Bader Y. Alhatlani. 2024. "In Silico Investigation of Taurodispacamide A and Strepoxazine A from Agelas oroides S. as Potential Inhibitors of Neuroblastoma Targets Reveals Promising Anticancer Activity" Applied Sciences 14, no. 20: 9306. https://doi.org/10.3390/app14209306
APA StyleLinani, A., Bensenouci, S., Hafsa, B. l., Benarous, K., Serseg, T., Bou-Salah, L., & Alhatlani, B. Y. (2024). In Silico Investigation of Taurodispacamide A and Strepoxazine A from Agelas oroides S. as Potential Inhibitors of Neuroblastoma Targets Reveals Promising Anticancer Activity. Applied Sciences, 14(20), 9306. https://doi.org/10.3390/app14209306







