Novel Understandings of Biomineralization in Backfill Materials: A Fundamental Investigation of Coal Gangue and Fly Ash Impact on B. pasteurii to Enhance Material Properties
Abstract
1. Introduction
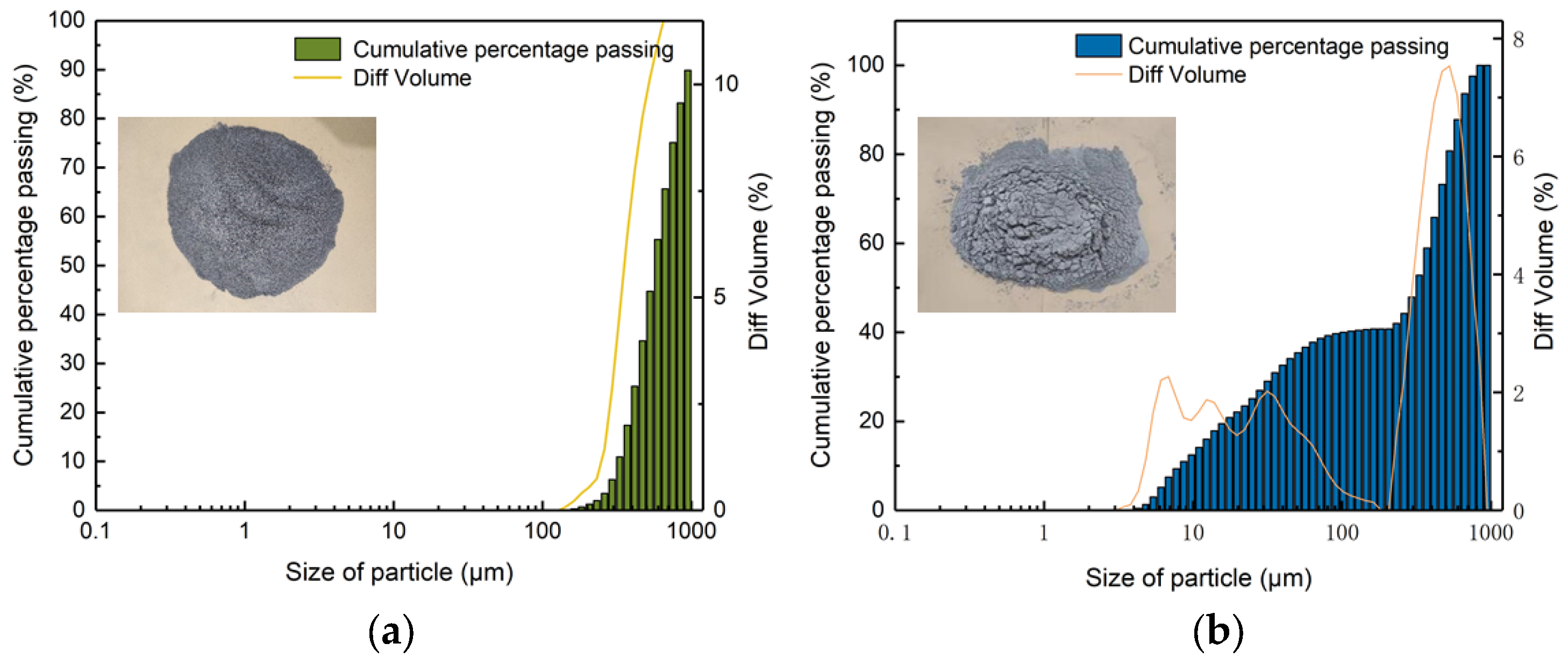
2. Materials
3. Methods
3.1. Micro-Tests after the Addition of B. pasteurii to CG and FA
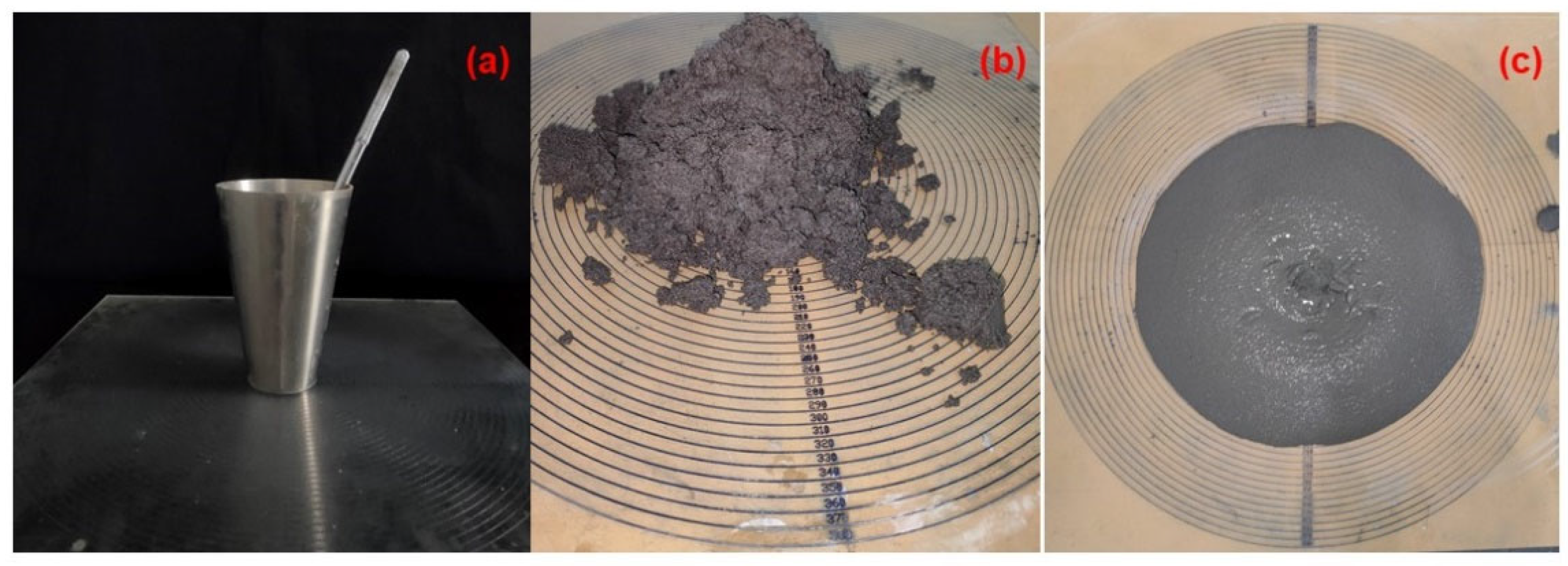
3.2. Macro-Test on Backfilling Material
| Components | A—Spread | B—Spread | B—Strength | C—Strength |
|---|---|---|---|---|
| CG (g) | 2000 | 2000 | 1200 | 1200 |
| FA (g) | 0 | 600 | 360 | 360 |
| B. pasteurii with mineralized solutions (mL) | 500 | 650 | 390 | 0 |
| Water (mL) | 0 | 0 | 0 | 390 |
4. Results and Discussion
4.1. Micro-Tests after the Addition of B. pasteurii to CG and FA
4.1.1. Bacterial Concentration Test
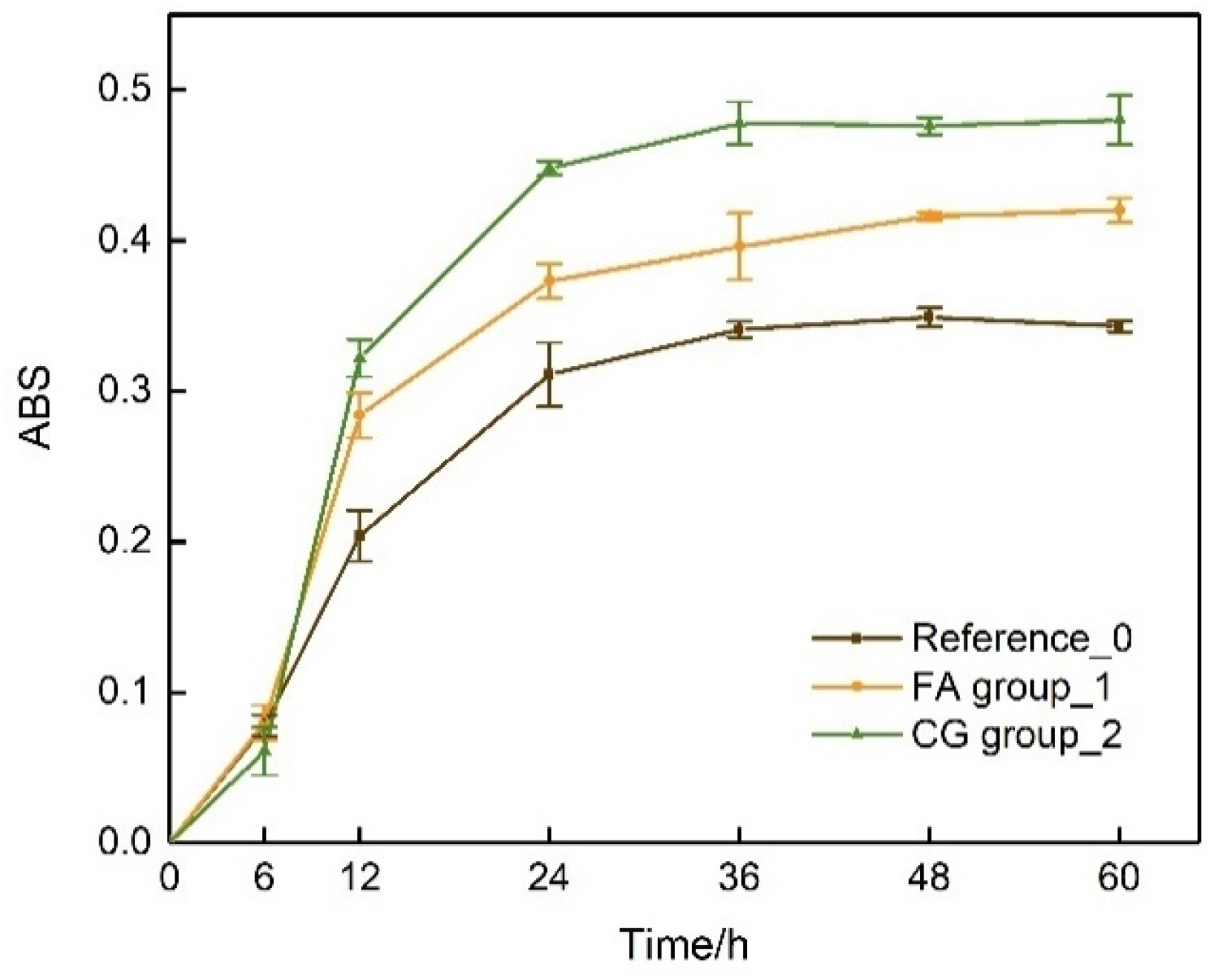
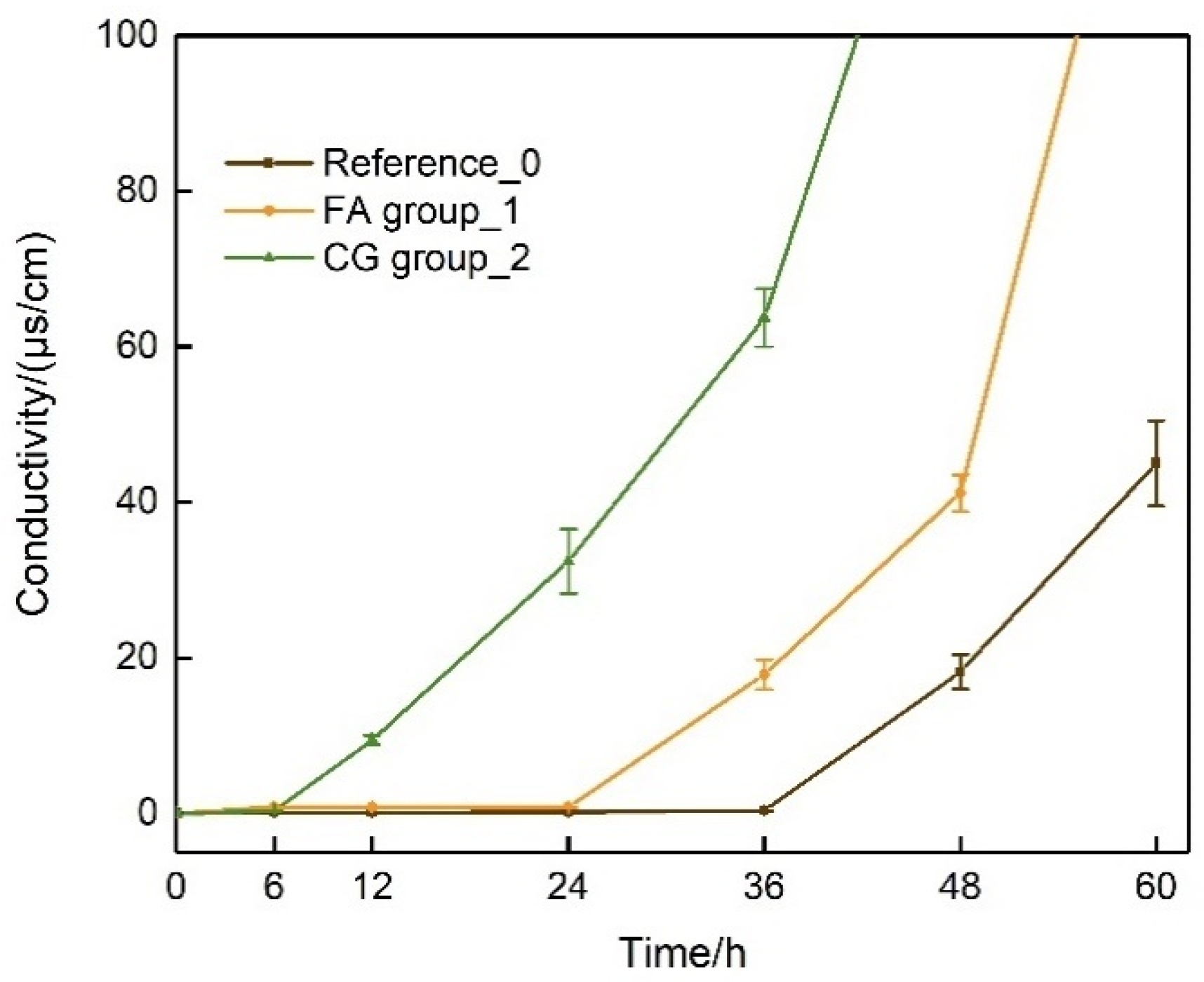
4.1.2. Urea Hydrolysis Test
4.1.3. In Situ Urea Hydrolysis Experiment

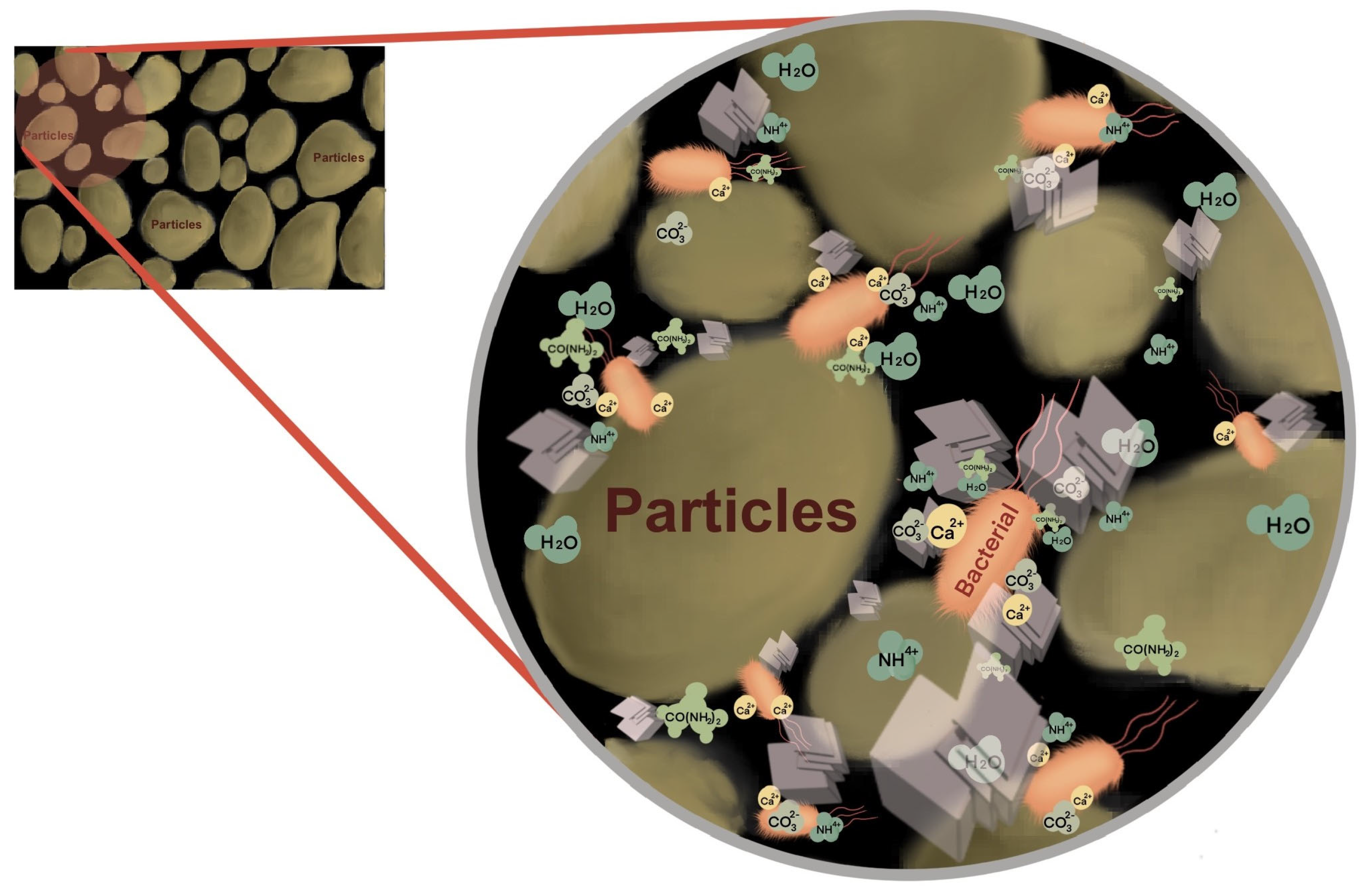
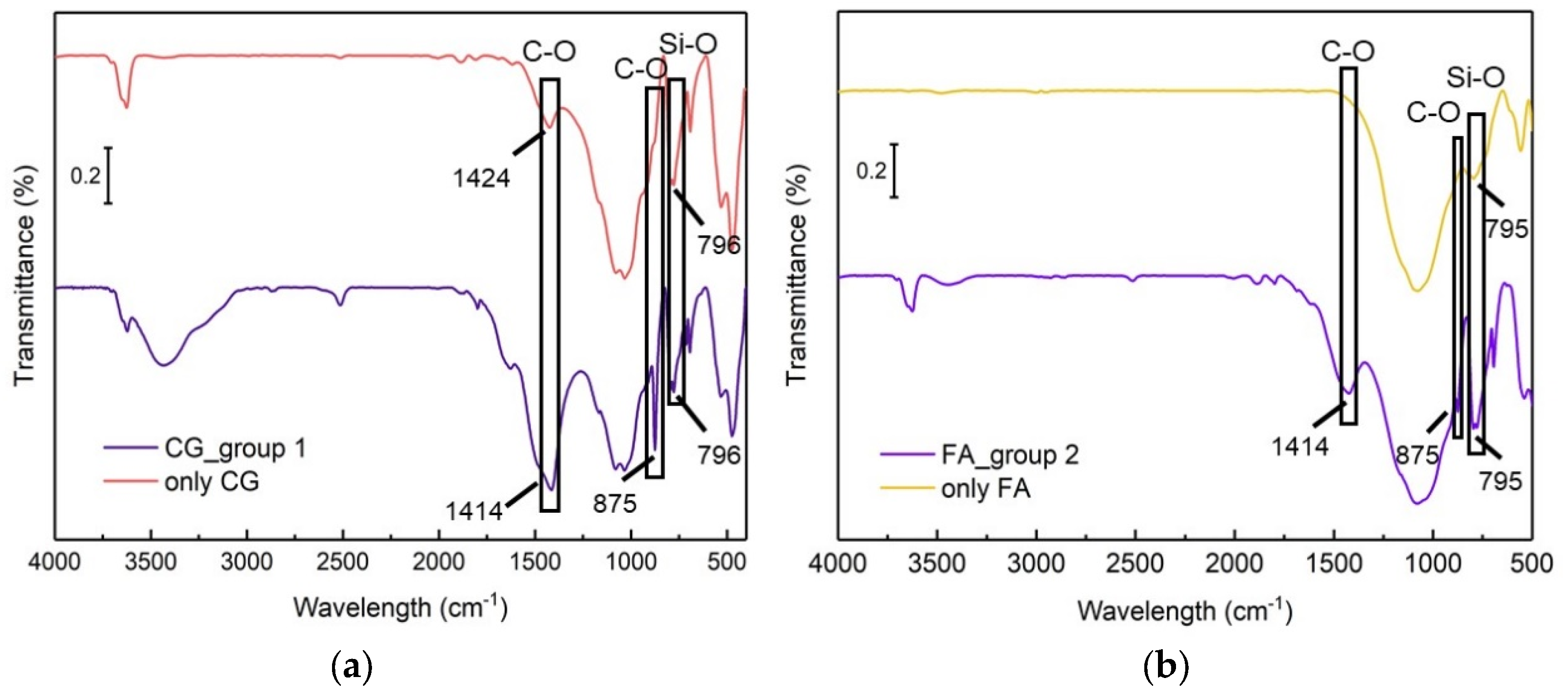
4.1.4. SEM/EDS and FTIR Precipitation Test
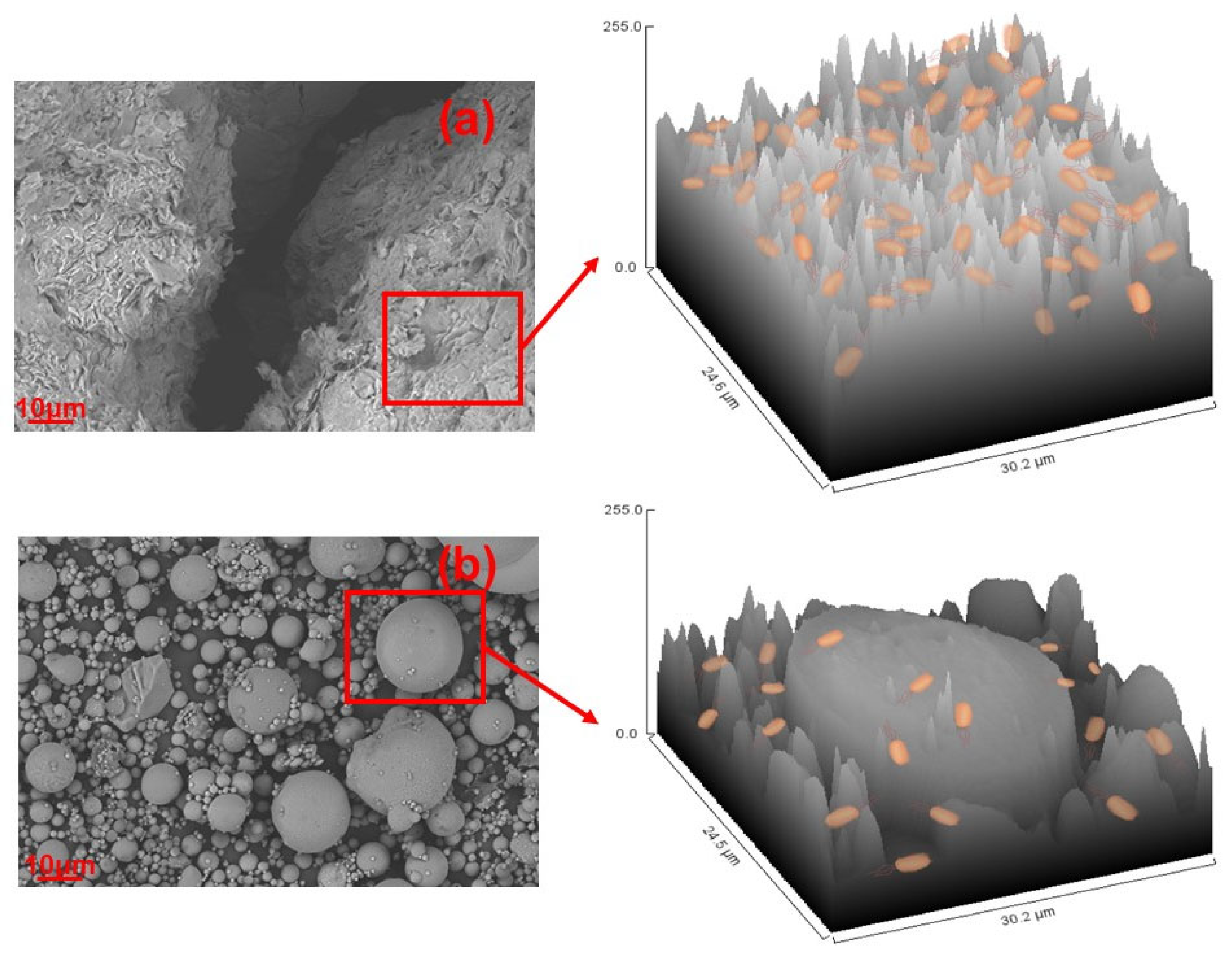
4.1.5. Effect of Solid Surface Roughness on B. pasteurii
4.2. Macro-Test on the Backfilling Material
5. Conclusions
- Both CG and FA environments are favorable for the growth of B. pasteurii.
- CG and FA environments can significantly shorten the initiation time for urea hydrolysis and mineralization. The time for the occurrence of B. pasteurii mineralization is reduced by 72% when using CG and by 28% in the presence of FA.
- A reason why B. pasteurii performs better in the CG environment than in FA may be ascribed to the substrate surface roughness, which offers more attachment sites.
- SEM and FTIR analyses confirmed that the mineralization precipitate, generated by the co-culture of B. pasteurii with CG or FA, is bio-CaCO3.
- Mortars with CG, 30% FA and B. pasteurii with mineralized solutions showed a better pumping performance than in the absence of FA.
- The compressive strength of biomineralized materials at 28 days is 5.34 times higher than that obtained without biomineralizing.
- Finally, our findings support the viability of using microbial mineralized coal gangue and fly ash for eco-friendly backfilling materials, demonstrating potential benefits for sustainable practices in coal mining operations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djamaluddin, I.; Mitani, Y.; Esaki, T. Evaluation of ground movement and damage to structures from Chinese coal mining using a new GIS coupling model. Int. J. Rock Mech. Min. Sci. 2011, 48, 380–393. [Google Scholar] [CrossRef]
- Pandey, V.C. Direct seeding offers affordable restoration for fly ash deposits. Energy Ecol. Environ. 2022, 7, 453–460. [Google Scholar] [CrossRef]
- Zierold, K.M.; Odoh, C. A review on fly ash from coal-fired power plants: Chemical composition, regulations, and health evidence. Rev. Environ. Health 2020, 35, 401–418. [Google Scholar] [CrossRef]
- Yakun, S.; Xingmin, M.; Kairong, L.; Hongbo, S. Soil characterization and differential patterns of heavy metal accumulation in woody plants grown in coal gangue wastelands in Shaanxi, China. Environ. Sci. Pollut. Res. 2016, 23, 13489–13497. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, I.; Yilmaz, E.; Yilmaz, A.O. Sodium silicate effect on setting properties, strength behavior and microstructure of cemented coal fly ash backfill. Powder Technol. 2021, 384, 17–28. [Google Scholar] [CrossRef]
- Du, X.; Feng, G.; Qi, T.; Guo, Y.; Zhang, Y.; Wang, Z. Failure characteristics of large unconfined cemented gangue backfill structure in partial backfill mining. Constr. Build. Mater. 2019, 194, 257–265. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Li, B.; Zhou, N.; Xiao, X.; Li, M.; Zhu, C. Environmental behavior of construction and demolition waste as recycled aggregates for backfilling in mines: Leaching toxicity and surface subsidence studies. J. Hazard. Mater. 2020, 389, 121870. [Google Scholar] [CrossRef] [PubMed]
- Bharathan, B.; McGuinness, M.; Kuhar, S.; Kermani, M.; Hassani, F.P.; Sasmito, A.P. Pressure loss and friction factor in non-Newtonian mine paste backfill: Modelling, loop test and mine field data. Powder Technol. 2019, 344, 443–453. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, J.; Ouyang, S.; Deng, X.; Dong, C.; Du, E. Feasibility study and performance optimization of sand-based cemented paste backfill materials. J. Clean. Prod. 2020, 259, 120798. [Google Scholar] [CrossRef]
- Li, B.; Ju, F. An experimental investigation into the compaction characteristic of granulated gangue backfilling materials modified with binders. Environ. Earth. Sci. 2018, 77, 1–9. [Google Scholar] [CrossRef]
- Etim, M.-A.; Babaremu, K.; Lazarus, J.; Omole, D. Health risk and environmental assessment of cement production in Nigeria. Atmosphere 2021, 12, 1111. [Google Scholar] [CrossRef]
- Calderón-Morales, B.R.; García-Martínez, A.; Pineda, P.; García-Tenório, R. Valorization of phosphogypsum in cement-based materials: Limits and potential in eco-efficient construction. J. Build. Eng. 2021, 44, 102506. [Google Scholar] [CrossRef]
- Shi, Y.; Cheng, L.; Tao, M.; Tong, S.; Yao, X.; Liu, Y. Using modified quartz sand for phosphate pollution control in cemented phosphogypsum (PG) backfill. J. Clean. Prod. 2021, 283, 124652. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, L.; Zheng, D.; Yang, F.; Wang, Q.; Wu, W.; Wang, C.; Cang, D. Research progress of geopolymer materials prepared from solid waste and their applications. Sci. Sin. Tech. 2022, 52, 529–546. [Google Scholar] [CrossRef]
- Tian, Z.; Tang, X.; Xiu, Z.; Zhou, H.; Xue, Z. The mechanical properties improvement of environmentally friendly fly ash-based geopolymer mortar using bio-mineralization. J. Clean. Prod. 2022, 332, 130020. [Google Scholar] [CrossRef]
- Karimian, A.; Hassanlourad, M. Mechanical behaviour of MICP-treated silty sand. Bull. Eng. Geol. Environ. 2022, 81, 285. [Google Scholar] [CrossRef]
- Farhadi, S.; Ziadloo, S. Self-healing microbial concrete—A review. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2020; pp. 8–12. [Google Scholar] [CrossRef]
- Proudfoot, D.; Brooks, L.; Gammons, C.H.; Barth, E.; Bless, D.; Nagisetty, R.M.; Lauchnor, E.G. Investigating the potential for microbially induced carbonate precipitation to treat mine waste. J. Hazard. Mater. 2022, 424, 127490. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shahin, M.A. Microbially induced calcite precipitation (MICP) for soil stabilization. In Ecological Wisdom Inspired Restoration Engineering; Springer: Singapore, 2019; pp. 47–68. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Mukherjee, A.; Huang, M.; Achal, V. Biomineralization in metakaolin modified cement mortar to improve its strength with lowered cement content. J. Hazard. Mater. 2017, 329, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, J.; Li, M.; Zhou, N.; Song, W.; Wang, Z.; Qi, S. A preliminary study of solid-waste coal gangue based biomineralization as eco-friendly underground backfill material: Material preparation and macro-micro analyses. Sci. Total Environ. 2021, 770, 145241. [Google Scholar] [CrossRef]
- Yu, X.; He, Z.; Li, X. Bio-cement-modified construction materials and their performances. Environ. Sci. Pollut. Res. 2022, 29, 11219–11231. [Google Scholar] [CrossRef]
- Nasser, A.A.; Sorour, N.M.; Saafan, M.A.; Abbas, R.N. Microbially-Induced-Calcite-Precipitation (MICP): A biotechnological approach to enhance the durability of concrete using Bacillus pasteurii and Bacillus sphaericus. Heliyon 2022, 8, e09879. [Google Scholar] [CrossRef] [PubMed]
- Van Wylick, A.; Monclaro, A.V.; Elsacker, E.; Vandelook, S.; Rahier, H.; De Laet, L.; David, C.; Peeters, E. A review on the potential of filamentous fungi for microbial self-healing of concrete. Fungal Biol. Biotechnol. 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation. Soils Found. 2020, 60, 840–855. [Google Scholar] [CrossRef]
- Tang, X.; Chen, L.; Zhang, G.; Jiang, P. Research and Application of Microbial Cement in Civil Engineering. J. Simul. 2022, 10, 35. [Google Scholar]
- Zeng, C.; Veenis, Y.; Hall, C.A.; Young, E.S.; van Der Star, W.R.; Zheng, J.J.; Van Paassen, L.A. Experimental and numerical analysis of a field trial application of microbially induced calcite precipitation for ground stabilization. J. Geotech. Geoenviron. Eng. 2021, 147, 05021003. [Google Scholar] [CrossRef]
- Song, H.; Kumar, A.; Ding, Y.; Wang, J.; Zhang, Y. Removal of Cd2+ from wastewater by microorganism induced carbonate precipitation (MICP): An economic bioremediation approach. Sep. Purif. Technol. 2022, 297, 121540. [Google Scholar] [CrossRef]
- Wang, M.; Wu, S.; Guo, J.; Zhang, X.; Yang, Y.; Chen, F.; Zhu, R. Immobilization of cadmium by hydroxyapatite converted from microbial precipitated calcite. J. Hazard. Mater. 2019, 366, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Han, B.; Song, H.; Yang, J.; Zhang, L.; Ning, P.; Lin, Z. Nonreductive biomineralization of uranium by Bacillus subtilis ATCC–6633 under aerobic conditions. J. Environ. Radioact. 2019, 208, 106027. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, Z.; Lyu, Q.; Cheng, Y.; Huan, C.; Jiang, X.; Yan, Z.; Tan, Z. Microbiologically induced calcite precipitation for in situ stabilization of heavy metals contributes to land application of sewage sludge. J. Hazard. Mater. 2023, 441, 129866. [Google Scholar] [CrossRef]
- Rajasekar, A.; Wilkinson, S.; Moy, C.K.S. MICP as a potential sustainable technique to treat or entrap contaminants in the natural environment: A review. Environ. Sci. Ecotechnol. 2021, 6, 100096. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y.; Wang, F.; Shi, X.; Yang, Y.; Xu, X.; Huang, Y.; de Wit, B. Experimental study on the mechanical properties and consolidation mechanism of microbial grouted backfill. Int. J. Min. Sci. Technol. 2022, 32, 271–282. [Google Scholar] [CrossRef]
- Gui, R.; Pan, Y.-X.; Ding, D.-X.; Liu, Y.; Zhang, Z.-J. Experimental study on the fine-grained uranium tailings reinforced by MICP. Adv. Civ. Eng. 2018, 2018, 2928985. [Google Scholar] [CrossRef]
- Pinheiro, V.E.; Michelin, M.; Vici, A.C.; de Almeida, P.Z.; Teixeira de Moraes Polizeli, M.d.L. Trametes versicolor laccase production using agricultural wastes: A comparative study in Erlenmeyer flasks, bioreactor and tray. Bioprocess Biosyst. Eng. 2020, 43, 507–514. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Li, M.; Guo, S.; Zhang, J.; Zhu, G. Experimental study of microorganism-induced calcium carbonate precipitation to solidify coal gangue as backfill materials: Mechanical properties and microstructure. Environ. Sci. Pollut. Res. 2022, 29, 45774–45782. [Google Scholar] [CrossRef]
- Yu, A.C.S.; Loo, J.F.C.; Yu, S.; Kong, S.K.; Chan, T.F. Monitoring bacterial growth using tunable resistive pulse sensing with a pore-based technique. Appl. Microbiol. Biotechnol. 2014, 98, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Chen, N.; Chen, J.; Zhou, J.; Li, J.; Wei, Y.; Bu, D. A new species of Desmodesmus sp. from high altitude area was discovered and its isolated, purification, amplification culture and species identification were studied. Preprint 2022. [Google Scholar] [CrossRef]
- An, H.; Fu, R.; Lu, Y.; Kong, D.; Luo, S.; Wang, L.; Lv, F. Effect of heat treatment time on splitting tensile properties of phosphogypsum-based composite cementitious materials. Bull. Silic. 2022, 41, 545–552. [Google Scholar] [CrossRef]
- Yaseri, S.; Mahdikhani, M.; Jafarinoor, A.; Verki, V.M.; Esfandyari, M.; Ghiasian, S.M. The development of new empirical apparatuses for evaluation fresh properties of self-consolidating mortar: Theoretical and experimental study. Constr. Build. Mater. 2018, 167, 631–648. [Google Scholar] [CrossRef]
- Committee, C.N.S. Standard for Geotechnical Testing Method; China Planning Press: Beijing, China, 2019. [Google Scholar]
- Committee, C.N.S. Methods for Determining the Physical and Mechanical Properties of Coal and Rock; China Planning Press: Beijing, China, 2009. [Google Scholar]
- Szalwinski, L.J.; Gonzalez, L.E.; Morato, N.M.; Marsh, B.M.; Cooks, R.G. Bacterial growth monitored by two-dimensional tandem mass spectrometry. Analyst 2022, 147, 940–946. [Google Scholar] [CrossRef]
- Paulton, R.J. The bacterial growth curve. J. Biol. Educ. 1991, 25, 92–94. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, S. High Strength In-Situ Biocementation of Soil by Calcite Precipitating Locally Isolated Ureolytic Bacteria. Ph.D. Dissertation, Murdoch University, Perth, Australia, 2008. Available online: https://researchrepository.murdoch.edu.au/id/eprint/721/ (accessed on 23 October 2023).
- Mempin, R.; Tran, H.; Chen, C.; Gong, H.; Kim Ho, K.; Lu, S. Release of extracellular ATP by bacteria during growth. BMC Microbiol 2013, 13, 301. [Google Scholar] [CrossRef]
- Harkes, M.P.; Van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Yu, X.; Rong, H. Seawater based MICP cements two/one-phase cemented sand blocks. Appl. Ocean Res. 2022, 118, 102972. [Google Scholar] [CrossRef]
- Gebru, K.A.; Kidanemariam, T.G.; Gebretinsae, H.K. Bio-cement production using microbially induced calcite precipitation (MICP) method: A review. Chem. Eng. Sci. 2021, 238, 116610. [Google Scholar] [CrossRef]
- Zhao, J.; Dyer, T.; Csetenyi, L.; Jones, R.; Gadd, G.M. Fungal colonization and biomineralization for bioprotection of concrete. J. Clean. Prod. 2022, 330, 129793. [Google Scholar] [CrossRef]
- Kang, B.; Zha, F.; Li, H.; Xu, L.; Sun, X.; Lu, Z. Bio-mediated method for immobilizing copper tailings sand contaminated with multiple heavy metals. Crystals 2022, 12, 522. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Wang, G.; Wang, P.; Zheng, J.-J.; Yan, J.; Xu, L.; Yan, Y. Effect of wool fiber addition on the reinforcement of loose sands by microbially induced carbonate precipitation (MICP): Mechanical property and underlying mechanism. Acta Geotech. 2021, 16, 1401–1416. [Google Scholar] [CrossRef]
- Rui, Y.; Qian, C. Characteristics of different bacteria and their induced biominerals. J. Ind. Eng. Chem. 2022, 115, 449–465. [Google Scholar] [CrossRef]
- Zehner, J.; Røyne, A.; Sikorski, P. Calcite seed-assisted microbial induced carbonate precipitation (MICP). PLoS ONE 2021, 16, e0240763. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Satyam, N.; Reddy, K.R. Investigation of various gram-positive bacteria for MICP in Narmada Sand, India. Int. J. Geotech. Eng. 2021, 15, 220–234. [Google Scholar] [CrossRef]
- Zheng, T.; Hou, D.; Leng, W.; Li, P.; Wei, W. Preparation, characterization, and formation mechanism of different biological calcium carbonate (CaCO3) induced by Bacillus mucilaginosus and Bacillus alcalophilus. J. Nanopart. Res. 2023, 25, 189. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X. Microbially induced calcite precipitation and synergistic mineralization cementation mechanism of Pisha sandstone components. Sci. Total Environ. 2023, 866, 161348. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Encinas, N.; Yang, C.-Y.; Geyer, F.; Kaltbeitzel, A.; Baumli, P.; Reinholz, J.; Mailänder, V.; Butt, H.-J.R.; Vollmer, D. Submicrometer-sized roughness suppresses bacteria adhesion. ACS Appl. Mater. Interfaces 2020, 12, 21192–21200. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]



| Chemical Composition (%) | K2O | Na2O | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | MnO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Coal gangue | 1.47 | 0.14 | 80.57 | 6.74 | 2.32 | 2.08 | 0.85 | 0.28 | 0.06 | 0.04 |
| Fly ash | 2.11 | 0.65 | 49.17 | 28.28 | 4.63 | 3.55 | 0.84 | 1.12 | 0.06 | 0.31 |
| Components | Reference_0 | FA Group_1 | CG Group_2 |
|---|---|---|---|
| CG (g) | 0 | 0 | 1 |
| FA (g) | 0 | 1 | 0 |
| Culture medium (mL) | 5 | 5 | 5 |
| B. pasteurii (mL) | 0.1 | 0.1 | 0.1 |
| Mortar | Compressive Strength (KPa) | Spread after 10 s (mm) |
|---|---|---|
| A | / | 100 |
| B | 471 | 243 |
| C | 88.22 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Fantilli, A.P.; Yan, H.; Sun, K.; Ding, L. Novel Understandings of Biomineralization in Backfill Materials: A Fundamental Investigation of Coal Gangue and Fly Ash Impact on B. pasteurii to Enhance Material Properties. Appl. Sci. 2024, 14, 799. https://doi.org/10.3390/app14020799
Guo S, Fantilli AP, Yan H, Sun K, Ding L. Novel Understandings of Biomineralization in Backfill Materials: A Fundamental Investigation of Coal Gangue and Fly Ash Impact on B. pasteurii to Enhance Material Properties. Applied Sciences. 2024; 14(2):799. https://doi.org/10.3390/app14020799
Chicago/Turabian StyleGuo, Shijie, Alessandro Pasquale Fantilli, Hao Yan, Kai Sun, and Luwei Ding. 2024. "Novel Understandings of Biomineralization in Backfill Materials: A Fundamental Investigation of Coal Gangue and Fly Ash Impact on B. pasteurii to Enhance Material Properties" Applied Sciences 14, no. 2: 799. https://doi.org/10.3390/app14020799
APA StyleGuo, S., Fantilli, A. P., Yan, H., Sun, K., & Ding, L. (2024). Novel Understandings of Biomineralization in Backfill Materials: A Fundamental Investigation of Coal Gangue and Fly Ash Impact on B. pasteurii to Enhance Material Properties. Applied Sciences, 14(2), 799. https://doi.org/10.3390/app14020799







