Effects of Photobiomodulation Using Near-Infrared Light on the Dentin and Periodontal Ligament in a Beagle Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Photobiomodulation (PBM)
2.3. Micro-Computed Tomography (Micro-CT)
2.4. Histological Procedure
2.5. Scanning Electron Microscopy (SEM)
3. Results
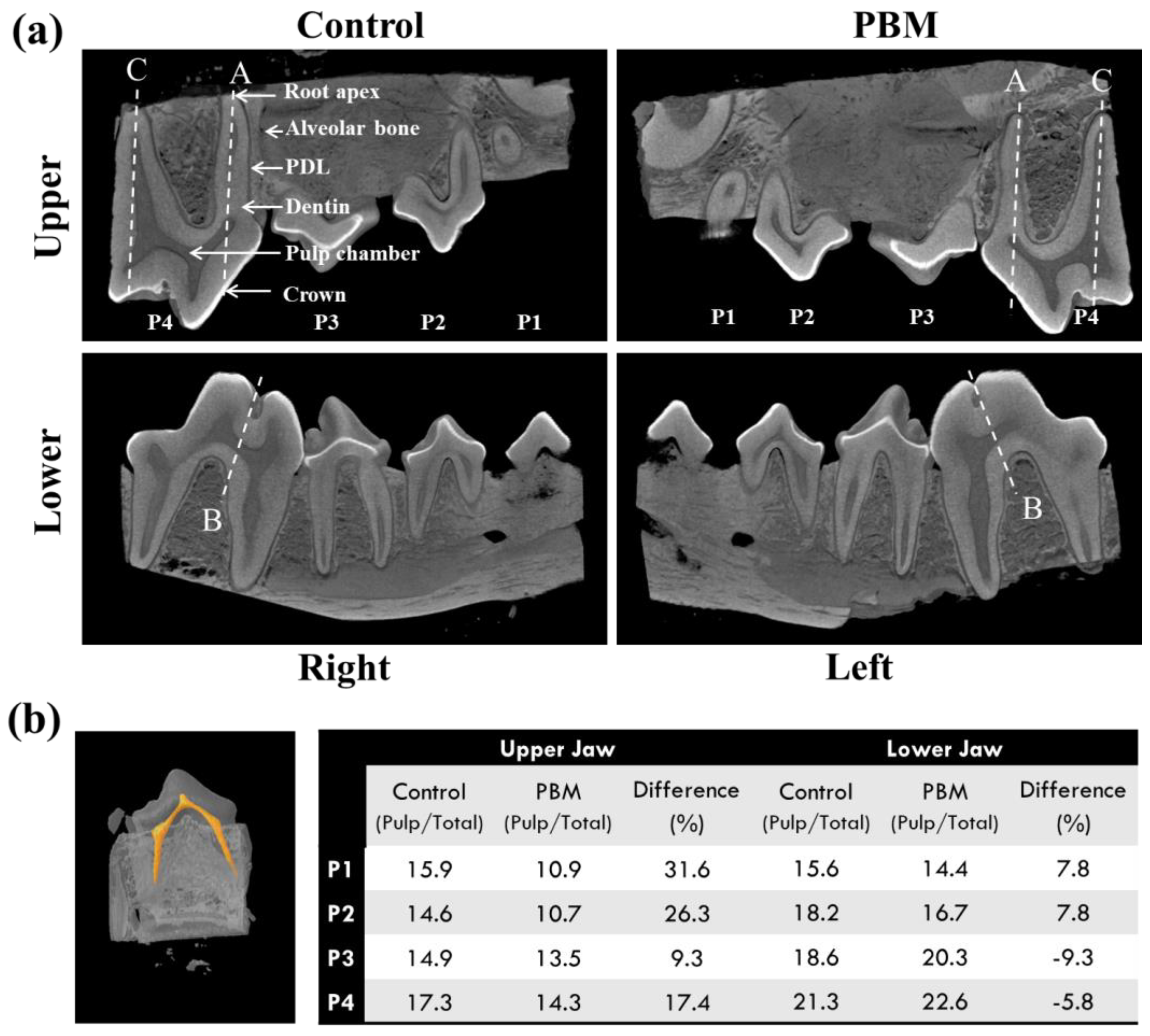
3.1. Micro-Computed Tomography (CT)
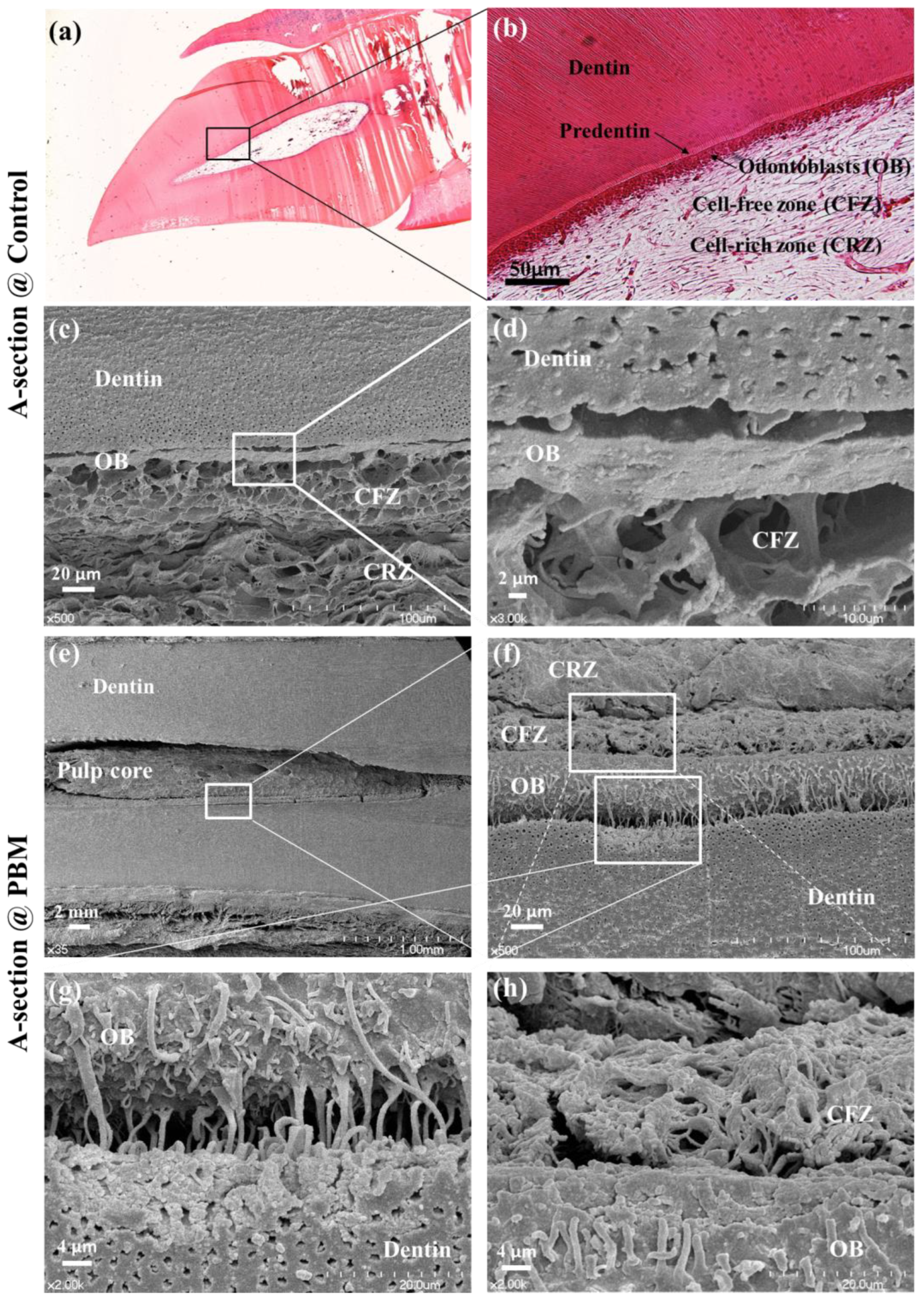
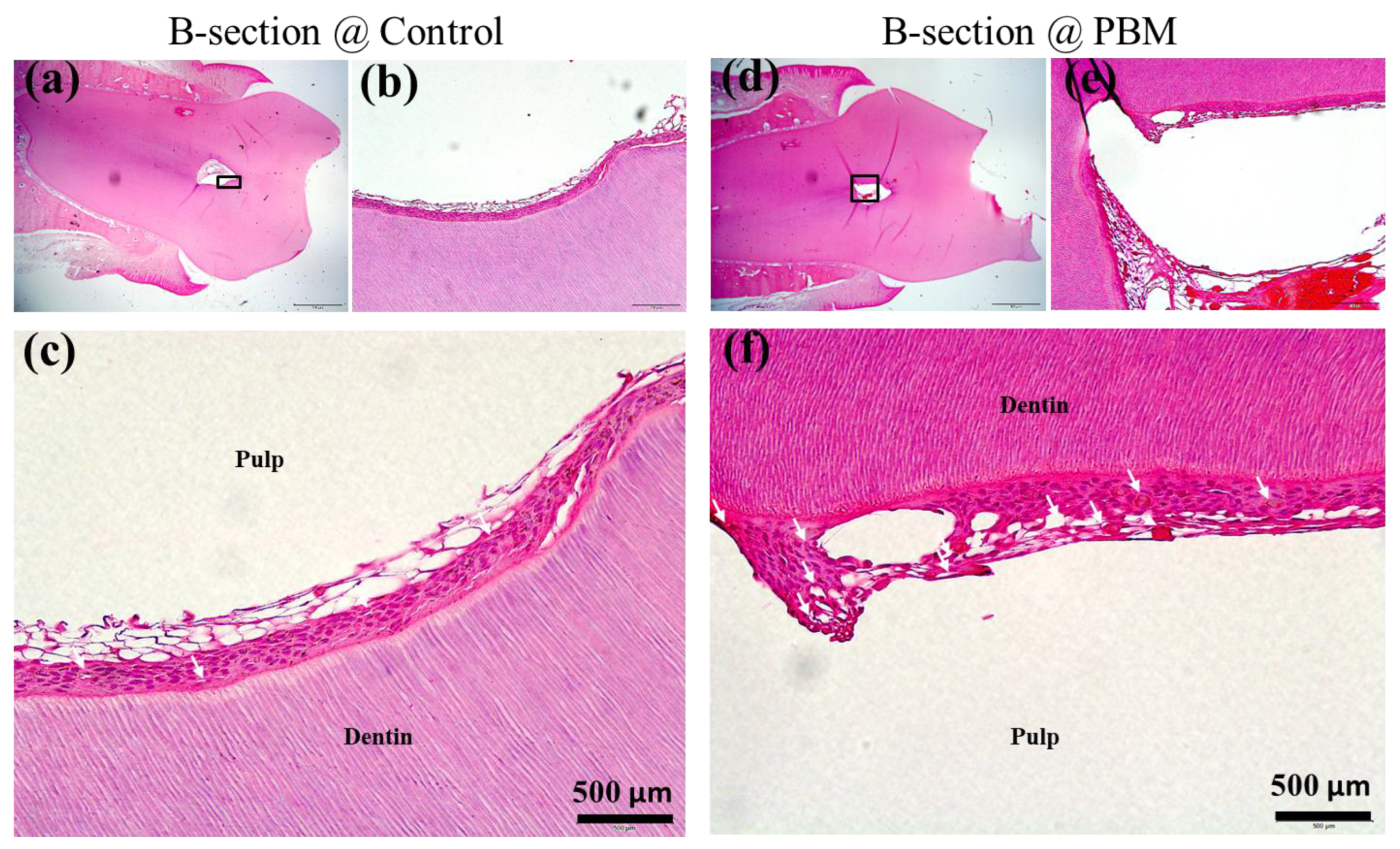
3.2. Structural Analysis of Dentin-Pulp Interface
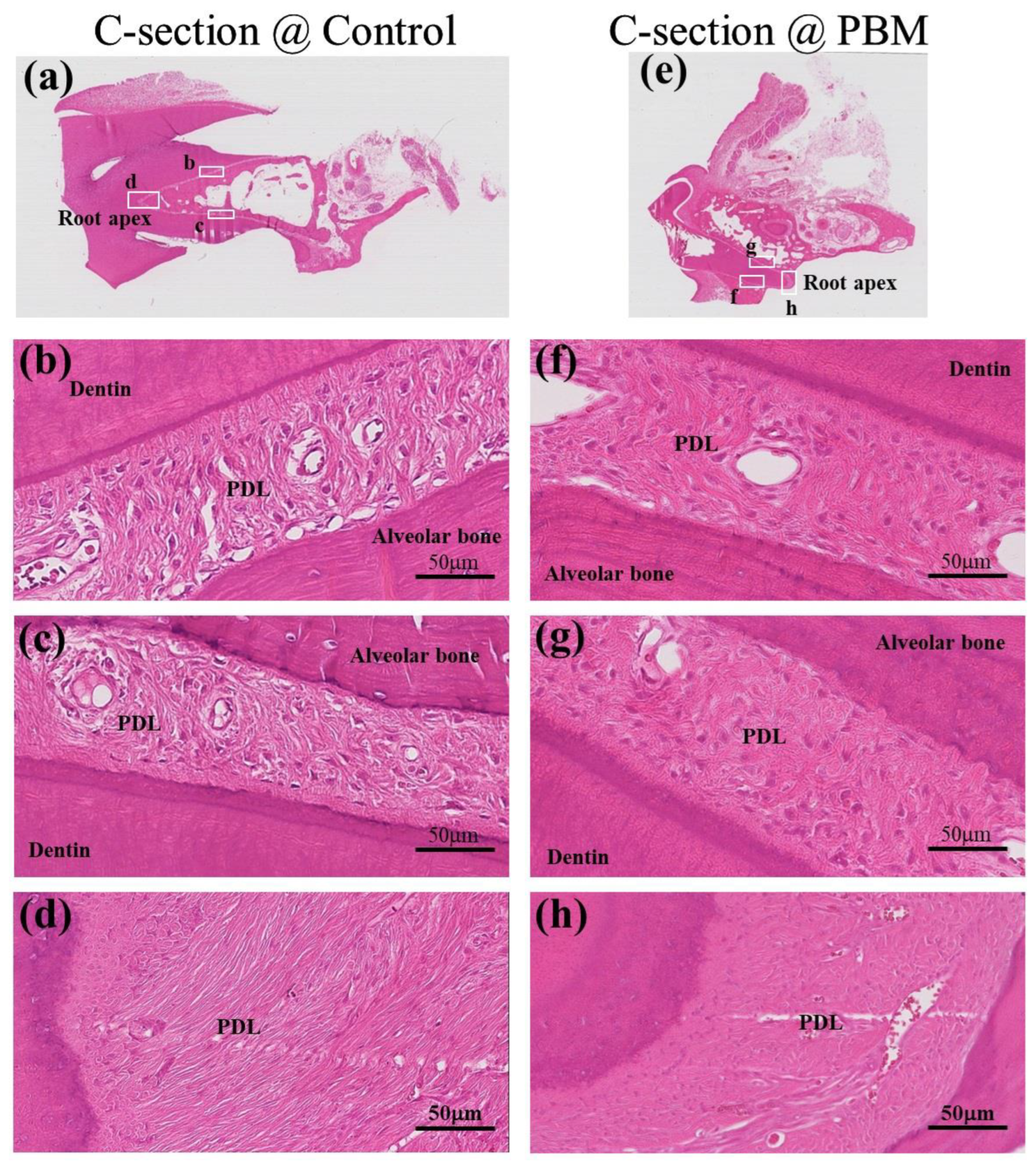
3.3. Structural Analysis of Periodontal Ligament (PDL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.H.; Jin, T.; Yu, Q.; Chen, F.M. Biological approaches toward dental pulp regeneration by tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e1–e16. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Gay, I.C.; Chen, S.; MacDougall, M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofac Res. 2007, 10, 149–160. [Google Scholar] [CrossRef]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef]
- Morsczeck, C.; Moehl, C.; Gotz, W.; Heredia, A.; Schaffer, T.E.; Eckstein, N.; Sippel, C.; Hoffmann, K.H. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol. Int. 2005, 29, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra69. [Google Scholar] [CrossRef]
- Mester, E.; Ludany, G.; Sellyei, M.; Szende, B. On the biologic effect of laser rays. Bull. Soc. Int. Chir. 1968, 27, 68–73. [Google Scholar]
- Yang, D.; Yi, W.; Wang, E.; Wang, M. Effects of light-emitting diode irradiation on the osteogenesis of human umbilical cord mesenchymal stem cells in vitro. Sci. Rep. 2016, 6, 37370. [Google Scholar] [CrossRef]
- Tuby, H.; Maltz, L.; Oron, U. Induction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Lasers Surg. Med. 2011, 43, 401–409. [Google Scholar] [CrossRef]
- Hashmi, J.T.; Huang, Y.Y.; Sharma, S.K.; Kurup, D.B.; De Taboada, L.; Carroll, J.D.; Hamblin, M.R. Effect of pulsing in low-level light therapy. Lasers Surg. Med. 2010, 42, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Mvula, B.; Mathope, T.; Moore, T.; Abrahamse, H. The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med. Sci. 2008, 23, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.F.; Zhang, H.; Yuan, X.; Li, J.; Wei, Y.J.; Hu, S.S. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: Proliferation, growth factors secretion and myogenic differentiation. Lasers Surg. Med. 2008, 40, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Benayahu, D.; Maltz, L.; Oron, U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed. Laser Surg. 2005, 23, 161–166. [Google Scholar] [CrossRef]
- Jones, D.L.; Wagers, A.J. No place like home: Anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008, 9, 11–21. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Kim, H.B.; Baik, K.Y.; Choung, P.H.; Chung, J.H. Pulse frequency dependency of photobiomodulation on the bioenergetic functions of human dental pulp stem cells. Sci. Rep. 2017, 7, 15927. [Google Scholar] [CrossRef]
- Verma, P.; Love, R.M. A micro CT study of the mesiobuccal root canal morphology of the maxillary first molar tooth. Int. Endod. J. 2011, 44, 210–217. [Google Scholar] [CrossRef]
- Egbuniwe, O.; Idowu, B.D.; Funes, J.M.; Grant, A.D.; Renton, T.; Di Silvio, L. P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell Cycle 2011, 10, 3912–3919. [Google Scholar] [CrossRef]
- Massler, M.; Schour, I. The appositional life span of the enamel and dentin-forming cells; human deciduous teeth and first permanent molars; introduction. J. Dent. Res. 1946, 25, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; De Bari, C.; About, I. Apoptosis in developmental and repair-related human tooth remodeling: A view from the inside. Exp. Cell Res. 2008, 314, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Park, J.W.; Seo, D.G. Intratubular crystal formation in the exposed dentin from nano-sized calcium silicate for dentin hypersensitivity treatment. Sci. Rep. 2023, 13, 14243. [Google Scholar] [CrossRef]
- Abuzinadah, S.H.; Alhaddad, A.J. A randomized clinical trial of dentin hypersensitivity reduction over one month after a single topical application of comparable materials. Sci. Rep. 2021, 11, 6793. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; Lussi, A.; Seong, J.; Hellwig, E. Dentin hypersensitivity: Pain mechanisms and aetiology of exposed cervical dentin. Clin. Oral. Investig. 2013, 17, S9–S19. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem cell-based bone and dental regeneration: A view of microenvironmental modulation. Int. J. Oral. Sci. 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, J.; Chen, M.; Lin, C.S.; Kim, S.G.; Zhou, Y.; Xiang, L.; Xie, M.; Bai, H.; Yao, H.; et al. Parenchymal and stromal tissue regeneration of tooth organ by pivotal signals reinstated in decellularized matrix. Nat. Mater. 2019, 18, 627–637. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohshima, H. Distribution and organization of peripheral capillaries in dental pulp and their relationship to odontoblasts. Anat. Rec. 1996, 245, 313–326. [Google Scholar] [CrossRef]
- Mir, M.; Mojahedi, S.M.; Tuner, J.; Shabani, M.; Darabi, F.; Rohban, A. The effectiveness of home-use photobiomodulation toothbrush for treating dentin hypersensitivity: A pilot study. Laser Ther. 2019, 28, 193–198. [Google Scholar] [CrossRef]
- Barros, A.P.O.; de Melo Alencar, C.; de Melo Pingarilho Carneiro, A.; da Silva Pompeu, D.; Barbosa, G.M.; Araujo, J.L.N.; Silva, C.M. Combination of two desensitizing protocols to control dentin hypersensitivity in non-carious lesions: A randomized, double-blind clinical trial. Clin. Oral. Investig. 2022, 26, 1299–1307. [Google Scholar] [CrossRef]
- Fossati, A.L.; Sobral, A.P.T.; Bruno, M.L.L.H.; Viarengo, N.O.; Sertaje, M.R.F.; Santos, E.M.; Gonçalves, M.L.L.; Ferrari, R.A.M.; Fernandes, K.P.S.; Horliana, A.C.R.T.; et al. Photobiomodulation and glass ionomer sealant as complementary treatment for hypersensitivity in molar incisor hypomineralisation in children: Protocol for a blinded randomised clinical trial. BMJ Open 2023, 13, e068102. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Groger, S.; Wu, Z.; Ruf, S.; Ye, Y.; Chen, X. Photobiomodulation Therapy and Pulp-Regenerative Endodontics: A Narrative Review. Bioengineering 2023, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhairy, F.; Vohra, F.; Naseem, M.; Ahmad, Z.H. Adhesive bond integrity of dentin conditioned by photobiomodulation and bonded to bioactive restorative material. Photodiagn. Photodyn. Ther. 2019, 28, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Baik, K.Y.; Seonwoo, H.; Jang, K.J.; Lee, M.C.; Choung, P.H.; Chung, J.H. Effects of pulsing of light on the dentinogenesis of dental pulp stem cells in vitro. Sci. Rep. 2018, 8, 2057. [Google Scholar] [CrossRef]
- Kim, H.B.; Kang, M.H.; Baik, K.Y.; Kim, J.E.; Park, S.B.; Choung, P.H.; Chung, J.H. Integration of blue light with near-infrared irradiation accelerates the osteogenic differentiation of human dental pulp stem cells. J. Photochem. Photobiol. B 2023, 245, 112752. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.B.; Baik, K.Y.; Kang, M.H.; Chung, J.H. Effects of Photobiomodulation Using Near-Infrared Light on the Dentin and Periodontal Ligament in a Beagle Model. Appl. Sci. 2024, 14, 724. https://doi.org/10.3390/app14020724
Kim HB, Baik KY, Kang MH, Chung JH. Effects of Photobiomodulation Using Near-Infrared Light on the Dentin and Periodontal Ligament in a Beagle Model. Applied Sciences. 2024; 14(2):724. https://doi.org/10.3390/app14020724
Chicago/Turabian StyleKim, Hong Bae, Ku Youn Baik, Moon Ho Kang, and Jong Hoon Chung. 2024. "Effects of Photobiomodulation Using Near-Infrared Light on the Dentin and Periodontal Ligament in a Beagle Model" Applied Sciences 14, no. 2: 724. https://doi.org/10.3390/app14020724
APA StyleKim, H. B., Baik, K. Y., Kang, M. H., & Chung, J. H. (2024). Effects of Photobiomodulation Using Near-Infrared Light on the Dentin and Periodontal Ligament in a Beagle Model. Applied Sciences, 14(2), 724. https://doi.org/10.3390/app14020724






