Impact of Different Solvents and Temperatures on the Extraction of Bioactive Compounds from Rose Fruits (Rosa rugosa) Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Rose Fruits Juice on a Laboratory Scale
2.4. Freeze-Drying of Rose Fruits Pomace
2.5. Preparation of Extracts from Rose Fruits Pomace Using Classic Extraction by Shaking with a Solvent
2.6. Determination of Total Phenolic Content
2.7. Determinations of L-Ascorbic Acid (AA) Content
2.8. Statistical Analysis
3. Results and Discussion
3.1. Impact of Process Variables on the Extraction of Total Phenolic Compounds (TPC) from Rose Fruits (Rosa rugosa) Pomace
3.2. Impact of Process Variables on the Extraction of L-Ascorbic Acid (AA) from Rose Fruits (Rosa rugosa) Pomace
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards circular economy in the food system. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Valorization of fruit wastes for circular bioeconomy: Current advances, challenges, and opportunities. Bioresour. Technol. 2022, 359, 127459. [Google Scholar] [CrossRef] [PubMed]
- Gumul, D.; Berski, W.; Zięba, T. The Influence of Fruit Pomaces on Nutritional, Pro-Health Value and Quality of Extruded Gluten-Free Snacks. Appl. Sci. 2023, 13, 4818. [Google Scholar] [CrossRef]
- Vagiri, M.; Jensen, M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017, 217, 409–417. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crop. Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Cybulska, J.; Mierczyńska, J.; Pieczywek, P.; Stasiak, M.; Zdunek, A. Effect of divalent metal ions on rheologigal properties of polysaccharide matrix from apple pomace. Żywność. Nauka. Technologia. Jakość 2015, 2, 103–113. (In Polish) [Google Scholar] [CrossRef]
- Raczkowska, E.; Nowicka, P.; Wojdyło, A.; Styczyńska, M.; Lazar, Z. Chokeberry Pomace as a Component Shaping the Content of Bioactive Compounds and Nutritional, Health-Promoting (Anti-Diabetic and Antioxidant) and Sensory Properties of Shortcrust Pastries Sweetened with Sucrose and Erythritol. Antioxidants 2022, 11, 190. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Bebak, A. Biological activity of selected and vegetable pomaces. Żywność. Nauka. Technologia. Jakość 2012, 4, 55–65. (In Polish) [Google Scholar]
- Klewicka, E.; Piekarska-Radzik, L.; Milala, J.; Klewicki, R.; Sójka, M.; Rosół, N.; Otlewska, A.; Matysiak, B. Antagonistic Activity of Lactic Acid Bacteria and Rosa rugosa Thunb. Pseudo-Fruit Extracts against Staphylococcus spp. Strains. Appl. Sci. 2022, 12, 4005. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Therapeutic applications of rose hips from different Rosa species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef]
- Cendrowski, A.; Królak, M.; Kalisz, S. Polyphenols, L-Ascorbic Acid, and Antioxidant Activity in Wines from Rose Fruits (Rosa rugosa). Molecules 2021, 26, 2561. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Brindza, J.; Ivanišová, E.; Juríková, T.; Schwarzová, M.; Horcinová Sedlácková, V.; Grygorieva, O. Morphological and antiradical characteristics of rugosa rose (Rosa rugosa Thunb.) Fruits canned in different kind ofhoneys and in beverages prepared from honey Potravinarstvo. Slovak. J. Food Sci. 2019, 13, 497–506. [Google Scholar] [CrossRef]
- Lu, J.; Wang, C. Medicinal components and pharmacological effects of Rosa rugosa. Rec. Nat. Prod. 2018, 15, 535–543. [Google Scholar] [CrossRef]
- Feng, L.; Chen, C.; Sheng, L.; Liu, P.; Tao, J.; Su, J.; Zhao, L. Comparative Analysis of Headspace Volatiles of Chinese Rosa rugosa. Molecules 2010, 15, 8390–8399. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Los, R.; Rzymowska, J.; Malm, A.; Chrusciel, K. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Cent. Eur. J. Biol. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- Cendrowski, A.; Kalisz, S.; Mitek, M. Properties and Application of Rose Hips in Food Processing. Żywność. Nauka. Technologia. Jakość 2012, 4, 24–31. (In Polish) [Google Scholar] [CrossRef]
- Dashbaldan, S.; Rogowska, A.; Paczkowski, C.; Szakiel, A. Distribution of Triterpenoids and Steroids in Developing Rugosa Rose (Rosa rugosa Thunb.) Accessory Fruit. Molecules 2021, 26, 5158. [Google Scholar] [CrossRef]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Kilinc, K.; Yaman, S.O.; Misir, S.; Kara, H.; Genc, B.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression. J. Pharm. Anal. 2018, 8, 394–399. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E.; Milala, J.; Klewicki, R.; Rosół, N.; Matysiak, B.; Sójka, M.; Markowski, J. Impact of polyphenols from Rosa rugosa Thunb. Pseudofruits pomace on growth of Lactobacillus bacteria. Żywność. Nauka. Technologia. Jakość 2019, 26, 73–87. (In Polish) [Google Scholar] [CrossRef]
- Yi, O.; Jovel, E.M.; Towers, G.H.; Wahbe, T.R.; Cho, D. Antioxidant and antimicrobial activities of native Rosa sp. from British Columbia, Canada. Int. J. Food Sci. Nutr. 2007, 58, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xue, P.; Sun, X.; Zhao, G. Determination of the volatile and polyphenol constituents and the antimicrobial, antioxidant, and tyrosinase inhibitory activities of the bioactive compounds from the by-product of Rosa rugosa Thunb. var. plena Regal tea. BMC Complement. Altern. Med. 2018, 18, 307. [Google Scholar] [CrossRef]
- Kamijo, M. Effects of Rosa rugosa Petals on Intestinal Bacteria. Biosci. Biotechnol. Biochem. 2007, 72, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trac. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Dailey, A.; Vuong, Q.V. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 2015, 1, 1115646. [Google Scholar] [CrossRef]
- Shalmashi, A.; Eliassi, A. Solubility of l-(+)-ascorbic acid in water, ethanol, methanol, propan-2-ol, acetone, acetonitrile, ethyl acetate, and tetrahydrofuran from (293 to 323) K. J. Chem. Eng. Data 2008, 53, 1332–1334. [Google Scholar] [CrossRef]
- Azrie, A.M.; Chuah, A.L.; Pin, K.Y.; Tan, H.P. Effect of solvents on the extraction of Kacip Fatimah (Labisia pumila) leaves. J. Chem. Pharm. Res. 2014, 6, 172–176. [Google Scholar]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Brunton, N. The optimisation of solid–liquid extraction of antioxidants from apple pomace by response surface methodology. J. Food Eng. 2010, 96, 134–140. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Suryadi, I.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.K.; Ng, S.Y.; Thoo, Y.Y.; Khoo, M.Z.; Wan, A.W.M.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. Int. Food Res. J. 2011, 18, 571–578. [Google Scholar]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Monograph 04/2022:50400; European Pharmacopoeia 11.4. EDQM: Strasbourg, France, 2022; pp. 5525–5532.
- Gao, X.; Ohlander, M.; Jeppson, N.; Bjork, L.; Trajkovski, V. Changes in antioxidant effect and their relationship to phytonutrients in fruit of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative Evaluation of UV-HPLC Methods and Reducing Agents to Determine Vitamin C in Fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Dent, M.; Dragovic-Uzelac, V.; Penic, M.; Brncic, M.; Bosiljkov, T.; Levaj, B. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage Extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Amendola, D.; Maggi, L.; Zalacain, A.; De Faveri, D.M.; Spigno, G. Microwave-assisted extraction of phenolic compounds from dried waste grape skins. Int. J. Food Eng. 2015, 11, 359–370. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Sielicka, M.; Pawlak, S. The influence of the solvent used for extraction on the determined content of phenolic compounds and the antioxidant activity of chokeberry pomace. In Management of By-Products of the Food Industry (Red. Górecka D, Pospiech E); Uniwersytet Przyrodniczy: Wrocław, Poland, 2016; pp. 25–35. ISBN 978-83-7160-836-0. (In Polish) [Google Scholar]
- Um, M.; Han, T.-H.; Lee, J.-W. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa Thunb.) fruit. Food Sci. Biotechnol. 2017, 27, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Drożdż, W.; Tomaszewska-Ciosk, E.; Zdybel, E.; Boruczkowska, H.; Boruczkowski, T.; Regiec, P. Effect of apple and rosehip pomaces on colour, total phenolics and antioxidant activity of corn extruded snacks. Pol. J. Chem. Technol. 2014, 16, 7–11. [Google Scholar] [CrossRef]
- Ilbay, Z.; Sahin, S.; Kirbaslar, S.I. Investigation of polyphenolic content of rose hip (Rosa canina L.) tea extracts: A comparative study. Foods 2013, 2, 43–52. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q.V. Impact of different solvents on the recovery of bioactive compounds and antioxidant properties from lemon (Citrus limon L.) pomace waste. Food Sci. Biotechnol. 2016, 25, 971–977. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, X.; Hao, Y.; Xu, G.; Xu, G.; Liu, D. Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrason. Sonochem. 2008, 15, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Hsu, Y.S.; Ho, C.T. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J. Food Drug Anal. 2014, 22, 290–295. [Google Scholar] [CrossRef]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the solvents on the extraction of major phenolic compounds (punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef]
- Cendrowski, A.; Ścibisz, I.; Kieliszek, M.; Kolniak-Ostek, J.; Mitek, M. UPLC-PDA-Q/TOF-MS Profile of Polyphenolic Compounds of Liqueurs from Rose Petals (Rosa rugosa). Molecules 2017, 22, 1832. [Google Scholar] [CrossRef]
- Marston, A.; Hostettmann, K. Separation and quantification of flavonoids. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; Taylor & Francis: Abingdon, UK, 2005. [Google Scholar]
- Abad-García, B.; Berrueta, L.A.; López-Márquez, D.M.; Crespo-Ferrer, I.; Gallo, B.; Vicente, F. Optimization and validation of a methodology based on solvent extraction and liquid chromatography for the simultaneous determination of several polyphenolic families in fruit juices. J. Chromatogr. A 2007, 1154, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological Significance of Ascorbic Acid (Vitamin C) in Human Health—A Review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar] [CrossRef]
- Milala, J.; Sójka, M.; Król, K.; Buczek, M. Charakterystyka składu chemicznego owoców Rosa pomifera “Karpatia”. Żywność. Nauka. Technologia. Jakość 2013, 5, 154–167. (In Polish) [Google Scholar]
- Adamczak, A.; Buchwald, W.; Zieliński, J.; Mielcarek, S. Flavonoid and organic acid content in rose hips (Rosa L., Sect. Caninae Dc. Em. Christ.). Acta Biol. Cracoviensia 2012, 54, 1–8. [Google Scholar] [CrossRef]
- Kayahan, S.; Ozdemir, Y.; Gulbag, F. Functional compounds and antioxidant activity of Rosa Species Grown in Turkey. Erwerbs-Obstbau 2023, 65, 1079–1086. [Google Scholar] [CrossRef]
- Paunović, D.; Kalušević, A.; Petrović, T.; Urošević, T.; Djinović, D.; Nedović, V.; Popović-Djordjević, J. Assessment of chemical and antioxidant properties of fresh and dried rosehip (Rosa canina L.). Not. Bot. Horti Agrobot. 2019, 47, 108–113. [Google Scholar] [CrossRef]
- Paul, R.; Ghosh, U. Effect of thermal treatment on ascorbic acid content of pomegranate juice. Indian J. Biotechnol. 2012, 11, 309–313. [Google Scholar]
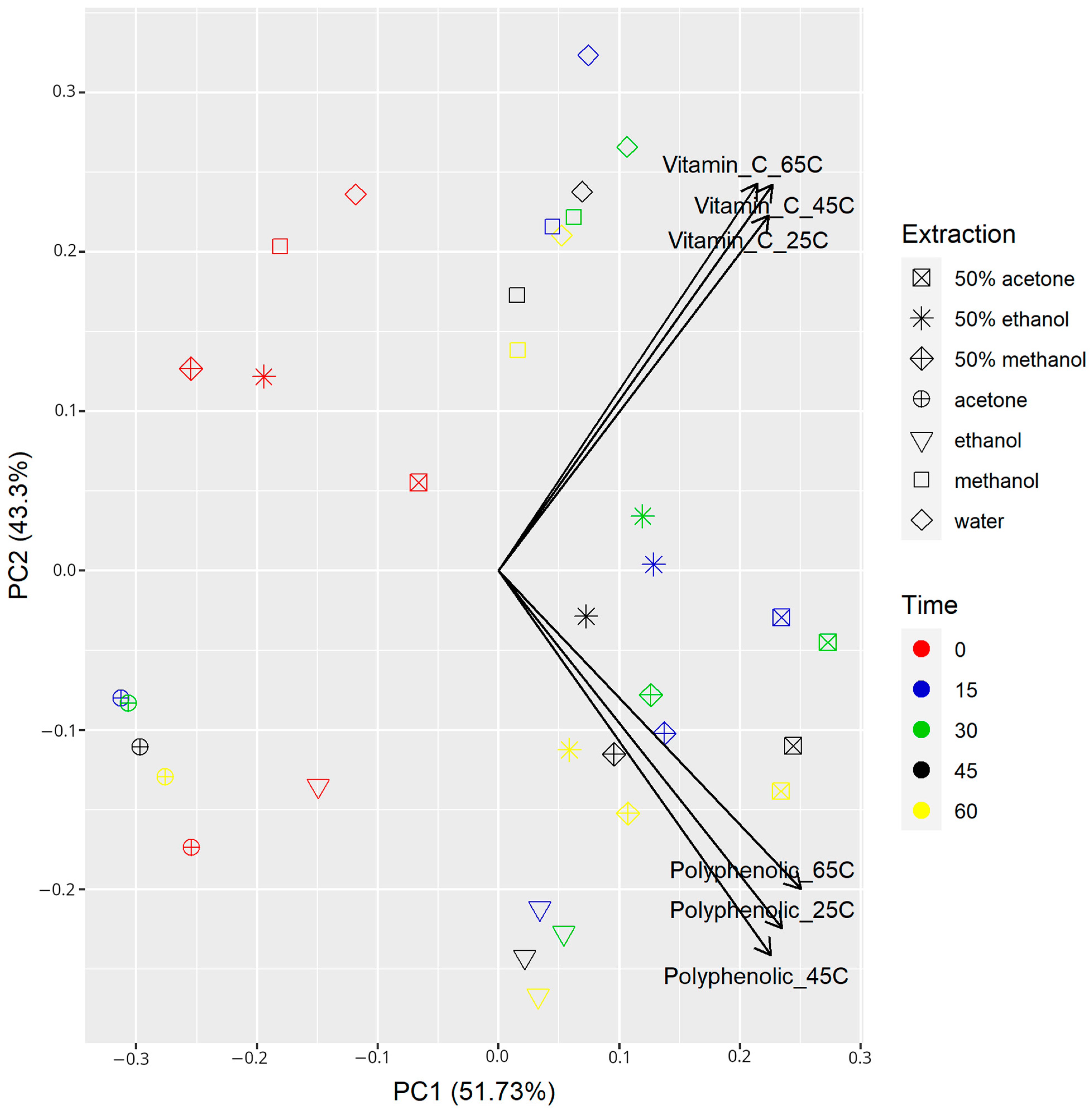
| Temp (°C) | Time (min) | Solvents | |||
|---|---|---|---|---|---|
| Water | Methanol | Ethanol | Acetone | ||
| TPC (mg GAE/g of Freeze-Dried Rose Fruits Pomace) | |||||
| 25 °C | 15 | 9.85 ± 0.40 Aa | 16.83 ± 0.18 Bb | 26.98 ± 0.39 Bc | 12.93 ± 0.22 BCb |
| 25 °C | 30 | 17.08 ± 0.62 Cb | 17.13 ± 0.13 Bb | 30.40 ± 0.41 Bc | 12.70 ± 0.51 BCa |
| 25 °C | 45 | 11.70 ± 0.72 Ba | 15.35 ± 0.62 Bb | 28.39 ± 0.24 Bc | 12.40 ± 0.33 Ba |
| 25 °C | 60 | 12.77 ± 0.69 Ba | 17.47 ± 0.75 Bb | 30.78 ± 0.27 Bc | 11.82 ± 0.29 Ba |
| 45 °C | 15 | 11.95 ± 0.71 Ba | 15.94 ± 0.72 Bb | 31.10 ± 1.12 BCc | 9.88 ± 0.52 Aa |
| 45 °C | 30 | 14.63 ± 0.08 Bb | 17.92 ± 0.66 Bc | 34.18 ± 0.35 Cd | 10.58 ± 0.42 Ba |
| 45 °C | 45 | 13.62 ± 0.13 Ca | 15.78 ± 0.58 Bb | 33.49 ± 0.24 Cc | 15.51 ± 0.41 Cb |
| 45 °C | 60 | 15.75 ± 0.15 Ca | 18.17 ± 0.99 BCa | 35.56 ± 0.48 Db | 15.33 ± 0.30 Ca |
| 65 °C | 15 | 21.36 ± 0.26 Db | 17.59 ± 0.61 Ba | 36.27 ± 0.26 Dc | 14.75 ± 0.69 Ca |
| 65 °C | 30 | 20.84 ± 0.26 Db | 17.17 ± 0.59 Ba | 33.55 ± 0.51 Cc | 15.42 ± 0.31 Ca |
| 65 °C | 45 | 25.94 ± 0.25 Dc | 19.97 ± 0.79 Cb | 33.80 ± 0.37 Cd | 14.86 ± 0.72 Ca |
| 65 °C | 60 | 23.26 ± 0.55 Db | 18.90 ± 0.47 Ca | 33.09 ± 0.46 Cc | 20.36 ± 0.48 Da |
| Reference sample | 9.34 ± 0.66 Aa | 7.94 ± 0.19 Aa | 12.09 ± 0.26 Ab | 8.16 ± 0.61 Aa | |
| Temp (°C) | Time (min) | Solvents | ||
|---|---|---|---|---|
| 50% Methanol | 50% Ethanol | 50% Acetone | ||
| TPC (mg GAE/g of Freeze-Dried Rose Fruits Pomace) | ||||
| 25 °C | 15 | 27.81± 0.34 Ba | 27.26 ± 0.25 Ca | 31.63 ± 0.66 Bb |
| 25 °C | 30 | 28.90 ± 0.39 Bb | 21.55 ± 0.44 Ba | 36.79 ± 0.60 Cc |
| 25 °C | 45 | 27.40 ± 0.26 Ba | 22.38 ± 0.24 Ba | 36.21 ± 0.31 Cb |
| 25 °C | 60 | 27.99 ± 0.17 Ba | 24.56 ± 0.35 Ba | 36.83 ± 0.13 Cb |
| 45 °C | 15 | 30.70 ± 0.23 Cb | 26.11 ± 0.20 Ba | 31.80 ± 0.58 Bb |
| 45 °C | 30 | 32.97 ± 0.25 Cb | 26.51 ± 0.42 Ba | 37.35 ± 0.45 CDc |
| 45 °C | 45 | 31.84 ± 0.15 Cb | 26.40 ± 0.43 Ba | 32.93 ± 0.31 Bb |
| 45 °C | 60 | 33.89 ± 0.51 Cb | 28.93 ± 0.43 BCa | 34.33 ± 0.93 Bb |
| 65 °C | 15 | 36.92 ± 0.52 Cb | 29.57 ± 0.19 Ca | 36.05 ± 0.57 Cb |
| 65 °C | 30 | 29.28 ± 0.34 BCa | 30.92 ± 0.41 Ca | 34.33 ± 0.69 Bb |
| 65 °C | 45 | 32.02 ± 0.52 Ca | 30.66 ± 0.24 Ca | 39.60 ± 0.35 Db |
| 65 °C | 60 | 34.65 ± 0.34 Ca | 32.90 ± 0.12 Ca | 39.12 ± 0.62 Da |
| Reference sample | 7.61 ± 0.16 Aa | 10.25 ± 0.22 Ab | 17.82 ± 0.25 Ac | |
| Source | Degrees of Freedom | Sum of Square | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Main effects | |||||
| temperature | 1 | 554 | 554.1 | 243.024 | <0.0001 |
| time | 4 | 7082 | 1770.5 | 776.535 | <0.0001 |
| solvent | 6 | 10,178 | 1696.3 | 744.005 | <0.0001 |
| temperature:time | 4 | 200 | 50.1 | 21.972 | <0.0001 |
| temperature:solvent | 6 | 163 | 27.2 | 11.915 | <0.0001 |
| time:solvent | 24 | 1477 | 61.5 | 26.993 | <0.0001 |
| temperature:time:solvet | 24 | 209 | 8.7 | 3.817 | <0.0001 |
| Residuals | 140 | 4648 | 33.2 |
| Temp (°C) | Time (min) | Solvents | |||
|---|---|---|---|---|---|
| Water | Methanol | Ethanol | Acetone | ||
| AA (mg/g Rose Fruit Pomace) | |||||
| 25 °C | 15 | 31.76 ± 1.89 Bd | 26.43 ±1.02 Ac | 10.67 ± 0.18 Bb | 3.01 ± 0.11 ABa |
| 25 °C | 30 | 33.75 ± 0.64 Bc | 28.33 ± 0.38 ABc | 11.39 ± 0.75 Ab | 3.32 ± 0.19 Ba |
| 25 °C | 45 | 35.54 ± 0.12 BCd | 30.54 ± 1.15 Bc | 12.26 ± 0.24 Bb | 3.55 ± 0.21 Ba |
| 25 °C | 60 | 36.65 ± 0.85 Cd | 31.65 ± 0.68 Bc | 13.08 ± 0.11 Bb | 4.00 ± 0.05 Ca |
| 45 °C | 15 | 37.80 ± 0.33 Cd | 32.50 ± 1.09 Bc | 13.65 ± 0.41 BCb | 3.96 ± 0.38 Ca |
| 45 °C | 30 | 38.90 ± 0.09 Cd | 33.76 ± 0.82 Bc | 13.88 ± 0.01 Cb | 4.21 ± 0.17 Ca |
| 45 °C | 45 | 33.50 ± 0.35 Bd | 28.44 ± 0.78 ABc | 11.44 ± 1.05 Ab | 3.34 ± 0.02 Ba |
| 45 °C | 60 | 30.54 ± 0.70 Bd | 26.08 ± 0.36 Ac | 10.56 ± 0.07 Ab | 3.06 ± 0.00 Ba |
| 65 °C | 15 | 38.90 ± 0.27 Cd | 33.32 ± 0.00 Bc | 13.92 ± 0.91 Cb | 4.04 ± 0.33 Ca |
| 65 °C | 30 | 33.21 ± 1.10 Bc | 33.21 ± 0.19 Bc | 14.05 ± 0.10 Cb | 3.89 ± 0.08 Ca |
| 65 °C | 45 | 28.21 ± 0.40 Ad | 24.26 ± 0.34 Ac | 9.68 ± 0.75 Ab | 2.80 ± 0.00 Aa |
| 65 °C | 60 | 25.01 ± 0.00 Ad | 21.65 ± 0.66 Ac | 8.41 ± 0.88 Ab | 2.45 ± 0.07 Aa |
| Reference sample | 24.83 ± 1.40 Ad | 20.89 ± 0.97 Ac | 8.39 ± 0.73 Ab | 2.43 ± 0.04 Aa | |
| Temp (°C) | Time (min) | Solvents | ||
|---|---|---|---|---|
| 50% Methanol | 50% Ethanol | 50% Acetone | ||
| AA (mg/g Rose Fruit Pomace) | ||||
| 25 °C | 15 | 18.53± 0.66 Ba | 21.43 ± 1.01 Bb | 25.19 ± 0.85 Ac |
| 25 °C | 30 | 19.83 ± 0.36 Ba | 23.00 ± 0.90 Bb | 26.87 ± 0.03 Bc |
| 25 °C | 45 | 21.45 ± 0.57 Ca | 24.45 ± 1.14 Bb | 28.17 ± 0.82 Cc |
| 25 °C | 60 | 23.78 ± 0.43 Ca | 26.06 ± 0.25 BCb | 28.97 ± 0.74 Cc |
| 45 °C | 15 | 22.75 ± 0.17 Ca | 27.43 ± 0.05 Cb | 29.88 ± 0.44 Cc |
| 45 °C | 30 | 23.61 ± 0.37 Ca | 27.85 ± 0.51 Cb | 30.76 ± 0.61 Cc |
| 45 °C | 45 | 19.98 ± 0.56 BCa | 23.43 ± 0.87 Bb | 26.54 ± 0.12 Bc |
| 45 °C | 60 | 18.23 ± 0.71 Bb | 12.82 ± 1.30 Aa | 24.66 ± 0.87 Bc |
| 65 °C | 15 | 23.24 ± 0.20 Ca | 27.81 ± 1.12 Cb | 30.75 ± 0.40 Cb |
| 65 °C | 30 | 23.47 ± 0.25 Ca | 28.25 ± 0.08 Cb | 31.48 ± 0.08 Cc |
| 65 °C | 45 | 16.82 ± 0.85 Ba | 17.81 ± 0.91 Aa | 22.39 ± 0.45 Bb |
| 65 °C | 60 | 13.19 ± 0.09 Aa | 14.82 ± 0.63 Aa | 18.91 ± 0.12 Ab |
| Reference sample | 14.66 ± 1.82 Aa | 16.98 ± 0.05 Ab | 19.63 ± 2.09 Ac | |
| Source | Degrees of Freedom | Sum of Square | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Main effects | |||||
| temperature | 1 | 41 | 40.6 | 17.008 | <0.0001 |
| time | 4 | 1688 | 421.9 | 176.634 | <0.0001 |
| solvent | 6 | 17,693 | 2948.8 | 1234.435 | <0.0001 |
| temperature:time | 4 | 872 | 218.1 | 91.306 | <0.0001 |
| temperature:solvent | 6 | 18 | 3 | 1.268 | 0.276 |
| time:solvent | 24 | 372 | 15.5 | 6.489 | <0.0001 |
| temperature:time:solvet | 24 | 165 | 6.9 | 2.876 | <0.0001 |
| Residuals | 140 | 334 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cendrowski, A.; Studnicki, M.; Kalisz, S. Impact of Different Solvents and Temperatures on the Extraction of Bioactive Compounds from Rose Fruits (Rosa rugosa) Pomace. Appl. Sci. 2024, 14, 691. https://doi.org/10.3390/app14020691
Cendrowski A, Studnicki M, Kalisz S. Impact of Different Solvents and Temperatures on the Extraction of Bioactive Compounds from Rose Fruits (Rosa rugosa) Pomace. Applied Sciences. 2024; 14(2):691. https://doi.org/10.3390/app14020691
Chicago/Turabian StyleCendrowski, Andrzej, Marcin Studnicki, and Stanisław Kalisz. 2024. "Impact of Different Solvents and Temperatures on the Extraction of Bioactive Compounds from Rose Fruits (Rosa rugosa) Pomace" Applied Sciences 14, no. 2: 691. https://doi.org/10.3390/app14020691
APA StyleCendrowski, A., Studnicki, M., & Kalisz, S. (2024). Impact of Different Solvents and Temperatures on the Extraction of Bioactive Compounds from Rose Fruits (Rosa rugosa) Pomace. Applied Sciences, 14(2), 691. https://doi.org/10.3390/app14020691






