Zinc Plating on Inkjet-Printed Ti3C2Tx MXene: Effect of Electrolyte and PEG Additive
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-Intercalated Manganese Dioxide Nanolayers as a High-Performance Cathode Material for an Aqueous Zinc-Ion Battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and Opportunities Facing Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Wang, J.; Zhang, Q.; Liu, Z.; Zhang, E.; Liu, Q.; Yu, X.; Lu, B. MoSe2/N-Doped Carbon as Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801477. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Yue, Y.; Wang, X.; Pakornchote, T.; Bovornratanaraks, T.; Zhang, X.; Wu, Z.-S.; Qin, J. Oxygen Defect Enriched (NH4)2V10O25·8H2O Nanosheets for Superior Aqueous Zinc-ion Batteries. Nano Energy 2021, 84, 105876. [Google Scholar] [CrossRef]
- He, P.; Chen, Q.; Yan, M.; Xu, X.; Zhou, L.; Mai, L.; Nan, C.-W. Building Better Zinc-Ion Batteries: A Materials Perspective. EnergyChem 2019, 1, 100022. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Dong, L. Stable Anode-Free Zinc-Ion Batteries Enabled by Alloy Network-Modulated Zinc Deposition Interface. J. Energy Chem. 2023, 76, 32–40. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Liu, Z.; Wang, D.; Guo, Y.; Li, X.; Tang, Y.; Li, H.; Dong, B.; Zhi, C. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 32, 2001854. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, K.; Pei, P.; Wei, M.; Liu, X.; Xiao, Y.; Zhang, P. Zinc Dendrite Growth and Inhibition Strategies. Mater. Today Energy 2021, 20, 100692. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.-P.; Fu, N.; Zhou, W.-B.; Liu, B.; Deng, Q.; Wu, X.-W. Comprehensive Review on Zinc-Ion Battery Anode: Challenges and Strategies. InfoMat 2022, 4, e12306. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the Material and Structural Design of Zinc Anode towards High-Performance Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Y.; Yao, L.; Yang, L.; Zhao, Q.; Ding, S.; Liu, L.; Pan, F. Progress in Interface Structure and Modification of Zinc Anode for Aqueous Batteries. Nano Energy 2022, 98, 107333. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Kim, Y.; Park, Y.; Kim, H.; Choi, J.W. Surface Overpotential as a Key Metric for the Discharge–Charge Reversibility of Aqueous Zinc-Ion Batteries. J. Am. Chem. Soc. 2023, 145, 15776–15787. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-B.; Ran, Q.; Yao, R.-Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X.-Y.; Jiang, Q. Lamella-Nanostructured Eutectic Zinc–Aluminum Alloys as Reversible and Dendrite-Free Anodes for Aqueous Rechargeable Batteries. Nat. Commun. 2020, 11, 1634. [Google Scholar] [CrossRef]
- Nie, W.; Cheng, H.; Sun, Q.; Liang, S.; Lu, X.; Lu, B.; Zhou, J. Design Strategies toward High-Performance Zn Metal Anode. Small Methods 2023, 2201572. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003065. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and Future Perspective on Zinc Metal Anode for Rechargeable Aqueous Zinc-Ion Batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Chladil, L.; Čech, O.; Smejkal, J.; Vanýsek, P. Study of Zinc Deposited in the Presence of Organic Additives for Zinc-Based Secondary Batteries. J. Energy Storage 2019, 21, 295–300. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Y.; Gao, C. Influence of Surfactants on Electrochemical Behavior of Zinc Electrodes in Alkaline Solution. J. Power Sources 1998, 72, 231–235. [Google Scholar] [CrossRef]
- Lan, C.J.; Lee, C.Y.; Chin, T.S. Tetra-Alkyl Ammonium Hydroxides as Inhibitors of Zn Dendrite in Zn-Based Secondary Batteries. Electrochim. Acta 2007, 52, 5407–5416. [Google Scholar] [CrossRef]
- Sorour, N.; Zhang, W.; Ghali, E.; Houlachi, G. A Review of Organic Additives in Zinc Electrodeposition Process (Performance and Evaluation). Hydrometallurgy 2017, 171, 320–332. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Qian, Y.; Zhang, W.; Jiang, L.; Shen, J. Effect of Additives on Electrodeposition of Nanocrystalline Zinc from Acidic Sulfate Solutions. J. Electrochem. Soc. 2007, 154, D567. [Google Scholar] [CrossRef]
- Wei, C.; Tao, Y.; An, Y.; Tian, Y.; Zhang, Y.; Feng, J.; Qian, Y. Recent Advances of Emerging 2D MXene for Stable and Dendrite-Free Metal Anodes. Adv. Funct. Mater. 2020, 30, 2004613. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Y.; Zhu, K.; Lian, R.; Gao, Y.; Yin, J.; Ye, K.; Cheng, K.; Yan, J.; Wang, G.; et al. Lithiophilic Three-Dimensional Porous Ti3C2Tx-rGO Membrane as a Stable Scaffold for Safe Alkali Metal (Li or Na) Anodes. ACS Nano 2019, 13, 14319–14328. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, C.; Wang, H.; Hu, X.; Matios, E.; Lu, X.; Zhang, W.; Tao, X.; Li, W. Pillared MXene with Ultralarge Interlayer Spacing as a Stable Matrix for High Performance Sodium Metal Anodes. Adv. Funct. Mater. 2019, 29, 1805946. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Flexible and Free-Standing Ti3C2Tx MXene@Zn Paper for Dendrite-Free Aqueous Zinc Metal Batteries and Nonaqueous Lithium Metal Batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef]
- Mateen, A.; Ansari, M.Z.; Hussain, I.; Eldin, S.M.; Albaqami, M.D.; Bahajjaj, A.A.A.; Javed, M.S.; Peng, K.-Q. Ti2CTx–MXene Aerogel Based Ultra–Stable Zn–Ion Supercapacitor. Compos. Commun. 2023, 38, 101493. [Google Scholar] [CrossRef]
- Shi, B.; Li, L.; Chen, A.; Jen, T.-C.; Liu, X.; Shen, G. Continuous Fabrication of Ti3C2Tx MXene-Based Braided Coaxial Zinc-Ion Hybrid Supercapacitors with Improved Performance. Nano-Micro Lett. 2021, 14, 34. [Google Scholar] [CrossRef]
- Xin, M.; Li, J.; Ma, Z.; Pan, L.; Shi, Y. MXenes and Their Applications in Wearable Sensors. Front. Chem. 2020, 8, 297. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Ferrara, C.; Gentile, A.; Marchionna, S.; Ruffo, R. Ti3C2Tx MXene Compounds for Electrochemical Energy Storage. Curr. Opin. Electrochem. 2021, 29, 100764. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at Every Step, from Their Precursors to Single Flakes and Assembled Films. Progress. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Essandoh, M.L.K.; Tan, J.; Cao, Z.; Zhang, X.; Dong, P.; Ajayan, P.M.; Ye, M.; Shen, J. Interface Engineering with Zincophilic MXene for Regulated Deposition of Dendrite-Free Zn Metal Anode. Energy Storage Mater. 2022, 50, 243–251. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Hou, Y.; Yang, Q.; Chen, Z.; Huang, Z.; Liang, G.; Zhao, Y.; Ma, L.; Li, M.; et al. Toward a Practical Zn Powder Anode: Ti3C2Tx MXene as a Lattice-Match Electrons/Ions Redistributor. ACS Nano 2021, 15, 14631–14642. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Liu, C.; Xiong, S.; Feng, J.; Qian, Y. Reversible Zinc-Based Anodes Enabled by Zincophilic Antimony Engineered MXene for Stable and Dendrite-Free Aqueous Zinc Batteries. Energy Storage Mater. 2021, 41, 343–353. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, X.; Xu, W.; Chen, W.; Zhao, K.; Wang, Y.; Hong, S.; Wu, Q.; Li, M.-C.; et al. Synergic Effect of Dendrite-Free and Zinc Gating in Lignin-Containing Cellulose Nanofibers-MXene Layer Enabling Long-Cycle-Life Zinc Metal Batteries. Adv. Sci. 2022, 9, 2202380. [Google Scholar] [CrossRef]
- Park, J.M.; Jana, M.; Baek, S.H.; Kang, T.; Xiong, P.; Park, J.H.; Kim, J.S.; Zeraati, A.S.; Shekhirev, M.; Braun, P.V.; et al. MXene Ink Hosting Zinc Anode for High Performance Aqueous Zinc Metal Batteries. J. Energy Chem. 2023, 76, 187–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Z.; Liu, S.; Du, Z.; Cui, Y.; Gu, J.; Shi, Y.; Li, B.; Yang, S. Charge-Enriched Strategy Based on MXene-Based Polypyrrole Layers Toward Dendrite-Free Zinc Metal Anodes. Adv. Energy Mater. 2022, 12, 2103979. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Luo, K.; Hou, Y.; Li, P.; Yang, Q.; Huang, Z.; Liang, G.; Chen, Z.; Du, S.; et al. Lattice Matching and Halogen Regulation for Synergistically Induced Uniform Zinc Electrodeposition by Halogenated Ti3C2 MXenes. ACS Nano 2022, 16, 813–822. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Qin, G.; Luo, K.; Lu, J.; Li, Y.; Liang, G.; Huang, Z.; Zhou, J.; Hultman, L.; et al. Halogenated Ti3C2 MXenes with Electrochemically Active Terminals for High-Performance Zinc Ion Batteries. ACS Nano 2021, 15, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk During Synthesis. ACS Chem. Health Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
- Gibertini, E.; Lissandrello, F.; Bertoli, L.; Viviani, P.; Magagnin, L. All-Inkjet-Printed Ti3C2 MXene Capacitor for Textile Energy Storage. Coatings 2023, 13, 230. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of Two-Dimensional Ti3C2Tx MXene Using HCl+LiF Etchant: Enhanced Exfoliation and Delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mhin, S.; Jeon, J.; Park, K.; Kim, J.; Choi, W. Stable and High-Energy-Density Zn-Ion Rechargeable Batteries Based on a MoS2-Coated Zn Anode. ACS Appl. Mater. Interfaces 2020, 12, 27249–27257. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhao, X.; Liu, S.; Hao, Y.; Tang, Q.; Hu, A.; Liu, Z.; Chen, X. Stabilizing Zinc Anodes by Regulating the Electrical Double Layer with Saccharin Anions. Adv. Mater. 2021, 33, 2100445. [Google Scholar] [CrossRef]
- Jin, S.; Yin, J.; Gao, X.; Sharma, A.; Chen, P.; Hong, S.; Zhao, Q.; Zheng, J.; Deng, Y.; Joo, Y.L.; et al. Production of Fast-Charge Zn-Based Aqueous Batteries via Interfacial Adsorption of Ion-Oligomer Complexes. Nat. Commun. 2022, 13, 2283. [Google Scholar] [CrossRef]
- Xie, X.; Liang, S.; Gao, J.; Guo, S.; Guo, J.; Wang, C.; Xu, G.; Wu, X.; Chen, G.; Zhou, J. Manipulating the Ion-Transfer Kinetics and Interface Stability for High-Performance Zinc Metal Anodes. Energy Environ. Sci. 2020, 13, 503–510. [Google Scholar] [CrossRef]
- Mitha, A.; Yazdi, A.Z.; Ahmed, M.; Chen, P. Surface Adsorption of Polyethylene Glycol to Suppress Dendrite Formation on Zinc Anodes in Rechargeable Aqueous Batteries. ChemElectroChem 2018, 5, 2409–2418. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, X.; Gao, S.; Xu, D.; Wang, Z.; Ye, Z.; Wang, L.; Chen, B.; Li, L.; Ye, M.; et al. Ultrastable Zinc Anode by Simultaneously Manipulating Solvation Sheath and Inducing Oriented Deposition with PEG Stability Promoter. Small 2022, 18, 2103345. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Wang, C.; Glazer, J.N.; Shan, Z.; Liu, N. Understanding and Controlling the Nucleation and Growth of Zn Electrodeposits for Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 32930–32936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, W.; Li, L.; Chang, C.; Yang, K.; Gao, L.; Pu, X. Nonepitaxial Electrodeposition of (002)-Textured Zn Anode on Textureless Substrates for Dendrite-Free and Hydrogen Evolution-Suppressed Zn Batteries. Adv. Mater. 2023, 35, 2300073. [Google Scholar] [CrossRef]
- Yuan, W.; Nie, X.; Ma, G.; Liu, M.; Wang, Y.; Shen, S.; Zhang, N. Realizing Textured Zinc Metal Anodes through Regulating Electrodeposition Current for Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2023, 62, e202218386. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bai, L.; Wang, C.; Wang, D.; Xu, K.; Dong, J.; Li, Y.; Shen, Q.; Yang, J. Synergistic Cooperation of Zn(002) Texture and Amorphous Zinc Phosphate for Dendrite-Free Zn Anodes. ACS Nano 2023, 17, 15113–15124. [Google Scholar] [CrossRef]
- Zhao, Z.; He, Y.; Yu, W.; Shang, W.; Ma, Y.; Tan, P. Revealing the Missing Puzzle Piece of Concentration in Regulating Zn Electrodeposition. Proc. Natl. Acad. Sci. USA 2023, 120, e2307847120. [Google Scholar] [CrossRef]
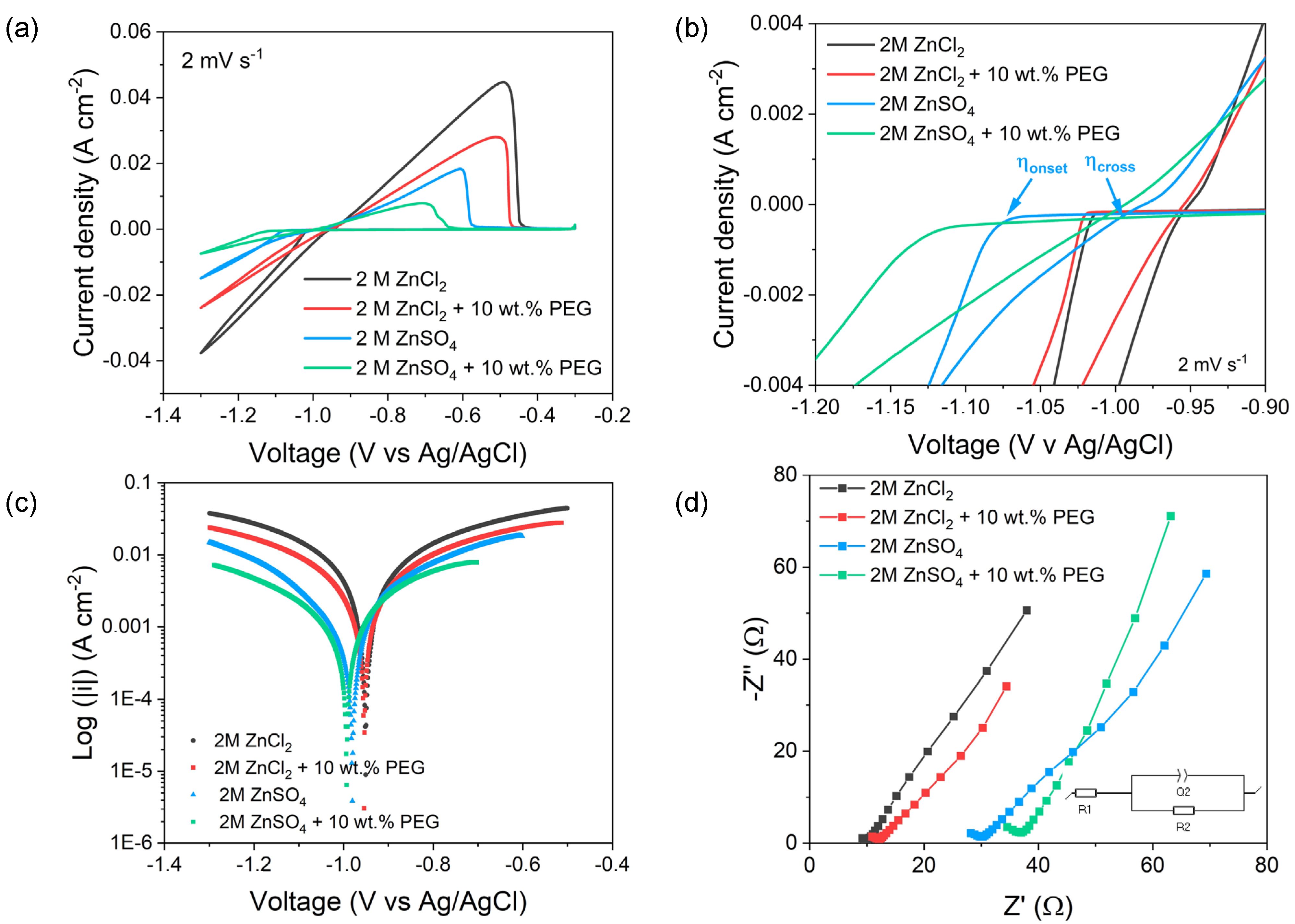
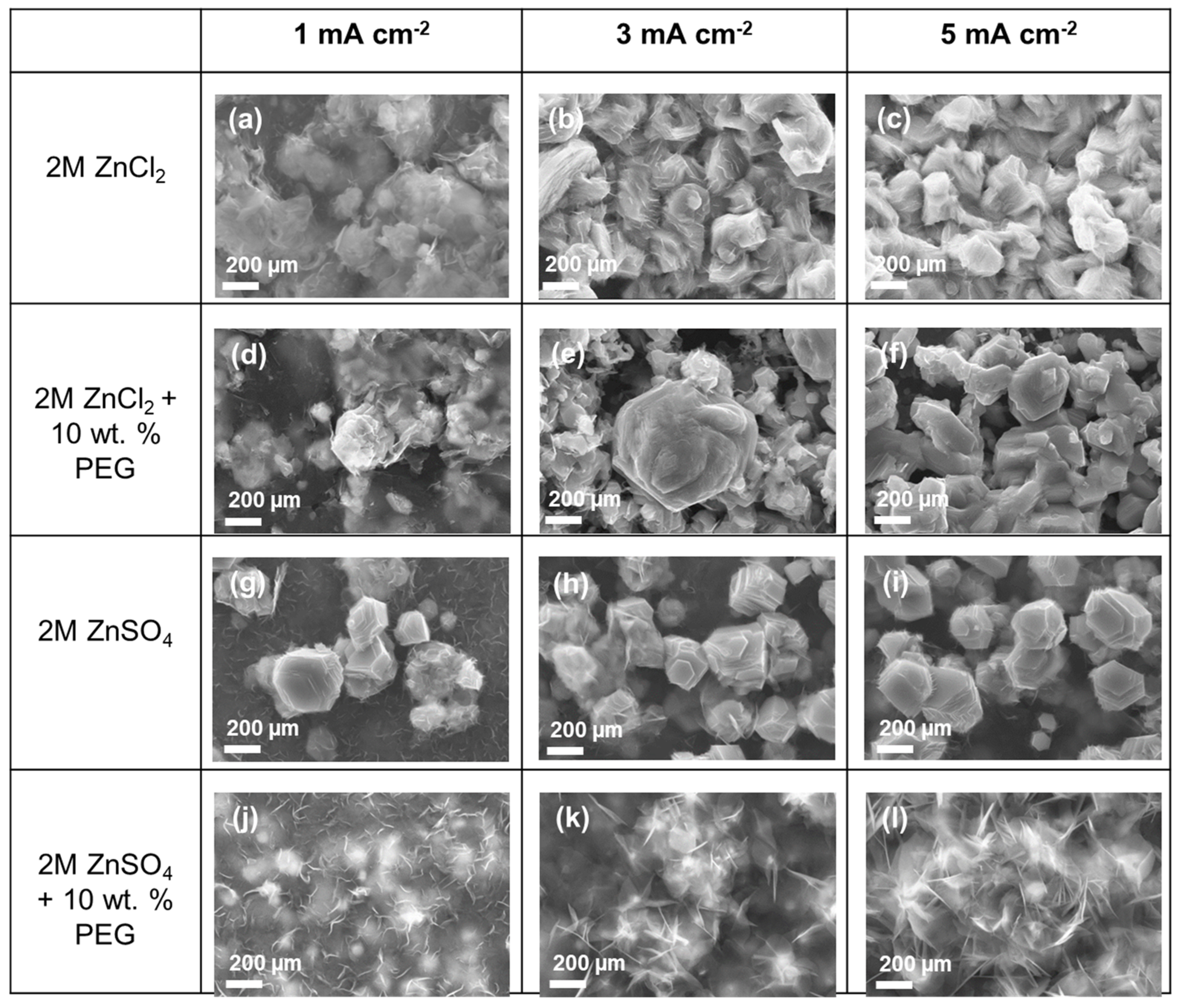
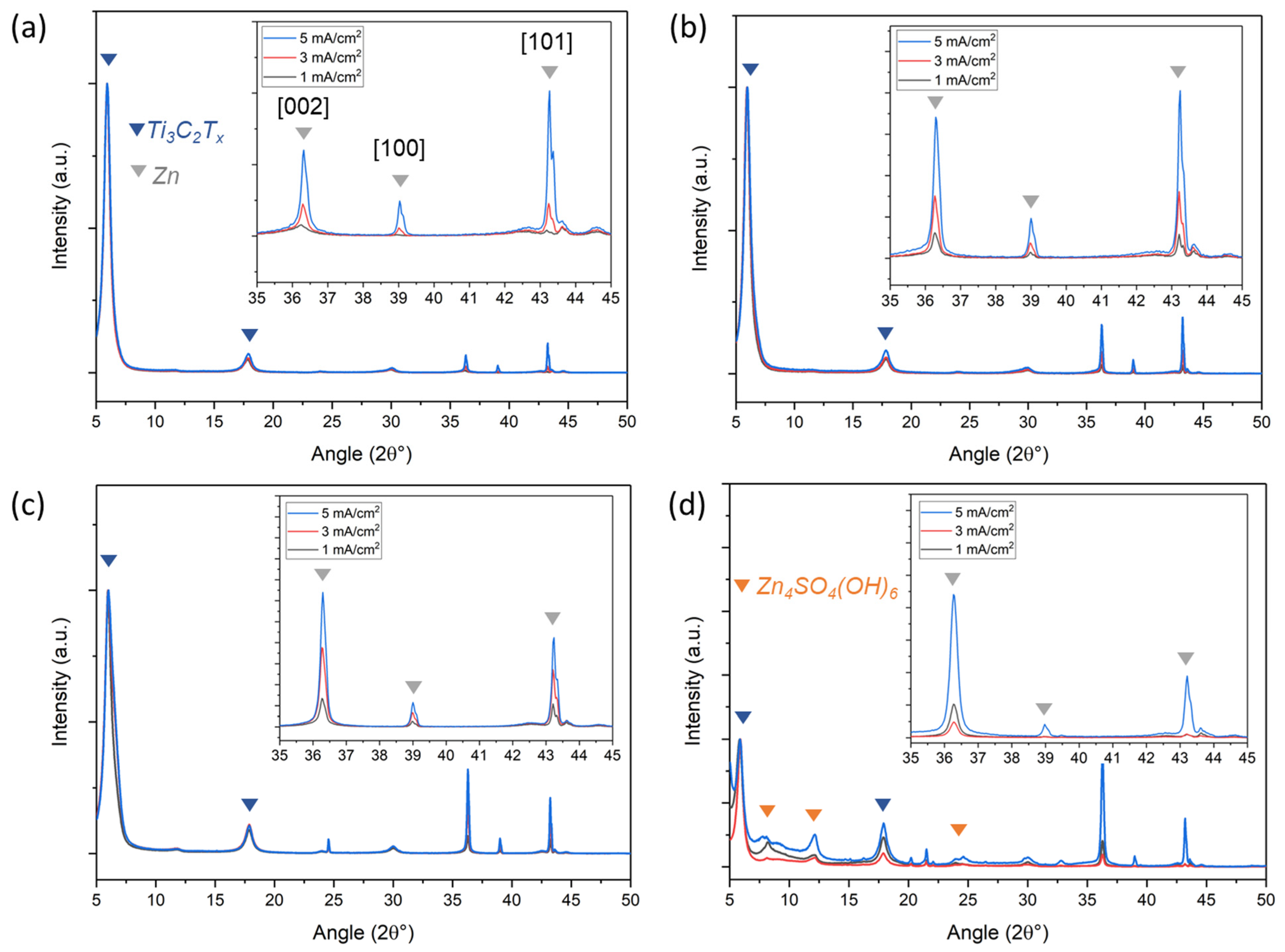
| 2M ZnCl2 | 2M ZnCl2 + 10 wt.% PEG400 | 2M ZnSO4 | 2M ZnSO4 + 10 wt.% PEG400 | |
|---|---|---|---|---|
| |ip,plat|(A cm−2) | 0.037 | 0.024 | 0.014 | 0.007 |
| Qplat (C) | 5.96 | 3.70 | 1.81 | 0.84 |
| Ip,strip (A cm−2) | 0.044 | 0.027 | 0.018 | 0.008 |
| Qstrip (C) | 5.86 | 3.70 | 1.75 | 0.78 |
| η (mV) | 62 | 71 | 80 | 121 |
| i0 (mA cm−2) | 10.94 | 6.66 | 2.95 | 1.47 |
| 2M ZnCl2 | 2M ZnCl2 + 10 wt.% PEG400 | 2M ZnSO4 | 2M ZnSO4 + 10 wt.% PEG400 | |
|---|---|---|---|---|
| σ (mS cm−1) | 100.2 | 68.1 | 54 | 29.1 |
| 2M ZnCl2 | 2M ZnCl2 + 10 wt.% PEG400 | 2M ZnSO4 | 2M ZnSO4 + 10 wt.% PEG400 | |
|---|---|---|---|---|
| R1 | 4.98 | 7.2 | 15.71 | 23.27 |
| R2 | 5.55 | 5.85 | 10.55 | 14.94 |
| R2, Eq.3 | 4.42 | 7.33 | 10.69 | 16.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viviani, P.; Gibertini, E.; Montanelli, V.; Magagnin, L. Zinc Plating on Inkjet-Printed Ti3C2Tx MXene: Effect of Electrolyte and PEG Additive. Appl. Sci. 2024, 14, 682. https://doi.org/10.3390/app14020682
Viviani P, Gibertini E, Montanelli V, Magagnin L. Zinc Plating on Inkjet-Printed Ti3C2Tx MXene: Effect of Electrolyte and PEG Additive. Applied Sciences. 2024; 14(2):682. https://doi.org/10.3390/app14020682
Chicago/Turabian StyleViviani, Prisca, Eugenio Gibertini, Vittorio Montanelli, and Luca Magagnin. 2024. "Zinc Plating on Inkjet-Printed Ti3C2Tx MXene: Effect of Electrolyte and PEG Additive" Applied Sciences 14, no. 2: 682. https://doi.org/10.3390/app14020682
APA StyleViviani, P., Gibertini, E., Montanelli, V., & Magagnin, L. (2024). Zinc Plating on Inkjet-Printed Ti3C2Tx MXene: Effect of Electrolyte and PEG Additive. Applied Sciences, 14(2), 682. https://doi.org/10.3390/app14020682





