Abstract
Patients sustaining a hip fracture experience reduced function and an increased risk of recurrent falls and institutionalization following surgical treatment. Rehabilitation programs that are feasible for home-based training could improve patient-reported outcomes and physical function while lowering the care need and social dependency of this patient group. In the present study, we designed and tested a home-based resistance training program on a group of patients with a femoral neck fracture (FNF) selected according to their poor post-operative functional recovery following an FNF. The results showed that the training program was feasible to perform for the patients, and after three months of training, the patients’ walking, physical activity, and patient-reported outcome measures improved. The patients were encouraged to continue walking and performing the training program, but twelve months after the FNF, the results were comparable to the baseline. Background: Femoral neck fracture (FNF) is associated with reduced function, often leading to an increased care need and a greater risk of recurrent falls. Thus, rehabilitation should be a priority. The present study investigated the training potential among fragile FNF patients with poor functional performance treated with total hip arthroplasty. Methods: In a prospective cohort study, 32 participants were included based on poor functional recovery following an FNF fracture. The participants completed a three-month, physiotherapy-guided, home-based resistance training program. At the baseline and three-month follow-up, physiotherapists performed functional tests and measured spatiotemporal parameters, muscle strength, and muscle mass. The Oxford hip score (OHS) questionnaire was administered and physical activity measurements were performed at baseline and at three-month and 12-month follow-ups. Results: Walking distance, step length, walking speed, and muscle strength increased at the three-month follow-up (p < 0.05). OHS scores increased from the baseline to the 12-month follow-up. Physical activity after three months showed more time spent standing (p = 0.02) and walks of 5–10 min (p = 0.002) compared to the baseline. At the 12-month follow-up, physical activity was similar to the baseline. Conclusions: Fragile patients with low functional performance following FNF displayed training potential with an improvement in function, strength, and physical activity. However, continued training is necessary in order to maintain the positive effects.
1. Introduction
Femoral neck fracture (FNF) is one of the major causes of trauma-related deaths among the elderly, and reduced function is common among survivors. As such, FNF patients are four times more likely to be homebound and three times more likely to be dependent on others for activities of daily living (ADL) two years after their FNF fracture surgery [1,2]. Additionally, patients who have suffered an FNF spend less time on their feet compared to age-matched controls [1,2]. The functional decline of FNF patients makes independent living more unlikely, and around 10% of patients are unable to return to their own homes after discharge due to increased care needs [2]. Furthermore, FNF patients face a high risk of recurrent falls and related injuries, which can degrade their mobility and increase their mortality. In order for FNF patients to return to their pre-fracture functional levels and reduce their risk of recurrent falls and related injuries, it is important to improve their gait function and balance, and resistance training is key in this rehabilitation [3,4].
Most research on the rehabilitation of FNF patients has been conducted in acute hospital or immediate post-discharge settings. However, patients in the acute phase of an FNF often experience pain, confusion, and amnesia, making rehabilitation a lower priority for these patients. Additionally, fragile patients typically experience multiple clinical issues that must be prioritized before and during the months after hospital discharge. Frequent transportation to training facilities is likely an obstacle to attending rehabilitation programs for several patients, and therefore, home-based training for frailer patients might be preferable. Although extensive research on the rehabilitation of FNF patients has been conducted, only a few studies have examined the training potential among fragile FNF patients years after their fractures [5].
The aim of the present study was to investigate the training potential of fragile patients with poor functional performance years after their FNFs and surgeries with total hip arthroplasty (THA) by evaluating their gait function, muscle strength and mass, patient-reported outcome, and physical activity after a three-month home-based resistance training program and to re-evaluate the effects of the intervention 1 year later.
2. Materials and Methods
From 2005 to 2011, 402 consecutive patients (291 women) at a mean age of 80 (30–98) years were admitted at a regional hospital in Denmark with a displaced medial FNF and operated upon with a THA using of a postero-lateral approach. The components were either a cementless hydroxyapatite-coated or a cemented Saturne® acetabular system (Amplitude, Valence, France) with 28 mm chrome-cobalt heads in dual-mobility UHMWPE and a cemented Exeter® stem (Stryker, Kalamazoo, MI, USA). In December 2012, all patients who were still alive were invited for an outpatient follow-up to evaluate their physical function, care needs, and social dependency. Two hundred and sixty-two patients had died or were unable to participate, mainly because of poor health. One hundred and forty patients participated in the follow-up and were screened for eligibility to participate in the training study. The inclusion criteria were as follows: a new mobility score (NMS) ≤8, a dementia score ≥7, and time needed to do 10 repetitions of sit-to-stand (STS10) of more than 30 s [6,7,8,9]. The exclusion criteria were as follows: polytrauma and pathological fractures. Thirty-eight patients met the inclusion criteria; however, 6 declined to participate in the study. The decliners were all female and of a similar mean age and follow-up time as the study group. Thirty-two patients (28 women) at a mean age of 80 (60–96) years were included at a mean of 3 (1–7) years after their FNF (Figure 1).
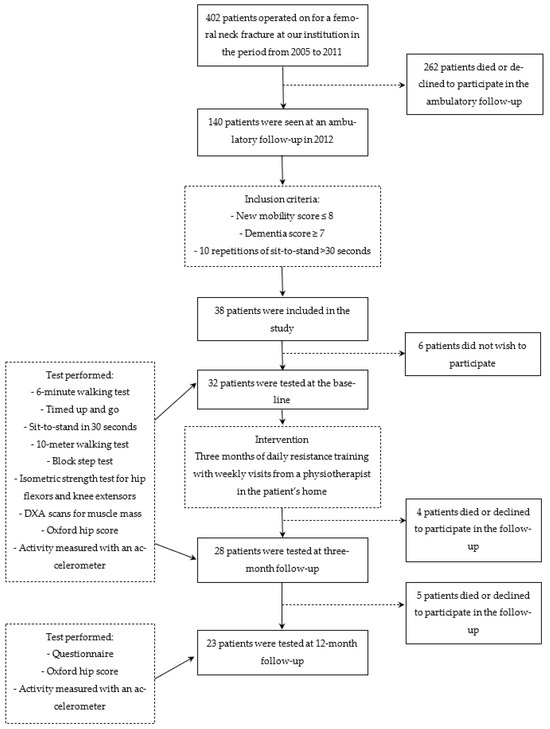
Figure 1.
Flow chart for the present study with inclusion criteria, the intervention, and the follow-up.
2.1. Intervention
Physiotherapists instructed the participants orally to perform a home-based resistance training program. Resistance training was chosen since it had previously been shown to increase functional outcomes, muscle mass, and muscle strength in patients with hip fractures [5,10]. The exercise program consisted of four resistance exercises and a daily walk (see Table 1 for details). A booklet with descriptions and illustrations of each exercise and a training journal were given to each participant (Supplementary Materials). Participants were instructed to exercise once daily, and all participants attended a weekly supervised session with a community-based physiotherapist during all three months of the intervention. Each training session was documented in a training journal with progression levels and the number of repetitions. The level of progression was determined by the physiotherapist in accordance with a standardized progression plan.

Table 1.
Description of the four exercises in the resistance training program used during the intervention.
2.2. Data Collection
The participants who met the inclusion criteria completed functional tests directed by a physiotherapist at the baseline and the three-month follow-up. The tests were performed in the same order at the baseline and the three-month follow-up. On the same day, the participants were administered a dual-energy X-ray absorptiometry (DXA) scan, completed questionnaires, and finally had an accelerometer mounted on their thighs to measure their physical activity. Furthermore, at three- and twelve-month follow-ups, the participants completed questionnaires and another physical activity measurement.
2.3. Measurement Outcomes
2.3.1. Functional Tests
The 6 min walking test (6 WT) was used to measure the participants’ endurance. The participants were instructed to walk as far as they could possibly and safely do during the 6 min [11,12]. The test was performed on a 30 m flat course. The distance was measured in meters. Walking aids were used if necessary, and this use was documented.
The timed up and go (TUG) test was performed with participants initially seated in a standard armchair. They were instructed to stand up and walk three meters, turn, walk back, and sit down as fast as they could safely do [13,14]. The participants performed three TUG trials. The fastest time and the use of walking aids were documented.
2.3.2. Spatiotemporal Tests
An inertia measurement unit (IMU) was used to evaluate the participants’ gait. The IMU contains both an accelerometer and a gyroscope, which measures velocity, orientation and gravitation, in order to assess the asymmetry, speed, and step length during tests [15,16]. The IMU-derived parameters were calculated using the manufacturer’s proprietary, non-disclosed algorithms based upon the algorithms by Zijlstra and Hof [17]. The parameters were adjusted by leg length. The following tests were performed with the IMU placed over the participants’ sacrums with double-adhesive tape:
- (1)
- A sit-to-stand test of 30 s (STS30) was performed with participants initially sitting in an armchair with a seat height of 45 cm, their backs placed against the backrest, and their arms crossed over their chests. The participants were instructed to stand up to a fully extended position and sit back down as many times as possible in 30 s without using the armrest for support [18]. If the participants were unable to perform a single repetition, they were allowed to use the armrest support, and this was documented.
- (2)
- A 10 m walking test was used to analyze the participants’ gait patterns. The participants walked a marked 10 m stretch on a flat surface with or without walking aids at a self-selected speed [19,20]. The distance by which the last step exceeded the 10 m marker was measured, and the exact distance was documented.
- (3)
- A block step test was performed with the participants ascending and descending onto a 30 cm block three times in a row at a self-selected speed. Both the legs were tested, always starting with the leg that had not been operated upon. The participants paused for three seconds at the top and bottom of the block.
2.3.3. Isometric Strength Test
An isometric strength test of the hip flexors and the knee extensors was performed bilaterally with a handheld dynamometer (HHD) [21,22].
Hip flexion was measured with the participants sitting, hips in 90 degree flexion, feet off the floor, and hands on the seat for balance. The HHD was placed five cm proximal to the patella. The participants were asked to raise the thighs of their test legs as hard as possible against the HDD.
Knee extension was measured with the participants sitting, hips in 90 degree flexion, knees in 60 degree flexion, and arms crossed over the chest. The test leg was fixed to the seat with a strap, and the HHD was placed five cm proximal to the lateral malleolus. The participants were asked to kick as hard as possible against the HDD with their test leg [21].
Both tests were repeated four times for each leg with 30 s of rest in between, and the best result was documented. The data were normalized according to the participants’ heights and weights.
2.3.4. DXA Scans
A DXA scan (iDXA, GE Healthcare, IL, USA) was performed at the baseline and the three-month follow-up to evaluate the participants’ muscle mass before and after the intervention. A total-body scan was performed, and the tissue was categorized using the DXA software (enCORE, GE Healthcare, IL, USA (Version 16)) as bone or soft tissue. The soft tissue was typed as fat tissue or fat-free tissue via the software. Fat-free tissue can be assumed to be equal to muscle mass [23], and it was measured in three regions of interest (ROI) in terms of the buttock, the thigh, and the calf areas of both the left and right lower extremities. The regions were marked on the total body scan using anatomical landmarks, as previously described [16].
2.3.5. Questionnaires
The participants filled out the Oxford hip score (OHS) questionnaire at the baseline and the three- and twelve-month follow-ups to assess their self-evaluated function and pain [24]. The OHS is a 12-question patient-reported outcome. Each question is ascribed one to five points, adding up to an overall score from 12 to 60 for each patient that quantifies their hip function, with 12 being the worst and 60 being excellent hip function. The OHS was chosen since it had been validated in Danish and had been shown to be sensitive to changes following a THA [25]. The OHS had, in previous studies, shown that a meaningful clinically important difference (MCID) is demonstrated by a score between five and nine [26,27].
The participants also filled out questionnaires regarding their training satisfaction, adverse events, and self-reported ADL at the three- and twelve-month follow-ups. Training satisfaction was evaluated using a questionnaire with a 5-point satisfaction score from not satisfied at all (1) to very satisfied (5).
2.3.6. Physical Activity
At the baseline, three months, and twelve months, a commercially available accelerometer (Ax3, Axivity Ltd., Newcastle upon Tyne, UK) was placed on the central part of the participants’ right lateral thighs using double-adhesive tape and used to measure physical activity during four consecutive days/nights. The data were imported to MatLab (version 2019b, The MathWorks Inc., Natick, MA, USA) and analyzed using a validated algorithm [28,29].
2.4. Statistical Analysis
A power calculation was performed with the primary outcome measure being the gait pattern, with an anticipated distribution of 7%, and the clinically relevant difference was set at 30%. The risk of committing a type 1 error was established at 5% with a power of 80% to detect a difference. The calculation indicated a requirement for 25 patients in the group. A total of 38 patients were included in the group to account for potential dropouts.
The secondary outcome measures were a functional test, muscle strength, muscle mass, and questionnaires.
Continuous data were expressed as means ± standard deviations (SDs), and categorical data were presented numerically or as percentages. Data were tested for normality using the Shapiro–Wilk test. When the data followed a Gaussian distribution, a paired t-test was used, and when not, non-parametric testing using a Mann–Whitney or Wilcoxon signed-rank test was used. For the accelerometer tests, an analysis of variance (ANOVA) test was used to evaluate changes in activity over time. For all tests, the statistical significance was defined as p < 0.05. Stata 14 was used for statistical comparisons.
3. Results
The baseline demographics of the study participants are listed in Table 2.

Table 2.
Summary of the demographic information of the study participants at the baseline.
At the 3-month follow-up, four participants were lost to follow-up (12.5%). After the 3-month training intervention, the participants showed a statistically significant increase in the number of repetitions per exercise in all four resistance exercises (Figure 2), and they achieved a significant increase in distance walked during the 6 WT (p = 0.01), but they showed no increase in TUG time (p = 0.89) (Table 3).
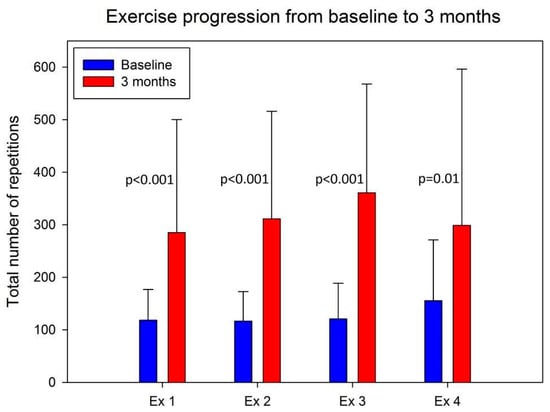
Figure 2.
The progression of the total number of repetitions of each of the four exercises from the start of the intervention to the final training during the intervention. Ex 1 = chair raises; Ex 2 = knee bends; Ex 3 = standing walk; Ex 4 = pelvic raises.

Table 3.
Results of the functional tests, spatiotemporal tests (IMU), isometric strength tests (HHD), and measured muscle mass (DXA scans) at the baseline and the three-month follow-up.
The spatiotemporal test at the 3-month follow-up showed a decrease in the time spent ascending and descending during the STS30 (p < 0.05) but did not show an increase in the numbers of STS taken during the 30 s (p = 0.30) (Table 3) or a change in the number of participants who needed to use the armrest. For the 10 m walking test, the participants increased their step length, walking speed, and cadence (p < 0.05) (Table 3), but the asymmetry data during the spatiotemporal test showed no general tendency from the baseline to the three-month follow-up (Table 3).
After three months of resistance training, an increase in muscle strength was found in the hip flexors of the leg that had not been operated upon and in the knee extensors of the leg that had been operated upon (p < 0.05) (Table 3). However, the participants showed no measurable increase in muscle mass in either of the three regions of interests according to the DXA scans (p > 0.56) (Table 3).
The OHS was 32 (15–49) at the baseline and 33 (12–51) at the three-month follow-up (p = 0.21) with no statistically significant increase.
The participants showed an increase in their number of 5–10 min walks from the baseline to the three-month follow-up (p = 0.002), but the overall time spent walking did not increase measurably (p = 0.55). The participants sat less and stood more after the three-month intervention (p = 0.02) (Figure 3).
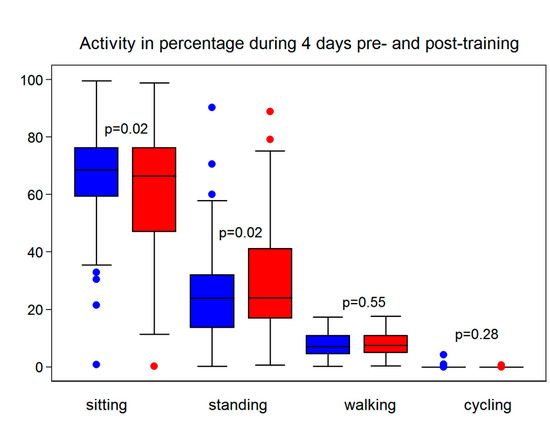
Figure 3.
Participants’ activity was measured using an accelerometer at the baseline (blue) and the 3-month follow-up (red), showing the participants standing more and sitting less but no significant difference in walking or cycling.
The three-month questionnaire showed that 59% of the participants had felt a prior need for more training following their FNFs. In total, 92% were satisfied with the training intervention. One adverse event was reported, with an accelerometer becoming unpleasantly warm on the thigh during data collection but no visible damage.
At the 12-month follow-up, there was no difference in the participants’ physical activity compared to the baseline as measured using the accelerometer. However, the OHS increased from 32 (15–49) at the baseline to 36 (12–37) at the 12-month follow-up (p = 0.02). At the 12-month follow-up, 30.5% of the participants reported a higher level of ADL, and 74% had continued the training on their own—primarily maintaining a daily 15 min walk. This was despite the fact that 48% of participants reported health issues that had affected their continued training abilities during the last 12 months.
4. Discussion
The present study showed that it is possible to engage a frail older population in a home-based resistance training program even years after an FNF, with a high level of completion and satisfaction. After completing the training program, the participants improved important parameters for balance and fall prevention, such as step length, walking speed, and muscle strength.
An impaired gait is one of the most prevalent and sensitive risk factors for falling [3,4]; a low walking speed and a short step length especially indicate a poor gait and an increased risk for falling among the elderly [4,30]. Conversely, an improvement in the gait parameters is an indirect sign of improved balance and a decreased fall risk. Previous studies have found 0.1 to 0.17 m/s to be a clinically significant change in walking speed [31,32]. In the present study, the participants achieved an increase of 0.23 m/s, indicating a clinically significant increase in their walking speed at the three-month follow-up. The participants had a baseline walking speed of 2.26 m/s, which was higher than that found in other studies despite their having focused on community-dwelling older adults [33,34,35]. This suggests that the participants were not as frail as anticipated. However, the participants had a much higher baseline TUG time of 15.6 s compared to the reported norm between 8.12 s and 12 s for frail women without FNFs between 65–85 years of age [36,37]. The step length of 0.41 m at the baseline was shorter than the 0.7 m reported by Bogen et al.; however, the participants in that study were community-dwelling older adults, which may indicate a better function [34]. A long TUG time and a short step length indicate poor balance and low function, and they confirm that the participants in the present study were in fact frail and at a greater fall risk.
Following the three-month resistance training intervention, the participants increased their walking distance on the 6 WT by 20 m, which correlates to a higher walking speed. Overgaard et al. found that a change in 6 WT should be at least 21.4 m to count as an improvement, rather than a learning effect [12]. However, Overgaard et al. [12] investigated hip fracture patients only a few weeks after their surgery, and pain improvement over time was a crucial factor for improvement in their walking abilities. The participants in the present study were a mean three years post-surgery and presumably way past their surgery-related pain. Casanova et al. tested healthy subjects and found that a learning effect alone could explain a 12 m improvement for the 6 WT [38]. Therefore, the 20 m improvement in our study group was most likely a real positive effect of the training, which was further supported by the increase in the step length and walking speed. Several studies have shown positive results for hip fracture patients after resistance training; however, most studies have been performed within a few months after surgery [39,40,41]. Edgren et al. showed that resistance training even years after a hip fracture can improve gait and function among these patients [5]. Like the present study, the participants in Edgren’s study were a mean of 3 years after their hip fractures and similar in baseline characteristics. Edgren et al., found, as we did in our study, that resistance training is feasible for elderly patients with a history of hip fracture, as well as increased physical function [5].
The spatiotemporal parameters showed no general decrease in asymmetry despite the participants being quicker to ascend and descend during the block step test and STS30. This might be explained by participants moving their upper bodies more during the tests simply to gain speed in order to perform the exercise.
The present study showed a significant increase in some of the muscle strength parameters, but it did not show any increase in muscle mass on the DXA scans. Possibly, the intensity of the exercises that could be achieved by this frail patient group with several comorbidities during the period of the intervention was not sufficient to be measurable as a change in muscle mass. Furthermore, the study group was small, and possibly the precision of DXA was not sufficient to detect an eventual change. Yet, Suetta et al. and Briggs et al. found improvements in muscle strength and muscle mass in patients with hip osteoarthritis and patients treated for hip fractures, respectively [42,43]. Our study population was older, especially compared to the patients in the study by Suetta et al. [42], and both studies’ interventions occurred closer to the participants’ surgeries than ours. With increasing age, the ability to improve muscle mass declines, and the process takes longer than for younger individuals. This stresses the importance of immediate and long-lasting physical rehabilitation after hip fracture surgery in order to lower the risk of new fall and fracture events, and it may explain some of the reasoning for the absence of increased muscle mass seen in our study. Several previous studies have shown training potential among elderly hip fracture patients and shown that they can perform an exercise program without adverse events [5,10,44,45]. Likewise, the participants in the present study showed progression in all four resistance exercises, and the participants also reported high satisfaction, high adherence to the training program, and improved self-reported ADL after the three-month training program. However, the increase in OHS did not meet the MCID.
After an FNF, the mean time to recovery in ADL function has been reported as six months (4–11 months), and the same has been reported for regaining muscle strength [46,47]. The participants in the present study were all a minimum of 12 months after their FNFs and, therefore, should have fully recovered from their fracture events. As such, the physical improvements found in the present study, we believe, are in fact a result of the training, rather than spontaneous improvements over time. However, considering the decline in activity at the 12-month follow-up, continued training seems important and could be completed at home with the training program described in the present study.
Limitations
This study faced some limitations, most importantly, the lack of a control group. The participants were identified as the frailest and weakest out of a larger examined patient group [48], and we found it unethical not to offer some fall prophylaxis treatment in terms of training to all these patients. Another limitation was the variation in the participants’ baseline function and the number of comorbidities, which may have limited the improvement for at least some of the participants. The diversity of this patient group was also reflected in the time elapsed since their surgeries (1–7 years). However, the heterogeneity of the patient group improved the external validity of the study’s results.
In order to evaluate the long-term effects of the resistance training program, it would have been an advantage to repeat the functional tests at the 12-month follow-up, but unfortunately, our resources did not allow for this.
The intervention was home-based, which had both advantages and disadvantages. The advantages of a home-based program include easy accessibility to facilities and the alleviation of transportation, which may have increased adherence to the program [44,45]. Another advantage is the easy progression and individualization of the training program. The disadvantages of a home-based training program are the cost of physiotherapists and their transport to the participants’ homes, the risk of limited training space and equipment at the participants’ homes, and the lack of support and encouragement from other participants to stick with the training.
5. Conclusions
The participants significantly improved their gaits, as demonstrated by an increase in walking speed and step length. Further, they were more active during the day after the three-month training program. The participants progressed significantly in their training and had high satisfaction with the intervention. The resistance program in this study is easily incorporated into current rehabilitation protocols due to the low number of exercises and minimal need for equipment. However, it is important to mention that some of the activity improvements found after completion of the three-month resistance training program were lost at the 12-month follow-up, indicating a need for continued training. More studies are needed in order to determine the training level that will sustain the improved function and ADL of this fragile group of patients for the purpose of lowering their fall and fracture risk and reducing their care needs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14020552/s1, Supplementary Materials—Training booklet with pelvic raise.
Author Contributions
Conceptualization, I.M., S.B., L.L., C.F.F., T.B.-H. and M.S.; methodology, I.M., S.B., C.F.F. and M.S.; software, I.M., S.B., C.F.F. and M.S.; validation, I.M., S.B., C.F.F. and M.S.; formal analysis, I.M., S.B., C.F.F. and M.S.; investigation, I.M., S.B., C.F.F. and M.S.; resources, L.L. and T.B.-H.; data curation, I.M., S.B., C.F.F. and M.S.; writing—original draft preparation, C.F.F.; writing—review and editing, I.M., C.F.F. and M.S.; visualization, I.M., C.F.F. and M.S.; supervision, I.M. and M.S.; project administration, M.S.; funding acquisition, T.B.-H. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was performed in accordance with the Helsinki Declaration II and was reported to and reviewed by the local ethical committee as a quality assurance investigation (inquiry 149/2012), and the data collection was registered by The Danish Data Protection Agency (number 2007-58-0010).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
Data can be made available upon reasonable written request. The data are not publicly available due to personal data.
Acknowledgments
The authors would like to thank the patients, municipalities, and physiotherapists that participated in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Norton, R.; Butler, M.; Robinson, E.; Lee-Joe, T.; Campbell, A.J. Declines in physical functioning attributable to hip fracture among older people: A follow-up study of case-control participants. Disabil. Rehabil. 2000, 22, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.M.; Crotty, M.; Fairhall, N.; Magaziner, J.; Beaupre, L.A.; Cameron, I.D.; Sherrington, C. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Thaler-Kall, K.; Peters, A.; Thorand, B.; Grill, E.; Autenrieth, C.S.; Horsch, A.; Meisinger, C. Description of spatio-temporal gait parameters in elderly people and their association with history of falls: Results of the population-based cross-sectional KORA-Age study. BMC Geriatr. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Stenhagen, M.; Ekström, H.; Nordell, E.; Elmståhl, S. Falls in the general elderly population: A 3- and 6- year prospective study of risk factors using data from the longitudinal population study ‘Good ageing in Skane’. BMC Geriatr. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Edgren, J.; Rantanen, T.; Heinonen, A.; Portegijs, E.; Alen, M.; Kiviranta, I.; Kallinen, M.; Sipila, S. Effects of progressive resistance training on physical disability among older community-dwelling people with history of hip fracture. Aginig Clin. Exp. Res. 2012, 24, 171–175. [Google Scholar] [CrossRef]
- Parker, M.J.; Palmer, C.R. A New Mobility After Score for Predicting Mortality. J. Bone Jt. Surg. Br. 1993, 75, 797–798. [Google Scholar] [CrossRef]
- Kristensen, M.T.; Bang, N.; Kehlet, H.; Centret, J.M. Timed Up & Go og New Mobility Score til prædiktion af funktion seks måneder efter hoftefraktur. [Timed up and go and new mobility score as predictors of function six months after hip fracture]. Ugeskr. Laeger 2005, 167, 3297–3300. [Google Scholar] [PubMed]
- Csuka, M.; Mccarty, D.J. Simple method for measurement of lower extremity muscle strength. Am. J. Med. 1985, 78, 77–81. [Google Scholar] [CrossRef]
- Hindsø, K. Prevention of Hip Fractures using External Hip Protectors. In Risk Factors for Falls, Hip Fractures, and Mortality, and Evaluation of the Consequences of Fear of Falling among Older Orthopaedic Patients; University of Copenhagen: Copenhagen, Denmark, 1998. [Google Scholar]
- Lee, S.Y.; Yoon, B.-H.; Beom, J.; Ha, Y.-C.; Lim, J.-Y. Effect of Lower-Limb Progressive Resistance Exercise After Hip Fracture Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Am. Med. Dir. Assoc. 2017, 18, 1096.e19–1096.e26. [Google Scholar] [CrossRef]
- Bautmans, I.; Lambert, M.; Mets, T. The six-minute walk test in community dwelling elderly: Influence of health status. BMC Geriatr. 2004, 4, 6. [Google Scholar] [CrossRef]
- Overgaard, J.A.; Larsen, C.M.; Holtze, S.; Ockholm, K.; Kristensen, M.T. Interrater Reliability of the 6-Minute Walk Test in Women with Hip Fracture. J. Geriatr. Phys. Ther. 2017, 40, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. JAGS 1991, 39, 142–148. [Google Scholar]
- Kristensen, M.; Bandholm, T.; Foss, N.; Ekdahl, C.; Kehlet, H. High inter-tester reliability of the new mobility score in patients with hip fracture. J. Rehabil. Med. 2008, 40, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Bolink, S.; Naisas, H.; Senden, R.; Essers, H.; Heyligers, I.; Meijer, K.; Grimm, B. Validity of an inertial measurement unit to assess pelvic orientation angles during gait, sit–stand transfers and step-up transfers: Comparison with an optoelectronic motion capture system. Med. Eng. Phys. 2015, 38, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Stilling, M.; Lorenzen, N.; Jakobsen, S.; Soballe, K.; Mechlenburg, I. Block-step asymmetry 5years after large-head metal-on-metal total hip arthroplasty is related to lower muscle mass and leg power on the implant side. Clin. Biomech. 2014, 29, 684–690. [Google Scholar] [CrossRef]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Mikkelsen, L.R.; Mikkelsen, S.; Søballe, K.; Mechlenburg, I.; Petersen, A.K. A study of the inter-rater reliability of a test battery for use in patients after total hip replacement. Clin. Rehabil. 2014, 29, 165–174. [Google Scholar] [CrossRef]
- Adell, E.; Wehmhörner, S.; Rydwik, E. The Test-Retest Reliability of 10 Meters Maximal Walking Speed in Older People Living in a Residential Care Unit. J. Geriatr. Phys. Ther. 2012, 36, 74–77. [Google Scholar] [CrossRef]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the Reliability and Validity of a Shorter Walk Test Compared With the 10-Meter Walk Test for Measurements of Gait Speed in Healthy, Older Adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Awwad, D.H.; Buckley, J.D.; Thomson, R.L.; O’connor, M.; Carbone, T.A.; Chehade, M.J. Testing the Hip Abductor Muscle Strength of Older Persons Using a Handheld Dynamometer. Geriatr. Orthop. Surg. Rehabil. 2017, 8, 166–172. [Google Scholar] [CrossRef]
- Roy, M.-A.G.; Doherty, T.J. Reliability of Hand-Held Dynamometry in Assessment of Knee Extensor Strength After Hip Fracture. Am. J. Phys. Med. Rehabil. 2004, 83, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Fuller, N.J.; Hardingham, C.R.; Graves, M.; Screaton, N.; Dixon, A.K.; Ward, L.C. Modeling Leg Sections by Bioelectrical Impedance Analysis, Dual-Energy X-ray Absorptiometry, and Anthropometry: Assessing Segmental Muscle Volume Using Magnetic Resonance Imaging as a Reference. Ann. N. Y. Acad. Sci. 2001, 904, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Fitzpatrick, R.; Murray, D.; Carr, A. Questionnaire on the perceptions of patients about total knee replacement. J. Bone Jt. Surg. Br. 2015, 78-B, 185–190. [Google Scholar] [CrossRef]
- Paulsen, A.; Odgaard, A.; Overgaard, S. Translation, cross-cultural adaptation and validation of the Danish version of the Oxford hip score: Assessed against generic and disease-specific questionnaires. Bone Jt. Res. 2012, 1, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Deckey, D.G.; Verhey, J.T.; Christopher, Z.K.; Gerhart, C.R.B.; Clarke, H.D.; Spangehl, M.J.; Bingham, J.S. Discordance Abounds in Minimum Clinically Important Differences in THA: A Systematic Review. Clin. Orthop. Relat. Res. 2023, 481, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.G.H.; Goh, G.S.; Chen, J.Y.; Lo, N.-N.; Yeo, S.-J.; Liow, M.H.L. Are Oxford Hip Score and Western Ontario and McMaster Universities Osteoarthritis Index Useful Predictors of Clinical Meaningful Improvement and Satisfaction After Total Hip Arthroplasty? J. Arthroplast. 2020, 35, 2458–2464. [Google Scholar] [CrossRef]
- Lipperts, M.; van Laarhoven, S.; Senden, R.; Heyligers, I.; Grimm, B. Clinical validation of a body-fixed 3D accelerometer and algorithm for activity monitoring in orthopaedic patients. J. Orthop. Transl. 2017, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- van Laarhoven, S.N.; Lipperts, M.; Bolink, S.A.; Senden, R.; Heyligers, I.C.; Grimm, B. Validation of a novel activity monitor in impaired, slow-walking, crutch-supported patients. Ann. Phys. Rehabil. Med. 2016, 59, 308–313. [Google Scholar] [CrossRef]
- Kwon, M.-S.; Kwon, Y.-R.; Park, Y.-S.; Kim, J.-W. Comparison of gait patterns in elderly fallers and non-fallers. Technol. Heal. Care 2018, 26, 427–436. [Google Scholar] [CrossRef]
- Palombaro, K.; Craik, R.; Mangione, K.; Tomlinson, J. Determining meaningful changes in gait speed after hip fracture. Phys. Ther. 2006, 6, 809–816. [Google Scholar] [CrossRef]
- Alley, D.E.; Hicks, G.E.; Shardell, M.; Hawkes, W.; Miller, R.; Craik, R.L.; Mangione, K.K.; Orwig, D.; Hochberg, M.; Resnick, B.; et al. Meaningful Improvement in Gait Speed in Hip Fracture Recovery. J. Am. Geriatr. Soc. 2011, 59, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Comfortable and maximum walking speed of adults aged 20–79 years: Reference values and determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bogen, B.; Aaslund, M.K.; Ranhoff, A.H.; Moe-Nilssen, R. Two-year changes in gait variability in community-living older adults. Gait Posture 2019, 72, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.; Fulk, G.D.; Herter, T.M.; Beets, M.W.; Donley, J.; Fritz, S.L. Self-selected and maximal walking speeds provide greater insight into fall status than walking speed reserve among community-dwelling older adults. Am. J. Phys. Med. Rehabil. 2017, 95, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Stähelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; Von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a cut-off point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chang, S.-F.; Kao, C.-Y.; Tsai, H.C. Muscle Strength, Physical Fitness, Balance, and Walking Ability at Risk of Fall for Prefrail Older People. BioMed Res. Int. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Casanova, C.; Celli, B.R.; Barria, P.; Casas, A.; Cote, C.; de Torres, J.P.; Jardim, J.; Lopez, M.V.; Marin, J.M.; de Oca, M.M.; et al. The 6-min walk distance in healthy subjects: Reference standards from seven countries. Eur. Respir. J. 2011, 37, 150–156. [Google Scholar] [CrossRef]
- Mangione, K.K.; Craik, R.L.; Palombaro, K.M.; Tomlinson, S.S.; Hofmann, M.T. Home-Based Leg Strengthening Exercise Improves Function One Year After Hip Fracture: A Randomized Controlled Study. J. Am. Geriatr. Soc. 2010, 58, 1911–1917. [Google Scholar] [CrossRef]
- Hauer, K.; Specht, N.; Schuler, M.; Bärtsch, P.; Oster, P. Intensive physical training in geriatric patients after severe falls and hip surgery. Age Ageing 2002, 31, 49–57. [Google Scholar] [CrossRef]
- Sylliaas, H.; Brovold, T.; Wyller, T.B.; Bergland, A. Prolonged strength training in older patients after hip fracture: A randomised controlled trial. Age Ageing 2012, 41, 206–212. [Google Scholar] [CrossRef]
- Suetta, C.; Magnusson, S.P.; Rosted, A.; Aagaard, P.; Jakobsen, A.K.; Larsen, L.H.; Duus, B.; Kjaer, M. Resistance Training in the Early Postoperative Phase Reduces Hospitalization and Leads to Muscle Hypertrophy in Elderly Hip Surgery Patients—A Controlled, Randomized Study. J. Am. Geriatr. Soc. 2004, 52, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.A.; Houck, J.R.; Drummond, M.J.; Fritz, J.M.; LaStayo, P.C.; Marcus, R.L. Muscle Quality Improves with Extended High-Intensity Resistance Training after Hip Fracture. J. Frailty Aging 2018, l7, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yu-Yahiro, J.A.; Resnick, B.; Orwig, D.; Hicks, G.; Magaziner, J. Design and Implementation of a Home-Based Exercise Program Post-Hip Fracture: The Baltimore Hip Studies Experience. PMR 2009, 1, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Portegijs, E.; Kallinen, M.; Rantanen, T.; Heinonen, A.; Sihvonen, S.; Alen, M.; Kiviranta, I.; Sipilä, S. Effects of Resistance Training on Lower-Extremity Impairments in Older People with Hip Fracture. Arch. Phys. Med. Rehabil. 2008, 89, 1667–1674. [Google Scholar] [CrossRef]
- Magaziner, J.; Hawkes, W.; Hebel, J.R.; Zimmerman, S.I.; Fox, K.M.; Dolan, M.; Felsenthal, G.; Kenzora, J. Recovery From Hip Fracture in Eight Areas of Function. J. Gerontol. Ser. A 2000, 55, M498–M507. [Google Scholar] [CrossRef]
- Fischer, K.; Trombik, M.; Freystätter, G.; Egli, A.; Theiler, R.; Bischoff-Ferrari, H. Timeline of functional recovery after hip fracture in seniors aged 65 and older: A prospective observational analysis. Osteoporos. Int. 2019, 30, 1371–1381. [Google Scholar] [CrossRef]
- Tabori-Jensen, S.; Hansen, T.B.; Bøvling, S.; Aalund, P.; Homilius, M.; Stilling, M. Good function and high patient satisfaction at mean 2.8 years after dual mobility THA following femoral neck fracture: A cross-sectional study of 124 patients. Clin. Interv. Aging 2018, 13, 615–621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).