An Emerging Role of Long Noncoding RNAs as Novel Biomarkers for Breast Cancer Metastasis
Abstract
1. Introduction
1.1. Breast Cancer
1.2. Epidemiology of Breast Cancer in Poland
1.3. Urban–Rural Differences
1.4. New Potential Biomarkers
2. Review Methods
3. Molecular and Functional Insights into Breast Cancer Metastasis
3.1. Mutational Background of Breast Cancer
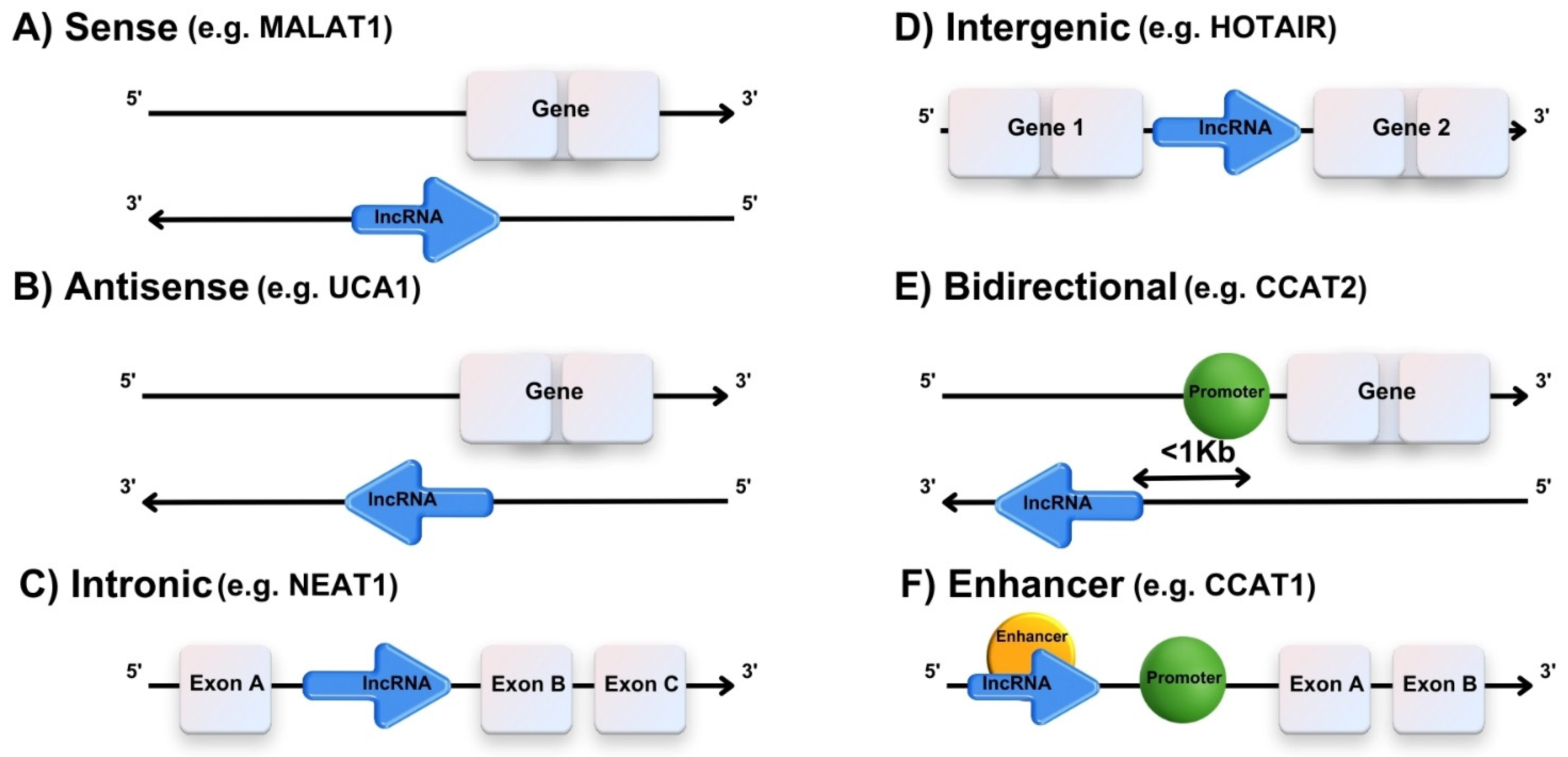
3.2. A Brief Biogenesis of lncRNAs
3.3. Role of lncRNAs Involved in Breast Cancer Metastasis Processes
4. The Role of lncRNAs beyond Metastasis
4.1. Tumor Environment
4.2. Cancer Stem Cell Maintenance
4.3. Immune Modulation
4.4. Drug Resistance
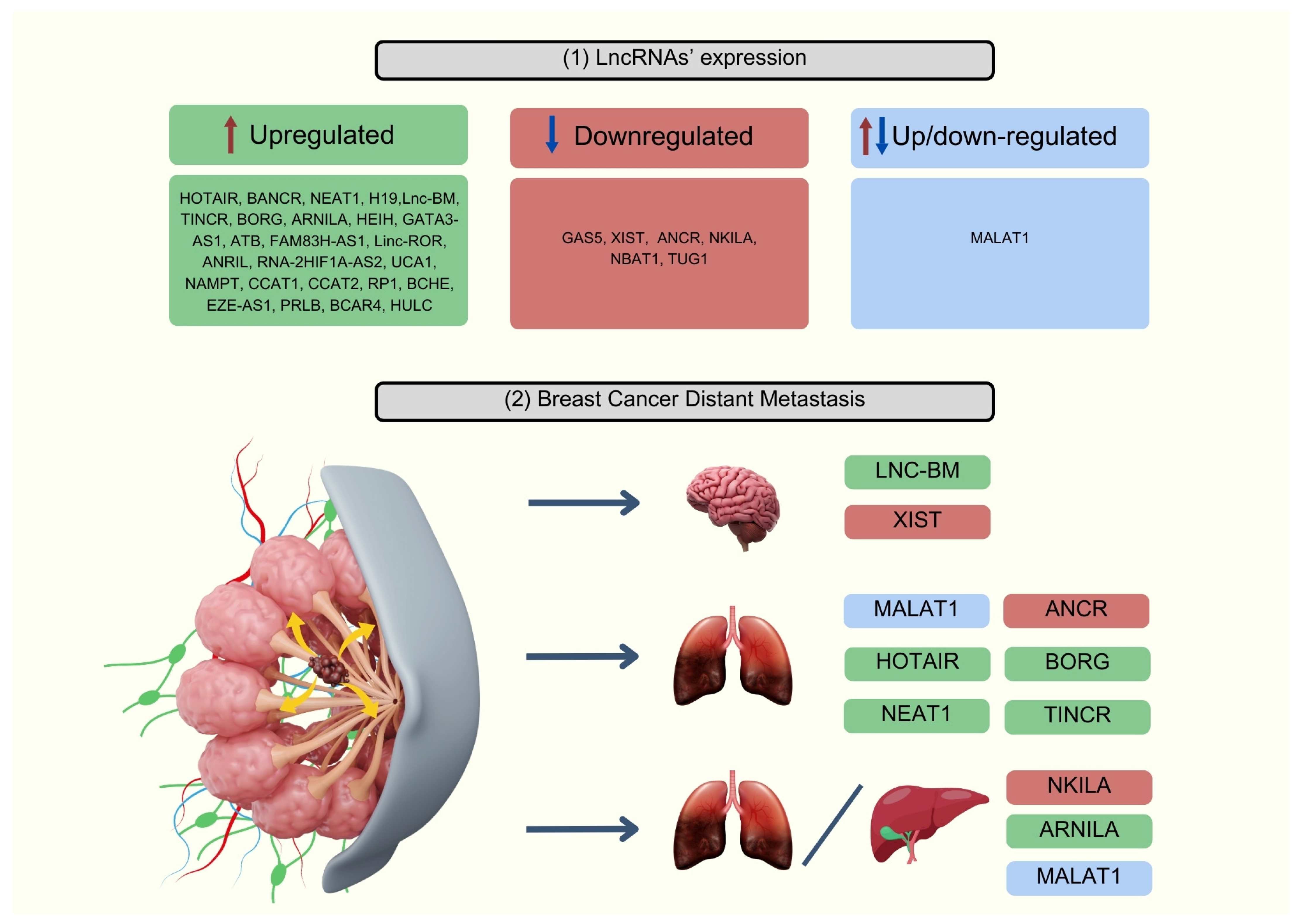
5. Potential lncRNA-Based Biomarkers
5.1. HOTAIR
5.2. MALAT1
5.3. BANCR
5.4. NEAT1
5.5. H19
5.6. GAS5
5.7. HEIH
5.8. TINCR
5.9. GATA3-AS1
5.10. ATB
5.11. Other Potential lncRNA-Based Biomarkers Involved in the Metastatic Progression of Breast Cancer
6. Clinical Utility
6.1. Diagnostic Biomarkers
6.2. Prognostic Indicators
6.3. Therapeutic Targets
7. Conclusions
7.1. Limitations and Challenges
7.2. Future Directions and Application into Clinical Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-thoubaity, F.K. Molecular classification of breast cancer: A retrospective cohort study. Ann. Med. Surg. 2019, 49, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lou, K.-X.; Li, Z.-H.; Wang, P.; Liu, Z.; Chen, Y.; Wang, X.-L.; Cui, H.-X. Long non-coding RNA BANCR indicates poor prognosis for breast cancer and promotes cell proliferation and invasion. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, U.; Barańska, K.; Miklewska, M.; Didkowska, J.A. Cancer incidence and mortality in Poland in 2020. Nowotw. J. Oncol. 2023, 73, 129–145. [Google Scholar] [CrossRef]
- Burzyńska, M.; Maniecka-Bryła, I.; Pikala, M. Trends of mortality due to breast cancer in Poland, 2000–2016. BMC Public Health 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Paszko, A.; Krzyżak, M.J.; Charkiewicz, A.E.; Ziembicka, D.; Żendzian-Piotrowska, M.; Szpak, A.S.; Florek-Łuszczki, M.; Maślach, D. Inequalities in breast cancer incidence and stage distribution between urban and rural female population in Świętokrzyskie Province, Poland. Ann. Agric. Environ. Med. 2019, 26, 159–164. [Google Scholar] [CrossRef]
- Sørensen, K.P.; Thomassen, M.; Tan, Q.; Bak, M.; Cold, S.; Burton, M.; Larsen, M.J.; Kruse, T.A. Long non-coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast Cancer Res. Treat. 2013, 142, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, G.; Li, X.; Ren, C.; Wang, Y.; Li, K.; Mok, H.; Cao, L.; Wen, L.; Jia, M.; et al. Comparison of BRCA versus non-BRCA germline mutations and associated somatic mutation profiles in patients with unselected breast cancer. Aging 2020, 12, 3140–3155. [Google Scholar] [CrossRef] [PubMed]
- Nik-Zainal, S.; Davies, H.; Staaf, J.; Ramakrishna, M.; Glodzik, D.; Zou, X.; Martincorena, I.; Alexandrov, L.B.; Martin, S.; Wedge, D.C.; et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 2016, 534, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mitri, Z.I.; Abuhadra, N.; Goodyear, S.M.; Hobbs, E.A.; Kaempf, A.; Thompson, A.M.; Moulder, S.L. Impact of TP53 mutations in Triple Negative Breast Cancer. NPJ Precis. Oncol. 2022, 6, 64. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Staaf, J.; Glodzik, D.; Bosch, A.; Vallon-Christersson, J.; Reuterswärd, C.; Häkkinen, J.; Degasperi, A.; Amarante, T.D.; Saal, L.H.; Hegardt, C.; et al. Whole-genome sequencing of triple-negative breast cancers in a population-based clinical study. Nat. Med. 2019, 25, 1526–1533. [Google Scholar] [CrossRef]
- Arshi, A.; Sharifi, F.S.; Khorramian Ghahfarokhi, M.; Faghih, Z.; Doosti, A.; Ostovari, S.; Mahmoudi Maymand, E.; Ghahramani Seno, M.M. Expression Analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in Breast Cancer Tissues from Young Women and Women over 45 Years of Age. Mol. Ther. Nucleic Acids 2018, 12, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Assaraf, Y.G.; Gacche, R.N. Long non-coding RNA mediated drug resistance in breast cancer. Drug Resist. Updat. 2022, 63, 100851. [Google Scholar] [CrossRef] [PubMed]
- Collina, F.; Aquino, G.; Brogna, M.; Cipolletta, S.; Buonfanti, G.; De Laurentiis, M.; Di Bonito, M.; Cantile, M.; Botti, G. LncRNA HOTAIR up-regulation is strongly related with lymph nodes metastasis and LAR subtype of Triple Negative Breast Cancer. J. Cancer 2019, 10, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Pádua Alves, C.; Fonseca, A.S.; Muys, B.R.; Barros e Lima Bueno, R.; Bürger, M.C.; Souza, J.E.; Valente, V.; Zago, M.A.; Silva, W.A., Jr. Brief report: The lincRNA Hotair is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells 2013, 31, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Yang, Z.; Chen, B.; Huang, W.; Liu, Y.; Zhang, Y. MiR-204/ZEB2 axis functions as key mediator for MALAT1-induced epithelial-mesenchymal transition in breast cancer. Tumour Biol. 2017, 39, 1010428317690998. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, C.-H.; Hu, H.-Z. LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2819–2824. [Google Scholar] [PubMed]
- Takahashi, K.; Yan, I.K.; Haga, H.; Patel, T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J. Cell Sci. 2014, 127 Pt 7, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jia, H.-H.; Xu, Y.-Q.; Zhou, X.; Zhao, X.-H.; Wang, Y.-F.; Song, X.; Zhu, Z.-Y.; Sun, T.; Dou, Y.; et al. Paracrine and epigenetic control of CAF-induced metastasis: The role of HOTAIR stimulated by TGF-ß1 secretion. Mol. Cancer 2018, 17, 5. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, Y.; Shang, L.; Qiu, Y.; Shen, N.; Wang, J.; Adam, T.; Wei, W.; Song, Q.; Li, J.; et al. LncRNA XIST regulates breast cancer stem cells by activating proinflammatory IL-6/STAT3 signaling. Oncogene 2023, 42, 1419–1437. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, P.; Wang, J.; Shu, Y.; Zhong, X.; Gao, Z.; Yang, J.; Jiang, Y.; Zhou, X.; et al. Long noncoding RNA HOTAIR regulates the stemness of breast cancer cells via activation of the NF-κB signaling pathway. J. Biol. Chem. 2022, 298, 102630. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Hou, Y.; Liu, S.; Zhu, P.; Wan, X.; Zhao, M.; Peng, M.; Zeng, H.; Li, Q.; Jin, T.; et al. A Novel Long Non-Coding RNA lnc030 Maintains Breast Cancer Stem Cell Stemness by Stabilizing SQLE mRNA and Increasing Cholesterol Synthesis. Adv. Sci. 2021, 8, 2002232. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Peng, X.; Shen, C. Identification and validation of immune-related lncRNA prognostic signature for breast cancer. Genomics 2020, 112, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Chen, M.; Chen, H.; Zhong, Q.; Liang, L.; Li, B. lncRNA TCL6 correlates with immune cell infiltration and indicates worse survival in breast cancer. Breast Cancer 2020, 27, 573–585. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Ma, X.; Liu, L.; Liu, J.; Yin, Y.; Li, H.; Chen, Y.; Zhang, X.; Zhang, L.; et al. LncRNA TINCR impairs the efficacy of immunotherapy against breast cancer by recruiting DNMT1 and downregulating MiR-199a-5p via the STAT1-TINCR-USP20-PD-L1 axis. Cell Death Dis. 2023, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yang, Y.A.; Zhang, A.; Fong, K.-W.; Kim, J.; Song, B.; Li, S.; Zhao, J.C.; Yu, J. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene 2016, 35, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qian, J.; Li, J.; Zhu, C. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2019, 18, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Wu, W.-Y.; Dong, M.; Guo, M. LncRNA MALAT1 promotes breast cancer progression and doxorubicin resistance via regulating miR-570-3p. Biomed. J. 2021, 44 (Suppl. S2), S296–S304. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Zang, R.; Zhang, E.; Liu, Y.; Shi, X.; Zhang, E.; Shao, L.; Li, A.; Yang, N.; Han, X.; et al. LncRNA H19 confers chemoresistance in ERα-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget 2016, 7, 81452–81462. [Google Scholar] [CrossRef]
- Contreras-Espinosa, L.; Alcaraz, N.; De La Rosa-Velázquez, I.A.; Díaz-Chávez, J.; Cabrera-Galeana, P.; Rebollar-Vega, R.; Reynoso-Noverón, N.; Maldonado-Martínez, H.A.; González-Barrios, R.; Montiel-Manríquez, R.; et al. Transcriptome Analysis Identifies GATA3-AS1 as a Long Noncoding RNA Associated with Resistance to Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer Patients. J. Mol. Diagn. 2021, 23, 1306–1323. [Google Scholar] [CrossRef]
- Arshi, A.; Raeisi, F.; Mahmoudi, E.; Mohajerani, F.; Kabiri, H.; Fazel, R.; Zabihian-Langeroudi, M.; Jusic, A. A Comparative Study of HOTAIR Expression in Breast Cancer Patient Tissues and Cell Lines. Cell J. 2020, 22, 178–184. [Google Scholar] [CrossRef]
- Gökmen-Polar, Y.; Vladislav, I.T.; Neelamraju, Y.; Janga, S.C.; Badve, S. Prognostic impact of HOTAIR expression is restricted to ER-negative breast cancers. Sci. Rep. 2015, 5, 8765. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; He, H.; Chen, Q. Estradiol induces HOTAIR levels via GPER-mediated miR-148a inhibition in breast cancer. J. Transl. Med. 2015, 13, 131. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Wang, X.; Xie, Y.; Wang, Z.; Xu, Y.; You, X.; Liang, Z.; Cao, H. Circulating DNA of HOTAIR in serum is a novel biomarker for breast cancer. Breast Cancer Res. Treat. 2015, 152, 199–208. [Google Scholar] [CrossRef]
- Lozano-Romero, A.; Astudillo-de la Vega, H.; Terrones-Gurrola, M.C.D.R.; Marchat, L.A.; Hernández-Sotelo, D.; Salinas-Vera, Y.M.; Ramos-Payan, R.; Silva-Cázares, M.B.; Nuñez-Olvera, S.I.; Hernández-de la Cruz, O.N.; et al. HOX Transcript Antisense RNA HOTAIR Abrogates Vasculogenic Mimicry by Targeting the AngiomiR-204/FAK Axis in Triple Negative Breast Cancer Cells. Noncoding RNA 2020, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhou, H.; Lu, K.; Lu, Y.; Wang, Y.; Feng, T. Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer. Onco Targets Ther. 2018, 11, 291–299. [Google Scholar] [CrossRef]
- Huang, X.; Xia, Y.; He, G.; Zheng, L.; Cai, Y.; Yin, Y.; Wu, Q. MALAT1 promotes angiogenesis of breast cancer. Oncol. Rep. 2018, 40, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Jadaliha, M.; Zong, X.; Malakar, P.; Ray, T.; Singh, D.K.; Freier, S.M.; Jensen, T.; Prasanth, S.G.; Karni, R.; Ray, P.S.; et al. Functional and prognostic significance of long non-coding RNA MALAT1 as a metastasis driver in ER negative lymph node negative breast cancer. Oncotarget 2016, 7, 40418–40436. [Google Scholar] [CrossRef]
- Ou, X.; Gao, G.; Bazhabayi, M.; Zhang, K.; Liu, F.; Xiao, X. MALAT1 and BACH1 are prognostic biomarkers for triple-negative breast cancer. J. Cancer Res. Ther. 2019, 15, 1597–1602. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Yang, S.; Yu, F.; Yang, H.; Cheng, Q.; Tang, B. Roles of MALAT1 in development and migration of triple negative and Her-2 positive breast cancer. Oncotarget 2017, 9, 2255–2267. [Google Scholar] [CrossRef]
- Miao, Y.; Fan, R.; Chen, L.; Qian, H. Clinical Significance of Long Non-coding RNA MALAT1 Expression in Tissue and Serum of Breast Cancer. Ann. Clin. Lab. Sci. 2016, 46, 418–424. [Google Scholar]
- Kim, J.; Piao, H.-L.; Kim, B.-J.; Yao, F.; Han, Z.; Wang, Y.; Xiao, Z.; Siverly, A.N.; Lawhon, S.E.; Ton, B.N.; et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat. Genet. 2018, 50, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Z.H.; Roche, V.; Chew, X.H.; Fadieieva, A.; Tay, Y. A non-canonical tumor suppressive role for the long non-coding RNA MALAT1 in colon and breast cancers. Int. J. Cancer 2018, 143, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sui, S.; Zhang, J.; Bai, N.; Shi, Q.; Zhang, G.; Gao, S.; You, Z.; Zhan, C.; Liu, F.; et al. Downregulation of long noncoding RNA MALAT1 induces epithelial-to-mesenchymal transition via the PI3K-AKT pathway in breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 4881–4891. [Google Scholar] [PubMed]
- Meseure, D.; Vacher, S.; Lallemand, F.; Alsibai, K.D.; Hatem, R.; Chemlali, W.; Nicolas, A.; De Koning, L.; Pasmant, E.; Callens, C.; et al. Prognostic value of a newly identified MALAT1 alternatively spliced transcript in breast cancer. Br. J. Cancer 2016, 114, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, S.-H.; Li, X.-J.; Sun, L.; Ge, Q.-D.; Li, C.; Zhang, W. Long non-coding RNA BRAF-regulated lncRNA 1 promotes lymph node invasion, metastasis and proliferation, and predicts poor prognosis in breast cancer. Oncol. Lett. 2018, 15, 9543–9552. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, E.; Lellahi, S.M.; Aure, M.R.; Nord, S.; Fismen, S.; Larsen, K.B.; Gabriel, M.T.; Hedberg, A.; Bjørklund, S.S.; Geisler, J.; et al. The expression of the long NEAT1_2 isoform is associated with human epidermal growth factor receptor 2-positive breast cancers. Sci. Rep. 2020, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Shin, V.Y.; Chen, J.; Cheuk, I.W.Y.; Siu, M.T.; Ho, C.W.; Wang, X.; Jin, H.; Kwong, A. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Li, Z.; Long, X.; Guo, Z.; Zhang, G.; Zu, J.; Chen, Y.; Wen, L. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int. J. Biol. Macromol. 2017, 105 Pt 1, 346–353. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Liu, X.; Cheng, X.; Zhang, Y.; Han, X.; Zhang, Y.; Liu, S.; Yang, J.; Xu, B.; et al. The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J. Clin. Investig. 2017, 127, 3421–3440. [Google Scholar] [CrossRef]
- Lo, P.-K.; Zhang, Y.; Wolfson, B.; Gernapudi, R.; Yao, Y.; Duru, N.; Zhou, Q. Dysregulation of the BRCA1/long non-coding RNA NEAT1 signaling axis contributes to breast tumorigenesis. Oncotarget 2016, 7, 65067–65089. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H.; Albukhari, A.; Morotti, M.; Haider, S.; Moralli, D.; Smythies, J.; Schödel, J.; Green, C.M.; Camps, C.; Buffa, F.; et al. Tumor hypoxia induces nuclear paraspeckle formation through HIF-2α dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene 2015, 34, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Peperstraete, E.; Lecerf, C.; Collette, J.; Vennin, C.; Raby, L.; Völkel, P.; Angrand, P.-O.; Winter, M.; Bertucci, F.; Finetti, P.; et al. Enhancement of Breast Cancer Cell Aggressiveness by lncRNA H19 and its Mir-675 Derivative: Insight into Shared and Different Actions. Cancers 2020, 12, 1730. [Google Scholar] [CrossRef]
- Lin, Y.; Fu, F.; Chen, Y.; Qiu, W.; Lin, S.; Yang, P.; Huang, M.; Wang, C. Genetic variants in long noncoding RNA H19 contribute to the risk of breast cancer in a southeast China Han population. Onco Targets Ther. 2017, 10, 4369–4378. [Google Scholar] [CrossRef] [PubMed]
- Vennin, C.; Spruyt, N.; Dahmani, F.; Julien, S.; Bertucci, F.; Finetti, P.; Chassat, T.; Bourette, R.P.; Le Bourhis, X.; Adriaenssens, E. H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-b. Oncotarget 2015, 6, 29209–29223. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Chen, P.; Li, H.; Wang, X. Long non-coding RNA H19 regulates cell growth and metastasis via miR-138 in breast cancer. Am. J. Transl. Res. 2019, 11, 3213–3225. [Google Scholar]
- Zhang, K.; Luo, Z.; Zhang, Y.; Zhang, L.; Wu, L.; Liu, L.; Yang, J.; Song, X.; Liu, J. Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 2016, 17, 187–194. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, K.; Li, J.; Xiao, S.; Wei, W.; Liu, J. Determination of Serum Exosomal H19 as a Noninvasive Biomarker for Breast Cancer Diagnosis. Onco Targets Ther. 2020, 13, 2563–2571. [Google Scholar] [CrossRef]
- Dong, L.; Li, G.; Li, Y.; Zhu, Z. Upregulation of Long Noncoding RNA GAS5 Inhibits Lung Cancer Cell Proliferation and Metastasis via miR-205/PTEN Axis. Med. Sci. Monit. 2019, 25, 2311–2319. [Google Scholar] [CrossRef]
- Li, W.; Zhai, L.; Wang, H.; Liu, C.; Zhang, J.; Chen, W.; Wei, Q. Downregulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer. Oncotarget 2016, 7, 27778–27786. [Google Scholar] [CrossRef]
- Li, S.; Zhou, J.; Wang, Z.; Wang, P.; Gao, X.; Wang, Y. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed. Pharmacother. 2018, 104, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, M.; Miao, K.; Xu, H. lncRNA GAS5-promoted apoptosis in triple-negative breast cancer by targeting miR-378a-5p/SUFU signaling. J. Cell Biochem. 2020, 121, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qian, L.; Tang, X.; Chen, Y.; Zhao, Z.; Zhang, C. Long non-coding RNA growth arrest-specific 5 (GAS5) acts as a tumor suppressor by promoting autophagy in breast cancer. Mol. Med. Rep. 2020, 22, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ma, P.; Liu, S.-M.; Zhou, X. Circulating long noncoding RNA GAS5 as a potential biomarker in breast cancer for assessing the surgical effects. Tumour Biol. 2016, 37, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; El-Wazir, A.; Ageeli, E.A.; Hussein, M.H.; Eltoukhy, M.M.; Killackey, M.T.; Kandil, E.; Fawzy, M.S. Unleash multifunctional role of long noncoding RNAs biomarker panel in breast cancer: A predictor classification model. Epigenomics 2020, 12, 1215–1237. [Google Scholar] [CrossRef] [PubMed]
- Kanabe, B.O.; Ozaslan, M.; Aziz, S.A.; Al-Attar, M.S.; Kılıç, İ.H.; Khailany, R.A. Expression patterns of LncRNA-GAS5 and its target APOBEC3C gene through miR-103 in breast cancer patients. Cell. Mol. Biol. 2021, 67, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Nafea, H.; Youness, R.A.; Abou-Aisha, K.; Gad, M.Z. LncRNA HEIH/miR-939-5p interplay modulates triple-negative breast cancer progression through NOS2-induced nitric oxide production. J. Cell. Physiol. 2021, 236, 5362–5372. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, B.; Lv, Y.; Qian, Q. LncRNA HEIH regulates cell proliferation and apoptosis through miR-4458/SOCS1 axis in triple-negative breast cancer. Hum. Cell 2019, 32, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Meng, R.; Yao, Q.; Wang, H.; Niu, J.; Cui, Y.U.; Chen, S.; Bai, Y. Long non-coding RNA HEIH promotes breast cancer development via negative modulation of microRNA-200b. Die Pharm.-Int. J. Pharm. Sci. 2019, 74, 471–476. [Google Scholar] [CrossRef]
- Chen, C.; Gu, C.; Ren, Q.; Ding, F.; Pan, Q.; Niu, Y.; Ma, D.; Wu, L. lncRNA HEIH, an indicator of high malignancy and poor prognosis, functions as an oncogene in breast cancer. Mol. Med. Rep. 2020, 22, 2869–2877. [Google Scholar] [CrossRef]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Azman, A.A.; Siok-Fong, C.; Rajab, N.F.; Md Zin, R.R.; Ahmad Daud, N.N.; Mohamad Hanif, E.A. The potential roles of lncRNA TINCR in triple negative breast cancer. Mol. Biol. Rep. 2023, 50, 7909–7917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; You, Z.; Yin, Y.; Liu, L.; Kang, Y.; Li, S.; Ning, S.; Li, H.; Gong, Y.; et al. LncRNA TINCR favors tumorigenesis via STAT3–TINCR–EGFR-feedback loop by recruiting DNMT1 and acting as a competing endogenous RNA in human breast cancer. Cell Death Dis. 2021, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.R.; Shaginurova, G.; Kim, L.C.; Chapman, N.; Spurlock, C.F.; Aune, T.M. Divergent lncRNA GATA3-AS1 Regulates GATA3 Transcription in T-Helper 2 Cells. Front. Immunol. 2018, 9, 2512. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Huang, W.; Yang, Y.; Qiu, R.; Zeng, Y.; Hou, Y.; Sun, G.; Shi, H.; Leng, S.; Feng, D.; et al. GATA3 recruits UTX for gene transcriptional activation to suppress metastasis of breast cancer. Cell Death Dis. 2019, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, N.; Song, P.; Fu, Y.; Ren, Y.; Li, Z.; Wang, J. LncRNA GATA3-AS1 facilitates tumour progression and immune escape in triple-negative breast cancer through destabilization of GATA3 but stabilization of PD-L1. Cell Prolif. 2020, 53, e12855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Jia, S.; Wang, Y.; Kang, Y.; Zhang, W. Down-regulation of lncRNA-ATB inhibits epithelial-mesenchymal transition of breast cancer cells by increasing miR-141-3p expression. Biochem. Cell Biol. 2019, 97, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-H.; Chen, M.; Liu, J.; Shao, C.-C.; Guo, C.-P.; Wei, X.-L.; Li, Y.-C.; Huang, W.-H.; Zhang, G.-J. Long noncoding RNA ATB promotes the epithelial-mesenchymal transition by upregulating the miR-200c/Twist1 axe and predicts poor prognosis in breast cancer. Cell Death Dis. 2018, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xiong, H.; Zhao, Y.; Dong, Z.; Liu, H.; Shi, F. Study on the Expression of lncRNA ATB and Nek9 in Breast Cancer Patients Based on Q-PCR Technology and Its Relationship with the Disease. Contrast Media Mol. Imaging 2022, 2022, 2634080. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Hussien, F.Z.; El-Feky, O.A.; Hamouda, S.M.; Al-Ashmawy, G.M. Serum LncRNA-ATB and FAM83H-AS1 as diagnostic/prognostic non-invasive biomarkers for breast cancer. Life Sci. 2020, 259, 118193. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Y.; Wu, S.-Y.; Wu, K.; Sharma, S.; Mo, Y.-Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, K.; Hu, Q.; Li, P.; Song, J.; Yang, Y.; Yao, J.; Mangala, L.S.; Li, C.; Yang, W.; et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J. Clin. Investig. 2017, 127, 4498–4515. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, P.; Fan, D.; Dong, M.; Ma, M.; Li, H.; Yao, R.; Li, Y.; Wang, G.; Geng, P.; et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017, 24, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Gooding, A.J.; Zhang, B.; Jahanbani, F.K.; Gilmore, H.L.; Chang, J.C.; Valadkhan, S.; Schiemann, W.P. The lncRNA BORG Drives Breast Cancer Metastasis and Disease Recurrence. Sci. Rep. 2017, 7, 12698. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.A.; Gooding, A.J.; Valadkhan, S.; Schiemann, W.P. lncRNA BORG:TRIM28 Complexes Drive Metastatic Progression by Inducing α6 Integrin/CD49f Expression in Breast Cancer Stem Cells. Mol. Cancer Res. MCR 2021, 19, 2068–2080. [Google Scholar] [CrossRef]
- Liu, B.; Sun, L.; Liu, Q.; Gong, C.; Yao, Y.; Lv, X.; Lin, L.; Yao, H.; Su, F.; Li, D.; et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 2015, 27, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, F.; Cui, X.; Yang, L.; Chen, J.; Zhao, J.; Huang, D.; Liu, J.; Yang, L.; Zeng, J.; et al. LncRNA NKILA suppresses TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB signaling in breast cancer. Int. J. Cancer 2018, 143, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shen, Y.; Zhang, W.; Jin, J.; Huang, D.; Fang, H.; Ji, W.; Shi, Y.; Tang, L.; Chen, W.; et al. An androgen receptor negatively induced long non-coding RNA ARNILA binding to miR-204 promotes the invasion and metastasis of triple-negative breast cancer. Cell Death Differ. 2018, 25, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhao, Y.; Li, Z.; Yao, R.; Ma, M.; Gao, Y.; Zhao, L.; Zhang, Y.; Huang, B.; Lu, J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014, 5, e1287. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Liu, Y.; Wei, H.-Y.; Lv, K.-Z.; Fu, P. Linc-ROR induces epithelial-mesenchymal transition and contributes to drug resistance and invasion of breast cancer cells. Tumour Biol. 2016, 37, 10861–10870. [Google Scholar] [CrossRef]
- Liu, M.; Xing, L.-Q.; Liu, Y.-J. A three-long noncoding RNA signature as a diagnostic biomarker for differentiating between triple-negative and non-triple-negative breast cancers. Medicine 2017, 96, e6222. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Wang, W.; Sun, J.-Y.; Xin, B.; Zhang, X.; Wang, T.; Zhang, Q.-F.; Yao, L.-B.; Han, H.; Fan, D.-M.; et al. Long non-coding RNAs AC026904.1 and UCA1: A “one-two punch” for TGF-β-induced SNAI2 activation and epithelial-mesenchymal transition in breast cancer. Theranostics 2018, 8, 2846–2861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Liu, Y.; Su, P.; Liang, Y.; Li, Y.; Wang, X.; Chen, T.; Song, X.; Sang, Y.; et al. Epigenetic Regulation of NAMPT by NAMPT-AS Drives Metastatic Progression in Triple-Negative Breast Cancer. Cancer Res. 2019, 79, 3347–3359. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, X.; Fan, Q.; Liu, G.; Yin, J. CCAT1 promotes triple-negative breast cancer progression by suppressing miR-218/ZFX signaling. Aging 2019, 11, 4858–4875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, T.; Li, Y.; Li, S. Overexpression of long non-coding RNA CCAT1 is a novel biomarker of poor prognosis in patients with breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9440–9445. [Google Scholar] [PubMed]
- Xu, Z.; Liu, C.; Zhao, Q.; Lü, J.; Ding, X.; Luo, A.; He, J.; Wang, G.; Li, Y.; Cai, Z.; et al. Long non-coding RNA CCAT2 promotes oncogenesis in triple-negative breast cancer by regulating stemness of cancer cells. Pharmacol. Res. 2020, 152, 104628. [Google Scholar] [CrossRef]
- Jia, X.; Shi, L.; Wang, X.; Luo, L.; Ling, L.; Yin, J.; Song, Y.; Zhang, Z.; Qiu, N.; Liu, H.; et al. KLF5 regulated lncRNA RP1 promotes the growth and metastasis of breast cancer via repressing p27kip1 translation. Cell Death Dis. 2019, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Wei, L.; Zhang, Y.-J.; Hayano, T.; Piñeiro Pereda, M.D.P.; Nakaoka, H.; Li, Q.; Barragán Mallofret, I.; Lu, Y.-Z.; Tamagnone, L.; et al. Long non-coding RNA p10247, high expressed in breast cancer (lncRNA-BCHE), is correlated with metastasis. Clin. Exp. Metastasis 2018, 35, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Chu, J.; Wu, Y.; Sun, L.; Lv, X.; Zhu, Y.; Li, J.; Guo, Q.; Gong, C.; Liu, B.; et al. NBAT1 suppresses breast cancer metastasis by regulating DKK1 via PRC2. Oncotarget 2015, 6, 32410–32425. [Google Scholar] [CrossRef]
- Fan, S.; Yang, Z.; Ke, Z.; Huang, K.; Liu, N.; Fang, X.; Wang, K. Downregulation of the long non-coding RNA TUG1 is associated with cell proliferation, migration, and invasion in breast cancer. Biomed. Pharmacother. 2017, 95, 1636–1643. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, X.; Huang, L.; Wan, Y.; Li, X.; Wang, Y. Long noncoding RNA EZR-AS1 promotes tumor growth and metastasis by modulating Wnt/β-catenin pathway in breast cancer. Exp. Ther. Med. 2018, 16, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, X.; Li, Y.; Sang, Y.; Zhang, N.; Zhang, H.; Liu, Y.; Duan, Y.; Chen, B.; Guo, R.; et al. A novel long non-coding RNA-PRLB acts as a tumor promoter through regulating miR-4766-5p/SIRT1 axis in breast cancer. Cell Death Dis. 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Lin, A.; Li, C.; Liang, K.; Wang, S.; Liu, Y.; Park, P.K.; Qin, L.; Wei, Y.; Hawke, D.H.; et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell 2014, 159, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhong, C.; Fu, M.; Li, L.; Wang, F.; Lv, P.; Zhu, M.; Xiong, Y.; Mi, H.; Gu, Y. Long Non-Coding RNA HULC Promotes the Development of Breast Cancer Through Regulating LYPD1 Expression by Sponging miR-6754-5p. Onco Targets Ther. 2019, 12, 10671–10679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, H.; Sun, T.; Wen, X.; Niu, C.; Li, M.; Li, W.; Hoffman, A.R.; Hu, J.-F.; Cui, J. HULC targets the IGF1R-PI3K-AKT axis in trans to promote breast cancer metastasis and cisplatin resistance. Cancer Lett. 2022, 548, 215861. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Teng, X.; Li, J.; Liang, X.-J. Antisense Oligonucleotide-Conjugated Nanostructure-Targeting lncRNA MALAT1 Inhibits Cancer Metastasis. ACS Appl. Mater. Interfaces 2019, 11, 37–42. [Google Scholar] [CrossRef]
- Shaath, H.; Vishnubalaji, R.; Elango, R.; Khattak, S.; Alajez, N.M. Single-cell long noncoding RNA (lncRNA) transcriptome implicates MALAT1 in triple-negative breast cancer (TNBC) resistance to neoadjuvant chemotherapy. Cell Death Discov. 2021, 7, 23. [Google Scholar] [CrossRef]
- Pickard, M.R.; Williams, G.T. Regulation of apoptosis by long non-coding RNA GAS5 in breast cancer cells: Implications for chemotherapy. Breast Cancer Res. Treat. 2014, 145, 359–370. [Google Scholar] [CrossRef]
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy. Bioconjug. Chem. 2019, 30, 907–919. [Google Scholar] [CrossRef]
- Milevskiy, M.J.G.; Al-Ejeh, F.; Saunus, J.M.; Northwood, K.S.; Bailey, P.J.; Betts, J.A.; McCart Reed, A.E.; Nephew, K.P.; Stone, A.; Gee, J.M.W.; et al. Long-range regulators of the lncRNA HOTAIR enhance its prognostic potential in breast cancer. Hum. Mol. Genet. 2016, 25, 3269–3283. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Liu, Y.; Sun, L.; Chen, B.; Wang, C.; Chen, T.; Wang, Y.; Li, Y.; Dong, Q.; et al. Individualized lncRNA differential expression profile reveals heterogeneity of breast cancer. Oncogene 2021, 40, 4604–4614. [Google Scholar] [CrossRef] [PubMed]
| Studied lncRNA | Authors, Year | Study Group | Study Material | lncRNA Change | Diagnostic Usefulness | References |
|---|---|---|---|---|---|---|
| HOTAIR | Zhang et al. (2015) | BC patients (n = 24) for the discovery; BC patients (n = 76) for validation | Serum | Upregulated | AUC: 0.799 | [34] |
| MALAT1 | Miao et al. (2016) | BC patients (n = 78); MDA-MB-231 cell line | Cancerous tissue samples; adjacent no-tumor samples; serum; cell line | Upregulated | AUC: 0.833 | [41] |
| MALAT1 | Xu et al. (2015) | BC patients (n = 78); MDA-MB-231, MDA-MB-453 cell lines | Cancerous tissue samples; adjacent no-tumor samples; cell lines | Downregulated | Strong correlation with axillary LNM | [44] |
| BANCR | Jiang et al. (2018) | BC patients (n = 65); MCF-7, MDA-MB-231, SKBR3, BT-20, MCF-10A cell lines | Cancerous tissue samples; adjacent no-tumor samples; cell lines | Upregulated | Larger tumor size, LNM, advanced TNM stage. | [46] |
| NEAT1 | Shin et al. (2019) | BC patients (n = 179) and normal controls (n = 192); MDA-MB-231 cell line | Serum; cell line | Upregulated | Particularly high expression in TNBC. | [48] |
| H19 | Zhong et al. (2020) | BC patients (n = 50), BBD patients (n = 50), healthy volunteers (n = 50) | Serum | Upregulated | AUC: 0.807 | [58] |
| GAS5 | Toraih et al. (2020) | BC patients (n = 50), non-cancer patients with at least one BC risk factor (n = 50), and healthy volunteers (n = 65) | Plasma | Downregulated | AUC: 0.754 | [65] |
| HEIH | Chen et al. (2020) | BC patients (n = 160), MCF-10A, MCF-7, SK-BR-3, MDA-MB-231, MDA-MB-468 cell lines | Cancerous tissue samples; adjacent no-tumor samples; cell lines | Upregulated | LNM, advanced TNM stage, independent 3-year OS indicator | [70] |
| TINCR | Wang et al. (2023) | BC patients; UACC812, MDA-MB-231, Hs578T, T47D, BT549, MCF-7, 4T1 cell lines; female BALB/c mice | Cancerous tissue samples; adjacent no-tumor samples; healthy breast tissues; cell lines; BALB/c mice | Upregulated | Positive correlation with PD-L1 expression and metastasis | [25] |
| GATA3-AS1 | Zhang et al. (2020) | TNBC patients (n = 68); MDA-MB-468, MDA-MB-436, MDA-MB-231, HCC1937, MCF-10A; Nude mice | Cancerous tissue samples; adjacent no-tumor samples; nude mice | Upregulated | Larger tumor size, LNM, advanced TNM stage, positive correlation with PD-L1 expression | [76] |
| ATB, FAM83H-AS1 | El-Ashmawy et al. (2020) | BC patients (n = 90), healthy volunteers | Serum | Upregulated | ATB: AUC of 0.844 FAM83H-AS1: larger tumor size, LNM, advanced TNM stage | [80] |
| XIST | Xing et al. (2018) | BC patients; MCF7, ZR75-1, SKBR3, MDA-MB-231, SIM-A9 cell lines; nude mice and transgenic mouse strains | Cancerous tissue samples; brain metastasis samples cell lines; nude mice | Downregulated | Negative correlation with brain metastasis | [81] |
| ANCR | Li et al. (2017) | BC patients (n = 32) MCF7, T47D, BT474, MDA-MB-436, MDA-MB-23 MDA-MB-231HM cell lines; BALB/c nude mice | Cancerous tissue samples; adjacent no-tumor samples; nude mice | Downregulated | Negative correlation with lung metastasis | [83] |
| NKILA | Liu et al. (2015) | BC patients (n = 1107), healthy volunteers; MDA-MB-231, MCF7, ZR-75-1, T47D, MDA-MB-453, and BT-474 cell lines; female athymic nude mice | Cancerous tissue samples; adjacent no tumor samples; healthy breast tissues; athymic nude mice | Downregulated | Negative correlation with lung and liver metastasis | [86] |
| ARNILA | Yang et al. (2018) | TNBC patients (n = 88); MDA-MB-231, MDA-MB-436, Hs578T cell lines; BALB/c nude mice | Cancerous tissue samples; adjacent no-tumor samples; BALB/c nude mice | Upregulated | Positive correlation with lung and liver metastasis; indicator of PFS | [88] |
| Linc-ROR | Chen et al. (2016) | BC patients (n = 142); MCF10A, SK-BR-3, MCF-7, Bcap-37, MDA-MB-231, T47D cell lines | Cancerous tissue samples; adjacent no-tumor samples | Upregulated | Strong correlation with LNM | [90] |
| ANRIL, RNA-2HIF1A-AS2, UCA1 | Liu et al. (2017) | BC patients (n = 60), healthy volunteers (n = 40) | Serum; cancerous tissue samples; adjacent no-tumor samples | Upregulated | AUC: ANRIL 0.785, RNA-2HIF1A-AS2 0.739, UCA1 0.817, combined 0.934 | [91] |
| NAMPT | Zhang et al. (2019) | BC patients; MDA-MB-231, MDA-MB-468, MCF-7, SKBR3 cell lines; BALB/c nude mice | Cancerous tissue samples; adjacent no-tumor samples; BALB/c nude mice | Upregulated | Significant association with younger age, larger tumors, LNM, negative ER/PR status, TNBC subtype, distant metastasis, and advanced stage | [93] |
| CCAT1 | Han et al. (2019) | BC patients (n = 92) | Cancerous tissue samples; adjacent no-tumor samples | Upregulated | Significant association with differentiation grade, TNM stage, LNM, independent predictor of OS and PFS | [95] |
| CCAT2 | Xu et al. (2020) | BC patients; MDA-MB-231, MCF-7, T-47D, Hs578t, 4173, 4175, Src-transformed MCF-10A; female nude mice | Cancerous tissue samples; adjacent no-tumor samples; healthy breast tissues; cell lines; nude mice | Upregulated | Indicator of tumor progression in TNBC | [96] |
| RP1 | Jia et al. (2019) | BC patients (n = 54); HEK-293T, MCF-7, T47D, SKBR3, MDA-MB-231, BT549, HCC38, HCC1937, MCF-10A cell lines; nude mice | Cancerous tissue samples; adjacent no-tumor samples; cell lines; nude mice | Upregulated | Higher expression in TNBC; positive correlation with tumor size, LNM, TNM stage, and distant metastasis | [97] |
| BCHE | Yang et al. (2018) | BC patients (n = 56); MDA-MB-231, MCF-7, MDA-MB-468 cell lines | Cancerous tissue samples; adjacent no-tumor samples; cell lines | Upregulated | Strong correlation with LNM and advanced stage | [98] |
| NBAT1 | Hu et al. (2015) | BC patients (n = 716 from TCGA database) | Cancerous tissue samples; adjacent no-tumor samples | Downregulated | Lower levels with significant correlation with LNM and post-menopausal status | [99] |
| TUG1 | Fan et al. (2017) | BC patients (n = 58); MDA-MB-231, MDA-MB-453, MDA-MB-468, T-47D, MCF-7, ZR-75, SK-BR-3, MCF-10A cell lines | Cancerous tissue samples; adjacent no-tumor samples; cell lines | Downregulated | Lower levels with significant correlation with LNM | [100] |
| EZR-AS1 | Bai et al. (2018) | BC patients (n = 50) MCF-10A, MCF7, MDA-MB-231, MDA-MB-468, SKBR-3 cell lines | Cancerous tissue samples; adjacent no-tumor samples; cell lines | Upregulated | Positive correlation with metastatic ability | [101] |
| PRLB | Liang et al. (2018) | BC patients (n = 18) MDA-MB-231, MDA-MB-468, MCF7 cell lines; female nude mice | Cancerous tissue samples; adjacent no-tumor samples; cell lines; nude mice | Upregulated | Positive correlation with lung metastasis | [102] |
| BCAR4 | Xing et al. (2014) | BC patients (n = 160); MDA-MB-231 LM2 cell line; mice | Cancerous tissue samples; adjacent no-tumor samples; cell lines; mice | Upregulated | Strong correlation with TNBC, LNM, and 5-year recurrence | [103] |
| HULC | Wang et al. (2019) | BC patients (n = 60); MCF-7, ZR-75-1, BT-20, MDA-MB-231, MCF-10A cell lines | Cancerous tissue samples; adjacent no-tumor samples; cell lines | Upregulated | Positive correlation with LNM and advanced stages | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derlatka, K.; Kulczycka, M.; Prendecka-Wróbel, M.; Homa-Mlak, I.; Małecka-Massalska, T. An Emerging Role of Long Noncoding RNAs as Novel Biomarkers for Breast Cancer Metastasis. Appl. Sci. 2024, 14, 6667. https://doi.org/10.3390/app14156667
Derlatka K, Kulczycka M, Prendecka-Wróbel M, Homa-Mlak I, Małecka-Massalska T. An Emerging Role of Long Noncoding RNAs as Novel Biomarkers for Breast Cancer Metastasis. Applied Sciences. 2024; 14(15):6667. https://doi.org/10.3390/app14156667
Chicago/Turabian StyleDerlatka, Kamila, Marika Kulczycka, Monika Prendecka-Wróbel, Iwona Homa-Mlak, and Teresa Małecka-Massalska. 2024. "An Emerging Role of Long Noncoding RNAs as Novel Biomarkers for Breast Cancer Metastasis" Applied Sciences 14, no. 15: 6667. https://doi.org/10.3390/app14156667
APA StyleDerlatka, K., Kulczycka, M., Prendecka-Wróbel, M., Homa-Mlak, I., & Małecka-Massalska, T. (2024). An Emerging Role of Long Noncoding RNAs as Novel Biomarkers for Breast Cancer Metastasis. Applied Sciences, 14(15), 6667. https://doi.org/10.3390/app14156667






