Impact of 50 Hz Electromagnetic Field on the Growth of Chlorella vulgaris
Abstract
1. Introduction
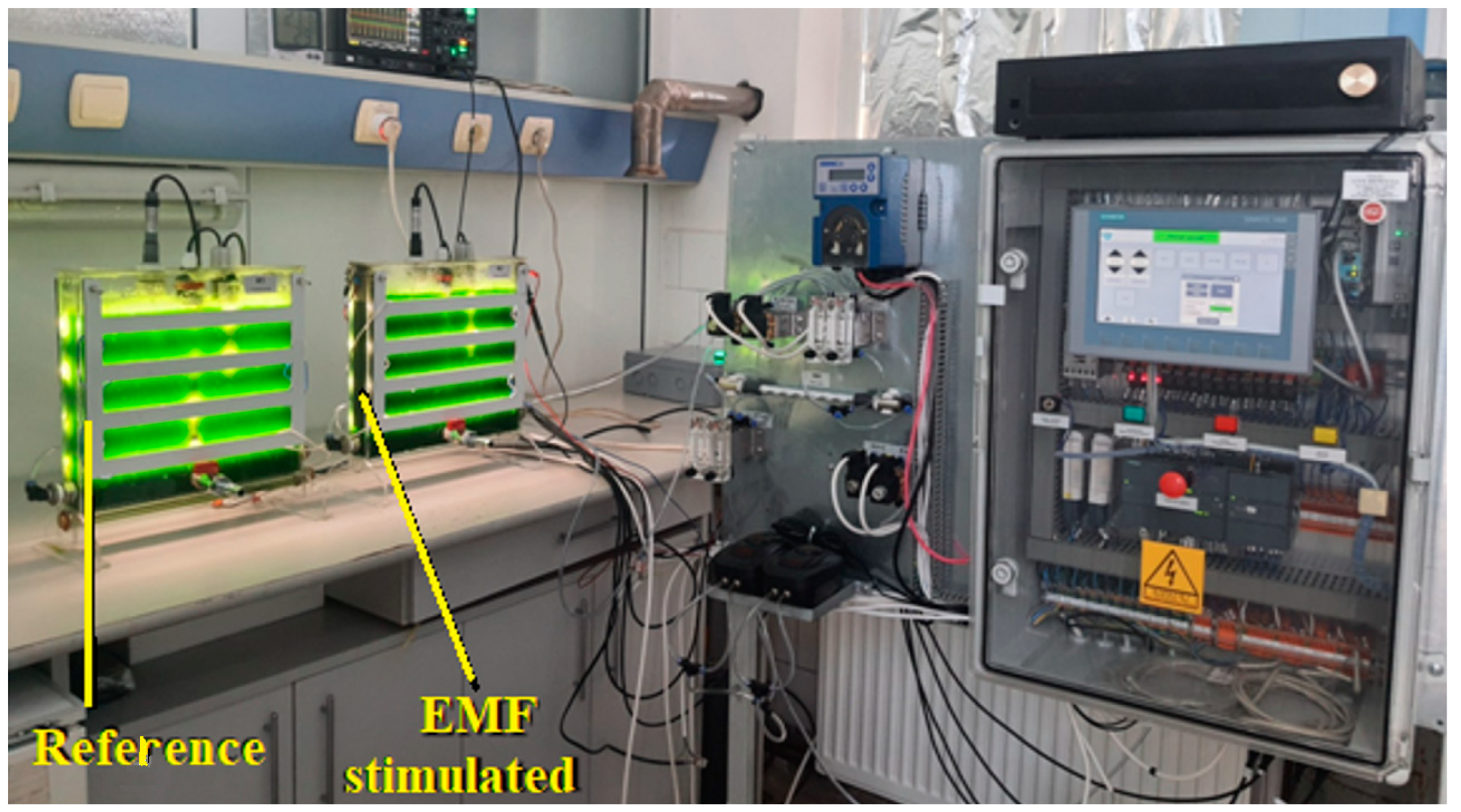
2. Materials and Methods
- -
- Evolution over time of pH was monitored by a pH sensor S401 DIG/N type, produced by Chemitec.
- -
- -
- Evolution over time of algal mass concentration by filtering 100 mL of algal suspension on pre-weighted glass fiber filter paper (ROTILABO®, CR261, Ø 47 mm, pore Ø 1.2 μm) washed with double distilled water, dried at 100 °C for 4 h, and cooled in a desiccator. The weight difference (measured with a Precisa balance, XR 125SM) after drying and cooling represented the dry weight (dw) of the microalgae.
- -
- The time evolution of dissolved oxygen in the culture medium by measurements performed with a type S423/C/OPT oxygen transducer manufactured by Chemitec, Florence, Italy.
- -
- The consumption of nutrients from the culture medium through analyses of samples collected before inoculation and after 96 h (4 days) of growth for the following: a. nitrogen concentrations in nitrate (N-NO3—Cuvette test LCK 340, measurement range 5–35 mg/L N-NO3, HACH, Berlin, Germany); b. total nitrogen (TNb—Cuvette test LCK 238—measurement range 5–40 mg/L TNb, HACH, Berlin, Germany); c. total phosphorus (P-PO4—Cuvette test LCK 350—range 2–20 mg/L P-PO4 and LCK 348—range 0.5–5 mg/L P-PO4, HACH, Berlin, Germany).
3. Results and Discussions
4. Conclusions
- -
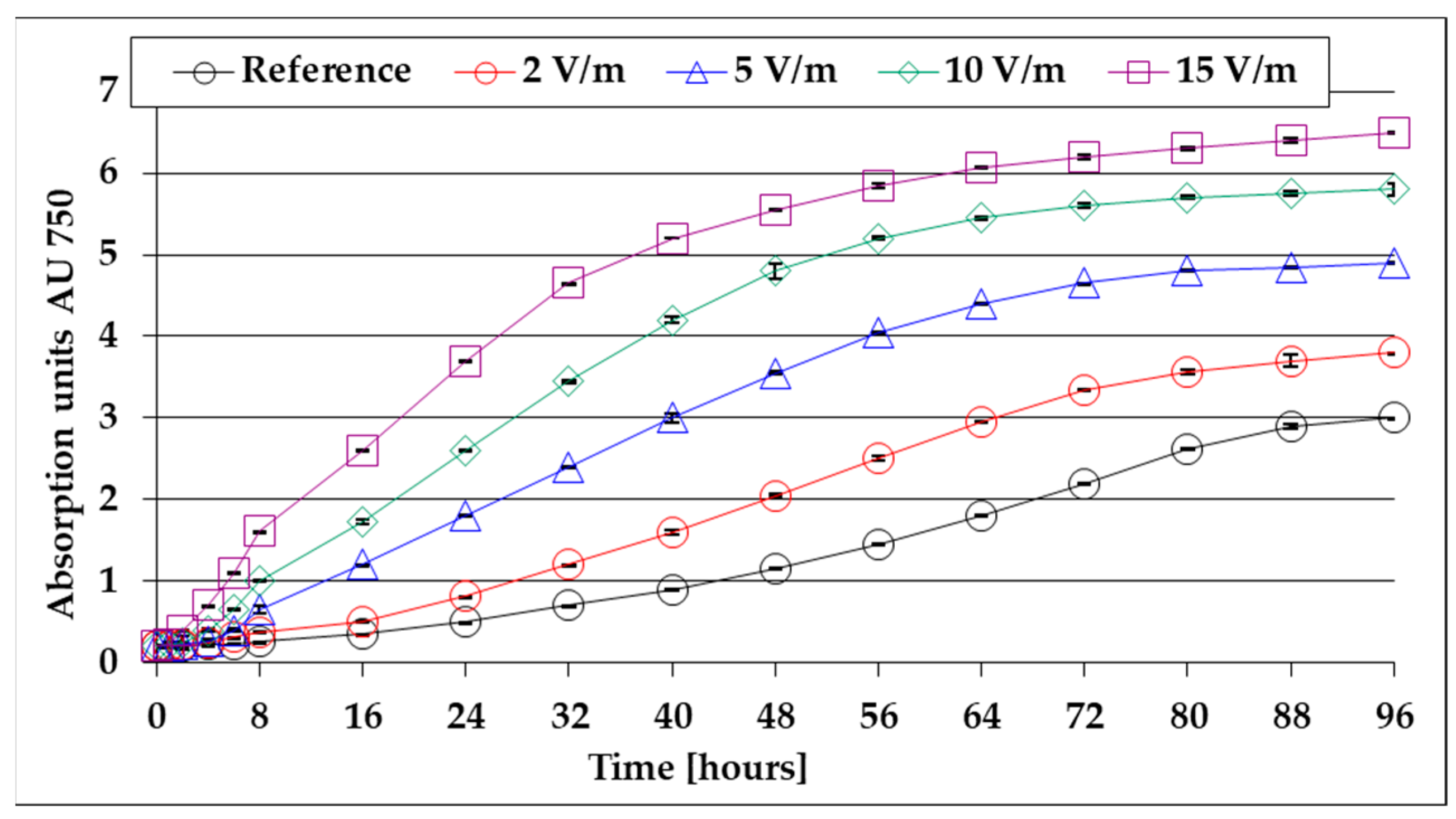
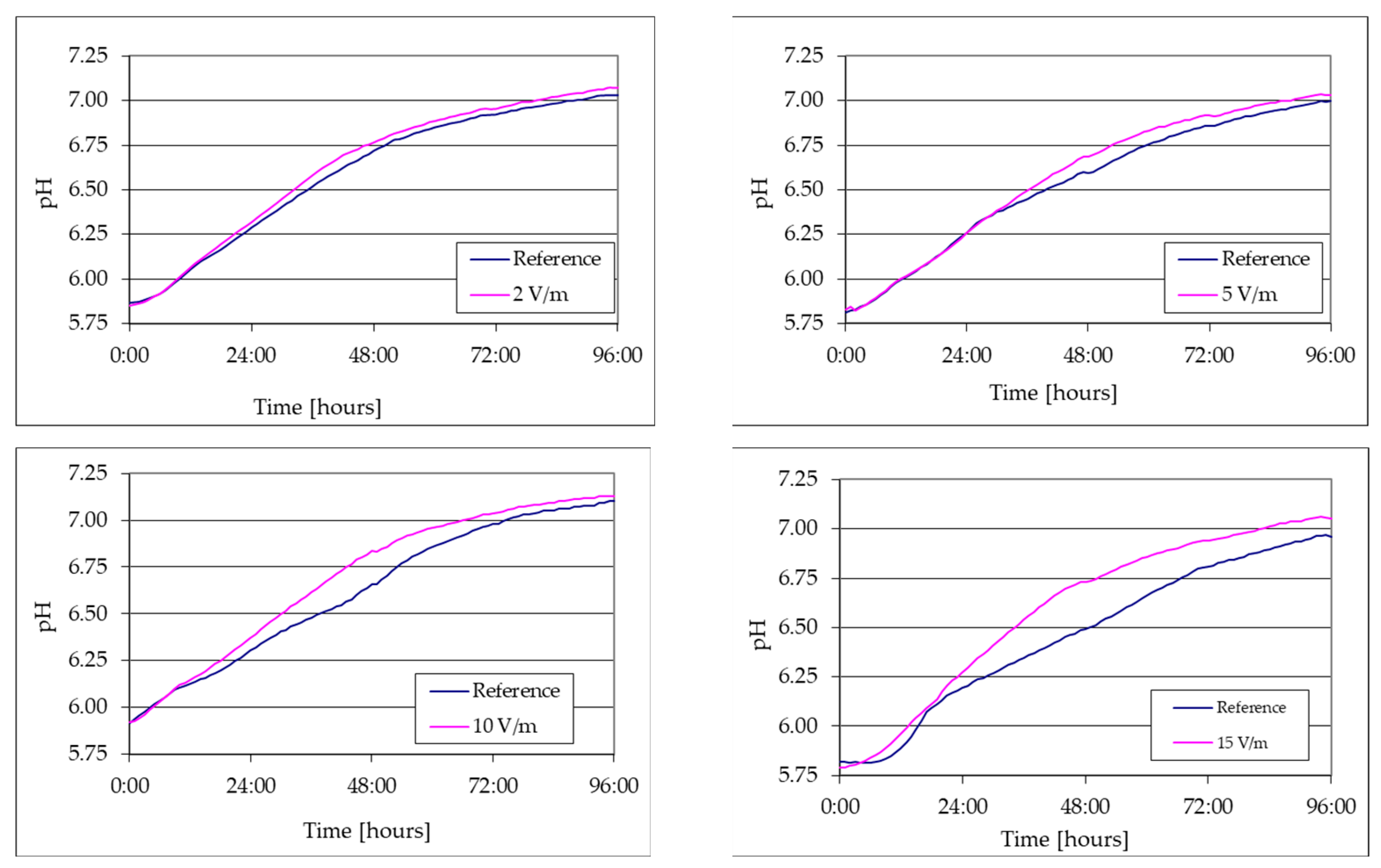
- The reference lag period lasts approximately 8 h and is reduced as the 50 Hz EMF intensity increases to approx. 6 h for EMF 2 V/m, 4.5 h for EMF 5 V/m, 3.2 h for EMF 10 V/m, and 2.5 h for EMF 15 V/m;
- -
- The average growth rates in the 96 h of cultivation were 0.47 gdw/L/day for EMF 2 V/m, 0.53 gdw/L/day for EMF 5 V/m, 0.57 gdw/L/day for EMF 10 V/m, and 0.60 gdw/L/day for EMF 15 V/m, compared to only 0.44 gdw/L/day obtained under reference conditions;
- -
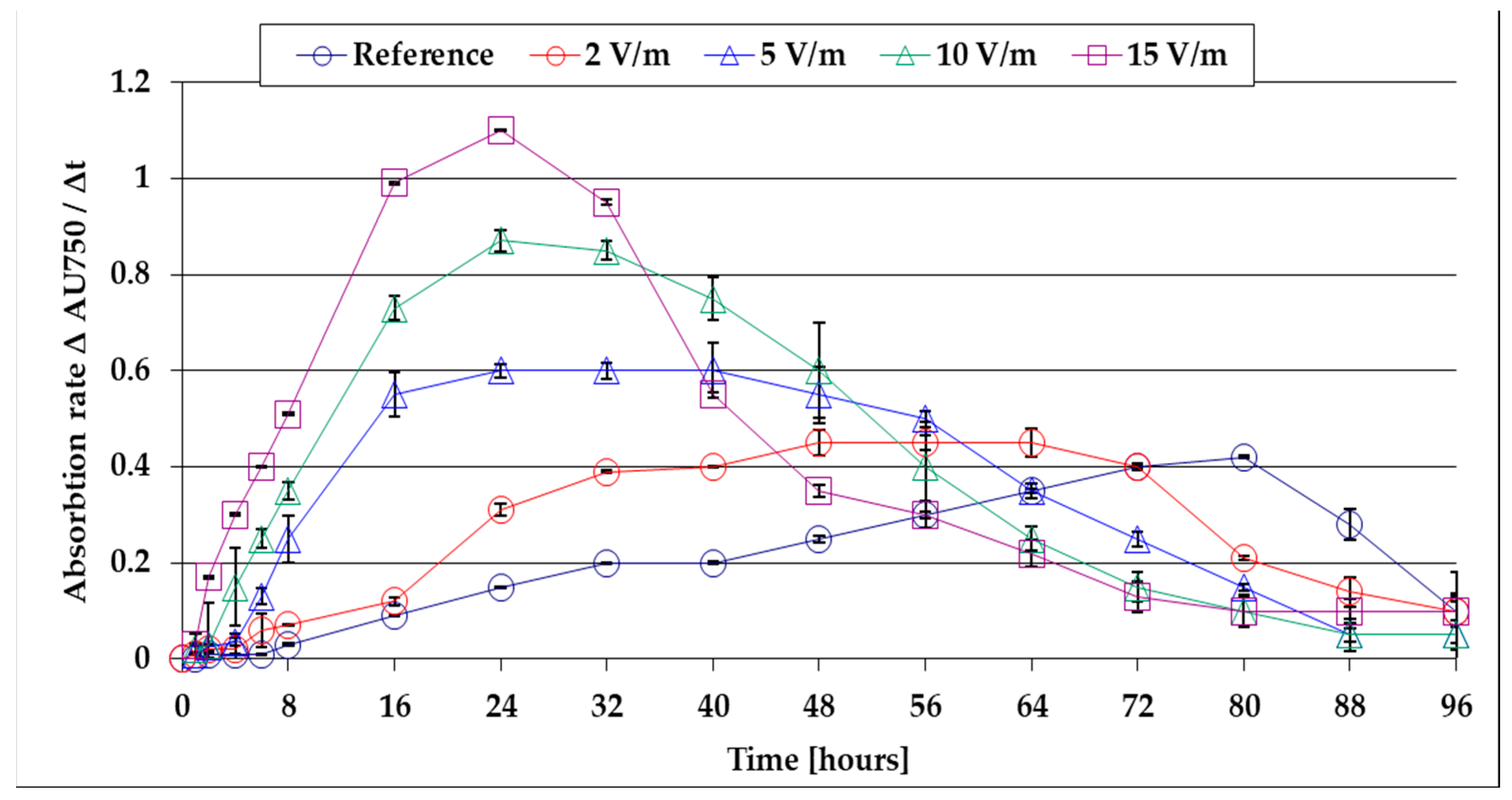
- After the lag period, the growth rates of the algal mass increase to a maximum at 80 h from inoculation to the reference and faster at 72 h for 2 V/m EMF, 40 h for 5 V/m EMF, 32 h for 10 V/m, and 24 h for EMF 15 V/cm;
- -
- The maximum growth rate of the algal mass at 15 V/m is 2.75 times higher than that recorded in the reference.
- -
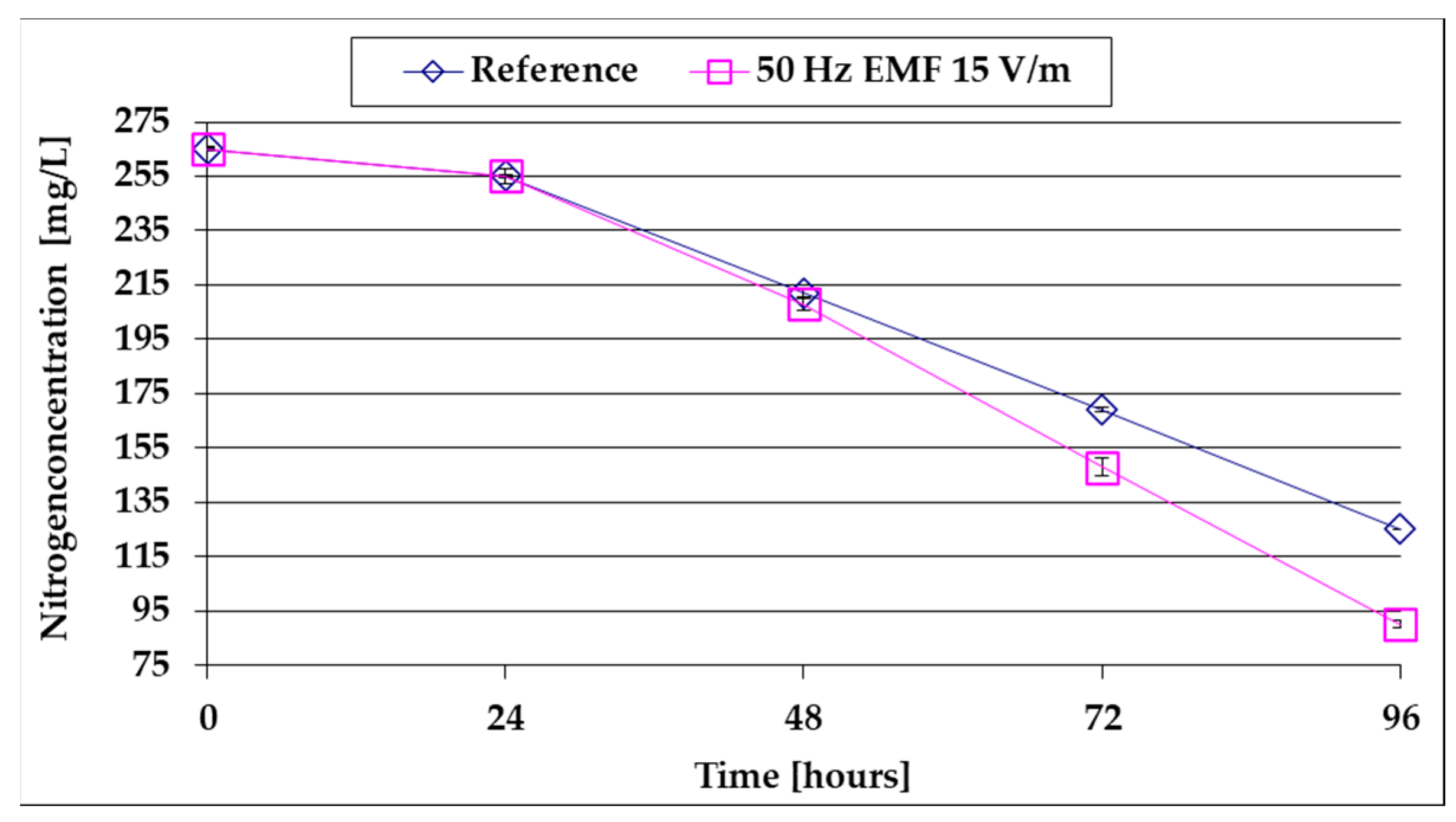
- Following the increase in algal mass, the N-NO3 content of the BG11 culture medium decreases by 58 mg/L/day at 15 V/m EMF compared to 43 mg/L in the reference;
- -
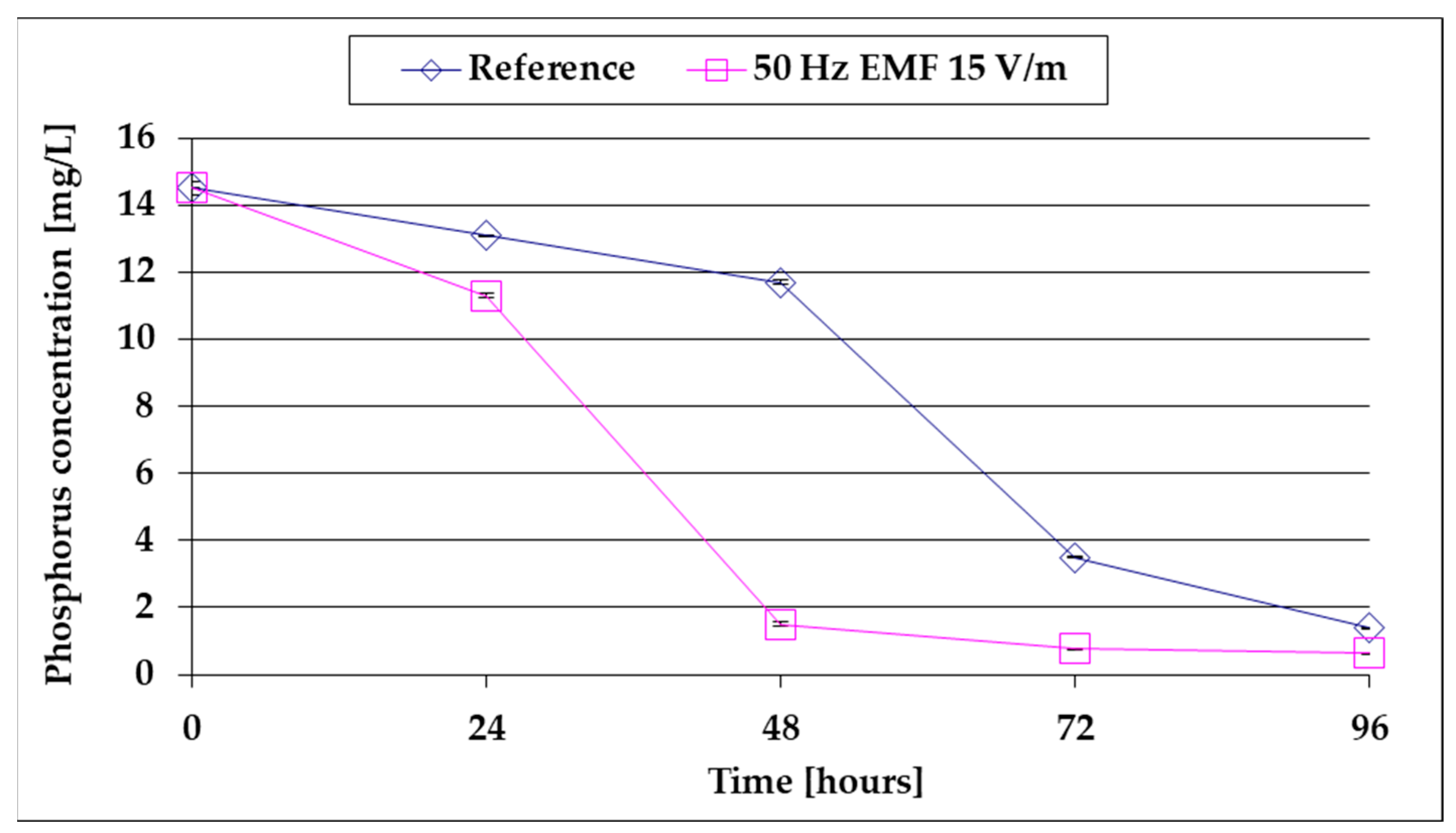
- Following the increase in algal mass, the Pt content of BG11 decreases to 90% depletion after approx. 80 h at baseline versus only 48 h of growth by exposure to 15 V/m ELF;
- -
- Following the increase in algal mass, the increase in TNb in 96 h was 8 mg/L and 115 mg/L at 15 V/m EMF (14 times higher), showing that the influence of the EMF increases with its intensity;
- -
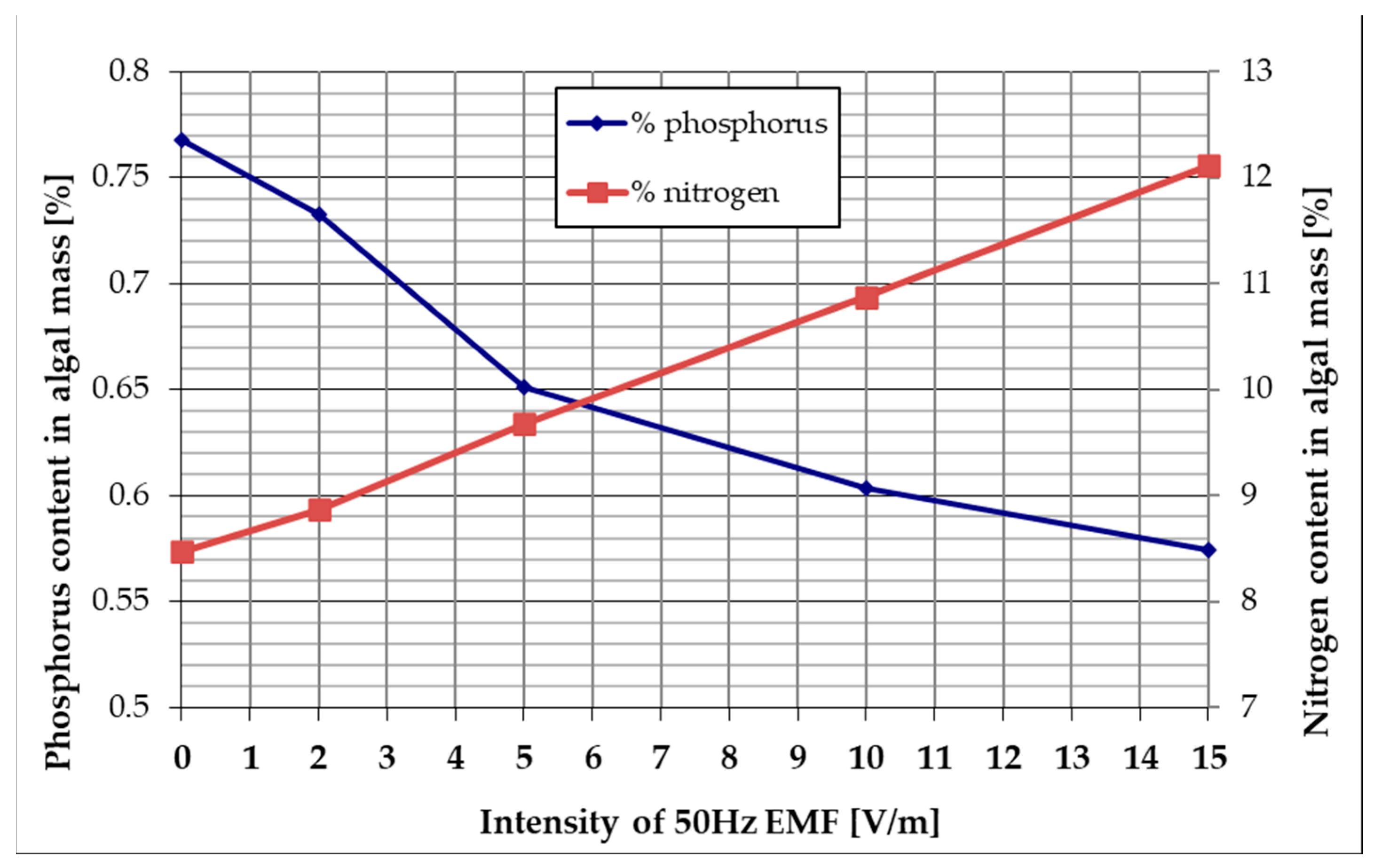
- The nitrogen content of the algal mass obtained after 96 h of culture increases linearly depending on the applied EMF intensity from 8.47% (reference) to 12.12% for 15 V/m EMF;
- -
- The phosphorus content of the algal mass obtained after 96 h of culture decreases from 0.77% (reference) to 0.57% for 15 V/m EMF.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bar-Even, A.; Noor, E.; Lewis, N.A.; Milo, R. Design and analysis of synthetic carbon fixation pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 8889–8894. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.H.; Huang, C.H.; Tan, C.S. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dunford, N.T. Algae: Nature’s Renewable Resource for Fuels and Chemicals. Biomass 2024, 4, 329–348. [Google Scholar] [CrossRef]
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.; Vasconcelos, V.; Vicente, A. Electrotechnologies applied to microalgal biotechnology—Applications, techniques and future trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668. [Google Scholar] [CrossRef]
- Fernandes, B.D.; Mota, A.; Teixeira, J.A.; Vicente, A. Continuous cultivation of photosynthetic microorganisms: Approaches, applications and future trends. Biotechnol. Adv. 2015, 33, 1228–1245. [Google Scholar] [CrossRef]
- Patras, D.; Moraru, C.V.; Socaciu, C. Screening of bioactive compounds synthesized by microalgae: A progress overview on extraction and chemical analysis. Stud. Univ. Babeș-Bolyai Ser. Chem. 2018, 63, 21–35. [Google Scholar] [CrossRef]
- James, A.O.; Bankole, A.O.; Pompei, C.M.E.; Dantas, G.A.S.A.; Ruas, G.; Silva, G.H.R. Exploration of Microalgae-Activated Sludge Growth Performance in Lab-Scale Photobioreactors under Outdoor Environmental Conditions for Wastewater Biotreatment. Phycology 2023, 3, 484–502. [Google Scholar] [CrossRef]
- Dammak, I.; Fersi, M.; Hachicha, R.; Abdelkafi, S. Current Insights into Growing Microalgae for Municipal Wastewater Treatment and Biomass Generation. Resources 2023, 12, 119. [Google Scholar] [CrossRef]
- Su, M.; Dell’Orto, M.; Scaglia, B.; D’Imporzano, G.; Bani, A.; Adani, F. Growth Performance, Biochemical Composition and Nutrient Recovery Ability of Twelve Microalgae Consortia Isolated from Various Local Organic Wastes Grown on Nano-Filtered Pig Slurry. Molecules 2022, 27, 422. [Google Scholar] [CrossRef]
- Aro, E.M. From first generation biofuels to advanced solar biofuels. Ambio 2016, 45 (Suppl. S1), 24–31. [Google Scholar] [CrossRef]
- Clippinger, J.; Davis, R. Techno-Economic Analysis for the Production of Algal Biomass via Closed Photobioreactors: Future Cost Potential Evaluated Across a Range of Cultivation System Designs; Technical Report NREL/TP-5100-72716; National Renewable Energy Lab: Golden, CO, USA, 2019. [Google Scholar] [CrossRef]
- Riffo, B.; Henríquez, C.; Chávez, R.; Peña, R.; Sangorrín, M.; Gil-Duran, C.; Rodríguez, A.; Ganga, M.A. Nonionizing Electromagnetic Field: A Promising Alternative for Growing Control Yeast. J. Fungi 2021, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Cellini, L.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Di Giulio, M.; Robuffo, I.; Trubiani, O.; A Mariggiò, M. Bacterial response to the exposure of 50 Hz electromagnetic fields. Bioelectromagnetics 2008, 29, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Tinoco, T.P.; Sánchez-Vázquez, V.; Del Carmen Fajardo-Ortiz, M.; González, I.; Beristain-Cardoso, R. How does a low-magnitude electric field influence anaerobic digestion in wastewater treatment? A review. Chemosphere 2023, 325, 138402. [Google Scholar] [CrossRef] [PubMed]
- Kubar, A.A.; Jin, N.; Cui, Y.; Hu, X.; Qian, J.; Zan, X.; Zhang, C.; Zhu, F.; Kumar, S.; Huo, S. Magnetic/electric field intervention on oil-rich filamentous algae production in the application of acrylonitrile butadiene styrene based wastewater treatment. Bioresour. Technol. 2022, 356, 127272. [Google Scholar] [CrossRef]
- Bartha, C.; Jipa, M.; Caramitu, A.-R.; Voina, A.; Tókos, A.; Circiumaru, G.; Micu, D.-D.; Lingvay, I. Behavior of Microorganisms from Wastewater Treatments in Extremely Low-Frequency Electric Field. Biointerface Res. Appl. Chem. 2022, 12, 5071–5080. [Google Scholar] [CrossRef]
- Ferencz, C.M.; Petrovszki, P.; Dér, A.; Sebők-Nagy, K.; Kóta, Z.; Páli, T. Oscillating Electric Field Measures the Rotation Rate in a Native Rotary Enzyme. Sci. Rep. 2017, 7, 45309. [Google Scholar] [CrossRef]
- Hunt, R.W.; Zavalin, A.; Bhatnagar, A.; Chinnasamy, S.; Das, K.C. Electromagnetic Biostimulation of Living Cultures for Biotechnology, Biofuel and Bioenergy Applications. Int. J. Mol. Sci. 2009, 10, 4515–4558. [Google Scholar] [CrossRef]
- Beretta, G.; Andrea Filippo Mastorgio, A.F.; Lisa Pedrali, L.; Sabrina Saponaro, S.; Sezenna, E. The effects of electric, magnetic and electromagnetic fields on microorganisms in the perspective of bioremediation. Rev. Environ. Sci. Bio/Technol. 2019, 18, 29–75. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Serov, D.A.; Gudkov, S.V. Biological Effects of Magnetic Storms and ELF Magnetic Fields. Biology 2023, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Chiaramello, E.; Parazzini, M.; Gajšek, P.; Ravazzani, P. Extremely Low Frequency Electric and Magnetic Fields Exposure: Survey of Recent Findings. IEEE J. Electromagn. RF Microw. Med. Biol. 2023, 7, 216–228. [Google Scholar] [CrossRef]
- Raz-Steinkrycer, L.S.; Dubnov, J.; Gelberg, S.; Jia, P.; Portnov, B.A. ELF-MF Exposure, Actual and Perceived, and Associated Health Symptoms: A Case Study of an Office Building in Tel Aviv-Yafo, Israel. Sustainability 2022, 14, 11065. [Google Scholar] [CrossRef]
- Rakoczy, R.; Konopacki, M.; Fijałkowski, K. The influence of a ferrofluid in the presence of an external rotating magnetic field on the growth rate and cell metabolic activity of a wine yeast strain. Biochem. Eng. J. 2016, 109, 43–50. [Google Scholar] [CrossRef]
- Raicu, V.; Feldman, Y. Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Applications; Oxford University Press: New York, NY, USA, 2015; Chapter 2. [Google Scholar] [CrossRef]
- Lingvay, M.; Caramitu, A.-R.; Borș, A.-M.; Lingvay, I. Dielectric Spectroscopic Evaluation in the Extremely Low Frequency Range of an Aspergillus Niger Culture. Stud. UBB Chem. 2019, 64, 279–288. [Google Scholar] [CrossRef]
- Bartha, C.; Caramitu, A.; Jipa, M.; Ignat, D.M.; Tókos, A. Dielectric Behavior of Sludge from Waste Water Treatment. Stud. UBB Chem. 2020, 65, 85–93. [Google Scholar] [CrossRef]
- Lingvay, I.; Radu, L.-E.; Caramitu, A.-R.; Mitrea, S.; Oprina, G.; Voina, A. Procedure for Determining Representative Frequencies in the Behavior of Microbial and Algal Cells. RO Patent 132117, 9 February 2016. [Google Scholar]
- Bartha, C.; Tókos, A.; Jipa, M.; Caramitu, A.; Voina, A.; Circiumaru, G.; Micu, D.-D.; Lingvay, I. Saving Energy in Biological Wastewater Treatment by Using Extremely Low-Frequency Electric Field—Pilot-Scale Study. Sustainability 2023, 15, 11670. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Zhang, L.; Xie, D.T.; Onwosi, C.O.; Muhammad, W.I.; Odoh, C.K.; Sam, K.; Idenyi, J.N. Bioaugmentation as a green technology for hydrocarbon pollution remediation. Problems and prospects. J. Environ. Manag. 2022, 304, 114313. [Google Scholar] [CrossRef] [PubMed]
- Zrimec, A.; Jerman, I.; Lahajnar, G. Alternating electric fields stimulate ATP synthesis in Escherichia coli. Cell. Mol. Biol. Lett. 2002, 7, 172–174. [Google Scholar] [PubMed]
- Zrimec, A.; Jerman, I.; Lahajnar, G. Low frequency alternating electric fields inhibit lactose uptake in Kluyveromyces marxianus. Bioelectrochem. Bioenerg. 1999, 48, 481–484. [Google Scholar] [CrossRef]
- He, Z.; Jin, W.; Zhou, X.; Han, W.; Gao, S.; Chen, C.; Chen, Y.; Yin, S.; Che, L.; Jiang, G. Enhancing biomass and lipid yield of microalga Scenedesmus obliquus by the periodic direct current. J. Water Process Eng. 2022, 48, 102872. [Google Scholar] [CrossRef]
- Voina, A.; Tokos, A.; Jipa, M.; Cîrciumaru, G.; Bartha, C.; Chihaia, R.A.; Tănase, N.; Lingvay, I. Equipment for Increasing the Phototrophic Microalgae Production at Lab Scale. In Proceedings of the ICECET 2023, Cape Town, South Africa, 16–17 November 2023; IEEE Xplore: Piscataway, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Insights into the physiology of Chlorella vulgaris cultivated in sweet sorghum bagasse hydrolysate for sustainable algal biomass and lipid production. Sci. Rep. 2021, 11, 6779. [Google Scholar] [CrossRef] [PubMed]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Psychol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Silkina, A.; Flynn, K.; Llewellyn, C.; Bayliss, C. (Eds.) Standard Operating Procedures for Analytical Methods and Data Collection in Support of Pilot-Scale Cultivation of Microalgae. In Public Output Report WP1A3.01 of the EnAlgae Project; EnAlgae, Swansea University, Centre for Sustainable Aquatic Research: Swansea, UK, 2015; p. 395. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Prats, C.; López, D.; Giró, A.; Ferrer, J.; Valls, J. Individual-based modelling of bacterial cultures to study the microscopic causes of the lag phase. Theor. Biol. 2006, 241, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.M.; Stanier, R.Y. Growth and Division of Some Unicellular Blue-green Algae. J. Gen. Microbiol. 1968, 51, 199–202. [Google Scholar] [CrossRef]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquatic Res. 2020, 46, 153–158. [Google Scholar] [CrossRef]
- Kim, D.; Vijayan, D.; Praveenkumar, R.; Han, J.; Lee, K.; Park, J.; Chang, W.; Lee, J.; Oh, Y. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef]
- Junge, W.; Nelson, N. ATP Synthase. Annu. Rev. Biochem. 2015, 84, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Vonck, J.; Mills, D.J.; Meier, T.; Kühlbrandt, W. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 2018, 360, 6389. [Google Scholar] [CrossRef]
- Szabó, T.; Magyar, M.; Hajdu, K.; Dorogi, M.; Nyerki, E.; Tóth, T.; Lingvay, M.; Garab, G.; Hernádi, K.; Nagy, L. Structural and Functional Hierarchy in Photosynthetic Energy Conversion—From Molecules to Nanostructures. Nanoscale Res. Lett. 2015, 10, 458. [Google Scholar] [CrossRef]
- Lingvay, M.; Akhtar, P.; Sebők-Nagy, K.; Páli, T.; Lambrev, P.H. Photobleaching of chlorophyll in light-harvesting complex II increases in lipid environment. Front. Plant Sci. 2020, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, P.; Lingvay, M.; Kiss, T.; Deák, R.; Bóta, A.; Ughy, B.; Garab, G.; Lambrev, P.H. Excitation energy transfer between Light-harvesting complex II and Photosystem I in reconstituted membranes. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, P.; Lindorfer, D.; Lingvay, M.; Pawlak, K.; Zsiros, O.; Siligardi, G.; Jávorfi, T.; Dorogi, M.; Ughy, B.; Garab, G.; et al. Anisotropic Circular Dichroism of Light-Harvesting Complex II in Oriented Lipid Bilayers: Theory Meets Experiment. J. Phys. Chem. B 2019, 123, 1090–1098. [Google Scholar] [CrossRef] [PubMed]


| Growth Time [hours] | Biomass Concentration c [gdw/L] and Stimulation Coefficient ks [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | EMF-2 V/m | EMF-5 V/m | EMF-10 V/m | EMF-15 V/m | |||||
| cref * | c | ks | c | ks | c | ks | c | ks | |
| 48 | 0.76 ± 0.037 | 0.78 ± 0.014 | 2.6 ± 0.14 | 0.82 ± 0.07 | 7.9 ± 0.78 | 0.96 ± 0.093 | 26.3 ± 2.85 | 1.02 ± 0.03 | 34.2 ± 1.95 |
| 72 | 1.29 ± 0.027 | 1.36 ± 0.015 | 5.4 ± 0.13 | 1.52 ± 0.02 | 17.8 ± 0.44 | 1.66 ± 0.017 | 28.8 ± 0.67 | 1.75 ± 0.08 | 35.7 ± 1.79 |
| 96 | 1.77 ± 0.11 | 1.88 ± 0.046 | 6.2 ± 0.42 | 2.12 ± 0.023 | 19.8 ± 1.25 | 2.29 ± 0.011 | 29.4 ± 1.83 | 2.41 ± 0.06 | 36.2 ± 2.42 |
| Parameter | Concentrations [mg/L] | |||||
|---|---|---|---|---|---|---|
| ci * | cf–reference * (0 V/m) | cf—2 V/m | cf—5 V/m | cf—10 V/m | cf—15 V/m | |
| N-NO3 | 265 ± 1.1 | 125 ± 0.71 | 123 ± 2.83 | 119 ± 0.4 | 114 ± 2.83 | 90 ± 1.4 |
| TNb (total nitrogen) | 267 ± 1.2 | 275 ± 7.78 | 288 ± 2.12 | 324 ± 0.53 | 363 ± 5.7 | 382 ± 2.83 |
| Organic nitrogen (Nalga) | 2 ± 1.6 | 150 ± 7.82 | 165 ± 3.5 | 205 ± 0.66 | 249 ± 6.3 | 292 ± 3.2 |
| Pt (total phosphorus) | 14.5 ± 0.1 | 0.91 ± 0.003 | 0.87 ± 0.1 | 0.7 ± 0.001 | 0.68 ± 0.008 | 0.65 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lingvay, I.; Vranceanu-Jipa, M.; Chihaia, R.-A.; Tókos, A.; Bartha, C.; Circiumaru, G. Impact of 50 Hz Electromagnetic Field on the Growth of Chlorella vulgaris. Appl. Sci. 2024, 14, 6506. https://doi.org/10.3390/app14156506
Lingvay I, Vranceanu-Jipa M, Chihaia R-A, Tókos A, Bartha C, Circiumaru G. Impact of 50 Hz Electromagnetic Field on the Growth of Chlorella vulgaris. Applied Sciences. 2024; 14(15):6506. https://doi.org/10.3390/app14156506
Chicago/Turabian StyleLingvay, Iosif, Monica Vranceanu-Jipa, Rares-Andrei Chihaia, Attila Tókos, Csaba Bartha, and Gabriela Circiumaru. 2024. "Impact of 50 Hz Electromagnetic Field on the Growth of Chlorella vulgaris" Applied Sciences 14, no. 15: 6506. https://doi.org/10.3390/app14156506
APA StyleLingvay, I., Vranceanu-Jipa, M., Chihaia, R.-A., Tókos, A., Bartha, C., & Circiumaru, G. (2024). Impact of 50 Hz Electromagnetic Field on the Growth of Chlorella vulgaris. Applied Sciences, 14(15), 6506. https://doi.org/10.3390/app14156506






