Abstract
The paper presents the experimental study of the influence of a 50 Hz extremely-low-frequency (ELF) electromagnetic field (EMF) on the growth of microalgae Chlorella vulgaris in a BG11 culture medium. Comparative experimental determinations carried out under reference conditions (microalgae growth without exposure to EMF) and with exposure to a homogeneous 50 Hz EMF of various intensities highlighted the fact that EMF has a major impact on both the growth speed and the nitrogen and phosphorus content of the obtained algal mass. Through spectrophotometry and gravimetric determinations, it was found that the lag time was reduced from approximately 8 h (reference) to approximately 6 h for EMF of 2 V/m, 4.5 h for EMF of 5 V/m, 3.2 h for EMF of 10 V/m, and 2.5 h for EMF of 15 V/m. In the stimulation with 15 V/m EMF, the maximum biomass growth rate was 2.75 times higher than the reference, leading to a 2-fold increase in the rate of exhaustion of nutrients, especially phosphorus, in the culture medium. The specific chemical analyses for N-NO3, total nitrogen TNb, and total phosphorus Pt highlighted that the N-NO3 content of the culture medium decreased by 58 mg/L/day at 15 V/m EMF compared to 43 mg/L for the reference. The Pt content decreased to 90% depletion after approximately 80 h for the reference culture medium, versus only 48 h of growth with exposure to 15 V/m ELF. The TNb content of the algal suspension in BG11 under the influence of 15 V/m EMF for 96 h of growth increased 14 times compared to the reference. This shows that nitrogen metabolization in the dispersed air was significantly stimulated. It was also found that the 50 Hz EMF also influences the nitrogen and phosphorus content of the increased algal mass. The results show the potential of EMF stimulation of Chlorella vulgaris growth, leading to an increased efficiency of algae growth reactors.
1. Introduction
Algae are phototrophic microorganisms that, through various carbon fixation pathways, have the ability to convert solar energy into chemical energy through photosynthesis, and to synthesize organic material from CO2 and water through enzymatically catalyzed photobiochemical processes. They are widespread in nature and make a major contribution to the retention of carbon from atmospheric CO2 and the release of oxygen in the biosphere [1,2,3].
Due to their high protein, lipid, and carbohydrate contents, microalgae—especially Chlorella, Spirulina, and Dunaliella—are used as a food source. Microalgae are natural raw materials in the pharmaceutical industry [4] where they are widely used to obtain food supplements with beneficial effects on digestion (Chlorella stimulates the growth of intestinal Lactobacillus). Spirulina sp. and Dunaliella sp., due to their high content in carotenoids, have demonstrated anticancer effects [5,6,7]. Different studies have explored the proper conditions for efficient algal biomass growth [8,9,10], with wastewater proving to be a suitable environment. On the other hand, through their oil content, oil-rich algae represent a renewable energy source. Thus, the third-generation biofuels are based on algal biomass production [11]. In this context, the demand for algal biomass—a product with increased added value—on the world market shows an upward trend [7,11].
The photobiochemical processes are relatively slow, which makes the productivity of bioreactors, including microalgae growth photobioreactors, relatively low, which has a substantial influence on production costs [2,12]. In this context, it is considered that research aimed at stimulating the processes of metabolism and growth of microalgae represents a way to reduce production costs, thus presenting economic importance.
Numerous studies have highlighted the fact that electromagnetic fields (EMFs) can influence the mechanism and kinetics of cellular biochemical processes of microorganisms [13,14,15,16,17,18]. The exhaustive investigations of Ryan W. Hunt et al. [19] and, more recently, Beretta, G et al. [20] show that by exposing biomass to an electromagnetic field, under certain specific conditions (waveform, frequency, applied field intensity), certain biochemical processes can be significantly modified. The excellently documented study of Ruslan M. Sarimov et al. [21] regarding the influences of magnetic fields of natural origin (magnetic storms) and/or anthropogenic causes (electromagnetic pollution of the environment [22,23,24] with an extremely-low-frequency EMF in the 0–1 kHz range) demonstrated complex biological effects.
The changes produced by ELF-EMF exposure in the mechanism and kinetics of biochemical processes are correlated with dielectric relaxation in biological systems. Thus, they are selectively produced at a proper frequency given by the relaxation frequency of certain chemical species (polarized molecules—with dipole moment) present in the system [21]. Dielectric spectroscopy is a useful tool for studying the behavior of biological systems in a sinusoidal electric field [24,25,26,27]. The rapid method of determining the proper frequency at which changes occur in the behavior of biochemical systems is based on the technique of dielectric spectroscopy [28]. Thus, by studying the dielectric behavior of the active sludge suspension from wastewater treatment plants, proper frequencies of 49.9, 99.9, 130.8, and 150.4 Hz were found [17,27]. Through specific chemical determinations, it was found that the rate of metabolism of wastewater pollutants increases significantly (doubles) following the exposure of the active sludge suspension to a 5 V/m electric field of 50 Hz [17]. Based on these results, a pilot installation was developed for the stimulation by exposure to ELF-EMF of microbiological activity in the biological treatment of wastewater. The comparative experimentation of the pilot plant highlighted the fact that, by stimulating the microbial activity with ELF-EMF, the specific electricity consumption of the wastewater biological treatment stage is reduced by approximately 50%; this represents an electricity saving of approximately 33% from the wastewater treatment plant’s total energy consumption [29]. Similar results of ELF-EMF stimulation of microbial cultures have been reported with applications to hydrocarbon pollution remediation [30], as well as in various biotechnologies for the production of biofuel and bioenergy [19].
It was experimentally found that the exposure to 100 Hz EMF of up to 250 V/m stimulates the ATP synthesis in Escherichia coli [31]. On the other hand, excessive EMF of 3000 V/m inhibits lactose uptake in the case of Kluyveromyces marxianus [32]. Regarding microalgae, it was shown that under the influence of a periodic direct current, the algal biomass growth of Scenedesmus obliquus and its lipid yield are significantly improved [33].
Given these considerations, a fully automated device for increasing phototrophic microalgae production by exposing the culture medium to ELF-EMF was recently designed and made [34]. In this context, the aim of the work is to study the behavior of the microalgae Chlorella vulgaris in the presence of ELF-EMF of 50 Hz. The selection of the microalgae strain for the experimental study was based on several advantageous characteristics, such as metabolic robustness, simple life cycle, rapid reproductive capacity, and industrial relevance within its genus [35,36].
2. Materials and Methods
In order to evaluate the influence of 50 Hz EMF on the growth of Chlorella vulgaris, comparative determinations were made on samples collected from two identical photobioreactors operating with identical parameters (illumination level, temperature, flow rate of air/CO2 mixture). A reference photobioreactor without exposing the culture medium to EMF was compared to another photobioreactor provided with polarizing electrodes for exposing the culture medium to 50 Hz EMF at various intensities. The experimental setup illustrated in Figure 1 was described in detail in [34]. The equipment diagram is shown in Figure 2.
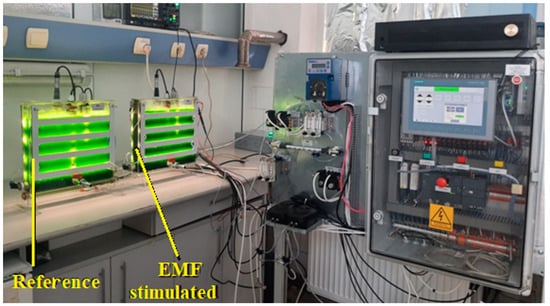
Figure 1.
The experimental setup.
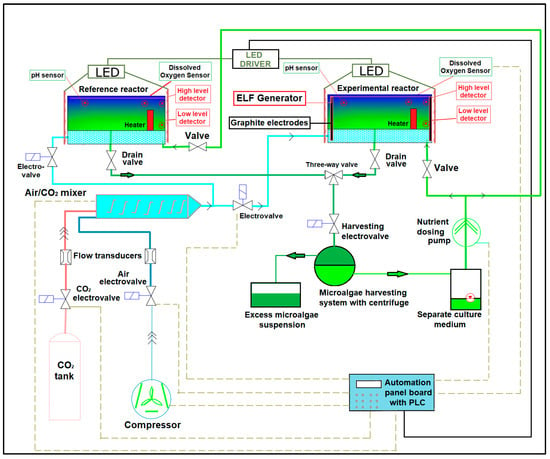
Figure 2.
Diagram of the microalgae growth equipment.
The culture medium used was BG11 with the following composition: 1.5 g/L NaNO3; 0.08 g/L K2HPO4; 0.075 g/L MgSO4 × 7H2O; 0.036 g/L CaCl2 × 2H2O; 0.006 g/L citric acid; 0.006 g/L Ferric ammonium citrate green 15% Fe; 0.001 g/L Na2EDTA; 0.002 g/L Na2CO3; and compounds for providing microelements: B from 0.00072 g/L H3BO3, Mn from 0.00045 g/L MnCl2 × 4H2O, Zn from 0.00045 g/lLZnSO4 × 7H2O, Mo from 0.000098 g/L Na2MoO4 × 2 H2O, Cu from 0.0002 g/L CuSO4 × 5H2O, and Co from 0.000013 g/L Co(NO3)2 × 6H2O).
The inoculum used to inoculate the culture medium from the two photobioreactors in Figure 1 was Chlorella vulgaris Bienjerinck CCALA 269 obtained from the Culture Collection of Autotrophic Microorganisms, Třeboň, Czech Republic. The working temperature was kept to 25 ± 0.5 °C and was monitored by the temperature sensor incorporated in the type S423/C/OPT oxygen transducer. The intensity of the luminous flux was measured with a portable luxmeter HD2102.1 (equipped with a PHOTON FLOW, PAR LP471 PAR probe, measurement range 0.01–10,000 μmol/m2/s—from Delta Ohm, Padova, Italy) and adjusted using an LED driver (Figure 2) at the level of 310 ± 10 µmol/m2/s, light 400 to 700 nm.
The bubbles were generated by injecting air mixed with 5% (by volume) CO2 through a porous hose at 2 L/min, thus resulting in a two-phase flow inside the reactors. The water represents the continuous phase, while the air/CO2 mixture represents the dispersed phase. The gaseous mixture flow rate was controlled by flow transducers and electrovalves.
The intensity and frequency of the EMF applied to the culture medium was controlled by measuring/monitoring the output voltage of the sin-way ELF generator (which was designed and made by the authors) connected to the plane-parallel mounted graphite polarizing electrodes (Figure 2).
The experiments were simultaneously performed in the reference reactor and in the experimental reactor, where different electromagnetic field intensities were applied in order to study their influence on the algae growth. Each experiment was carried out on fresh (new) culture medium, both in the reference and in the experimental reactor, and lasted for 96 h. For each of the 4 EMF intensities, 3 determinations were made in the two reactors simultaneously. Thus, under reference conditions, 12 determinations were made.
During the growth of Chlorella vulgaris, the following parameters were monitored:
- -
- Evolution over time of pH was monitored by a pH sensor S401 DIG/N type, produced by Chemitec.
- -
- Evolution over time of the culture medium turbidity was achieved through the measurement of light absorption in suspension (optical density DO at a wavelength of 750 nm [37,38]) with a portable spectrophotometer (HACH, Berlin, Germany, DR3900).
- -
- Evolution over time of algal mass concentration by filtering 100 mL of algal suspension on pre-weighted glass fiber filter paper (ROTILABO®, CR261, Ø 47 mm, pore Ø 1.2 μm) washed with double distilled water, dried at 100 °C for 4 h, and cooled in a desiccator. The weight difference (measured with a Precisa balance, XR 125SM) after drying and cooling represented the dry weight (dw) of the microalgae.
- -
- The time evolution of dissolved oxygen in the culture medium by measurements performed with a type S423/C/OPT oxygen transducer manufactured by Chemitec, Florence, Italy.
- -
- The consumption of nutrients from the culture medium through analyses of samples collected before inoculation and after 96 h (4 days) of growth for the following: a. nitrogen concentrations in nitrate (N-NO3—Cuvette test LCK 340, measurement range 5–35 mg/L N-NO3, HACH, Berlin, Germany); b. total nitrogen (TNb—Cuvette test LCK 238—measurement range 5–40 mg/L TNb, HACH, Berlin, Germany); c. total phosphorus (P-PO4—Cuvette test LCK 350—range 2–20 mg/L P-PO4 and LCK 348—range 0.5–5 mg/L P-PO4, HACH, Berlin, Germany).
In order to quantify the effect of the EMF influence on Chlorella vulgaris, a growth stimulation coefficient ks was calculated as (1):
ks = 100 · (c − cref)/cref
3. Results and Discussions
The results of the respective spectrophotometric determinations, and the comparative absorbance evolution of the algal mass suspension in the liquid culture medium taken from the reference photobioreactor and from that exposed to growth stimulation by EMF of 2, 5, and 10, at 15 V/m, in the first 96 h after inoculation, are synthetically presented in Figure 3. Its analysis shows that at the beginning, in the first hours after inoculation, the absorbance did not change significantly during the lag period [39] (in the reference photobioreactor it lasted approximately 8 h) and was reduced as the 50 Hz EMF intensity increased, to approximately 6 h for EMF 2 V/m, 4.5 h for EMF 5 V/m, 3.2 h for EMF 10 V/m, and 2.5 h for EMF 15 V/m.
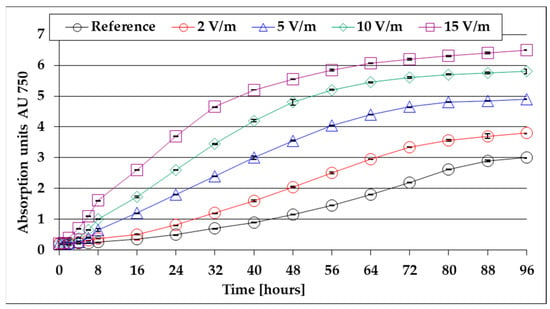
Figure 3.
Evolution of absorbance in the first 96 h after inoculation (average values for three determinations each).
A similar observation of lag time shortening under the influence of EMF 50 Hz was also reported for the particularly diverse flora of microorganisms from active sludge from wastewater treatment plants [17]. After the lag period, following the multiplication and growth of microalgae, the absorbance of the medium increases accordingly, with the most pronounced increases being recorded in the case of the stimulation with 15 V/m EMF. It was also found that at 40–80 h after inoculation (depending on the intensity of the EMF 50 Hz applied) a limiting trend appears; this can be explained by the decrease in the nutrients’ concentration in the culture medium (approximately proportional to the biomass formed).
The absorbance is approximately proportional to the concentration of biomass in the suspension. By calculating the variation rate of the absorbance VAU750 = Δ AU750/Δt, information is obtained regarding the growth rate evolution of the algal mass during the 96 h of cultivation. Based on the data presented in Figure 3, the evolution of the absorbance variation rate during the first 96 h of the algal mass growth was obtained and is shown in Figure 4.
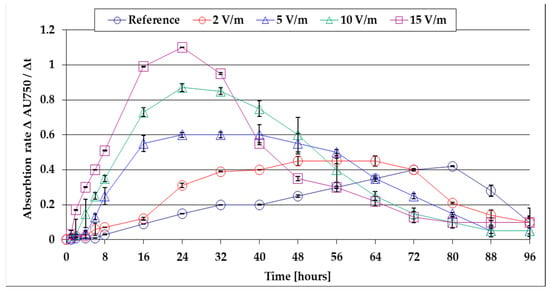
Figure 4.
The evolution of the absorbance variation rate during the first 96 h of growth of the algal mass.
Figure 4 shows that at the end of the lag period, the algal mass growth rate increases up to a maximum plateau, after which—as the concentration of nutrients in the culture medium decreases—it gradually decreases. The algal mass growth rate is minimal in the case of the reference bioreactor (VAU750 = 0.41), where growth limitation begins after 80 h from inoculation. By applying ELF-EMF of 50 Hz, the maximum recorded values increase, the algal mass formed increases, and, as a consequence, the nutrient consumption from the culture medium is higher. In these conditions, the decreases in the algal mass growth rates are established faster—after 72 h for 2 V/m EMF, after 40 h for 5 V/m EMF, after 32 h for 10 V/m EMF, and after 24 h for 15 V/m EMF. By comparing the maximum growth rate, it is found that at 15 V/m the EMF is 2.75 times higher than the reference. These findings are important for the optimization of the operating parameters of the experimental setup for continuous flow production of algal mass. The results show that, for maximum productivity, it is suggested to harvest the algal mass daily in the case of growth stimulation with 15 V/m EMF. At each harvest/sample, it is suggested that the exhausted culture medium be compositionally corrected (by nutrient dosing and pH correction) and revalued by re-introduction into the photobioreactor. The results of the gravimetric determinations, regarding the weighting of the algal mass obtained by filtering 100 mL of suspension and drying the filter, are presented comparatively in Table 1.

Table 1.
The evolution of the biomass concentration (% dried mass) as a function of time at various intensities of stimulating fields.
By analyzing the data in Table 1, it is found that after 96 h of culture, by stimulation with 15 V/m EMF, the algal mass concentration in the culture medium is 36.2% higher than that of the reference. Based on the data in Table 1, the average growth rates during the 96 h (4 days) of cultivation can be calculated as 0.47 gdw/L/day for EMF 2 V/m, 0.53 gdw/L/day for EMF 5 V/m, 0.57 gdw/L/day for EMF 10 V/m, and 0.60 gdw/L/day for EMF 15 V/m, compared to only 0.44 gdw/L/day obtained under reference conditions (without exposure of the culture medium to 50 Hz ELF-EMF).
The phototropic growth of the algal mass involves the metabolization of CO2 available in the culture medium—an endothermic process that takes place through enzymatic photocatalysis (hυ) and through which the conversion of CO2 into organic (algal) mass takes place with the release of O2 (2):
2x · CO2 + 2y · H2O + hυ → 2CxHyOz + (2x + y − z) · O2↑
The oxygen formed by the process described by Equation (2) is dissolved in the culture medium—the total dissolved oxygen OD (limited by the maximum solubility at the given temperature of 25 ± 0.5 °C) being given by the oxygen released by (2) and part of the oxygen from the air/CO2 bubbles. Under these conditions, the OD from the algal suspension in liquid culture medium is a parameter that characterizes the speed of the process (2). The results of the recordings regarding the time evolution of dissolved oxygen OD in the culture medium under experimental conditions are presented in Figure 5.
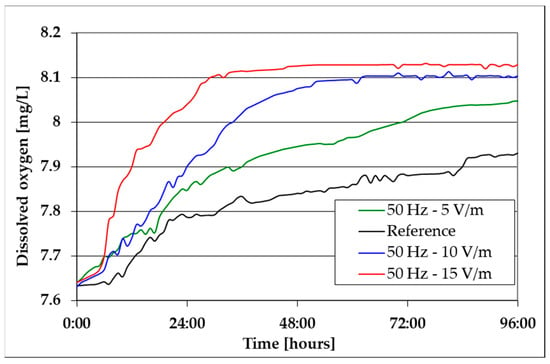
Figure 5.
The evolution of dissolved oxygen in the culture medium.
By analyzing Figure 5, it can be noticed that before the inoculation of the liquid culture medium, OD (given by the oxygen supply from the air/CO2 bubbles) is approximately 7.64 mg/L. After the inoculation of the culture medium, due to the increase in the algal mass, following the development of the processes described by Equation (2), the OD increase is more pronounced in the first approximately 30 h; as it approaches the maximum solubility of oxygen under the given conditions, it shows a limiting trend. This limitation of OD is more pronounced in the case of algal mass suspension exposed to 15 V/m EMF (appears after approx. 24 h limitation to approx. 8.13 mg/L), explained by the maximum growth rate of algal mass in these conditions, as is also the case of the results from Figure 4. The bioreactor is open at the top. Thus, under the current experiment conditions, by injecting bubbles of an air/CO2 mixture, a turbulent flow is generated throughout the reactor and at its surface, intensifying the oxygen transfer from the atmosphere to the culture environment. Thus, it is difficult to assume that the equilibrium is reached. The value of 8.13 mg O2/L is actually the result of the two opposing processes occurring in the reactor: algal photosynthesis that generates O2 and the bubbling system that injects a mixture of air and 5% CO2.
In order to evaluate the consumption of nutrients from the culture medium according to the growth of the algal mass through specific chemical analyses, the initial concentrations ci (before inoculation) and the final concentrations cf after 96 h of growth of the algal mass for inorganic nitrogen (N-NO3) were determined, with total nitrogen (TNb) and total phosphorus (Pt). The difference between total nitrogen TNb and inorganic nitrogen N-NO3 represents the amount of nitrogen retained by the algal mass (organic nitrogen). The results of the comparative chemical analysis performed on the culture medium before inoculation ci and after inoculation and 96 h of culture cf (25 ± 0.5 °C, continuous lighting with 300 μmol/m2/s) are presented comparatively in Table 2.

Table 2.
Chemical analysis results *.
By analyzing the values in Table 2, it can be noticed that during the growth of Chlorella vulgaris Bienjerinck CCALA 269, a significant part of the nitrogen (N-NO3) and phosphorus content of the culture medium is retained/incorporated into the formed algal mass. Unlike the prokaryotes [40], green microalgae are unable to utilize atmospheric N2 because it would require the usage of a consortium of nitrogen-fixing bacteria and microalgae to access such nitrogen species. As it is known, nitrogen serves as a crucial macronutrient that governs the metabolism, and consequently, the growth and biochemical composition of microalgae. As microalgae proliferate, they uptake nitrogen present in the growth medium to facilitate the biosynthesis of essential biological macromolecules like proteins, chlorophylls, and DNA [41]. The reproductive mechanism of the microalga C. vulgaris involves the production of asexual autospores through the division of the mother cell, resulting in 2–32 autospores, a process influenced by a range of environmental factors [36,42]. The objective of the study was to investigate the impact of 50 Hz sinusoidal alternating electromagnetic field intensity on the growth of C. vulgaris under non-limiting growth conditions, including temperature, nutrients, and light. The noticed enhancement in total nitrogen (TNb) levels in the culture subjected to varying field intensities could potentially be attributed to the stimulated influence of the electromagnetic field on the metabolic processes of C. vulgaris (Table 2). It is interesting to note that TNb increases as algal mass increases. Thus, in the 96 h of growth in the case of the reference, an increase in TNb of only 8 mg/L was recorded, and in the case of growth stimulation by exposure to 50 Hz EMF, the nitrogen surplus increased significantly (21 mg/L at 2 V/m EMF, 57 mg/L at 5 V/m EMF, 96 mg/L at 10 V/m EMF, and 115 mg/L at 15 V/m EMF). These data indicate that, by exposing the culture medium mixed with air/CO2 dispersed gas to 50 Hz EMF, the ability of Chlorella vulgaris to metabolize nitrogen from culture media increases significantly (more than 14 times at 15 V/m EMF).
In order to evaluate the depletion rate of Pt nutrients and inorganic nitrogen N-NO3 during the four days of growth, periodic comparative determinations were made (from 24 to 24 h). The recorded results are presented in Figure 6 (for nitrogen) and Figure 7 (for phosphorus).
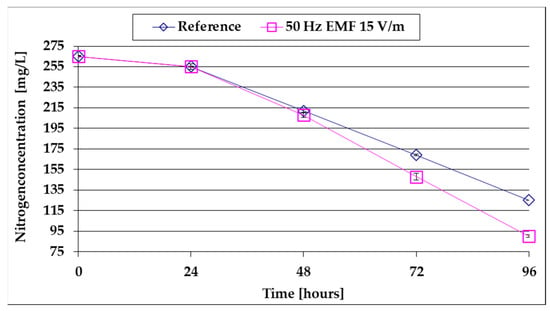
Figure 6.
The comparative evolution of the N-NO3 content of the culture medium.
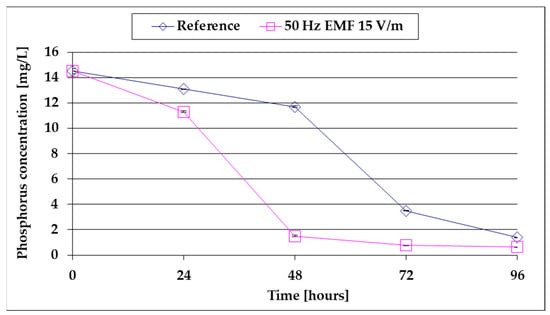
Figure 7.
The comparative evolution of the Pt content of the culture medium.
From the analysis of Figure 6, it is observed that after a first slow decrease during the lag period, the N-NO3 concentration decreases linearly as the algal mass increases. The decrease is more accelerated (58 mg/L/day compared to 43 mg/L for the reference) under the influence of 15 V/m EMF when the algae growth rate is higher than the reference (Table 1).
From the analysis of Figure 7, it is observed that after a first slow decrease during the LAG period, the Pt concentration drops sharply as the algal mass increases, and the decrease is more pronounced under the influence of 15 V/m EMF when the algae growth rate is higher than the reference (Table 1). It is also found that after approx. 48 h of growth by stimulation with 15 V/m ELF, 90% of the Pt reserve of the culture medium is depleted (compared to the reference, when 90% depletion is reached after approx. 80 h of growth). Comparing Figure 6 and Figure 7 with Figure 4 leads to the conclusion that the limitations of the biomass growth rate in Figure 1 are due to the depletion in N-NO3 and especially in Pt of the culture medium. It is noted that the presence of the phosphorus resource in the culture medium is determined in the biosynthesis of phospholipids that enter the structure of the cell membrane, the native rotary enzyme [18], and ATP [43], including chloroplast ATP [44], which together with the light harvesting complex have a determined role in the photosynthesis process (2) [45,46,47,48]. Under these conditions, the rapid decrease in the phosphorus content of the culture medium for growth stimulated in 15 V/m EMF (Figure 7) can be explained by the increase in the growth rate of the algal mass following the stimulation of ATP synthesis. This is in good agreement with the results reported in [31] for Escherichia coli.
By comparing the values from Table 1 (the concentration of biomass formed) with the values from Table 2, the nitrogen and phosphorus contents of the algal mass formed are calculated as:
CNitrogen = 100 · calgal mass/Nalgae [%]
CfPhosphorus = 100 · calgal mass/(ciPt − cf Pt) [%]
The main source of nitrogen in the culture medium is NaNO3 and that for phosphorus is K2 HPO4. Through the metabolism of nitrogen and phosphorus from these nutrients, the culture medium becomes alkaline. The comparative evolution of pH during the 96 h of growth at various intensities of the applied electric field is shown in Figure 8.
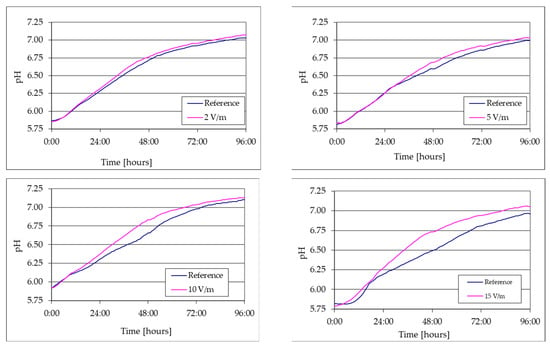
Figure 8.
Comparative evolution of pH during the 96 h of growth at various intensities of the applied electromagnetic field.
The pH measurement during the 96 h of cultivation allowed the observation of the effect of 50 Hz EMF on the growth of C. vulgaris based on N-NO3 and CO2 consumption. The guidelines for preparing the BG11 medium included adjusting the pH to 7.1. Based on the data collected by the pH sensor, it was observed that following the commencement of aeration using a mixture consisting of 95% air and 5% CO2 by volume, the pH values in each comparative experiment decreased from the initial range of 7.1–7.2 and stabilized within the range of 5.82–5.91. During the cultivation of C. vulgaris, the process of photosynthesis resulted in a gradual increase in the alkalinity of the culture medium and subsequent elevation of the pH. This phenomenon was consistently noted irrespective of the presence or absence of the electromagnetic field. However, under the influence of the 50 Hz EMF of 10 and 15 V/m, the pH evolution exhibited increased values in comparison to the reference.
Figure 9 shows the nitrogen and phosphorus contents of the algal mass grown under reference conditions (EMF intensity = 0) and at various applied 50 Hz EMF intensities, calculated according to (3) and (4), respectively.
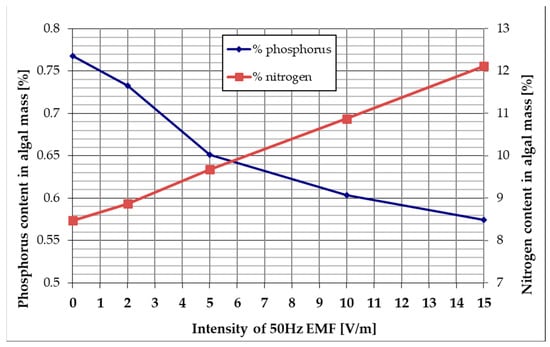
Figure 9.
The influence of 50 Hz EMF intensity on the nitrogen and phosphorus content of the algal mass.
By analyzing Figure 9, it can be noticed that by stimulating the algal mass growth with a 50 Hz ELF in the investigated culture medium, the nitrogen content obtained after 96 h of culture increases linearly depending on the applied EMF intensity from 8.47% (reference—without electric field (ELF)) to 12.12% (ELF stimulation 50 Hz—at 15 V/m). According to the data in Table 2, this increase of about 3% can be explained by the fact that the exposure to 15 V/m EMF of 50 Hz determines an increase in the ability of Chlorella vulgaris to metabolize nitrogen from air. Also, Figure 9 shows that by stimulating the algal mass growth with 50 Hz in the investigated culture medium, the phosphorus content obtained after 96 h of culture decreases depending on the applied EMF intensity from 0.77% (reference—without electric field) to 0.57% (ELF stimulation 50 Hz—at 15 V/m). This finding, correlated with data from Figure 7, suggests that the decrease in phosphorus content of the algal mass grown in 15 V/m EMF 50 Hz is due to the rapid depletion of the phosphorus resource in the culture medium.
4. Conclusions
Through experimental determinations, the influence of ELF-EMF of 50 Hz on the microalgae Chlorella vulgaris growth in BG11 culture medium was studied. The experimental determinations were made simultaneously in two photobioreactors with identical geometry at identical operating parameters (25 ± 0.5 °C temperature, 310 ± 10 µmol/m2/s—light 400 to 700 nm, 2 L/minute dispersed mixture of air with 5% CO2 by volume), one reference and one equipped with graphite electrodes placed in parallel planes connected to a sin-way ELF generator with adjustable output voltage to create a controlled and homogeneous EMF on the culture medium.
The results of spectrophotometric and gravimetric determinations showed the following:
- -
- The reference lag period lasts approximately 8 h and is reduced as the 50 Hz EMF intensity increases to approx. 6 h for EMF 2 V/m, 4.5 h for EMF 5 V/m, 3.2 h for EMF 10 V/m, and 2.5 h for EMF 15 V/m;
- -
- The average growth rates in the 96 h of cultivation were 0.47 gdw/L/day for EMF 2 V/m, 0.53 gdw/L/day for EMF 5 V/m, 0.57 gdw/L/day for EMF 10 V/m, and 0.60 gdw/L/day for EMF 15 V/m, compared to only 0.44 gdw/L/day obtained under reference conditions;
- -
- After the lag period, the growth rates of the algal mass increase to a maximum at 80 h from inoculation to the reference and faster at 72 h for 2 V/m EMF, 40 h for 5 V/m EMF, 32 h for 10 V/m, and 24 h for EMF 15 V/cm;
- -
- The maximum growth rate of the algal mass at 15 V/m is 2.75 times higher than that recorded in the reference.
The registered values of dissolved oxygen concentration (OD) in the culture medium showed that after LAG time, OD has a period of growth in which the growth rates are consistent with the growth speed of the algal mass, after which it shows a trend of limitation to the maximum solubility of oxygen.
The results of the chemical analysis for the concentrations of N-NO3, TNb (total nitrogen), and Pt (total phosphorus) showed the following:
- -
- Following the increase in algal mass, the N-NO3 content of the BG11 culture medium decreases by 58 mg/L/day at 15 V/m EMF compared to 43 mg/L in the reference;
- -
- Following the increase in algal mass, the Pt content of BG11 decreases to 90% depletion after approx. 80 h at baseline versus only 48 h of growth by exposure to 15 V/m ELF;
- -
- Following the increase in algal mass, the increase in TNb in 96 h was 8 mg/L and 115 mg/L at 15 V/m EMF (14 times higher), showing that the influence of the EMF increases with its intensity;
- -
- The nitrogen content of the algal mass obtained after 96 h of culture increases linearly depending on the applied EMF intensity from 8.47% (reference) to 12.12% for 15 V/m EMF;
- -
- The phosphorus content of the algal mass obtained after 96 h of culture decreases from 0.77% (reference) to 0.57% for 15 V/m EMF.
These results lead to the conclusion that the EMF of 50 Hz stimulates the growth of Chlorella vulgaris (maximum growth rate 2.75 times higher than the reference) in a BG11 culture medium, whose Pt reserves are depleted approx. 2 times faster than in reference conditions. The depletion of 90% of the Pt resource in the culture medium results in the cessation of the proliferation of algal biomass. The 15 V/m EMF stimulates the ability to accelerate the nitrate assimilation, the increase in TNb being 14 times higher than in the reference. Exposure to EMF 50 Hz leads to changes in the phosphorus and nitrogen content (concentration) of the algal mass. Considering these conclusions, in order to establish the technological parameters of an industrial installation for the intensive production of algal mass, it is considered appropriate to continue the research to establish the consumption of microelements and nutrients from the culture medium, to perform elemental analyses on algal mass, and to establish the EMF intensity of stimulation for a given composition of the culture medium under continuous dosing of nutrients and microelements.
5. Patents
The work reported in this manuscript led to the elaboration of two Romanian patent applications: A/00600/2023, Growth stimulation process and photobioreactor for the phototropic growth of microalgae and A/00292/2024, Method and installation for reducing CO2 consumption in the phototropic growth of microalgae.
Author Contributions
Conceptualization, I.L. and M.V.-J.; methodology, I.L., M.V.-J. and C.B.; software, A.T.; validation, M.V.-J., R.-A.C. and G.C.; formal analysis, I.L., R.-A.C. and G.C.; investigation, I.L., M.V.-J., R.-A.C., A.T., C.B. and G.C.; data curation, M.V.-J.; writing—original draft preparation, I.L.; writing—review and editing, R.-A.C. and G.C.; project administration, M.V.-J. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian Ministry of Education, Research and Digitalization, UEFISCDI, project number PNIII-P2-2.1-PTE-2021-0075 as well as Nucleu contract no. 42N/2023, project no. PN 23140101/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon reasonable request.
Acknowledgments
This work was supported by the Romanian Ministry of Education, Research and Digitalization, CCCDI—UEFISCDI, project number PNIII-P2-2.1-PTE-2021-0075 as well as Nucleu contract no. 42N/2023, project no. PN 23140101/2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bar-Even, A.; Noor, E.; Lewis, N.A.; Milo, R. Design and analysis of synthetic carbon fixation pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 8889–8894. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.H.; Huang, C.H.; Tan, C.S. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dunford, N.T. Algae: Nature’s Renewable Resource for Fuels and Chemicals. Biomass 2024, 4, 329–348. [Google Scholar] [CrossRef]
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.; Vasconcelos, V.; Vicente, A. Electrotechnologies applied to microalgal biotechnology—Applications, techniques and future trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668. [Google Scholar] [CrossRef]
- Fernandes, B.D.; Mota, A.; Teixeira, J.A.; Vicente, A. Continuous cultivation of photosynthetic microorganisms: Approaches, applications and future trends. Biotechnol. Adv. 2015, 33, 1228–1245. [Google Scholar] [CrossRef]
- Patras, D.; Moraru, C.V.; Socaciu, C. Screening of bioactive compounds synthesized by microalgae: A progress overview on extraction and chemical analysis. Stud. Univ. Babeș-Bolyai Ser. Chem. 2018, 63, 21–35. [Google Scholar] [CrossRef]
- James, A.O.; Bankole, A.O.; Pompei, C.M.E.; Dantas, G.A.S.A.; Ruas, G.; Silva, G.H.R. Exploration of Microalgae-Activated Sludge Growth Performance in Lab-Scale Photobioreactors under Outdoor Environmental Conditions for Wastewater Biotreatment. Phycology 2023, 3, 484–502. [Google Scholar] [CrossRef]
- Dammak, I.; Fersi, M.; Hachicha, R.; Abdelkafi, S. Current Insights into Growing Microalgae for Municipal Wastewater Treatment and Biomass Generation. Resources 2023, 12, 119. [Google Scholar] [CrossRef]
- Su, M.; Dell’Orto, M.; Scaglia, B.; D’Imporzano, G.; Bani, A.; Adani, F. Growth Performance, Biochemical Composition and Nutrient Recovery Ability of Twelve Microalgae Consortia Isolated from Various Local Organic Wastes Grown on Nano-Filtered Pig Slurry. Molecules 2022, 27, 422. [Google Scholar] [CrossRef]
- Aro, E.M. From first generation biofuels to advanced solar biofuels. Ambio 2016, 45 (Suppl. S1), 24–31. [Google Scholar] [CrossRef]
- Clippinger, J.; Davis, R. Techno-Economic Analysis for the Production of Algal Biomass via Closed Photobioreactors: Future Cost Potential Evaluated Across a Range of Cultivation System Designs; Technical Report NREL/TP-5100-72716; National Renewable Energy Lab: Golden, CO, USA, 2019. [Google Scholar] [CrossRef]
- Riffo, B.; Henríquez, C.; Chávez, R.; Peña, R.; Sangorrín, M.; Gil-Duran, C.; Rodríguez, A.; Ganga, M.A. Nonionizing Electromagnetic Field: A Promising Alternative for Growing Control Yeast. J. Fungi 2021, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Cellini, L.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Di Giulio, M.; Robuffo, I.; Trubiani, O.; A Mariggiò, M. Bacterial response to the exposure of 50 Hz electromagnetic fields. Bioelectromagnetics 2008, 29, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Tinoco, T.P.; Sánchez-Vázquez, V.; Del Carmen Fajardo-Ortiz, M.; González, I.; Beristain-Cardoso, R. How does a low-magnitude electric field influence anaerobic digestion in wastewater treatment? A review. Chemosphere 2023, 325, 138402. [Google Scholar] [CrossRef] [PubMed]
- Kubar, A.A.; Jin, N.; Cui, Y.; Hu, X.; Qian, J.; Zan, X.; Zhang, C.; Zhu, F.; Kumar, S.; Huo, S. Magnetic/electric field intervention on oil-rich filamentous algae production in the application of acrylonitrile butadiene styrene based wastewater treatment. Bioresour. Technol. 2022, 356, 127272. [Google Scholar] [CrossRef]
- Bartha, C.; Jipa, M.; Caramitu, A.-R.; Voina, A.; Tókos, A.; Circiumaru, G.; Micu, D.-D.; Lingvay, I. Behavior of Microorganisms from Wastewater Treatments in Extremely Low-Frequency Electric Field. Biointerface Res. Appl. Chem. 2022, 12, 5071–5080. [Google Scholar] [CrossRef]
- Ferencz, C.M.; Petrovszki, P.; Dér, A.; Sebők-Nagy, K.; Kóta, Z.; Páli, T. Oscillating Electric Field Measures the Rotation Rate in a Native Rotary Enzyme. Sci. Rep. 2017, 7, 45309. [Google Scholar] [CrossRef]
- Hunt, R.W.; Zavalin, A.; Bhatnagar, A.; Chinnasamy, S.; Das, K.C. Electromagnetic Biostimulation of Living Cultures for Biotechnology, Biofuel and Bioenergy Applications. Int. J. Mol. Sci. 2009, 10, 4515–4558. [Google Scholar] [CrossRef]
- Beretta, G.; Andrea Filippo Mastorgio, A.F.; Lisa Pedrali, L.; Sabrina Saponaro, S.; Sezenna, E. The effects of electric, magnetic and electromagnetic fields on microorganisms in the perspective of bioremediation. Rev. Environ. Sci. Bio/Technol. 2019, 18, 29–75. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Serov, D.A.; Gudkov, S.V. Biological Effects of Magnetic Storms and ELF Magnetic Fields. Biology 2023, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Chiaramello, E.; Parazzini, M.; Gajšek, P.; Ravazzani, P. Extremely Low Frequency Electric and Magnetic Fields Exposure: Survey of Recent Findings. IEEE J. Electromagn. RF Microw. Med. Biol. 2023, 7, 216–228. [Google Scholar] [CrossRef]
- Raz-Steinkrycer, L.S.; Dubnov, J.; Gelberg, S.; Jia, P.; Portnov, B.A. ELF-MF Exposure, Actual and Perceived, and Associated Health Symptoms: A Case Study of an Office Building in Tel Aviv-Yafo, Israel. Sustainability 2022, 14, 11065. [Google Scholar] [CrossRef]
- Rakoczy, R.; Konopacki, M.; Fijałkowski, K. The influence of a ferrofluid in the presence of an external rotating magnetic field on the growth rate and cell metabolic activity of a wine yeast strain. Biochem. Eng. J. 2016, 109, 43–50. [Google Scholar] [CrossRef]
- Raicu, V.; Feldman, Y. Dielectric Relaxation in Biological Systems: Physical Principles, Methods, and Applications; Oxford University Press: New York, NY, USA, 2015; Chapter 2. [Google Scholar] [CrossRef]
- Lingvay, M.; Caramitu, A.-R.; Borș, A.-M.; Lingvay, I. Dielectric Spectroscopic Evaluation in the Extremely Low Frequency Range of an Aspergillus Niger Culture. Stud. UBB Chem. 2019, 64, 279–288. [Google Scholar] [CrossRef]
- Bartha, C.; Caramitu, A.; Jipa, M.; Ignat, D.M.; Tókos, A. Dielectric Behavior of Sludge from Waste Water Treatment. Stud. UBB Chem. 2020, 65, 85–93. [Google Scholar] [CrossRef]
- Lingvay, I.; Radu, L.-E.; Caramitu, A.-R.; Mitrea, S.; Oprina, G.; Voina, A. Procedure for Determining Representative Frequencies in the Behavior of Microbial and Algal Cells. RO Patent 132117, 9 February 2016. [Google Scholar]
- Bartha, C.; Tókos, A.; Jipa, M.; Caramitu, A.; Voina, A.; Circiumaru, G.; Micu, D.-D.; Lingvay, I. Saving Energy in Biological Wastewater Treatment by Using Extremely Low-Frequency Electric Field—Pilot-Scale Study. Sustainability 2023, 15, 11670. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Zhang, L.; Xie, D.T.; Onwosi, C.O.; Muhammad, W.I.; Odoh, C.K.; Sam, K.; Idenyi, J.N. Bioaugmentation as a green technology for hydrocarbon pollution remediation. Problems and prospects. J. Environ. Manag. 2022, 304, 114313. [Google Scholar] [CrossRef] [PubMed]
- Zrimec, A.; Jerman, I.; Lahajnar, G. Alternating electric fields stimulate ATP synthesis in Escherichia coli. Cell. Mol. Biol. Lett. 2002, 7, 172–174. [Google Scholar] [PubMed]
- Zrimec, A.; Jerman, I.; Lahajnar, G. Low frequency alternating electric fields inhibit lactose uptake in Kluyveromyces marxianus. Bioelectrochem. Bioenerg. 1999, 48, 481–484. [Google Scholar] [CrossRef]
- He, Z.; Jin, W.; Zhou, X.; Han, W.; Gao, S.; Chen, C.; Chen, Y.; Yin, S.; Che, L.; Jiang, G. Enhancing biomass and lipid yield of microalga Scenedesmus obliquus by the periodic direct current. J. Water Process Eng. 2022, 48, 102872. [Google Scholar] [CrossRef]
- Voina, A.; Tokos, A.; Jipa, M.; Cîrciumaru, G.; Bartha, C.; Chihaia, R.A.; Tănase, N.; Lingvay, I. Equipment for Increasing the Phototrophic Microalgae Production at Lab Scale. In Proceedings of the ICECET 2023, Cape Town, South Africa, 16–17 November 2023; IEEE Xplore: Piscataway, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Insights into the physiology of Chlorella vulgaris cultivated in sweet sorghum bagasse hydrolysate for sustainable algal biomass and lipid production. Sci. Rep. 2021, 11, 6779. [Google Scholar] [CrossRef] [PubMed]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Psychol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Silkina, A.; Flynn, K.; Llewellyn, C.; Bayliss, C. (Eds.) Standard Operating Procedures for Analytical Methods and Data Collection in Support of Pilot-Scale Cultivation of Microalgae. In Public Output Report WP1A3.01 of the EnAlgae Project; EnAlgae, Swansea University, Centre for Sustainable Aquatic Research: Swansea, UK, 2015; p. 395. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Prats, C.; López, D.; Giró, A.; Ferrer, J.; Valls, J. Individual-based modelling of bacterial cultures to study the microscopic causes of the lag phase. Theor. Biol. 2006, 241, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.M.; Stanier, R.Y. Growth and Division of Some Unicellular Blue-green Algae. J. Gen. Microbiol. 1968, 51, 199–202. [Google Scholar] [CrossRef]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquatic Res. 2020, 46, 153–158. [Google Scholar] [CrossRef]
- Kim, D.; Vijayan, D.; Praveenkumar, R.; Han, J.; Lee, K.; Park, J.; Chang, W.; Lee, J.; Oh, Y. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef]
- Junge, W.; Nelson, N. ATP Synthase. Annu. Rev. Biochem. 2015, 84, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Vonck, J.; Mills, D.J.; Meier, T.; Kühlbrandt, W. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 2018, 360, 6389. [Google Scholar] [CrossRef]
- Szabó, T.; Magyar, M.; Hajdu, K.; Dorogi, M.; Nyerki, E.; Tóth, T.; Lingvay, M.; Garab, G.; Hernádi, K.; Nagy, L. Structural and Functional Hierarchy in Photosynthetic Energy Conversion—From Molecules to Nanostructures. Nanoscale Res. Lett. 2015, 10, 458. [Google Scholar] [CrossRef]
- Lingvay, M.; Akhtar, P.; Sebők-Nagy, K.; Páli, T.; Lambrev, P.H. Photobleaching of chlorophyll in light-harvesting complex II increases in lipid environment. Front. Plant Sci. 2020, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, P.; Lingvay, M.; Kiss, T.; Deák, R.; Bóta, A.; Ughy, B.; Garab, G.; Lambrev, P.H. Excitation energy transfer between Light-harvesting complex II and Photosystem I in reconstituted membranes. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, P.; Lindorfer, D.; Lingvay, M.; Pawlak, K.; Zsiros, O.; Siligardi, G.; Jávorfi, T.; Dorogi, M.; Ughy, B.; Garab, G.; et al. Anisotropic Circular Dichroism of Light-Harvesting Complex II in Oriented Lipid Bilayers: Theory Meets Experiment. J. Phys. Chem. B 2019, 123, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).