Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review
Abstract
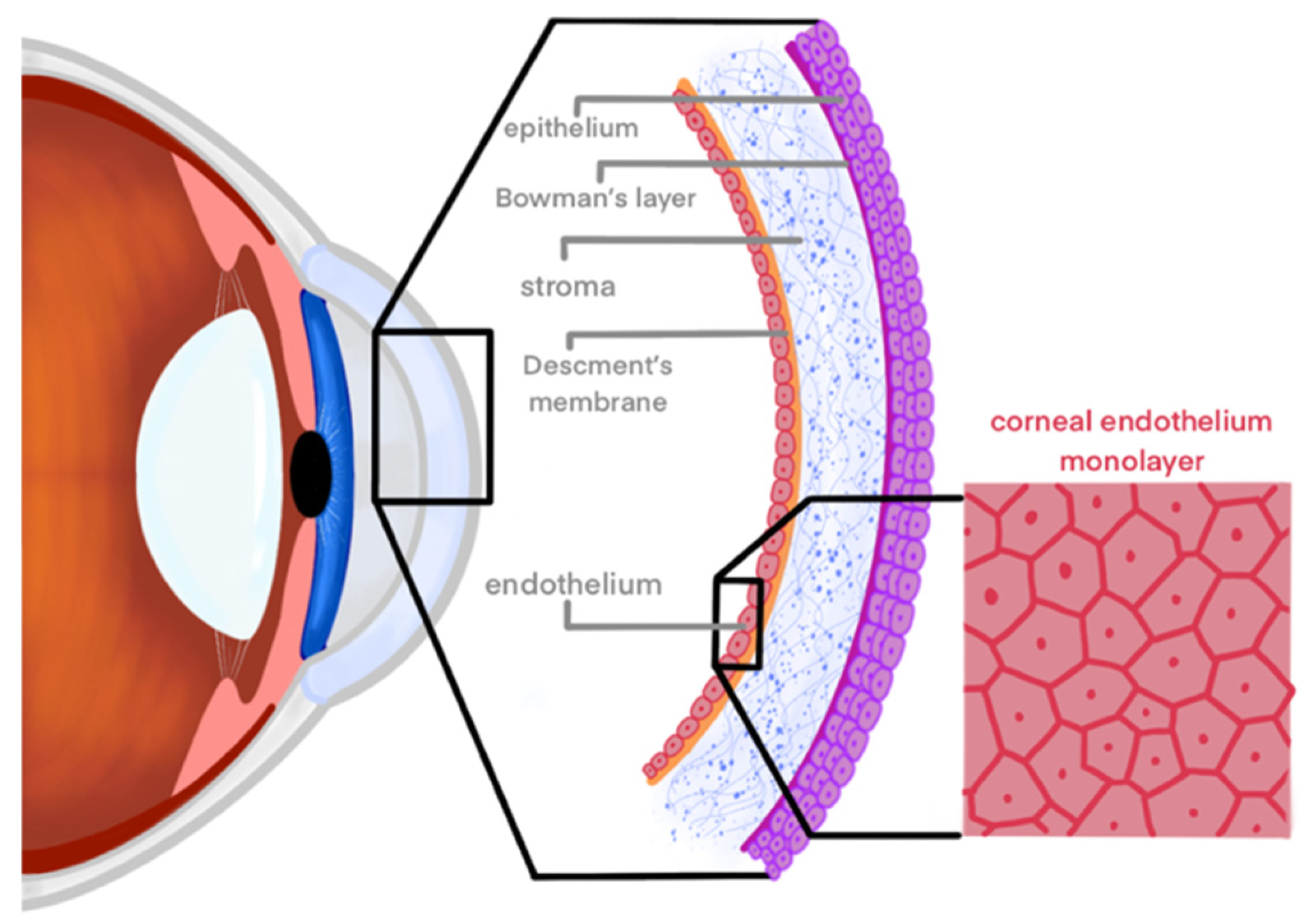
1. Introduction
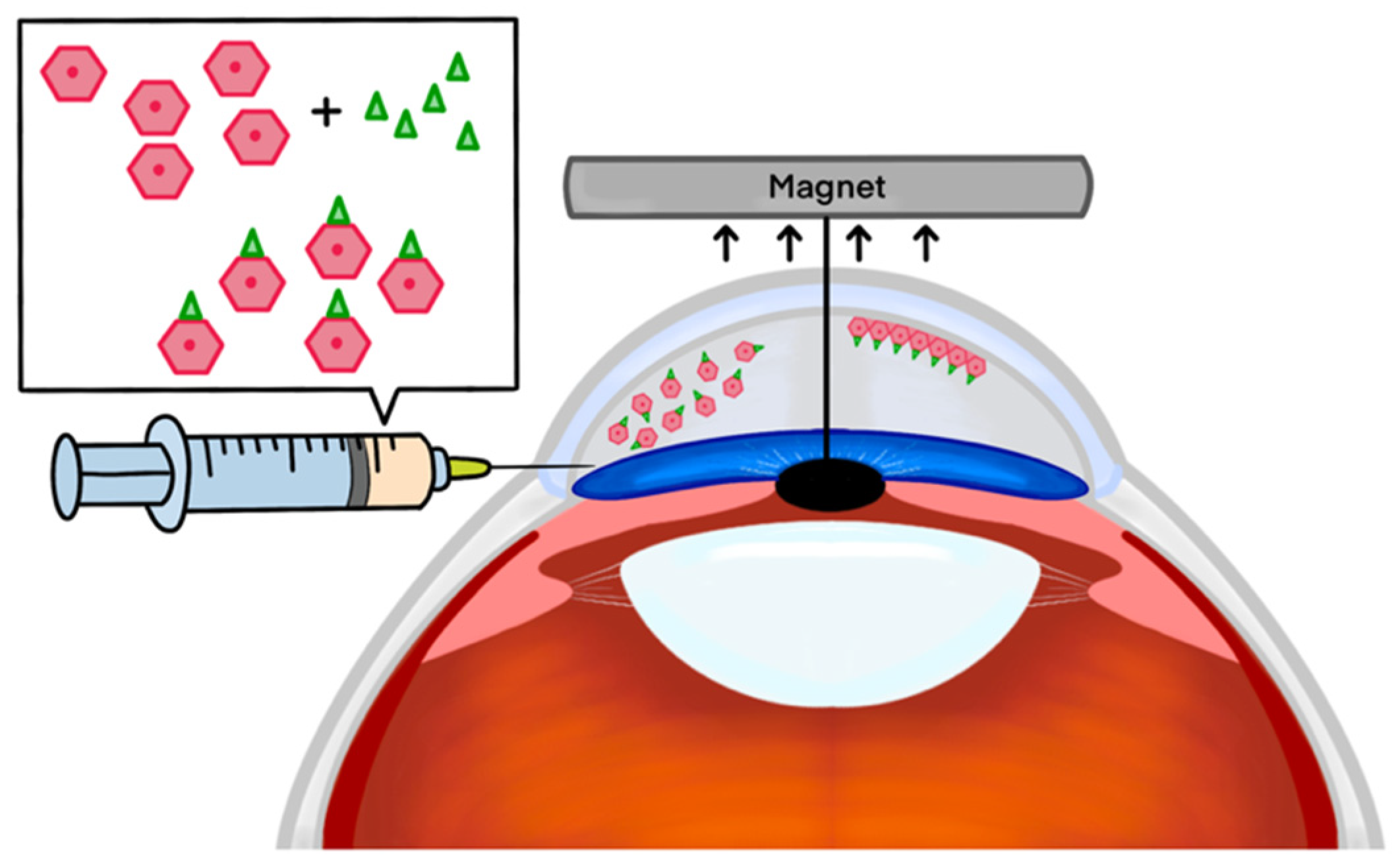
1.1. Magnetic Nanotechnology
1.2. Culturing CECs
1.3. Options for Delivery of Cultured CECs
2. Discussion
2.1. Effectiveness of Magnetic Nanoparticles in Enhanced Delivery of HCECs
| Moysidis et al. [80] | Xia et al. [81] | Zhao et al. [83] | |
|---|---|---|---|
| Cell type | Cadaveric donor HCECs—50,000 | Cadaveric donor primary HCECs—200,000–600,000 | Donor CECs (origin not specified)—100,000 |
| Experimental Model | In vitro (contact lens model) | Rabbit model (corneal endothelial dysfunction; endothelial cell or Descemet stripping) | Rabbit model (corneal endothelium injury by mechanical destruction) |
| MNPs used | 50 nm diameter superparamagnetic nanoparticles | 50 nm diameter superparamagnetic nanoparticles | Superparamagnetic Fe3O4 nanoparticles in a HA gel matrix (size not specified) |
| Magnet used | Custom made magnet | External neodymium magnet (diameter = 12 mm and height 20 mm) | External neodymium magnet (diameter = 5 mm and height = 20 mm) |
| Control | HCECs without nanoparticles | BSS+ solution | Magnetic PBS solution |
| Results | 2.4-fold increase in cell density compared to gravity | Improved post-operative corneal clarity and reduced corneal thickness | Increased delivery efficiency with HA gel, challenges in uniform distribution |
| Histological findings | Tight junction formation (ZO-1) and functional integration into a monolayer (preserved corneal morphology of transplanted cells) | Tight junction protein expression (ZO-1 and NCAM) and functional integration into a monolayer (preserved corneal morphology of transplanted cells) | Functional integration, irregular cellular distribution |
| Adverse events | N/A as in vitro study | No migration of cells to the iris or trabecular meshwork and no acute fluctuations in IOP | IOP was not investigated as an adverse event |
| Challenges | Saturation effect at higher | Limited follow-up period | Gel degradation impacting cell distribution, impact on IOP not reported |
2.2. Limitations, Safety and Challenges
3. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Dua, H.S.; Faraj, L.A.; Said, D.G.; Gray, T.; Lowe, J. Human Corneal Anatomy Redefined: A Novel Pre-Descemet’s Layer (Dua’s Layer). Ophthalmology 2013, 120, 1778–1785. [Google Scholar] [CrossRef]
- Klyce, S.D. 12. Endothelial pump and barrier function. Exp. Eye Res. 2020, 198, 108068. [Google Scholar] [CrossRef]
- He, Z.; Forest, F.; Gain, P.; Rageade, D.; Bernard, A.; Acquart, S.; Peoc’h, M.; Defoe, D.M.; Thuret, G. 3D map of the human corneal endothelial cell. Sci. Rep. 2016, 6, 29047. [Google Scholar] [CrossRef]
- Okumura, N.; Hirano, H.; Numata, R.; Nakahara, M.; Ueno, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Cell surface markers of functional phenotypic corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7610–7618. [Google Scholar] [CrossRef]
- Parekh, M.; Peh, G.; Mehta, J.S.; Ahmad, S.; Ponzin, D.; Ferrari, S. Effects of corneal preservation conditions on human corneal endothelial cell culture. Exp. Eye Res. 2019, 179, 93–101. [Google Scholar] [CrossRef]
- Polisetti, N.; Joyce, N.C. The culture of limbal stromal cells and corneal endothelial cells. Corneal Regen. Med. Methods Protoc. 2013, 1014, 131–139. [Google Scholar]
- Joyce, N.C.; Meklir, B.; Joyce, S.J.; Zieske, J.D. Cell cycle protein expression and proliferative status in human corneal cells. Investig. Ophthalmol. Vis. Sci. 1996, 37, 645–655. [Google Scholar]
- Chen, K.H.; Harris, D.L.; Joyce, N.C. TGF-beta2 in aqueous humor suppresses S-phase entry in cultured corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2513–2519. [Google Scholar]
- Murphy, C.; Alvarado, J.; Juster, R.; Maglio, M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Investig. Ophthalmol. Vis. Sci. 1984, 25, 312–322. [Google Scholar]
- Age-Related Changes and Diseases of the Ocular Surface and Cornea |IOVS| ARVO Journals. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2127383 (accessed on 5 May 2024).
- Stiemke, M.M.; Edelhauser, H.F.; Geroski, D.H. The developing corneal endothelium: Correlation of morphology, hydration and Na/K ATPase pump site density. Curr. Eye Res. 1991, 10, 145–156. [Google Scholar] [CrossRef]
- Bourne, W.M. Cellular changes in transplanted human corneas. Cornea 2001, 20, 560–569. [Google Scholar] [CrossRef]
- Zhu, C.; Joyce, N.C. Proliferative Response of Corneal Endothelial Cells from Young and Older Donors. Investig. Opthalmol. Vis. Sci. 2004, 45, 1743. [Google Scholar] [CrossRef]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef]
- Vaiciuliene, R.; Rylskyte, N.; Baguzyte, G.; Jasinskas, V. Risk factors for fluctuations in corneal endothelial cell density (Review). Exp. Ther. Med. 2022, 23, 129. [Google Scholar] [CrossRef]
- Adamis, A.P.; Filatov, V.; Tripathi, B.J. Fuchs’ endothelial dystrophy of the cornea. Surv. Ophthalmol. 1993, 38, 149–168. [Google Scholar] [CrossRef]
- Krachmer, J.H. Posterior polymorphous corneal dystrophy: A disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans. Am. Ophthalmol. Soc. 1985, 83, 413–475. [Google Scholar]
- Chan, C.C.; Green, W.R.; Barraquer, J.; Barraquer-Somers, E.; de la Cruz, Z.C. Similarities between Posterior Polymorphous and Congenital Hereditary Endothelial Dystrophies: A Study of 14 Buttons of 11 Cases. Cornea 1982, 1, 155. [Google Scholar] [CrossRef]
- Shields, M.B. Progressive essential iris atrophy, Chandler’s syndrome, and the iris nevus (Cogan-Reese) syndrome: A spectrum of disease. Surv. Ophthalmol. 1979, 24, 3–20. [Google Scholar] [CrossRef]
- Carlson, K.H.; Ilstrup, D.M.; Bourne, W.M.; Dyer, J.A. Effect of silicone elastomer contact lens wear on endothelial cell morphology in aphakic eyes. Cornea 1990, 9, 45–47. [Google Scholar] [CrossRef]
- Bourne, W.M.; Hodge, D.O.; McLaren, J.W. Estimation of corneal endothelial pump function in long-term contact lens wearers. Investig. Ophthalmol. Vis. Sci. 1999, 40, 603–611. [Google Scholar]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Continued endothelial cell loss ten years after lens implantation. Ophthalmology 1994, 101, 1014–1022; discussion 1022–1023. [Google Scholar] [CrossRef]
- Price, M.O.; Feng, M.T.; Price, F.W.J. Endothelial Keratoplasty Update 2020. Cornea 2021, 40, 541. [Google Scholar] [CrossRef]
- Price, M.O.; Price, F.W., Jr. Endothelial keratoplasty—A review. Clin. Exp. Ophthalmol. 2010, 38, 128–140. [Google Scholar] [CrossRef]
- Melles, G.R.; Eggink, F.A.; Lander, F.; Pels, E.; Rietveld, F.J.; Beekhuis, W.H.; Binder, P.S. A surgical technique for posterior lamellar keratoplasty. Cornea 1998, 17, 618–626. [Google Scholar] [CrossRef]
- Melles, G.R.J.; Wijdh, R.H.J.; Nieuwendaal, C.P. A technique to excise the descemet membrane from a recipient cornea (descemetorhexis). Cornea 2004, 23, 286–288. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 6, CD012097. [Google Scholar] [CrossRef]
- Grottone, G.T.; Pereira, N.C.; Gomes, J.Á.P. Endothelial keratoplasty: Evolution and horizons. Arq. Bras. Oftalmol. 2012, 75, 439–446. [Google Scholar] [CrossRef]
- Lee, W.B.; Jacobs, D.S.; Musch, D.C.; Kaufman, S.C.; Reinhart, W.J.; Shtein, R.M. Descemet’s stripping endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology 2009, 116, 1818–1830. [Google Scholar] [CrossRef]
- Hurley, D.J.; Murtagh, P.; Guerin, M. Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (UT-DSAEK) versus Descemet Membrane Endothelial Keratoplasty (DMEK)—A systematic review and meta-analysis. Eye 2023, 37, 3026–3032. [Google Scholar] [CrossRef]
- Zafar, S.; Parker, J.S.; de Kort, C.; Melles, G.; Sikder, S. Perceived difficulties and barriers to uptake of Descemet’s membrane endothelial keratoplasty among surgeons. Clin. Ophthalmol. 2019, 13, 1055–1061. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic Characterization of Iron Oxide Nanoparticles for Biomedical Applications. Biomed. Nanotechnol. Methods Protoc. 2017, 1570, 47–71. [Google Scholar]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Misra, R.D.K. Magnetic nanoparticle carrier for targeted drug delivery: Perspective, outlook and design. Mater. Sci. Technol. 2008, 24, 1011–1019. [Google Scholar] [CrossRef]
- Edelman, E.R.; Langer, R. Optimization of release from magnetically controlled polymeric drug release devices. Biomaterials 1993, 14, 621–626. [Google Scholar] [CrossRef]
- Raju, H.B.; Hu, Y.; Vedula, A.; Dubovy, S.R.; Goldberg, J.L. Evaluation of magnetic micro- and nanoparticle toxicity to ocular tissues. PLoS ONE 2011, 6, e17452. [Google Scholar] [CrossRef]
- Giannaccini, M.; Pedicini, L.; De Matienzo, G.; Chiellini, F.; Dente, L.; Raffa, V. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci. Rep. 2017, 7, 43092. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Zhang, S.; Zhong, M.; Fan, H. Ferrite Nanoparticles-Based Reactive Oxygen Species-Mediated Cancer Therapy. Front. Chem. 2021, 9, 651053. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Y.X.; Gao, Y.J.; Gao, F.P.; Fan, Y.S.; Li, X.J.; Duan, Z.Y.; Wang, H. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 5100–5107. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, K.; Subramanian, S.; Korde, A.; Singh, R.; Sawant, K. Heterogeneous surface architectured pH responsive Metal-Drug Nano-conjugates for mitochondria targeted therapy of Glioblastomas: A multimodal intranasal approach. Chem. Eng. J. 2020, 394, 124419. [Google Scholar] [CrossRef]
- Pankhurst, Q.; Connolly, J.; Jones, S.; Dobson, J. TOPICAL REVIEW: Applications of magnetic nanoparticles in biomedicine. J. Phys. Appl. Phys. 2003, 36. [Google Scholar] [CrossRef]
- Plank, C.; Scherer, F.; Schillinger, U.; Bergemann, C.; Anton, M. Magnetofection: Enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. J. Liposome Res. 2003, 13, 29–32. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in Vitro Gene Delivery: It is Now Time to Set the Bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef]
- Bartakova, A.; Kunzevitzky, N.J.; Goldberg, J.L. Regenerative Cell Therapy for Corneal Endothelium. Curr. Ophthalmol. Rep. 2014, 2, 81–90. [Google Scholar] [CrossRef]
- Baum, J.L.; Niedra, R.; Davis, C.; Yue, B.Y. Mass culture of human corneal endothelial cells. Arch. Ophthalmol. 1979, 97, 1136–1140. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Toh, K.P.; Wu, F.Y.; Tan, D.T.; Mehta, J.S. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS ONE 2011, 6, e28310. [Google Scholar] [CrossRef]
- Frausto, R.F.; Swamy, V.S.; Peh, G.S.L.; Boere, P.M.; Hanser, E.M.; Chung, D.D.; George, B.L.; Morselli, M.; Kao, L.; Azimov, R.; et al. Phenotypic and functional characterization of corneal endothelial cells during in vitro expansion. Sci. Rep. 2020, 10, 7402. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Chng, Z.; Ang, H.P.; Cheng, T.Y.D.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.F.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Bartakova, A.; Kuzmenko, O.; Alvarez-Delfin, K.; Kunzevitzky, N.J.; Goldberg, J.L. A Cell Culture Approach to Optimized Human Corneal Endothelial Cell Function. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1617–1629. [Google Scholar] [CrossRef]
- Takamizawa, S.; Maehata, Y.; Imai, K.; Senoo, H.; Sato, S.; Hata, R.I. Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol. Int. 2004, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Schweigerer, L.; Neufeld, G.; Friedman, J.; Abraham, J.A.; Fiddes, J.C.; Gospodarowicz, D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature 1987, 325, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Shima, N.; Kimoto, M.; Yamaguchi, M.; Yamagami, S. Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with L-ascorbic acid 2-phosphate. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8711–8717. [Google Scholar] [CrossRef]
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J. Biol. Chem. 2018, 293, 3758–3769. [Google Scholar] [CrossRef]
- Ko, M.K.; Kay, E.P. Regulatory role of FGF-2 on type I collagen expression during endothelial mesenchymal transformation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4495–4503. [Google Scholar] [CrossRef]
- Engelmann, K.; Böhnke, M.; Friedl, P. Isolation and long-term cultivation of human corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1656–1662. [Google Scholar]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Ishizaki, T.; Uehata, M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000, 325, 273–284. [Google Scholar] [PubMed]
- Ishizaki, T.; Uehata, M.; Tamechika, I.; Keel, J.; Nonomura, K.; Maekawa, M.; Narumiya, S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000, 57, 976–983. [Google Scholar] [PubMed]
- Park, J.H.; Lee, K.; Park, C.Y. Effect of Magnetic Microparticles on Cultivated Human Corneal Endothelial Cells. Transl. Vis. Sci. Technol. 2023, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Wongvisavavit, R.; Parekh, M.; Ahmad, S.; Daniels, J.T. Challenges in corneal endothelial cell culture. Regen. Med. 2021, 16, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.L.; Ong, H.S.; Adnan, K.; Ang, H.P.; Lwin, C.N.; Seah, X.Y.; Lin, S.J.; Mehta, J.S. Functional Evaluation of Two Corneal Endothelial Cell-Based Therapies: Tissue-Engineered Construct and Cell Injection. Sci. Rep. 2019, 9, 6087. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Qu, J.; Xie, H.; Zhao, J.; Fan, T.; Liu, X.; Zhang, M. Tissue-Engineered Corneal Endothelial Sheets Using Ultrathin Acellular Porcine Corneal Stroma Substrates for Endothelial Keratoplasty. ACS Biomater. Sci. Eng. 2022, 8, 1301–1311. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Park, S.; Leonard, B.C.; Raghunathan, V.K.; Kim, S.; Li, J.Y.; Mannis, M.J.; Murphy, C.J.; Thomasy, S.M. Animal models of corneal endothelial dysfunction to facilitate development of novel therapies. Ann. Transl. Med. 2021, 9, 1271. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK Inhibitor Converts Corneal Endothelial Cells into a Phenotype Capable of Regenerating In Vivo Endothelial Tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.-I.; Ueno, M.; Hagiya, M.; Hamuro, J.; et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef] [PubMed]
- Bostan, C.; Thériault, M.; Forget, K.J.; Doyon, C.; Cameron, J.D.; Proulx, S.; Brunette, I. In Vivo Functionality of a Corneal Endothelium Transplanted by Cell-Injection Therapy in a Feline Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1620–1634. [Google Scholar] [CrossRef]
- Mimura, T.; Shimomura, N.; Usui, T.; Noda, Y.; Kaji, Y.; Yamgami, S.; Amano, S.; Miyata, K.; Araie, M. Magnetic attraction of iron-endocytosed corneal endothelial cells to Descemet’s membrane. Exp. Eye Res. 2003, 76, 745–751. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Yanagi, Y.; Usui, T.; Ono, K.; Araie, M.; Amano, S. Sphere Therapy for Corneal Endothelium Deficiency in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3128–3135. [Google Scholar] [CrossRef][Green Version]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Aurion Biotech. CLARA: A Phase 1/2 Multi-Center, Randomized, Double-Masked, Prospective, Parallel-Arm Study of AURN001 in Subjects with Corneal Edema Secondary to Corneal Endothelial Dysfunction (ABA-1). 2024; Report No.: NCT06041256. Available online: https://clinicaltrials.gov/study/NCT06041256 (accessed on 1 January 2024).
- Worthylake, R.A.; Burridge, K. RhoA and ROCK Promote Migration by Limiting Membrane Protrusions. J. Biol. Chem. 2003, 278, 13578–13584. [Google Scholar] [CrossRef]
- Cornell, L.E.; Wehmeyer, J.L.; Johnson, A.J.; Desilva, M.N.; Zamora, D.O. Magnetic Nanoparticles as a Potential Vehicle for Corneal Endothelium Repair. Mil. Med. 2016, 181 (Suppl. S5), 232–239. [Google Scholar] [CrossRef]
- Moysidis, S.N.; Alvarez-Delfin, K.; Peschansky, V.J.; Salero, E.; Weisman, A.D.; Bartakova, A.; Raffa, G.A.; Merkhofer, R.M.; Kador, K.E.; Kunzevitzky, N.J.; et al. Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 499–509. [Google Scholar] [CrossRef]
- Xia, X.; Atkins, M.; Dalal, R.; Kuzmenko, O.; Chang, K.C.; Sun, C.B.; Benatti, C.A.; Rak, D.J.; Nahmou, M.; Kunzevitzky, N.J.; et al. Magnetic Human Corneal Endothelial Cell Transplant: Delivery, Retention, and Short-Term Efficacy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2438–2448. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Usui, T.; Ishii, Y.; Ono, K.; Yokoo, S.; Funatsu, H.; Araie, M.; Amano, S. Long-term outcome of iron-endocytosing cultured corneal endothelial cell transplantation with magnetic attraction. Exp. Eye Res. 2005, 80, 149–157. [Google Scholar] [CrossRef]
- Zhao, S.; Hou, S.; Li, D.; Li, L.; Ding, X.; Huang, Y.; Li, Y.; Ji, J.; Wang, L.; Fan, Y. Injectable magnetic hyaluronic acid gel for corneal endothelial cells efficient delivery and retention. Appl. Mater. Today 2024, 37, 102090. [Google Scholar] [CrossRef]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Jeong, S.H.; Logan, C.M.; Le, P.; Mundy, D.; Chen, F.; Chen, K.M.; Kim, M.; Lee, G.-H.; Na, K.-S.; et al. Supramolecular host-guest hyaluronic acid hydrogels enhance corneal wound healing through dynamic spatiotemporal effects. Ocul. Surf. 2022, 23, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Karvinen, J.; Sorsa, E.; Jönkkäri, I.; Väliaho, J.; Kallio, P.; Ilmarinen, T.; Miettinen, S.; Skottman, H.; Kellomäki, M. Hydrazone crosslinked hyaluronan-based hydrogels for therapeutic delivery of adipose stem cells to treat corneal defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Hong, Y.M.; Chung, W.G.; Park, W.; Lee, J.; Kim, H.K.; Byeon, S.H.; Kim, D.W.; Park, J.-U. Real-time in vivo monitoring of intraocular pressure distribution in the anterior chamber and vitreous chamber for diagnosis of glaucoma. Sci. Adv. 2024, 10, eadk7805. [Google Scholar] [CrossRef] [PubMed]
- Benozzi, J.; Nahum, L.P.; Campanelli, J.L.; Rosenstein, R.E. Effect of hyaluronic acid on intraocular pressure in rats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2196–2200. [Google Scholar]
- Emmecell. A Phase 1, Prospective, Multi-Center, Open-Label, Dose-Escalation Study to Assess the Safety, and Tolerability of EO2002 with and without Endothelial Brushing or Descemet Stripping in the Treatment of Corneal Edema (EMME-001). 2024; Report No.: NCT04894110. Available online: https://clinicaltrials.gov/study/NCT04894110 (accessed on 1 January 2024).
- Asociación para Evitar la Ceguera en México. Phase 1, Multiple Dose, Open-Label Study to Assess the Safety and Tolerability of EO2002 Intracameral Injections with or without Topical Ripasudil in the Treatment of Corneal Edema. 2022. Report No.: NCT05636579. Available online: https://clinicaltrials.gov/study/NCT05636579 (accessed on 1 January 2024).
- Markides, H.; Rotherham, M.; Haj, A. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Raju, H.B.; Hu, Y.; Padgett, K.R.; Rodriguez, J.E.; Goldberg, J.L. Investigation of nanoparticles using magnetic resonance imaging after intravitreal injection. Clin. Exp. Ophthalmol. 2012, 40, 100–107. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Meena, R.; Rajamani, P. Iron oxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in lymphocytes. J. Appl. Toxicol. JAT 2017, 37, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.R.; Yue, X.; Zou, B.; Shi, H.; Yu, H.; Liu, K.; Lin, X.; Xu, J.; Yang, C.; Zhao, J.; et al. Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int. J. Nanomed. 2014, 9, 1393–1402. [Google Scholar]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef] [PubMed]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of nanoparticles from cells: Role in cellular retention and toxicity. Adv. Colloid Interface Sci. 2013, 201–202, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic Hyperthermia with Magnetic Nanoparticles: A Status Review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, S.A.; Rajaei Jafarabadi, M.; Abrishami, M. Temperature Distribution Simulation of the Human Eye Exposed to Laser Radiation. J. Lasers Med. Sci. 2013, 4, 175–181. [Google Scholar] [PubMed]
- Demirci, H.; Slimani, N.; Pawar, M.; Kumon, R.E.; Vaishnava, P.; Besirli, C.G. Magnetic Hyperthermia in Y79 Retinoblastoma and ARPE-19 Retinal Epithelial Cells: Tumor Selective Apoptotic Activity of Iron Oxide Nanoparticle. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef]
- Shu, D.Y.; Chaudhary, S.; Cho, K.S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and Gold Nanoparticles Exposure to In Vitro Cultured Retina—Studies on Nanoparticle Internalization, Apoptosis, Oxidative Stress, Glial- and Microglial Activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B.K. Nanoceria Prevents Glucose-Induced Protein Glycation in Eye Lens Cells. Nanomaterials 2021, 11, 1473. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B. Treatment of Human Lens Epithelium with High Levels of Nanoceria Leads to Reactive Oxygen Species Mediated Apoptosis. Molecules 2020, 25, 441. [Google Scholar] [CrossRef] [PubMed]
| Corneal Endothelial Disease | Current Treatments |
|---|---|
| Fuchs’ Endothelial Corneal Dystrophy | Conservative Management: Hypertonic saline drops Surgical Options: Endothelial keratoplasty (EK), Descemet’s stripping endothelial keratoplasty (DSEK), Descemet’s membrane endothelial keratoplasty (DMEK) |
| Bullous Keratopathy | Conservative Management: Hypertonic saline drops, bandage contact lenses Surgical Options: EK, DSEK, DMEK |
| Endothelial Decompensation due to Contact Lens Wear or Infections | Antimicrobial Therapy: Treatment of underlying infections Surgical Options: EK, DSEK, DMEK |
| Congenital Hereditary Endothelial Dystrophy (CHED) | Medical Management: Symptomatic relief with lubricating drops Surgical Options: EK, DSEK, DMEK |
| Posterior Polymorphous Corneal Dystrophy (PPCD) | Conservative Management: Monitoring for progression Medical Management: Hypertonic saline drops Surgical Options: Glaucoma Drainage Implants, EK, DSEK, DMEK |
| Iridocorneal Endothelial Syndrome (ICE) | Medical Management: Hypertonic saline drops, Topical medications to control intraocular pressure Surgical Options: Trabeculectomy, Glaucoma Drainage Implants, EK, DSEK, DMEK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkelyte, V.; Thompson, P.; Coelho, M.; Woronkowicz, M.; Skopinski, P.; Roberts, H. Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review. Appl. Sci. 2024, 14, 5877. https://doi.org/10.3390/app14135877
Vilkelyte V, Thompson P, Coelho M, Woronkowicz M, Skopinski P, Roberts H. Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review. Applied Sciences. 2024; 14(13):5877. https://doi.org/10.3390/app14135877
Chicago/Turabian StyleVilkelyte, Virginija, Polly Thompson, Maria Coelho, Małgorzata Woronkowicz, Piotr Skopinski, and Harry Roberts. 2024. "Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review" Applied Sciences 14, no. 13: 5877. https://doi.org/10.3390/app14135877
APA StyleVilkelyte, V., Thompson, P., Coelho, M., Woronkowicz, M., Skopinski, P., & Roberts, H. (2024). Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review. Applied Sciences, 14(13), 5877. https://doi.org/10.3390/app14135877






