The Influence of Propolis Nonwoven Scaffolds on Burn Wound’s Heparan Sulfates and Hyaluronan
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Development of Experimental Dressings
2.3. Heparan Sulfate and Hyaluronan Extraction
2.4. Heparan Sulfate and Hyaluronan Quantitative Assessment
- The competitive inhibition enzyme immunoassay kit for Heparan Sulfate (HS), no. CEA161Ge—from Cloud-Clone Corp.
- The competitive inhibition enzyme immunoassay kit for Hyaluronic Acid (HA), no. CEA182Ge—from Cloud-Clone Corp.
2.5. Statistical Analysis
3. Results
3.1. Heparan Sulfates
3.2. Hyaluronan
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Artem Ataide, J.; Caramori Cefali, L.; Machado Croisfelt, F.; Arruda Martins Shimojo, A.; Oliveira-Nascimento, L.; Gava Mazzola, P. Natural actives for wound healing: A review. Phytother. Res. 2018, 32, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Kwiatkowska, A.; Hołderna-Kędzia, E.; Sosnowska, K.; Mrówczyńska, L.; Ratajczak, I. Aktywność biologiczna ekstraktów z propolisu. Postęp. Fitoter. 2021, 22, 8–13. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. Diverse roles of heparan sulfate and heparin in wound repair. BioMed Res. Int. 2015, 2015, 549417. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Komosinska-Vassev, K.; Wisowski, G.; Mencner, L.; Stojko, J.; Kozma, E.M. Propolis modulates fibronectin expression in the matrix of thermal injury. BioMed Res. Int. 2014, 2014, 748101. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Koźma, E.M.; Wisowski, G.; Stojko, J.; Klimek, K.; Olczyk, K. Propolis modulates vitronectin, laminin, and heparan sulfate/heparin expression during experimental burn healing. J. Zhejiang Univ. Sci. B 2012, 13, 932–941. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosinska-Vassev, K.; Winsz-Szczotka, K.; Stojko, J.; Klimek, K.; Kozma, E.M. Propolis induces chondroitin/dermatan sulphate and hyaluronic acid accumulation in the skin of burned wound. Evid.-Based Complement. Altern. Med. 2013, 2013, 290675. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Wisowski, G.; Komosinska-Vassev, K.; Stojko, J.; Klimek, K.; Olczyk, M.; Kozma, E.M. Propolis modifies collagen types I and III accumulation in the matrix of burnt tissue. Evid.-Based Complement. Altern. Med. 2013, 2013, 423809. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Stojko, J.; Klimek, K.; Olczyk, K. Wpływ Propolu T na akumulację lamininy i witronektyny w macierzy doświadczalnych ran oparzeniowych—Badania wstępne. Farm Pol. 2011, 67, 804–808. [Google Scholar]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. BioMed Res. Int. 2014, 2014, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, R.J.D.; Coutinho-Netto, J. Cellular aspects of wound healing. An. Bras. Dermatol. 2009, 84, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Rodwell, V.W.; Bender, D.; Botham, K.M.; Kennelly, P.J.; Weil, P.A. Biochemia Harpera; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2018. [Google Scholar]
- Sufleta, A.; Mazur-Zielińska, H. Glikozaminoglikany—Budowa, właściwości biochemiczne i znaczenie kliniczne. Ann. Acad. Med. Silesiensis 2010, 64, 64–68. [Google Scholar]
- Daroszewski, J.; Rybka, J.; Gamian, A. Glikozaminoglikany w patogenezie i diagnostyce oftalmopatii Gravesa Postepy. Postep. Hig. Med. Dosw. 2006, 60, 370–378. [Google Scholar]
- Kroma, A.; Feliczak-Guzik, A.; Nowak, I. Zastosowanie glikozaminoglikanów w preparatach kosmetycznych. Chemik 2012, 66, 136–139. [Google Scholar]
- Toole, B.P.; Slomiany, M.G. Hyaluronan: A constitutive regulator of chemoresistance and malignancy in cancer cells. Semin. Cancer Biol. 2008, 18, 244–250. [Google Scholar] [CrossRef]
- Sadowski, M.; Borzyn-Kłuczyk, M.; Stypułkowska, A.; Wiełgat, P.; Zwierz, K. Macierz międzykomórkowa ściany żyły. Przegl. Flebol. 2006, 14, 141–149. [Google Scholar]
- Abaterusso, C.; Gambaro, G. The role of glycosaminoglycans and sulodexide in the treatment of diabetic nephropathy. Treat. Endocrinol. 2006, 5, 211–222. [Google Scholar] [CrossRef]
- Koźma, E.M.; Olczyk, K.; Głowacki, A.; Bobiński, R. An accumulation of proteoglycans in scarred fascia. Mol. Cell. Biochem. 2000, 203, 103–112. [Google Scholar] [CrossRef]
- Im, A.R.; Kim, Y.S. Role of glycosaminoglycans in wound healing. Arch. Pharm. Sci. Res. 2009, 2, 106–114. [Google Scholar]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Zeyland, J.O.; Lipiński, D.A.; Juzwa, W.O.; Pławski, A.; Słomski, R. Budowa i zastosowanie wybranych glikozoaminoglikanów. Med. Weter. 2006, 62, 139–144. [Google Scholar]
- Han, J.; Zhang, F.; Xie, J.; Linhardt, R.J.; Hiebert, L.M. Changes in cultured endothelial cell glycosaminoglycans under hyperglycemic conditions and the effect of insulin and heparin. Cardiovasc. Diabetol. 2009, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Pitt, S.J.; Stewart, A.J. Glycosaminoglycan Neutralization in Coagulation Control. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1258–1270. [Google Scholar] [CrossRef]
- Li, J.-P.; Kusche-Gullberg, M. Heparan Sulfate: Biosynthesis, Structure, and Function. Int. Rev. Cell Mol. Biol. 2016, 325, 215–273. [Google Scholar] [CrossRef]
- Collins, L.E.; Troeberg, L. Heparan sulfate as a regulator of inflammation and immunity. J. Leukoc. Biol. 2018, 105, 81–92. [Google Scholar] [CrossRef]
- Mende, M.; Bednarek, C.; Wawryszyn, M.; Sauter, P.; Biskup, M.B.; Schepers, U.; Bräse, S. Chemical synthesis of glycosaminoglycans. Chem. Rev. 2016, 116, 8193–8255. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, B.L.; Lord, M.S.; Melrose, J.; Whitelock, J.M. The role of heparan sulfate in inflammation, and the development of biomimetics as anti-inflammatory strategies. J. Histochem. Cytochem. 2018, 66, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Cecora, A.; Chwała, M. Czy glikozaminoglikany zmieniają właściwości ściany żylnej w warunkach zastoju krwi u chorych z przewlekłą niewydolnością żylną? Przegl. Flebol. 2003, 11, 85–89. [Google Scholar]
- Ravera, M.; Re, M.; Weiss, U.; Deferrari, L.; Deferrari, G. Emerging therapeutic strategies in diabetic nephropathy. J. Nephrol. 2007, 20 (Suppl. S12), 23–32. [Google Scholar]
- Sasarman, F.; Maftei, C.; Campeau, P.M.; Brunel-Guitton, C.; Mitchell, G.A.; Allard, P. Biosynthesis of glycosaminoglycans: Associated disorders and biochemical tests. J. Inherit. Metab. Dis. 2016, 39, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ly, M.; Linhardt, R.J. Proteoglycan sequence. Mol. Biosyst. 2012, 8, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska, D.; Milner-Krawczyk, M.; Kazanecka, M. Kwas hialuronowy—Charakterystyka, otrzymywanie i zastosowanie. Biotechnol. Food Sci. 2011, 75, 55–70. [Google Scholar]
- Heinegård, D. Proteoglycans and more—From molecules to biology. Int. J. Exp. Pathol. 2009, 90, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Rügheimer, L. Hyaluronan: A matrix component. Proc. AIP Conf. 2008, 1049, 126–132. [Google Scholar]
- Kablik, J.; Monheit, G.D.; Yu, L.; Chang, G.; Gershkovich, J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009, 35, 302–312. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Olczyk, K. Hyaluronan: Structure, metabolism, functions, and role in wound healing. Postep. Hig. Med. Dosw. 2008, 62, 651–659. [Google Scholar]
- Musiał, C. Rola i zastosowanie glikozaminoglikanów w trychologii i kosmetologii. Aesthetic Cosmetol. Med. 2021, 10, 33–37. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2006, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kruglikov, I.L.; Akgul, Y.; Scherer, P.E. Hyaluronan in adipogenesis, adipose tissue physiology and systemic metabolism. Matrix Biol. 2019, 78–79, 284–291. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M. Właściwości i zastosowanie kwasu hialuronowego w kosmetologii i medycynie estetycznej. Kosmetol. Estet. 2017, 4, 329–335. [Google Scholar]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Hoekstra, M.J.; Hupkens, P.; Dutrieux, R.P.; Bosch, M.M.C.; Brans, T.A.; Kreis, R.W. A comparative burn wound model in the New Yorkshire pig for the histopathological evaluation of local therapeutic regimens: Silver sulfadiazine cream as a standard. Br. J. Plast. Surg. 1993, 46, 585–589. [Google Scholar] [CrossRef]
- Brans, T.A.; Dutrieux, R.; Hoekstra, M.; Kreis, R.; du Pont, J. Histopathological evaluation of scalds and contact burns in the pig model. Burns 1994, 20, S48–S51. [Google Scholar] [CrossRef]
- Scott, J. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. In Methods of Biochemical Analysis; Glick, D., Ed.; Wiley: New York, NY, USA, 1960. [Google Scholar]
- Van Amerongen, J.P.; Lemmens, A.G.; Tonino, G.J.M. Glycosaminoglycans in dental pulp. In Dynamic Aspects of Dental Pulp: Molecular Biology, Pharmacology and Pathophysiology; Springer: Dordrecht, The Netherlands, 1990; pp. 259–276. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F.; Petrella, A. Effects of Prisma® Skin dermal regeneration device containing glycosaminoglycans on human keratinocytes and fibroblasts. Cell Adhes. Migr. 2018, 12, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Esko, J.D. Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 2014, 83, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Zbinden, M.M.; Hekking, I.J.; Vermeij, M.; Barritault, D.; Van Neck, J.W. RGTA OTR 4120, a heparan sulfate proteoglycan mimetic, increases wound breaking strength and vasodilatory capability in healing rat full-thickness excisional wounds. Wound Repair Regen. 2008, 16, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Gallo, R.L. Glycosaminoglycans and their proteoglycans: Host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006, 20, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Rodriguez, R.M.; Markwald, R.R.; Misra, S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed]
- Keil, S.; Gupta, M.; Brand, M.; Knopf, F. Heparan sulfate proteoglycan expression in the regenerating zebrafish fin. Dev. Dyn. 2021, 250, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, J.; Cao, R.; Morita, H.; Soininen, R.; Chan, K.M.; Liu, B.; Cao, Y.; Tryggvason, K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004, 64, 4699–4702. [Google Scholar] [CrossRef]
- Siméon, A.; Wegrowski, Y.; Bontemps, Y.; Maquart, F.-X. Expression of glycosaminoglycans and small proteoglycans in wounds: Modulation by the tripeptide–copper complex glycyl-L-histidyl-L-lysine-Cu2+. J. Investig. Dermatol. 2000, 115, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Bennett, C.L.; Demiot, C.; Ullmann, Y.; Teot, L.; Desmoulière, A. Erythropoietin, a novel repurposed drug: An innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen. 2014, 22, 23–33. [Google Scholar] [CrossRef]
- Hamed, S.; Ullmann, Y.; Egozi, D.; Keren, A.; Daod, E.; Anis, O.; Kabha, H.; Belokopytov, M.; Ashkar, M.; Shofti, R.; et al. Topical erythropoietin treatment accelerates the healing of cutaneous burn wounds in diabetic pigs through an aquaporin-3–dependent mechanism. Diabetes 2017, 66, 2254–2265. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Oksala, O.; Salo, T.; Tammi, R.; Häkkinen, L.; Jalkanen, M.; Inki, P.; Larjava, H. Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 1995, 43, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J.S. The role of hyaluronan in wound healing. Int. Wound J. 2014, 11, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Weigel, P.H.; Frost, S.J.; LeBoeuf, R.D.; McGary, C.T. The specific interaction between fibrin(ogen) and hyaluronan: Possible consequences in haemostasis, inflammation and wound healing. In Ciba Foundation Symposium 143-The Biology of Hyaluronan: The Biology of Hyaluronan: Ciba Foundation Symposium; John Wiley & Sons, Ltd.: Chichester, UK, 1989; Volume 143, pp. 248–261. [Google Scholar]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Stojko, M.; Wolny, D.; Włodarczyk, J. Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules 2021, 26, 5701. [Google Scholar] [CrossRef]
- Stojko, M.; Włodarczyk, J.; Sobota, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Dobrzyński, P.; Starczynowska, G.; Orchel, A.; Stojko, J.; et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics 2020, 12, 883. [Google Scholar] [CrossRef]
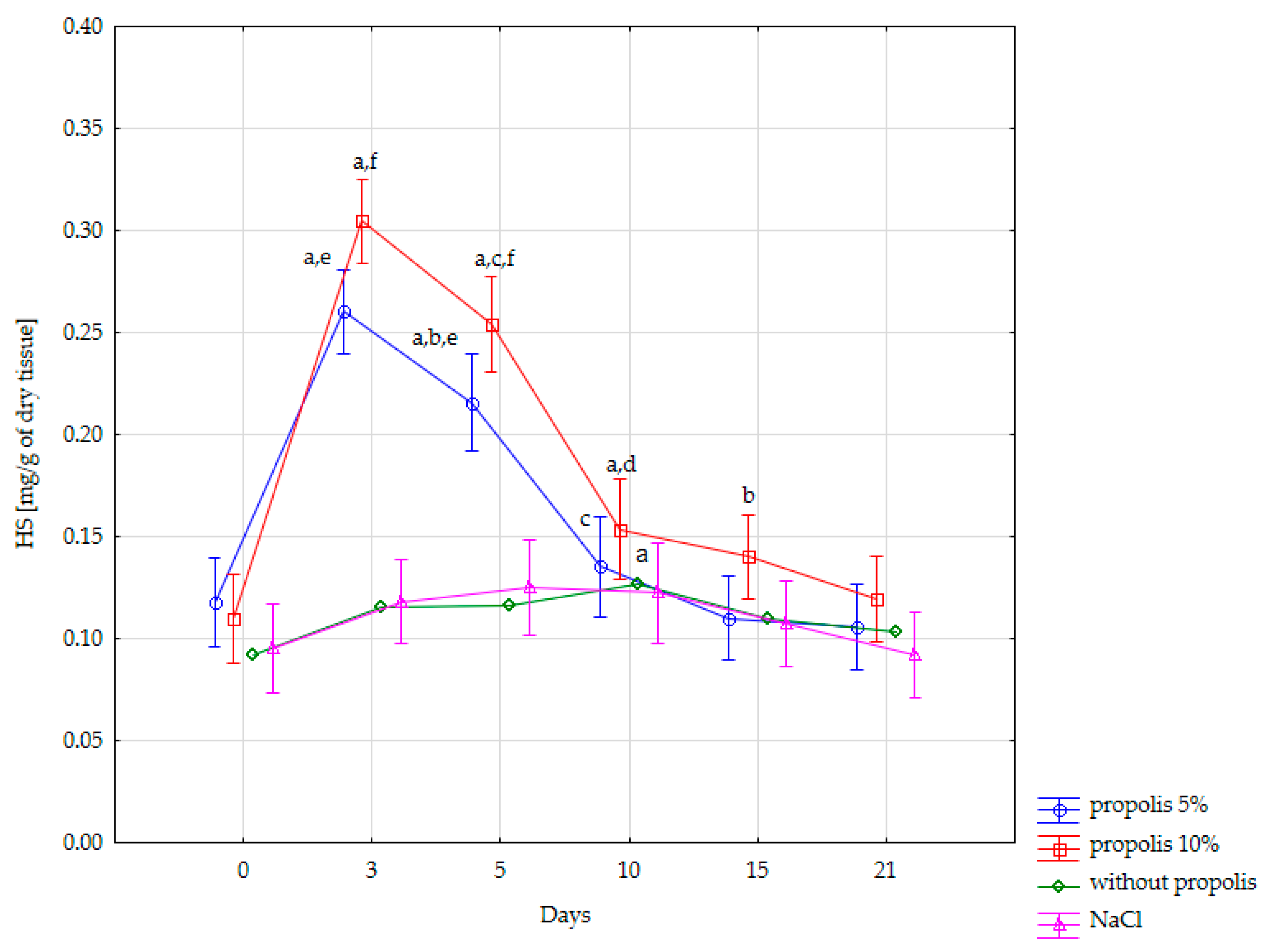
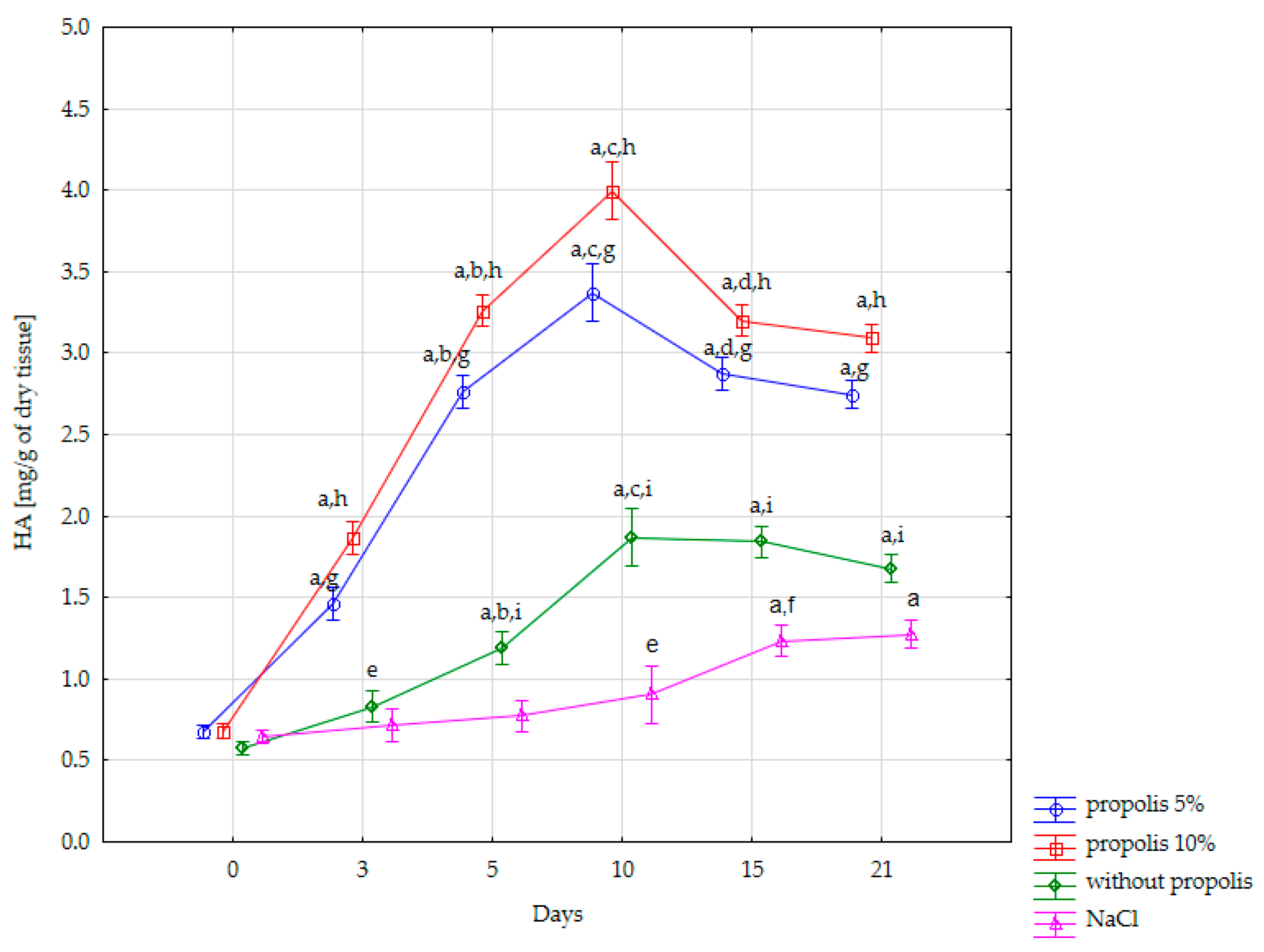
| Content of HS [mg/g of Dry Tissue] in the Burn Wound | ||||
|---|---|---|---|---|
| Days of Experiment | NaCl (n = 3) | Nonwoven without Propolis (n = 3) | Nonwoven with 5% Propolis (n = 3) | Nonwoven with 10% Propolis (n = 3) |
| Day 0 (normal skin) | 0.095 ± 0.01 | 0.092 ± 0.015 | 0.118 ± 0.018 | 0.11 ± 0.02 |
| Day 3 | 0.118 ± 0.022 | 0.115 ± 0.015 | 0.26 ± 0.015 | 0.305 ± 0.008 |
| Day 5 | 0.125 ± 0.029 | 0.116 ± 0.013 | 0.216 ± 0.009 | 0.254 ± 0.013 |
| Day 10 | 0.122 ± 0.024 | 0.126 ± 0.012 | 0.135 ± 0.015 | 0.154 ± 0.021 |
| Day 15 | 0.107 ± 0.022 | 0.11 ± 0.014 | 0.11 ± 0.01 | 0.14 ± 0.014 |
| Day 21 | 0.092 ± 0.014 | 0.1 ± 0.016 | 0.106 ± 0.01 | 0.120 ± 0.021 |
| Content of HA [mg/g of Dry Tissue] in a Burn Wound | ||||
|---|---|---|---|---|
| Days of Experiment | NaCl (n = 3) | Nonwoven without Propolis (n = 3) | Nonwoven with 5% Propolis (n = 3) | Nonwoven with 10% Propolis (n = 3) |
| Day 0 (normal skin) | 0.646 ± 0.036 | 0.575 ± 0.02 | 0.677 ± 0.029 | 0.680 ± 0.04 |
| Day 3 | 0.716 ± 0.072 | 0.83 ± 0.055 | 1.464 ± 0.076 | 1.865 ± 0.087 |
| Day 5 | 0.773 ± 0.04 | 1.187 ± 0.033 | 2.767 ± 0.09 | 3.261 ± 0.11 |
| Day 10 | 0.903 ± 0.038 | 1.869 ± 0.125 | 3.371 ± 0.076 | 3.996 ± 0.222 |
| Day 15 | 1.234 ± 0.041 | 1.842 ± 0.064 | 2.874 ± 0.094 | 3.199 ± 0.086 |
| Day 21 | 1.275 ± 0.038 | 1.675 ± 0.042 | 2.746 ± 0.049 | 3.093 ± 0.104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlińska, K.M.; Stocerz, K.; Kuczera, M.A.; Stojko, M.; Włodarczyk, J.; Kasperczyk, J.; Skalicka-Woźniak, K.; Kulinowski, Ł.; Tasinov, O.; Ivanova, D.; et al. The Influence of Propolis Nonwoven Scaffolds on Burn Wound’s Heparan Sulfates and Hyaluronan. Appl. Sci. 2024, 14, 5872. https://doi.org/10.3390/app14135872
Orlińska KM, Stocerz K, Kuczera MA, Stojko M, Włodarczyk J, Kasperczyk J, Skalicka-Woźniak K, Kulinowski Ł, Tasinov O, Ivanova D, et al. The Influence of Propolis Nonwoven Scaffolds on Burn Wound’s Heparan Sulfates and Hyaluronan. Applied Sciences. 2024; 14(13):5872. https://doi.org/10.3390/app14135872
Chicago/Turabian StyleOrlińska, Kinga Maria, Klaudia Stocerz, Mariusz Adam Kuczera, Mateusz Stojko, Jakub Włodarczyk, Janusz Kasperczyk, Krystyna Skalicka-Woźniak, Łukasz Kulinowski, Oskan Tasinov, Diana Ivanova, and et al. 2024. "The Influence of Propolis Nonwoven Scaffolds on Burn Wound’s Heparan Sulfates and Hyaluronan" Applied Sciences 14, no. 13: 5872. https://doi.org/10.3390/app14135872
APA StyleOrlińska, K. M., Stocerz, K., Kuczera, M. A., Stojko, M., Włodarczyk, J., Kasperczyk, J., Skalicka-Woźniak, K., Kulinowski, Ł., Tasinov, O., Ivanova, D., Janik, P., Kulej, M., Pudełko, A., Gorecka, A., Komosińska-Vassev, K., Olczyk, K., Stojko, J., & Olczyk, P. (2024). The Influence of Propolis Nonwoven Scaffolds on Burn Wound’s Heparan Sulfates and Hyaluronan. Applied Sciences, 14(13), 5872. https://doi.org/10.3390/app14135872










