The Effects of Frass and Vermicompost Fertilization on the Biometrical Parameters of Plant and Soil Quality, and the Rhizobiome, in Red Beet (Beta vulgaris L.) Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Preparation of Fertilizers
2.2. Conducting a Greenhouse Experiment
2.3. Soil Chemical Analysis
2.4. Soil DNA Isolation and Nanopore Sequencing
2.5. Statistical Analysis
3. Results
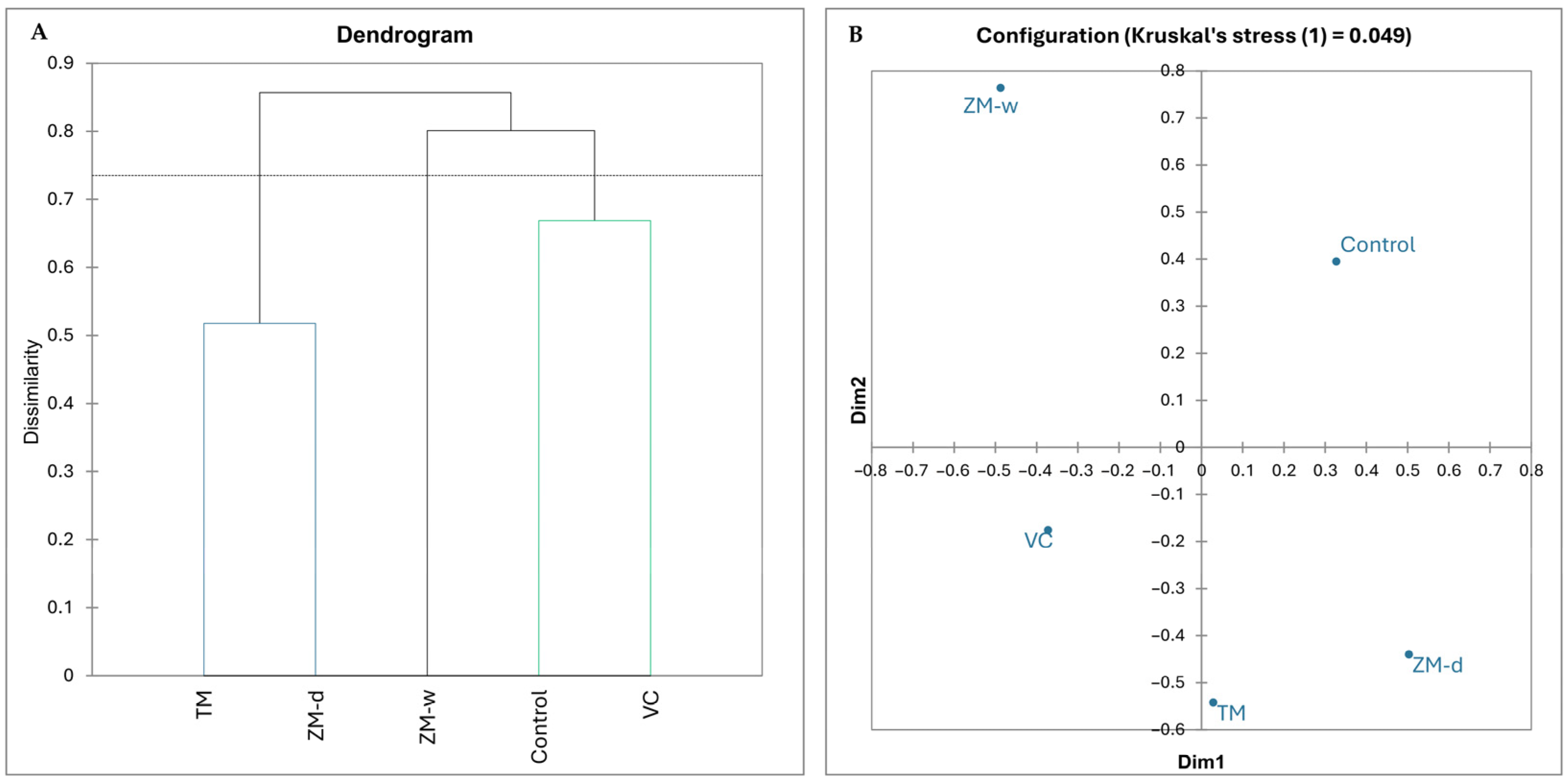
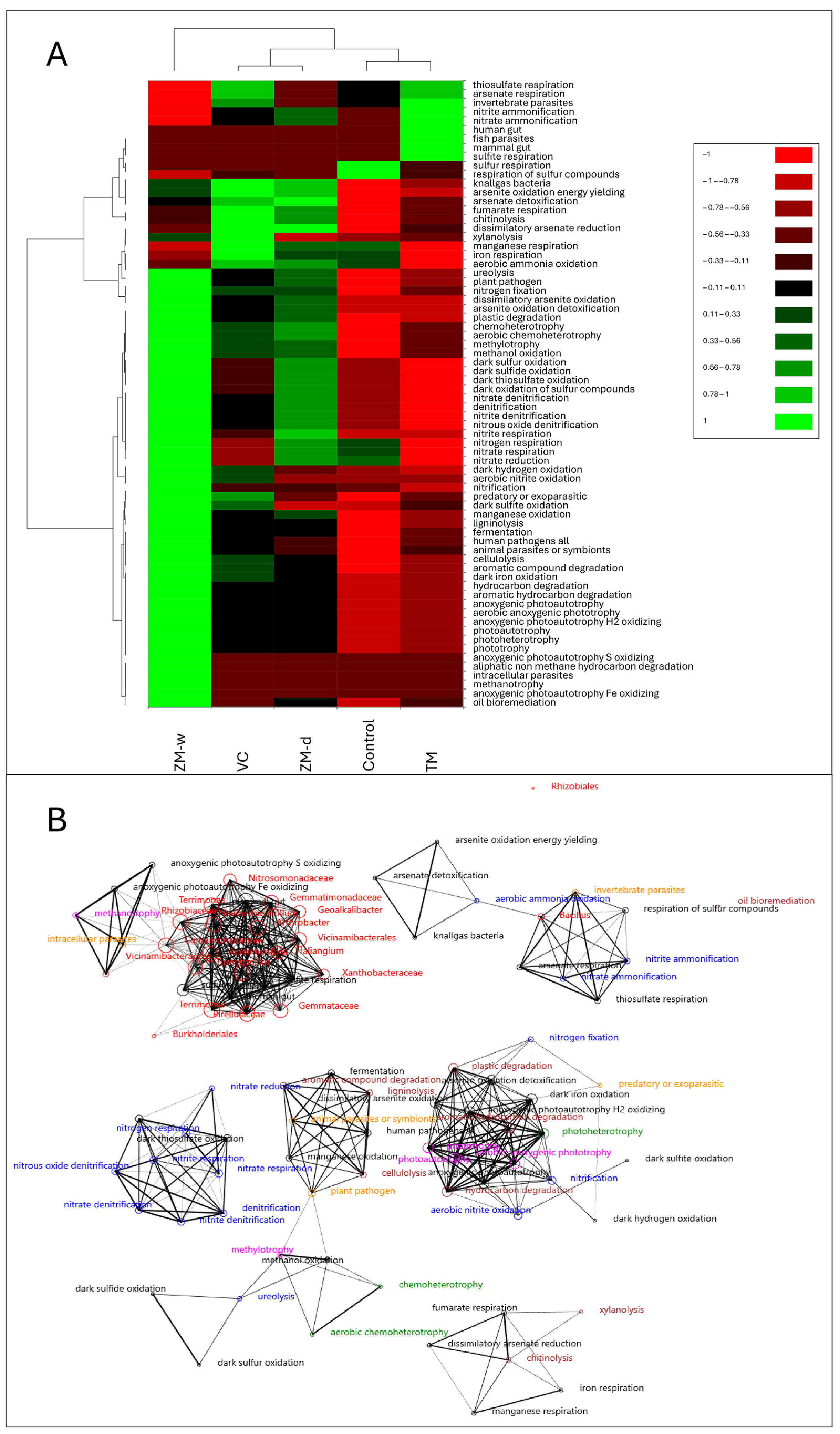
3.1. Structure of the Rhizosphere Bacteriobiome
3.2. Chemical Properties of Soils
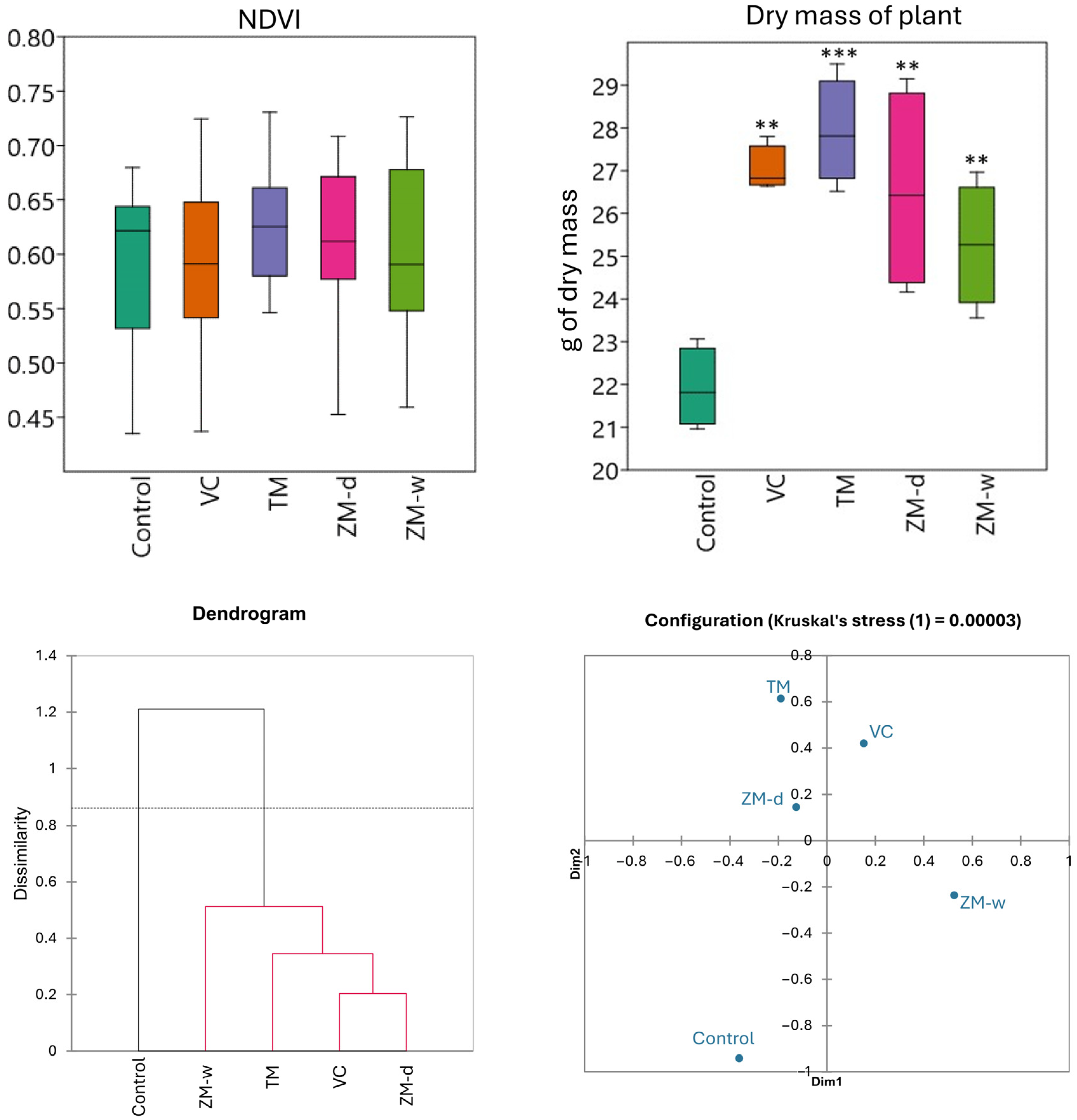
3.3. Biometrical Parameters of Plants
3.4. Overall Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gableta, M.; Dziuba, S. Organic Food Production from the Standpoint of Employee Potential. Mark. Manag. 2018, 51, 63–72. [Google Scholar] [CrossRef]
- Góralczyk, K. Is Organic Food Really the Best? Stud. Ecol. Et Bioethicae 2018, 16, 51–56. [Google Scholar] [CrossRef]
- Fehse, K.; Simmank, F.; Gutyrchik, E.; Sztrókay-Gaul, A. Organic or Popular Brands—Food Perception Engages Distinct Functional Pathways. An FMRI Study. Cogent. Psychol. 2017, 4, 1284392. [Google Scholar] [CrossRef]
- Żelezik, M. Why Ecological Agriculture? Świętokrzyskie Yearbook. B-Nat. Sci. 2009, 30, 155–166. [Google Scholar]
- Golik, D.; Żmija, D. Organic Agriculture and Prospects of Its Development in Poland in the Light of EU Experience. Sci. J. Krakow Univ. Econ. 2017, 1, 117–129. [Google Scholar] [CrossRef][Green Version]
- Zuba, M. Chances and Barriers in the Integration of the Chain of the Organic Food in Poland. WSEI Sci. J. Econ. Ser. 2011, 3, 261–288. [Google Scholar]
- Kazimierczak, R.; Salach, K.; Rembiałkowska, E. Distribution Channels of Organic Agricultural Products in Poland—An Example of the Producers from the Masovian Voivodeship. J. Res. Appl. Agric. Eng. 2014, 59, 103–107. [Google Scholar]
- Pawlewicz, A.; Gotkiewicz, W. Channels of distribution of raw materials from organic farms in the voivodeship of Warmia and Mazury. Logistyka 2012, 4, 1168–1174. [Google Scholar]
- Skłodowski, P.; Bielska, A. Properties and Fertility of Polish Soils—The Basis for Shaping Agri-Environmental Relations. Water-Environment-Rural 2009, 9, 203–214. [Google Scholar]
- Harinarayanan, U.N.D.; Lakshmanan, P. Organic Vegetable Cultivation. In Vegetable Crops; Yildirim, E., Ekinci, M., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Akan, S.; Tuna Gunes, N.; Erkan, M. Red Beetroot: Health Benefits, Production Techniques, and Quality Maintaining for Food Industry. J. Food Process. Preserv. 2021, 45, e15781. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a Functional Food with Huge Health Benefits: Antioxidant, Antitumor, Physical Function, and Chronic Metabolomics Activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef] [PubMed]
- Hlisnikovský, L.; Menšík, L.; Křížová, K.; Kunzová, E. The Effect of Farmyard Manure and Mineral Fertilizers on Sugar Beet Beetroot and Top Yield and Soil Chemical Parameters. Agronomy 2021, 11, 133. [Google Scholar] [CrossRef]
- Alves, A.U.; Prado, R.d.M.; Gondim, A.R.d.O.; Fonseca, I.M.; Cecílio Filho, A.B. Desenvolvimento e Estado Nutricional Da Beterraba Em Função Da Omisão de Nutrientes. Hortic. Bras. 2008, 26, 292–295. [Google Scholar] [CrossRef]
- Martin, H.L. Management of Soilborne Diseases of Beetroot in Australia: A Review. Aust. J. Exp. Agric. 2003, 43, 1281–1292. [Google Scholar] [CrossRef]
- Cervenski, J.; Gvozdanović-Varga, J.; Vasić, M.; Stojanović, A.; Medić-Pap, S.; Danojević, D.; Savić, A. Home Gardens and Backyards-Suitable Area for Vegetable Production. Acta Hortic. 2016, 1142, 179–186. [Google Scholar] [CrossRef]
- Kosewska, A.; Nijak, K.; Nietupski, M.; Kędzior, R.; Ludwiczak, E. Effect of Plant Protection on Assemblages of Ground Beetles (Coleoptera, Carabidae) in Sugar Beet Crops in Four-Year Rotation. Acta Zool. Acad. Sci. Hung. 2020, 66, 49–68. [Google Scholar] [CrossRef]
- Menino, R.; Murta, D. The Insects as a Workforce for Organic Fertilizers Production–Insect Frass. New Gener. Org. Fertil. 2022, 1–20. [Google Scholar] [CrossRef]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia Illucens Larvae Meal as a Supplement for Swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Medvedev, A.Y.; Volgina, N.V.; Smetankina, V.G.; Matkovskaya, A.A.; Zelenkov, A.P.; Zelenkova, G.A. Biological Features of Tenebrio molitor, Zophobas morio and Hermetia illucens Larvae as the Source of Feed Protein for Animals. Vet. Pathol. 2023, 22, 19–25. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential Use of Mealworm Frass as a Fertilizer: Impact on Crop Growth and Soil Properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef] [PubMed]
- Menino, R.; Felizes, F.; Castelo-Branco, M.A.; Fareleira, P.; Moreira, O.; Nunes, R.; Murta, D. Agricultural Value of Black Soldier Fly Larvae Frass as Organic Fertilizer on Ryegrass. Heliyon 2021, 7, e05855. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; de Vries, W. Potential Benefits of Using Hermetia illucens Frass as a Soil Amendment on Food Production and for Environmental Impact Reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect Frass as a Novel Organic Soil Fertilizer for the Cultivation of Spinach (Spinacia oleracea): Effects on Soil Properties, Plant Physiological Parameters, and Nutrient Status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Załuski, D.; Kosewska, A.; Skwierawska, M.; Sienkiewicz, S. The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment. Int. J. Environ. Res. Public Health 2023, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Przemieniecki, S.W.; Ruraż, K.; Kosewska, O.; Oćwieja, M.; Gorczyca, A. The Impact of Various Forms of Silver Nanoparticles on the Rhizosphere of Wheat (Triticum aestivum L.)–Shifts in Microbiome Structure and Predicted Microbial Metabolic Functions. Sci. Total Environ. 2024, 914, 169824. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere Bacteriome Structure and Functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Purwin, C.; Zapałowska, A.; Mastalerz, J.; Kotlarz, K.; Kolaczek, K. Biometric, Chemical, and Microbiological Evaluation of Common Wheat (Triticum aestivum L.) Seedlings Fertilized with Mealworm (Tenebrio molitor L.) Larvae Meal. Appl. Soil Ecol. 2021, 167, 104037. [Google Scholar] [CrossRef]
- Schachtschabel, P. The magnesium of the soil available to plants and its purpose. J. Plant Nutr. Fert. Soil Sci. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- Egńer, H.; Riehm, H.; Domingo, W.R. Studies on chemical soil analysis as a basis for assessing the nutrient status of the soil. II. Chemical extraction methods for determining phosphorus and potassium. K. Lantbrukshogskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Kjeldahl, J. New Method for the Determination of Nitrogen in Organic Substances. Z. Anal. Chem. 1883, 22, 366–383. [Google Scholar] [CrossRef]
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia. Electronica. 2001, 4, 1–9. [Google Scholar]
- Przemieniecki, S.W.; Damszel, M.; Ciesielski, S.; Kubiak, K.; Mastalerz, J.; Sierota, Z.; Gorczyca, A. Bacterial Microbiome in Armillaria Ostoyae Rhizomorphs Inhabiting the Root Zone during Progressively Dying Scots Pine. Appl. Soil Ecol. 2021, 164, 103929. [Google Scholar] [CrossRef]
- Le Boulch, M.; Déhais, P.; Combes, S.; Pascal, G. The MACADAM database: A MetAboliC pAthways DAtabase for Microbial taxonomic groups for mining potential metabolic capacities of archaeal and bacterial taxonomic groups. Database. 2019, 2019, baz049. [Google Scholar] [CrossRef] [PubMed]
- Lumivero XLSTAT Basic Solutions. Available online: https://www.xlstat.com/en/solutions/basic (accessed on 20 June 2023).
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kong, H.G.; Song, G.C.; Ryu, C.M. Disruption of Firmicutes and Actinobacteria Abundance in Tomato Rhizosphere Causes the Incidence of Bacterial Wilt Disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, D.P.; Tiwari, R.; Kumar, K.; Singh, R.V.; Singh, S.; Prasanna, R.; Saxena, A.K.; Nain, L. Taxonomic and Functional Annotation of Gut Bacterial Communities of Eisenia Foetida and Perionyx Excavatus. Microbiol. Res. 2015, 175, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm Frass as a Potential Biofertilizer and Abiotic Stress Tolerance-Inductor in Plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Gudeta, K.; Bhagat, A.; Julka, J.M.; Sinha, R.; Verma, R.; Kumar, A.; Kumari, S.; Ameen, F.; Bhat, S.A.; Amarowicz, R.; et al. Vermicompost and Its Derivatives against Phytopathogenic Fungi in the Soil: A Review. Horticulturae 2022, 8, 311. [Google Scholar] [CrossRef]
- Raja Sekar, K.; Karmegam, N. Earthworm Casts as an Alternate Carrier Material for Biofertilizers: Assessment of Endurance and Viability of Azotobacter chroococcum, Bacillus megaterium and Rhizobium leguminosarum. Sci. Hortic. 2010, 124, 286–289. [Google Scholar] [CrossRef]
- Basco, M.J.; Bisen, K.; Keswani, C.; Singh, H.B. Biological Management of Fusarium Wilt of Tomato Using Biofortified Vermicompost. Mycosphere 2017, 8, 467–483. [Google Scholar] [CrossRef]
- Zusková, E.; Konradyová, V.; Ryšánek, P.; Kazda, J. Bioproducts and Their Potential in Protection of Brassica napus L. against Verticillium Longisporum. Plant Soil Environ. 2024, 70, 188–194. [Google Scholar] [CrossRef]
- Sridevi, M.; Mallaiah, K.V. Factors Effecting Chitinase Activity of Rhizobium Sp. from Sesbania Sesban. Biologia 2008, 63, 307–312. [Google Scholar] [CrossRef]
- Adnan, M.; Joshi, N. The Uniqueness of Microbial Diversity from the Gut of Earthworm and Its Importance. J. Microbiol. Biotechnol. Res. 2013, 3, 111–115. [Google Scholar]
- Zhang, W.; Wang, C.; Liu, M.; Yu, Y. Integrated Reclamation of Saline Soil Nitrogen Transformation in the Hyphosphere by Earthworms and Arbuscular Mycorrhizal Fungus. Appl. Soil Ecol. 2019, 135, 137–146. [Google Scholar] [CrossRef]
- Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Kosewska, A.; Załuski, D.; Kozera, W.J.; Żarczyński, P.J. Farmed Insect Frass as a Future Organic Fertilizer. Appl. Sci. 2024, 14, 2380. [Google Scholar] [CrossRef]
- Zhang, B.-G.; Li, G.-T.; Shen, T.-S.; Wang, J.-K.; Sun, Z. Changes in Microbial Biomass C, N, and P and Enzyme Activities in Soil Incubated with the Earthworms Metaphire guillelmi or Eisenia fetida. Soil Biol. Biochem. 2000, 32, 2055–2062. [Google Scholar] [CrossRef]
- Cao, Y.H.; Zhao, X.W.; Nie, G.; Wang, Z.Y.; Song, X.; Zhang, M.X.; Hu, J.P.; Zhao, Q.; Jiang, Y.; Zhang, J.L. The Salt-Tolerance of Perennial Ryegrass Is Linked with Root Exudate Profiles and Microflora Recruitment. Sci. Total Environ. 2024, 916, 170205. [Google Scholar] [CrossRef]
- Młodzińska-Michta, E. Regulation of Growth and Development of the Roots by Selected Environmental and Endogenous Factors. Kosmos 2019, 67, 801–811. [Google Scholar] [CrossRef]
- Blakstad, J.I.; Strimbeck, R.; Poveda, J.; Bones, A.M.; Kissen, R. Frass from Yellow Mealworm (Tenebrio molitor) as Plant Fertilizer and Defense Priming Agent. Biocatal. Agric. Biotechnol. 2023, 53, 102862. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Lee, S.; Edwards, C.A.; Arancon, N.Q.; Metzger, J.D. The Influence of Humic Acids Derived from Earthworm-Processed Organic Wastes on Plant Growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G.; Vikmane, M.; Iirse, A.; Karlsons, A. Effect of Vermicompost Extract and Vermicompostderived Humic Acids on Seed Germination and Seedling Growth of Hemp. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2017, 71, 286–292. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Debaene, G.; Smreczak, B. Particle and structure characterization of fulvic acids from agricultural soils. J. Soils Sediments 2018, 18, 2833–2843. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost Significantly Affects Plant Growth. A Meta-Analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Rostami, M.; Karegar, A.; Taghavi, S.M.; Ghasemi-Fasaei, R.; Ghorbani, A. Effective Combination of Arugula Vermicompost, Chitin and Inhibitory Bacteria for Suppression of the Root-Knot Nematode Meloidogyne javanica and Explanation of Their Beneficial Properties Based on Microbial Analysis. PLoS ONE 2023, 18, e0289935. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Von-Wobeser, E.; Rocha-Estrada, J.; Shapiro, L.R.; De La Torre, M. Enrichment of Verrucomicrobia, Actinobacteria and Burkholderiales Drives Selection of Bacterial Community from Soil by Maize Roots in a Traditional Milpa Agroecosystem. PLoS ONE 2018, 13, e0208852. [Google Scholar] [CrossRef]
- Datta, B.; Chakrabartty, P.K. Siderophore Biosynthesis Genes of Rhizobium sp. Isolated from Cicer Arietinum L. 3 Biotech. 2014, 4, 391–401. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Oćwieja, M.; Ciesielski, S.; Halecki, W.; Matras, E.; Gorczyca, A. Chemical Structure of Stabilizing Layers of Negatively Charged Silver Nanoparticles as an Effector of Shifts in Soil Bacterial Microbiome under Short-Term Exposure. Int. J. Environ. Res. Public Health 2022, 19, 14438. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Park, S.B.; Jegal, J.; Park, S.A.; Park, J.; Oh, D.X.; Kim, H.J. Circular Waste Management: Superworms as a Sustainable Solution for Biodegradable Plastic Degradation and Resource Recovery. Waste Manag. 2023, 171, 568–579. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Ciesielski, S.; Kosewska, O. Changes in the Gut Microbiome and Enzymatic Profile of Tenebrio Molitor Larvae Biodegrading Cellulose, Polyethylene and Polystyrene Waste. Environ. Pollut. 2020, 256, 113265. [Google Scholar] [CrossRef] [PubMed]

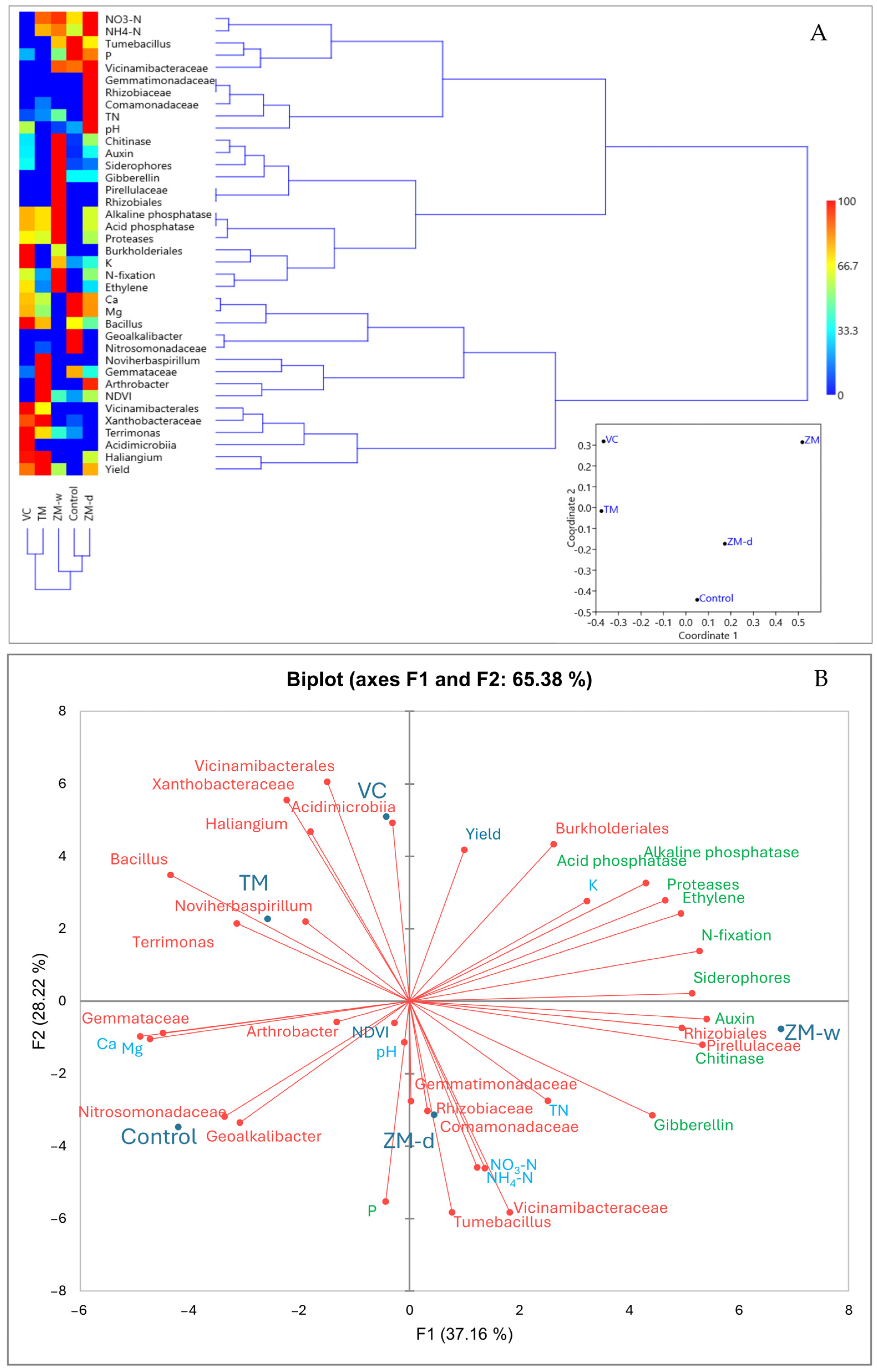
| #OTU ID | Control | VC | TM | ZM-d | ZM-w |
|---|---|---|---|---|---|
| g__Bacillus | 15.8 | 18.2 | 16.4 | 14.2 | 10.7 |
| o__Rhizobiales | 0.0 | 0.0 | 0.0 | 0.0 | 52.2 * |
| o__Burkholderiales | 0.1 | 26.6 * | 0.0 | 0.1 | 16.2 * |
| f__Gemmataceae | 9.9 | 10.9 | 12.7 | 4.8 | 0 * |
| f__Xanthobacteraceae | 10.4 | 11.4 | 12.7 | 0.2 * | 0.1 * |
| f__Comamonadaceae | 0.0 | 0.0 | 3.1 | 24.9 * | 0.0 |
| g__Haliangium | 0.0 | 9.0 * | 9.2 * | 5.5 * | 0.0 |
| f__Nitrosomonadaceae | 21.1 | 0 * | 2.3 * | 0 * | 0 * |
| f__Vicinamibacteraceae | 7.3 | 0 * | 0 * | 8.5 | 7.5 |
| g__Geoalkalibacter | 21.8 | 0 * | 0 * | 0 * | 0 * |
| g__Terrimonas | 10.1 | 8.7 | 6.0 | 0.1 * | 3.4 |
| f__Rhizobiaceae | 0.0 | 0.0 | 0.0 | 17.5 * | 0.0 |
| o__Vicinamibacterales | 0.0 | 8.2 * | 5.5 * | 0.0 | 0.0 |
| g__Arthrobacter | 0.0 | 0.0 | 5.9 * | 5.7 * | 0.0 |
| g__Paenibacillus | 0.1 | 0.0 | 10.4 * | 0.0 | 0.0 |
| g__Flavitalea | 0.0 | 0.0 | 0.0 | 10.2 * | 0.0 |
| g__Tumebacillus | 3.8 | 0.1 | 0.2 | 2.6 | 2.8 |
| g__Noviherbaspirillum | 0.0 | 0.0 | 7.5 * | 0.0 | 0.0 |
| f__Pirellulaceae | 0.0 | 0.0 | 0.0 | 0.0 | 7.2 * |
| c__Acidimicrobiia | 0.0 | 7.1 * | 0.0 | 0.0 | 0.0 |
| f__Gemmatimonadaceae | 0.0 | 0.0 | 0.0 | 6.1 * | 0.0 |
| g__Pantoea | 0.3 | 0.0 | 3.5 | 0.1 | 0.1 |
| g__Luteimonas | 0.0 | 0.0 | 2.5 | 0.0 | 0.0 |
| c__Gammaproteobacteria | 0.0 | 0.0 | 2.2 | 0.0 | 0.0 |
| Dominance class | Eudomination | Domination | Subdomination | Rare or occasionally | |
| >10.0% | 5.1–10.0% | 2.1–5.0% | <2.0% | ||
| Dominance_D | 0.1534 | 0.155 | 0.1017 * | 0.1431 | 0.3221 * |
| Shannon_H | 1.964 | 1.976 | 2.405 * | 2.107 * | 1.475 * |
| Evenness_e^H/S | 0.8908 | 0.9019 | 0.8522 * | 0.822 * | 0.6246 * |
| Treatment | Ntotal | NH4-N | NO3-N | P | K | Mg | Ca | pH | NDVI | Yield | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 3.31 | e | 203.58 | c | 949.01 | c | 167.50 | a | 3750.00 | bc | 1800.00 | a | 7375.00 | a | 6.28 | c | 0.60 | 21.91 | b |
| VC | 3.54 | d | 2.24 | d | 6.81 | d | 85.00 | bc | 4525.00 | a | 1550.00 | ab | 6275.00 | b | 6.48 | b | 0.59 | 27.02 | a |
| TM | 3.73 | c | 259.21 | b | 1168.50 | b | 60.00 | c | 3550.00 | c | 1312.50 | b | 5600.00 | b | 6.15 | c | 0.62 | 27.91 | a |
| ZM-w | 4.39 | b | 283.29 | b | 1249.82 | ab | 113.75 | b | 4287.50 | a | 762.50 | c | 3062.50 | c | 6.20 | c | 0.60 | 25.27 | a |
| ZM-d | 5.63 | a | 336.38 | a | 1341.80 | a | 150.00 | a | 3925.00 | b | 1600.00 | ab | 6525.00 | ab | 6.73 | a | 0.61 | 26.54 | a |
| F | 674.84 | 157.73 | 277.93 | 44.87 | 32.87 | 21.27 | 55.57 | 29.77 | 0.54 | 10.89 | |||||||||
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.71 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przemieniecki, S.W.; Damszel, M.; Kosewska, O.; Porzuc, B.; Wiśniewska, K.; Borsuk-Stanulewicz, M.; Kosewska, A. The Effects of Frass and Vermicompost Fertilization on the Biometrical Parameters of Plant and Soil Quality, and the Rhizobiome, in Red Beet (Beta vulgaris L.) Cultivation. Appl. Sci. 2024, 14, 5539. https://doi.org/10.3390/app14135539
Przemieniecki SW, Damszel M, Kosewska O, Porzuc B, Wiśniewska K, Borsuk-Stanulewicz M, Kosewska A. The Effects of Frass and Vermicompost Fertilization on the Biometrical Parameters of Plant and Soil Quality, and the Rhizobiome, in Red Beet (Beta vulgaris L.) Cultivation. Applied Sciences. 2024; 14(13):5539. https://doi.org/10.3390/app14135539
Chicago/Turabian StylePrzemieniecki, Sebastian Wojciech, Marta Damszel, Olga Kosewska, Bartłomiej Porzuc, Karolina Wiśniewska, Marta Borsuk-Stanulewicz, and Agnieszka Kosewska. 2024. "The Effects of Frass and Vermicompost Fertilization on the Biometrical Parameters of Plant and Soil Quality, and the Rhizobiome, in Red Beet (Beta vulgaris L.) Cultivation" Applied Sciences 14, no. 13: 5539. https://doi.org/10.3390/app14135539
APA StylePrzemieniecki, S. W., Damszel, M., Kosewska, O., Porzuc, B., Wiśniewska, K., Borsuk-Stanulewicz, M., & Kosewska, A. (2024). The Effects of Frass and Vermicompost Fertilization on the Biometrical Parameters of Plant and Soil Quality, and the Rhizobiome, in Red Beet (Beta vulgaris L.) Cultivation. Applied Sciences, 14(13), 5539. https://doi.org/10.3390/app14135539







