Effects of Congested Matches and Training Schedules on Salivary Markers in Elite Futsal Players
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach to the Problem
2.2. Subjects
2.3. Saliva Collection
2.4. Assays
2.4.1. Salivary Immunoglobulin A
2.4.2. Oxidative Stress Biomarker Quantifications
2.4.3. Total Protein Content Determination
2.5. Statistical Analysis
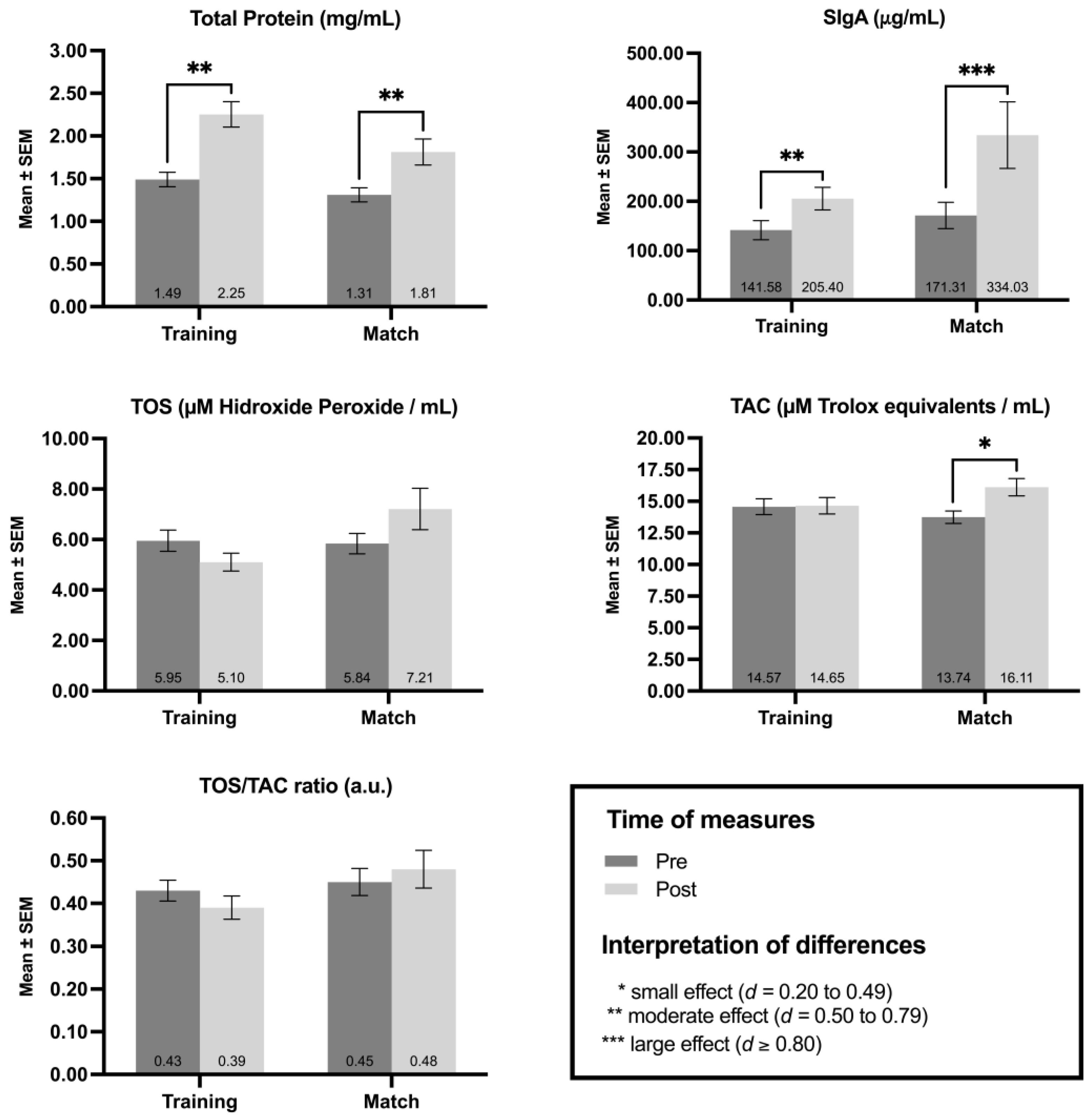
3. Results
3.1. Acute Load
3.2. Chronic Load
4. Discussion
4.1. Acute Load
4.2. Chronic Load
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemente, F.M.; Martinho, R.; Calvete, F.; Mendes, B. Training Load and Well-Being Status Variations of Elite Futsal Players across a Full Season: Comparisons between Normal and Congested Weeks. Physiol. Behav. 2019, 201, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.N.; Monteiro, D.; Gonçalves, B.; Brito, J.; Sampaio, J.; Travassos, B. Variation in Physical Performance of Futsal Players During Congested Fixtures. Int. J. Sports Physiol. Perform. 2022, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, K.; Freitas, T.T.; Marín-Cascales, E.; Alcaraz, P.E. Physical and Physiological Match-Play Demands and Player Characteristics in Futsal: A Systematic Review. Front. Psychol. 2020, 11, 569897. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, H.; Ekstrand, J.; Hägglund, M. Muscle Injury Rates in Professional Football Increase with Fixture Congestion: An 11-Year Follow-up of the UEFA Champions League Injury Study. Br. J. Sports Med. 2013, 47, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. The Influence of Soccer Playing Actions on the Recovery Kinetics after a Soccer Match. J. Strength Cond. Res. 2014, 28, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Yiannaki, C.; Barron, D.; Collins, D.; Carling, C. Match Performance in a Reference Futsal Team during an International Tournament–Implications for Talent Development in Soccer. Biol. Sport 2020, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.; Rampinini, E.; Sassi, R.; Beato, M. Workload Monitoring in Top-Level Soccer Players During Congested Fixture Periods. Int. J. Sports Med. 2020, 41, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Monitoring Training Load to Understand Fatigue in Athletes. Sports Med. 2014, 44 (Suppl. S2), S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Lockie, R.G.; Knight, T.J.; Clark, A.C.; Janse de Jonge, X.A.K. A Comparison of Methods to Quantify the In-Season Training Load of Professional Soccer Players. Int. J. Sports Physiol. Perform. 2013, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Lozano, J.M.; Muyor, J.M.; Puche Ortuño, D.; Rico-González, M.; Pino-Ortega, J. Analysis of Key External and Internal Load Variables in Professional Female Futsal Players: A Longitudinal Study. Res. Sports Med. Print 2021, 31, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, F.M.; Marcora, S.M.; Coutts, A.J. Internal and External Training Load: 15 Years On. Int. J. Sports Physiol. Perform. 2019, 14, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Spence, L.; Brown, W.J.; Pyne, D.B.; Nissen, M.D.; Sloots, T.P.; McCormack, J.G.; Locke, A.S.; Fricker, P.A. Incidence, Etiology, and Symptomatology of Upper Respiratory Illness in Elite Athletes. Med. Sci. Sports Exerc. 2007, 39, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.; Oliveira, M.; McCauley, T.; Tauler, P.; Muhamad, A.S. Respiratory Infection Risk in Athletes: Association with Antigen-Stimulated IL-10 Production and Salivary IgA Secretion. Scand. J. Med. Sci. Sports 2012, 22, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; de Moura, N.R.; Coutts, A.; Costa, E.C.; Kempton, T.; Aoki, M.S. Monitoring Internal Training Load and Mucosal Immune Responses in Futsal Athletes. J. Strength Cond. Res. 2013, 27, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Kamarauskas, P.; Conte, D. The Effect of Basketball Matches on Salivary Markers: A Systematic Review. Biol. Sport 2022, 39, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Bacurau, R.; Napimoga, M.; Arruda, A.; Freitas, C.; Drago, G.; Aoki, M. Salivary IL-21 and IgA responses to a competitive match in elite basketball players. Biol. Sport 2013, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Kamarauskas, P.; Conte, D. Changes in Salivary Markers during Basketball Long-Term and Short-Term Training Periods: A Systematic Review. Biol. Sport 2022, 39, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Lamm, M.E. Interaction of Antigens and Antibodies at Mucosal Surfaces. Annu. Rev. Microbiol. 1997, 51, 311–340. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Pyne, D.B.; Renshaw, G.; Cripps, A.W. Antimicrobial Peptides and Proteins, Exercise and Innate Mucosal Immunity. FEMS Immunol. Med. Microbiol. 2006, 48, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; McDonald, W.A.; Pyne, D.B.; Cripps, A.W.; Francis, J.L.; Fricker, P.A.; Clancy, R.L. Salivary IgA Levels and Infection Risk in Elite Swimmers. Med. Sci. Sports Exerc. 1999, 31, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.B.; McDonald, W.A.; Gleeson, M.; Flanagan, A.; Clancy, R.L.; Fricker, P.A. Mucosal Immunity, Respiratory Illness, and Competitive Performance in Elite Swimmers. Med. Sci. Sports Exerc. 2001, 33, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Arsati, F.; de Oliveira Lima-Arsati, Y.B.; de Freitas, C.G.; de Araújo, V.C. Salivary Immunoglobulin A Responses in Professional Top-Level Futsal Players. J. Strength Cond. Res. 2011, 25, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, L.T.; Ginn, E.; Seymour, G.J. Decreased Salivary Immunoglobulin A Secretion Rate after Intense Interval Exercise in Elite Kayakers. Eur. J. Appl. Physiol. 1993, 67, 180–184. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, L.T.; Jenkins, D.G. Decreased Salivary Immunoglobulins after Intense Interval Exercise before and after Training. Med. Sci. Sports Exerc. 1993, 25, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Fagoaga, O.R.; Utter, A.C.; Vinci, D.M.; Davis, J.M.; Nehlsen-Cannarella, S.L. Change in Salivary IgA Following a Competitive Marathon Race. Int. J. Sports Med. 2002, 23, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mortatti, A.L.; Moreira, A.; Aoki, M.S.; Crewther, B.T.; Castagna, C.; de Arruda, A.F.S.; Filho, J.M. Effect of Competition on Salivary Cortisol, Immunoglobulin A, and Upper Respiratory Tract Infections in Elite Young Soccer Players. J. Strength Cond. Res. 2012, 26, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Sari-Sarraf, V.; Reilly, T.; Doran, D.A. Salivary IgA Response to Intermittent and Continuous Exercise. Int. J. Sports Med. 2006, 27, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Arsati, F.; Cury, P.R.; Franciscon, C.; de Oliveira, P.R.; de Araújo, V.C. Salivary Immunoglobulin a Response to a Match in Top-Level Brazilian Soccer Players. J. Strength Cond. Res. 2009, 23, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Nehlsen-Cannarella, S.L.; Nieman, D.C.; Fagoaga, O.R.; Kelln, W.J.; Henson, D.A.; Shannon, M.; Davis, J.M. Saliva Immunoglobulins in Elite Women Rowers. Eur. J. Appl. Physiol. 2000, 81, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Neville, V.; Gleeson, M.; Folland, J.P. Salivary IgA as a Risk Factor for Upper Respiratory Infections in Elite Professional Athletes. Med. Sci. Sports Exerc. 2008, 40, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Crewther, B.T.; Heke, T.; Keogh, J.W.L. The Effects of Training Volume and Competition on the Salivary Cortisol Concentrations of Olympic Weightlifters. J. Strength Cond. Res. 2011, 25, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Franchini, E.; de Freitas, C.G.; Schultz de Arruda, A.F.; de Moura, N.R.; Costa, E.C.; Aoki, M.S. Salivary Cortisol and Immunoglobulin A Responses to Simulated and Official Jiu-Jitsu Matches. J. Strength Cond. Res. 2012, 26, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, A.F.S.; Aoki, M.S.; Drago, G.; Moreira, A. Salivary Testosterone Concentration, Anxiety, Perceived Performance and Ratings of Perceived Exertion in Basketball Players during Semi-Final and Final Matches. Physiol. Behav. 2019, 198, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Laing, S.J.; Oliver, S.J.; Montague, J.C.; Walters, R.; Bilzon, J.L.J. Saliva Parameters as Potential Indices of Hydration Status during Acute Dehydration. Med. Sci. Sports Exerc. 2004, 36, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Montague, J.C.; Callow, N.; Rowlands, A.V. Saliva Flow Rate, Total Protein Concentration and Osmolality as Potential Markers of Whole Body Hydration Status during Progressive Acute Dehydration in Humans. Arch. Oral Biol. 2004, 49, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Feng, J.-F.; Zeng, P.; Yang, Y.-H.; Luo, J.; Yang, Y.-W. Total Oxidant/Antioxidant Status in Sera of Patients with Thyroid Cancers. Endocr. Relat. Cancer 2011, 18, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; SAGE: London, UK, 2013; ISBN 978-1-4462-4917-8. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Chapter 8. The Analysis of Variance and Covariance. In Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988; pp. 273–406. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science: Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Mucosal Immunity and Respiratory Illness in Elite Athletes. Int. J. Sports Med. 2000, 21 (Suppl. S1), S33–S43. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Dumke, C.I.; Henson, D.A.; McAnulty, S.R.; McAnulty, L.S.; Lind, R.H.; Morrow, J.D. Immune and Oxidative Changes during and Following the Western States Endurance Run. Int. J. Sports Med. 2003, 24, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, M.M.; Engels, H.J.; Morgan, A.L.; Kolokouri, I. Mucosal IgA Response to Repeated Wingate Tests in Females. Int. J. Sports Med. 2001, 22, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Blannin, A.K.; Robson, P.J.; Walsh, N.P.; Clark, A.M.; Glennon, L.; Gleeson, M. The Effect of Exercising to Exhaustion at Different Intensities on Saliva Immunoglobulin A, Protein and Electrolyte Secretion. Int. J. Sports Med. 1998, 19, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.J.; Wherry, A.D.; Petersen, M.C.; Johnson, J.C.; Stuart, M.K.; Sexton, W.L. Salivary Immunoglobulin A Response to a Collegiate Rugby Game. J. Strength Cond. Res. 2007, 21, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Tharp, G.D. Basketball Exercise and Secretory Immunoglobulin A. Eur. J. Appl. Physiol. 1991, 63, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, T.B.; Trudeau, F.B.; Czerwinski, D.; Erredge, S. Immune Parameters in Athletes before and after Strenuous Exercise. J. Clin. Immunol. 1982, 2, 173–178. [Google Scholar] [CrossRef]
- Walsh, N.P.; Blannin, A.K.; Clark, A.M.; Cook, L.; Robson, P.J.; Gleeson, M. The Effects of High-Intensity Intermittent Exercise on Saliva IgA, Total Protein and Alpha-Amylase. J. Sports Sci. 1999, 17, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Schipper, R.G.; Silletti, E.; Vingerhoeds, M.H. Saliva as Research Material: Biochemical, Physicochemical and Practical Aspects. Arch. Oral Biol. 2007, 52, 1114–1135. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Immune Function in Sport and Exercise; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006; ISBN 978-0-443-10118-2. [Google Scholar]
- Fahlman, M.M.; Engels, H.-J. Mucosal IgA and URTI in American College Football Players: A Year Longitudinal Study. Med. Sci. Sports Exerc. 2005, 37, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Pyne, D.B. Special Feature for the Olympics: Effects of Exercise on the Immune System: Exercise Effects on Mucosal Immunity. Immunol. Cell Biol. 2000, 78, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Novas, A.M.P.; Rowbottom, D.G.; Jenkins, D.G. Tennis, Incidence of URTI and Salivary IgA. Int. J. Sports Med. 2003, 24, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; Leithäuser, R.M.; Ochentel, O. Blood Lactate Diagnostics in Exercise Testing and Training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Week | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| 1 | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | - | |
| TT//PT | TT//PT | TT//PT | TT//PT | TT//PT | |||
| 2 | Session 6 | Session 7 | Session 8 | Session 9 | Session 10 | Session 11 | - |
| TT//PT SS1 | TT//PT | TT//PT | TT//PT SS2 | TT | FM SS3 | ||
| 3 | Session 12 | Session 13 | Session 14 | Session 15 | - | Session 16 | - |
| TT//PT SS4 | TT//PT | TT//PT | TT//PT SS5 | FM SS6 | |||
| 4 | Session 17 | Session 18 | Session 19 | Session 20 | - | Session 21 | - |
| FM SS7 | TT//PT SS8 | FM SS9 | TT//PT SS10 | FM SS11 |
| Training M ± SD | Match M ± SD | t | p-Value | Cohen’s d (Effect) | |
|---|---|---|---|---|---|
| Total Protein | 0.76 ± 1.04 | 0.50 ± 0.97 | 1.55 | 0.13 | 0.15 (trivial) |
| SIgA | 63.81 ± 122.46 | 162.73 ± 149.01 | −1.96 | 0.04 | 0.77 (moderate) |
| TAC | 0.09 ± 4.73 | 2.37 ± 5.12 | −2.84 | <0.01 | 0.47 (low) |
| TOS | −0.85 ± 3.37 | 1.37 ± 5.66 | −2.37 | 0.02 | 0.49 (low) |
| TOS/TAC ratio | −0.04 ± 0.20 | 0.03 ± 0.32 | −1.18 | 0.24 | 0.13 (trivial) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soler-López, A.; Gómez-Carmona, C.D.; Moreno-Villanueva, A.; Gutiérrez, A.M.; Pino-Ortega, J. Effects of Congested Matches and Training Schedules on Salivary Markers in Elite Futsal Players. Appl. Sci. 2024, 14, 4968. https://doi.org/10.3390/app14124968
Soler-López A, Gómez-Carmona CD, Moreno-Villanueva A, Gutiérrez AM, Pino-Ortega J. Effects of Congested Matches and Training Schedules on Salivary Markers in Elite Futsal Players. Applied Sciences. 2024; 14(12):4968. https://doi.org/10.3390/app14124968
Chicago/Turabian StyleSoler-López, Alejandro, Carlos D. Gómez-Carmona, Adrián Moreno-Villanueva, Ana M. Gutiérrez, and José Pino-Ortega. 2024. "Effects of Congested Matches and Training Schedules on Salivary Markers in Elite Futsal Players" Applied Sciences 14, no. 12: 4968. https://doi.org/10.3390/app14124968
APA StyleSoler-López, A., Gómez-Carmona, C. D., Moreno-Villanueva, A., Gutiérrez, A. M., & Pino-Ortega, J. (2024). Effects of Congested Matches and Training Schedules on Salivary Markers in Elite Futsal Players. Applied Sciences, 14(12), 4968. https://doi.org/10.3390/app14124968









