Abstract
The incessant threat posed by the Varroa destructor mite to bee colonies has spurred extensive research into control strategies. One of these strategies involves ultraviolet radiation, aiming to harness the damaging effects that this type of radiation induces in arthropods. This study focuses on investigating the potential influence of near ultraviolet (UVA) radiation on the surface damage incurred by Varroa destructor. To address this inquiry, multiple specimens were continuously irradiated with UVA while digital holograms were recorded. To assess surface damage, these holographic records were processed and analyzed. It was found that exposure to radiation induces subtle swelling, around a few tenths of micrometers, which is more pronounced around the anal shield and genital shield of the mite. These alterations could impact the health and viability of this parasitic mite. This is the first time that the measurement and quantification of this superficial damage is reported, contributing to the understanding of the impact of UVA irradiation on the external structure of the mite.
1. Introduction
In recent years, Varroa destructor has emerged as a major contributor to the decline in honeybee populations worldwide [1]. The mite not only feeds on the bodily fluids of adult and developing bees but also acts as a vector for various pathogens [1,2]. Recent studies by Vandame et al. [3] suggest that the chemical attraction to the brood is the primary factor causing infestation. It is proposed that the fatty acid esters (such as methyl palmitate), naturally emitted by bee larvae to induce cell sealing by nurse bees, are the promoters of this attraction. While feeding, V. destructor can transmit various viruses: deformed wing virus, acute bee paralysis virus, Israeli acute paralysis virus, and Kashmir bee virus, among others. Without treatment of the honey bee colony, the number of parasites increases continuously with the growth in the bee population and its brood-rearing activity, leading to the colony’s collapse within 1–4 years [3].
Traditional control methods primarily include the application of chemical acaricides, with pyrethroids such as fluvalinate (Apistan®) and flumethrin (Bayvarol®) being the most commonly used [2,4,5,6,7]. These acaricides have demonstrated high efficiency in combating Varroa mites, especially in their plastic strip formulations. However, underdosing and homemade preparations of these acaricides have led to the development of resistance in the mites [4]. Additionally, chemical acaricides can contaminate hive products, such as honey and wax, with residues that could affect consumer health [3]. These limitations have driven the exploration of innovative approaches, such as the use of airborne ultrasound and ultraviolet radiation [2,8,9].
Ultraviolet radiation is known to have detrimental effects on various organisms [10], and its potential as a control measure for arthropod pests has been investigated in different contexts [11]. However, the specific impact of UVA radiation on Varroa destructor remains relatively unexplored. Understanding the impact of UVA irradiation on this parasitic mite could open new avenues for integrated pest management in beekeeping practices, thereby promoting the well-being and resilience of honeybee colonies. This study seeks to address this gap by examining the consequences of UVA exposure on the external structure of Varroa destructor. Digital Holography (DH) represents a recent and innovative advancement in imaging technology. A digital hologram is an interference pattern created optically through the superposition of object and reference beams. This pattern is captured by a charge-coupled device (CCD) camera and digitized, transferring it to a computer as a numerical array [12]. To quantify surface damage, phase maps derived from various series of holograms were processed and analyzed. Changes in phase distribution provided a quantitative measure of alterations in the mite’s surface topography caused by UVA exposure. The utilization of Digital Holographic Interferometry (DHI) allows for a precise assessment of surface damage, providing valuable insights into morphological changes. Through this investigation, we aim to contribute to the development of effective and sustainable strategies for Varroa destructor control. This research holds significance not only for apiculture but also for the broader ecological balance, as healthy honeybee populations play a crucial role in pollination and ecosystem stability.
2. Materials and Methods
2.1. Digital Holographic Interferometry
DHI is an advanced optical technique that combines the principles of holography and interferometry to capture the phase difference information between two holograms recorded before and after an object undergoes deformation [13]. This phase difference is related to the deformation by the following equation [14,15,16]:
In this last equation, represents the wavelength of the laser used, is the sensitivity vector, and is the displacement field. Thus, any external deformation experienced by the mite due to UVA irradiation is contained within the phase difference.
Experimental Setup
The experimental setup consisted of a laser beam with a wavelength of 355 nm, a pco.ultraviolet CCD camera, two 50⁄50 beam splitters, an optical fiber, and a set of UV lenses. The experimental setup is schematically shown in Figure 1. The laser beam was split into a reference beam (Rb) and an object beam (Ob) by a beam splitter (Bs1). The reference beam was directed into the optical fiber (Of) and transmitted to a second beam splitter (Bs2), which reflected 50% of the beam intensity toward the CCD camera. The object beam was collimated using special UV lenses (L1 and L2) and directed toward the specimen. The CCD camera collected the backscattered light at an angle of . A special UV lens (L3) and an aperture (A) in front of it were used to capture an image on the CCD camera.
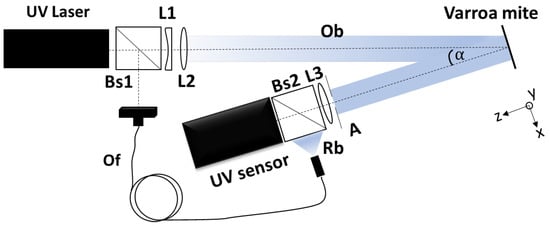
Figure 1.
Schematic representation of the experimental setup.
The laser used in this study was the Cobolt Zouk, a high-performance continuous-wave diode-pumped solid-state laser. This laser operates at a wavelength of 355 nm and delivers an output power of up to 20 mW. For the camera, the authors used a pco.ultraviolet, a high-sensitivity, high-speed scientific camera designed for ultraviolet (UV) imaging applications. This UV camera has a resolution of 1392 × 1040 (h × v) and a 14-bit dynamic range. Additionally, a single-mode optical fiber was used in the experimental setup. The lenses used in the experimental setup were the UVFS Plano-Convex Lens Set–UV Coated, with a range of 290–370 nm.
Specimens were carefully mounted on adhesive slide sheets and continuously exposed during 1 h to UVA irradiation.
Holograms were recorded with an exposure time of 10 ms, and the CCD camera (Sae Manufacturing Specialties Corp., Bayville, NY, USA) was set to record one hologram every 1.6 min, during which a UVA irradiation dose of W/cm2 per second was measured. The holographic setup ensured the acquisition of high-resolution holograms, capturing the intricate details of the mite’s surface. Digital reconstruction of the holograms was performed using image-processing algorithms through MATLAB R2023 software. This process enabled the generation of phase maps of the mite, allowing precise measurements of surface deformations.
2.2. Sample Collection
Varroa destructor specimens were donated by the Secretaría de Agricultura y Desarrollo Rural (SADER). Sampling was conducted during the spring of the year 2023, ensuring representation across different developmental stages of the mite. Mites were carefully collected using the David de Jong bee wash method (following NOM-001-ZOO-1994 [17], last modified in 1998) to avoid causing stress or damage to the specimens. Special care was taken to select mites that exhibited typical features of a healthy population. The careful collection and handling of specimens were essential steps in obtaining reliable data on the effects of UVA irradiation. In total, 35 samples were donated for studies about the Varroa mites, and in this study, five specimens were used.
3. Calculations
The procedure carried out in this study is graphically depicted in Figure 2. For each specimen, a series of holograms was recorded, evenly spaced with a time difference of 1.6 min. This approach ensures that any surface damage is captured across the hologram series, enabling a detailed assessment of potential alterations.
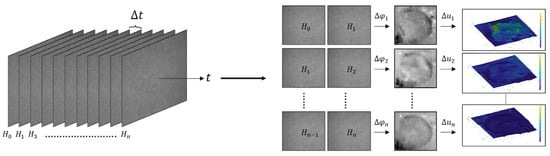
Figure 2.
Graphical representation of the measurement of surface damages.
From the hologram series, the phase difference between consecutive holograms, mathematically expressed by Equation (1), was extracted through digital processing. Finally, each of the phase maps was processed to obtain the displacement maps in the z-axis. These displacement maps provided a quantitative measure of the variations in the geometry that the Varroa destructor undergoes due to exposure to UVA radiation.
4. Results and Discussion
In Figure 3a–d, the amplitude of four holograms reconstructed through digital processing is depicted. In Figure 3a, it is observed that the mite reflects less intensity than its surroundings, suggesting the significant absorption of UVA radiation by the mite. To improve measurement results, attempts were made to reduce the reflection from the surroundings by adding a dark background and optimizing the exposure time (10 ms). The results are shown in Figure 3a–c.
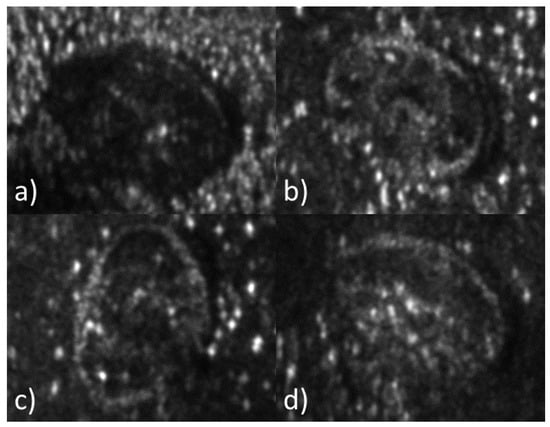
Figure 3.
Amplitude reconstruction of four Varroa destructor specimens. Holograms were recorded with a UVA laser at nm.
Figure 4 displays the first nine phase difference maps extracted from a series of holograms. Each of the images represents the phase difference between two consecutive holograms whose registration differs by a time corresponding to one dose of UVA irradiation (1.6 min). In Figure 4b, it is possible to observe a subtle but meaningful deformation around the mite’s genital shield (GS).
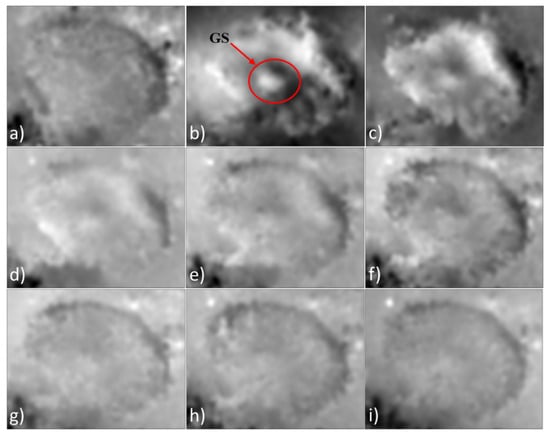
Figure 4.
Difference phase maps extracted from a series of holograms recorded with a UVA laser at nm. Each map of phase difference was extracted between two consecutive holograms. (a) Phase difference at 1.6 min. (b) Phase difference at 3.2 min. (c) Phase difference at 4.8 min. (d) Phase difference at 6.4 min. (e) Phase difference at 8.0 min. (f) Phase difference at 9.6 min. (g) Phase difference at 11.2 min. (h) Phase difference at 12.8 min. (i) Phase difference at 14.4 min.
Figure 5a depicts the displacement map with the highest amplitude (approximately m). Areas enclosed by a red circle (referred to as anal shield (AS) and genital shield (GS) as designated in [18]) indicate regions of the Varroa specimen undergoing more pronounced swelling. Figure 5b displays the displacement maps extracted from the phase maps of Figure 4. These displacement maps show the progression of alterations in the mite over time. The difference in amplitude among the maps in Figure 5b suggests a deceleration of alterations after the third dose of irradiation.
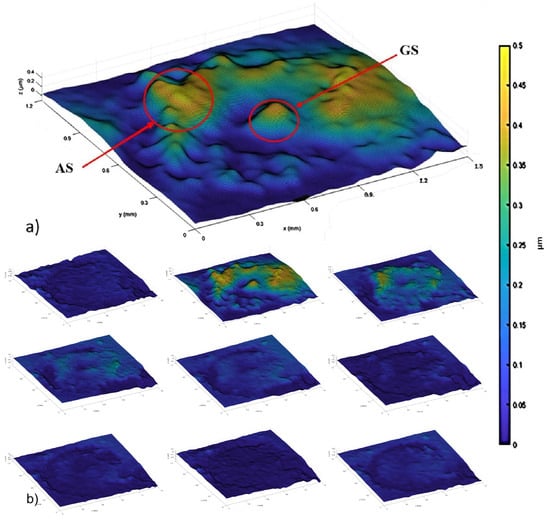
Figure 6 shows a graph of the average displacement value over time for each specimen. In this plot, it can be observed that all specimens exhibit the same behavior; however, the time at which they reach maximum displacement varies across experiments. This suggests that each specimen may absorb a different amount of UVA radiation.
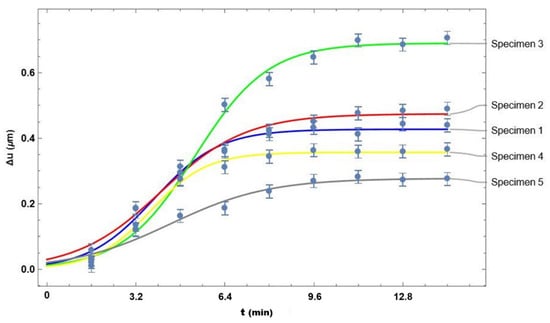
Figure 6.
Graph of the average displacement value over time for each specimen.
The curves in the graph of Figure 6 were generated using the equation
where a, b, and k are constants selected in such a way that the curves best fit the experimental data. The values of these constants are shown in Table 1.

Table 1.
Parameters of Equation (2) that provide the best fit to the experimental data.
The current study shows, through the use of DHI, the effects of UVA irradiation on Varroa mites. These effects were observed by measuring the changes in phase maps derived from a series of holograms. The experimental methodology presented in the study demonstrates a novel approach for measuring alterations in the Varroa mite. The measured changes occur on a small scale and are captured in real time. The research also highlights positive aspects in the fight against the Varroa mite by introducing new methods that reduce reliance on chemical products and minimize the risk of resistance development in mites. The changes on the surface of the mite specimens indicate that the applied irradiation has adverse effects that directly impact the mite’s integrity. Building on the aforementioned, this research is expected to contribute to the lines of knowledge applied to bee conservation, as well as to topics related to the applications of digital holographic interferometric techniques.
5. Conclusions
Our investigation into the effects of UVA irradiation on Varroa destructor has provided valuable insights into the responses of this parasitic mite to UVA. The observation that specimens reflect minimal UVA radiation underscores its pronounced absorption of this form of electromagnetic energy. This behavior may be attributed to the mite’s unique structural and physiological characteristics. The application of increasing doses of UVA irradiation resulted in a subtle yet discernible inflammation with maximum alterations around the anal shield and genital shield. This inflammation, observed within the initial doses, suggests a rapid response of the mite to UVA exposure that could have a direct impact on the mite’s integrity, with potential implications for its overall health and viability. The fact that these alterations are not easily visible to the naked eye highlights the precision of the technique. These findings contribute to the ongoing efforts to mitigate the impact of this parasitic mite on honeybee colonies, thereby supporting the health and stability of bee populations. This methodology not only enhances our understanding of the effects of UVA irradiation on Varroa destructor but also establishes a foundation for the application of DHI in studying other arthropod pests and their responses to environmental stressors. The insights gained into these effects have both theoretical and practical implications. Beyond contributing to our foundational understanding of this parasitic mite’s biology, our findings open up possibilities for practical applications in control strategies. Future research could explore phototoxic reactions in Varroa specimens exposed to chemicals that trigger these reactions more rapidly, optimizing UVA irradiation doses for maximum effectiveness, assessing the sustained impact on Varroa destructor post-exposure, and expanding the investigation to a broader spectrum of environmental conditions.
Author Contributions
J.L.S.-A.: data curation, formal analysis, methodology, writing—original draft preparation, writing—review and editing. T.S.-A.: conceptualization. F.M.-S.: project administration, supervision. M.D.S.H.-M.: resources, validation. C.G.-M.: software. D.G.-S.: investigation. B.S.-O.: visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
Authors acknowledge the support provided by Consejo Nacional de Humanidades Ciencia y Tecnología (CONAHCYT), México.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flores, J.M.; Gámiz, V.; Jiménez-Marín, Á.; Flores-Cortés, A.; Gil-Lebrero, S.; Garrido, J.J.; Hern, O.M.D. Impact of Varroa destructor and associated pathologies on the colony collapse disorder affecting honey bees. Res. Vet. Sci. 2021, 135, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Rangel, J. Understanding the enemy: A review of the genetics, behavior and chemical ecology of Varroa destructor, the parasitic mite of Apis mellifera. J. Insect Sci. 2022, 22, 18. [Google Scholar]
- Vandame, R.; Morand, S.; Colin, M.E.; Belzunces, L.P. Parasitism in the social bee Apis mellifera: Quantifying costs and benefits of behavioral resistance to Varroa destructor mites. Apidologie 2002, 33, 433–445. [Google Scholar] [CrossRef]
- Calderón, R.A.; Ramírez, M.; Ramírez, F.; Villalobos, E. Efectividad del ácido fórmico y el timol en el control del ácaro Varroa destructor en colmenas de abejas africanizadas. Agron. Costarric. 2014, 38, 175–188. [Google Scholar] [CrossRef]
- Isaev, Y.G.; Sotnikov, A.N.; Gulyukin, M.I.; Stepanova, T.V. Modern Approaches in Diagnostics and the Fight against Varroosis of Bees. Russ. Agric. Sci. 2021, 47.2, 172–176. [Google Scholar] [CrossRef]
- Berry, J.A.; Owens, W.B.; Delaplane, K.S. Small-cell comb foundation does not impede Varroa mite population growth in honey bee colonies. Apidologie 2021, 41, 40–44. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.C.; Verstraten, L.; Butler, F.T.; Whelan, P.M.; Wright, W.M. The use of airborne ultrasound for Varroa destructor mite control in beehives. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2008. [Google Scholar]
- Hall, H.; Bencsik, M.; Newton, M.I.; Chandler, D.; Prince, G.; Dwyer, S. Varroa destructor mites regularly generate ultra-short, high magnitude vibrational pulses. Entomol. Gen. 2022, 42, 375–388. [Google Scholar] [CrossRef]
- Ben-Yakir, D.; Fereres, A. The effects of UV radiation on arthropods: A review of recent publications (2010–2015). Acta Hortic. 2016, 1134, 335–342. [Google Scholar] [CrossRef]
- Romanchenko, M.; Kundenko, N.; Sanin, Y. Analysis of the effect of ultraviolet irradiation on varroa mite. East.-Eur. J. Enterp. Technol. 2018, 1, 42–52. [Google Scholar] [CrossRef][Green Version]
- Kim, M.K. Principles and techniques of digital holographic microscopy. SPIE Rev. 2010, 1, 018005. [Google Scholar] [CrossRef]
- Hernández-Montes, M.D.S.; Mendoza-Santoyo, F.; Flores Moreno, M.; de la Torre-Ibarra, M.; Silva Acosta, L.; Palacios-Ortega, N. Macro to nano specimen measurements using photons and electrons with digital holographic interferometry: A review. J. Eur. Opt. Soc.-Rapid Publ. 2020, 16, 1–31. [Google Scholar] [CrossRef]
- del Socorro Hernández-Montes, M.; Santoyo, F.M.; López, C.P.; Solís, S.M.; Esquivel, J. Digital holographic interferometry applied to the study of tympanic membrane displacements. Opt. Lasers Eng. 2011, 49, 698–702. [Google Scholar] [CrossRef]
- Solís, S.M.; Santoyo, F.M.; del Socorro Hernández-Montes, M. 3D displacement measurements of the tympanic membrane with digital holographic interferometry. Opt. Express 2012, 20, 5613–5621. [Google Scholar] [CrossRef]
- Saucedo-A, T.; De la Torre-Ibarra, M.H.; Santoyo, F.M.; Moreno, I. Digital holographic interferometer using simultaneously three lasers and a single monochrome sensor for 3D displacement measurements. Opt. Express 2010, 18, 19867–19875. [Google Scholar] [CrossRef]
- SAGARPA (Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Modificación a la Norma Oficial Mexicana NOM-001-ZOO-1994, Campaña Contra la Varroasis de las Abejas. 2005. Available online: https://www.agricultura.gob.mx/sites/default/files/sagarpa/document/2018/08/07/1274/180919-nom-001-zoo-1994-y-nom-002-zoo-1994.pdf (accessed on 16 May 2024).
- Farjamfar, M.; Saboori, A.; Nozari, J. Morphometric analysis in different geographical populations of Varroa destructor (Acari: Varroidae) associated with Apis mellifera colonies in Iran. Syst. Appl. Acarol. 2018, 23, 1915–1930. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).