Abstract
It is not yet clear how adding silicon foliar fertilisation affects olive leaf (OL) phenolics and their potential to impact different cancer cells. Thus, we conducted a field trial to study the effect of foliar Si biostimulant fertilisation on the OL phenolic content of the ‘Leccino’ (LE) and ‘Istarska Bjelica’ (IB) cultivars. The experiment compared untreated Control (C) and three distinct levels of silicon (Si1, Si2, Si3) with Si concentrations of 0.55 g/L, 1.1 g/L, and 2.2 g/L, respectively. Si3 application resulted in the highest levels of oleuropein, apigenin-7-O-glucoside, luteolin-4-O-glucoside, rutin, and tyrosol compared to the C treatment. The polyphenols showed high cytotoxic activity in three cancer cell lines tested: cervical adenocarcinoma (HeLa), colon cancer (HCT116), and osteosarcoma (U2OS). The strongest inhibition of cell growth was observed in the HCT116 cell line. All cancer cells tested were more sensitive to treatment with polyphenols isolated from plants with added Si than those without added Si. The cytotoxic activity of the extracts on the healthy cell line RPE1 was similar to that on the cancer cell line HCT116 and U2OS.
1. Introduction
Olive leaves (OL) contain high levels of phenolic compounds, which are essential for human health and agriculture. These compounds, such as oleuropein and hydroxytyrosol, possess strong antioxidant properties that can help protect our bodies from oxidative stress and inflammation, thereby reducing the risk of chronic diseases like cancer and heart disease. In addition to their health benefits, phenolic compounds act as natural defence mechanisms against diseases and pests in olive trees, making them significant in promoting sustainable agriculture [1]. These dual benefits underscore the importance of olive leaves and their phenolic compounds, highlighting their potential to positively impact our health and the environment.
Several research studies have revealed that the phenolic compounds found in olive leaves, such as oleuropein and hydroxytyrosol, have the potential to prevent and manage colon cancer [2]. These bioactive compounds have significant anti-cancer properties as they can inhibit the growth of colon cancer cells and promote programmed cell death or apoptosis [3,4]. Moreover, their potent antioxidant [5] and anti-inflammatory effects [6] also make them effective in fighting cancer. Chronic inflammation and oxidative stress are known to be linked to the development of colon cancer [7]. Additionally, phenolic compounds from olive leaves are believed to help regulate gene expression related to tumour suppression [8]. Although more studies are needed to understand their full impact, these findings suggest that incorporating OL extracts or olive oil rich in these compounds into one’s diet may be a valuable strategy in the fight against colon cancer [9].
Fertilisation is a process that can increase the phenolic content in various plants, including fruits, vegetables, and herbs [10]. This process, known as biofortification, involves enriching the soil with essential nutrients, which are then absorbed by the plants and utilised in synthesising phenolic compounds [11]. Specific fertilisation strategies, such as organic or mineral-based fertilisers, can be tailored to optimise the phenolic content in crops [12]. Adequate nutrient availability, particularly nitrogen and phosphorus, can boost plant metabolism and stimulate the production of phenolic compounds, essential for the plant’s defence mechanisms [13]. By enhancing the phenolic content, we not only improve the nutritional quality of the plants but also their resistance to pests and diseases. This makes fertilisation important for promoting healthy plant growth [14,15].
Fertilisation is essential for olive trees and can improve the phenolic content in their leaves by providing necessary nutrients that support the synthesis of these valuable compounds. Like other plants, olive trees also require essential nutrients for their growth and development. Nitrogen, phosphorus, and potassium are vital elements, but micronutrients like iron, manganese, and copper also play crucial roles in producing phenolic compounds [16,17]. When olive trees receive appropriate fertilisation, they can maintain vigorous growth and overall health, enabling them to allocate more energy and resources toward the biosynthesis of phenolic compounds. Balanced and well-timed fertilisation practices not only improve nutrient availability to the trees but also help regulate the pH of the soil, which can influence the bioavailability of certain nutrients crucial for phenolic synthesis [17,18]. Furthermore, organic fertilisers, like compost and organic matter, can introduce a diverse range of beneficial microorganisms to the soil, further supporting the production of phenolic compounds [19]. In this way, proper fertilisation can lead to an increase in phenolic content in olive leaves.
Silicon foliar fertilisation has been found to positively impact the phenolic content in plant leaves [20]. Silicon is not an essential nutrient for most plants, including olive trees, but when applied as a foliar spray, it can boost the production of phenolic compounds. This treatment strengthens the plant’s natural defence mechanisms and can increase the synthesis of phenolic compounds [21,22]. Hence, silicon foliar application could be important in olives as a low Si-accumulating species [23]. Indeed, silicon helps improve plant cell walls’ structural integrity and rigidity, making the olive tree more resistant to drought, disease, and pests [22,24].
Applying silicon foliar fertilisation on olive trees can help increase the accumulation of phenolic compounds, which are beneficial for human health due to their antioxidant and anti-inflammatory properties. This study aims to investigate how silicon as a foliar fertiliser can impact the production of phenolic compounds, especially oleuropein, in olive leaves. Furthermore, the study will evaluate the potential effects of oleuropein accumulation on cervical adenocarcinoma, colon cancer and osteosarcoma cells in vitro.
2. Materials and Methods
2.1. Location, Treatments and Olive Leaf Sampling
The experiment was set up at an orchard in Sikovo, Zadar County, Croatia. Trees in full maturity were selected within two olive cultivars, Leccino (L) and Istarska Bjelica (IB). Standard agricultural practices according to Integrated Pest Management (IPM) were implemented, including soil fertiliser application in the form of 1 kg NPK (7:20.28) per tree in autumn (2020) and 1 kg of KAN per tree in spring (2021) to ensure optimal growth conditions. The following foliar treatments were conducted until runoff, at 15, 30, and 45 days after anthesis, with each application of approx. 5 L solution per olive tree:
- -
- Control treatment (C): water
- -
- Si1 treatment: water and Silitec (Kimitec Agro®) at a concentration of 4.25 mL per litre of water (0.55 g Si/L)
- -
- Si2 treatment: water and Silitec at a concentration of 8.5 mL per litre of water (1.1 g Si/L)
- -
- Si3 treatment: water and Silitec at a concentration of 17 mL per litre of water (2.2 g Si/L).
At harvest time, during October of 2021, leaves were collected from both cultivars from the middle portion of the current season shoots as described in our previous research [25].
All samples were transferred to the laboratory and rinsed with tap water, a 1% acetic acid solution with deionised water, and, finally, two times with deionised water. Plant material was then air dried until constant mass and milled.
2.2. Chemicals
Analytical grade standards of phenolic compounds were obtained from Extrasynthese (Genay, France). HPLC grade purity Methanol (MeOH) and acetonitrile (AcN) were obtained from Merck (Darmstadt, Germany), and phosphoric acid was obtained from Sigma–Aldrich (St. Louis, MO, USA). Hydrochloric acid (Suprapure) was purchased from Merck (Darmstadt, Germany). HPLC grade deionised water was obtained from Siemens UltraClear (Siemens AG, München, Germany). The multielement standard solution was obtained from Perkin Elmer (NexION Setup Solution, Waltham, MA, USA). Argon used to form plasma for ICP-MS was supplied by Messer (Messer Croatia Plin d.o.o., Zaprešić, Croatia) and was of purity 6.0 as well as acetylene.
2.3. Identification and Quantification of Phenolic Compounds by High-Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography analysis was performed for the analytical qualification and quantification of phenolic compounds in each olive leaf extract according to our previously published method [26]. Briefly, ground leaves (30 mg) were suspended in methanol/water (80:20 v/v, 1.5 mL) and sonicated in an ultrasonic bath (Sonorex Digitec; Bandelin electronic, Berlin, Germany) for 15 min, centrifuged for 5 min at 5000 rpm (Domel Centric 350; Železniki, Slovenia), and filtered through 0.45 μm filters before HPLC analysis. The separation of phenolic compounds was performed on the HPLC instrument (Shimadzu Nexera LC-40DX3, Kyoto, Japan) using a C18, 2.1 mm × 150 mm, 2.7 µm core-shell column (Agilent, Palo Alto, CA, USA) held at 30 °C. The elution was carried out in gradient mode using a binary solvent mixture composed of water acidified with 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B). A linear gradient was run as follows: 95% (A) and 5% (B) was held for the first 2 min., from 95% (A) and 5% (B) to 50% (A) and 50% (B) gradient changed during 18 min; then, it changed to 5% (A) and 95% (B) in 1 min and remained unchanged for the next 2 min, after which the system was reequilibrated for 7 min to initial composition. The mobile phase flow rate was 0.35 mL/min, and the injection volume of each sample was 5 μL. All phenolic compounds were identified and quantified by comparing their retention times and peaks area with those of analytical standards. The UV/Vis detection was set at 360 nm for luteolin-4-O-glucoside, luteolin-7-O-glucoside, apigenin-7-O-glucoside, apigenin, luteolin, and rutin, at 280 nm for oleuropein, oleuropein aglycone, oleacein, catechin, tyrosol, hydroxytyrosol, and verbascoside, and at 210 nm for oleanolic acid. Quantification was performed using the external standard method, while the calibration curves for individual polyphenols were obtained by serial dilutions of the corresponding stock standard solutions [26].
2.4. Elemental Analysis
Approximately 500 mg of air-dried and finely ground olive leaves were weighed in porcelain dishes and dry ashed at 550 °C for 8 h. Ash was then dissolved in 5 mL of 0.6 M hydrochloric acid (Merck, Darmstadt, Germany) with heating for 15 min at 60 °C. Munsell No. 388 filter paper was used to filter out the solution to PE graduated tubes, where it was diluted to 50 mL with deionised water [27].
Analyses of Cu, Zn, Se, Si, and I were performed using an inductively coupled plasma mass spectrometer (ICP-MS) NexION 300× (PerkinElmer Instruments, Waltham, MA, USA) equipped with an S10 autosampler. As a tuning solution, a multi-element solution which covers a wide range of masses of the elements was used (NexION Setup Solution, PerkinElmer, Waltham, MA, USA). The oxide ratio and double charged species were maintained below 3%. Ca, Mg, and K were analysed by a flame atomic absorption spectrometer (FAAS) PerkinElmer AAS800 (PerkinElmer Instruments, Waltham, MA, USA) using an acetylene–air oxidant. At least six (ICP-MS) or five calibration levels (FAAS) were used to obtain each calibration curve made by appropriate dilutions of the multi-element standard solution using the same acid matrix. The calibration curves with R2 ≥ 0.999 were accepted for concentration calculation, with ranges suitable for the selected analytes. For each sample/element, a mean of five runs was obtained. Reagent blanks were prepared and determined in the same way as the samples.
2.5. MTS-Based Cell Proliferation Assay
The cytotoxic activity of polyphenols extracted from non-Si-treated and Si-treated Leccino and Istarska Bjelica plants was evaluated on the human cervical cancer cell line (HeLa), the colon cancer cell line (HCT116), the osteosarcoma cell line (U2OS), and the human retinal pigment epithelial-1 (RPE1) cell line using the MTS-based CellTiter 96® Aqueous Assay (Promega, Madison, WI, USA), as described by Fredotović et al. [28]. The cells were kindly provided by Prof. Janoš Terzić from the School of Medicine, University of Split. The cells were grown in a CO2 incubator at 37 °C and 5% CO2. When they reached the desired confluence, they were counted with the automatic handheld cell counter (Merck, Darmstadt, Germany), seeded in 96-well plates, and treated with serially diluted extracts. The cells were cultured for an additional 48 h. Then, 20 µL of MTS tetrazolium reagent (Promega, Madison, WI, USA) was added to each well and left for 3 h at 37 °C and 5% CO2. The absorbance was measured at 490 nm using a 96-well plate reader (Infinite M Plex, Tecan, AG, Männedorf, Switzerland). Measurements were performed in quadruplets for each dilution, and IC50 values were calculated from three independent experiments.
2.6. Statistical Analysis
The experiment was set up as a completely randomised design with four replications where one olive tree represented one replication. A two-way analysis of variance (ANOVA) was performed on polyphenol and mineral data, as well as a cytotoxicity study, with cultivar and different Si foliar application levels as the main factors. ANOVA was followed by Tukey’s multiple comparison test with p ≤ 0.05. ANOVA and post-hoc comparisons were performed using Statistica v. 14.1.0.8. software [29].
Principal component analysis (PCA) was performed on polyphenols and elemental data from a correlation matrix. Principal components with eigenvalues larger than 1 and explained variance larger than 10% were used. PCA analysis was performed in PAST software 4.16c [30].
3. Results
3.1. Effect of Si Foliar Biostimulant Application on Phenolic Content in Olive Leaf
Effects of Si foliar biostimulant application on thirteen different polyphenols are shown in Table 1.

Table 1.
The phenolic compound concentration in leaves of two olive cultivars (IB, L) under four different Si foliar fertilisation levels (C, Si1, Si2, Si3).
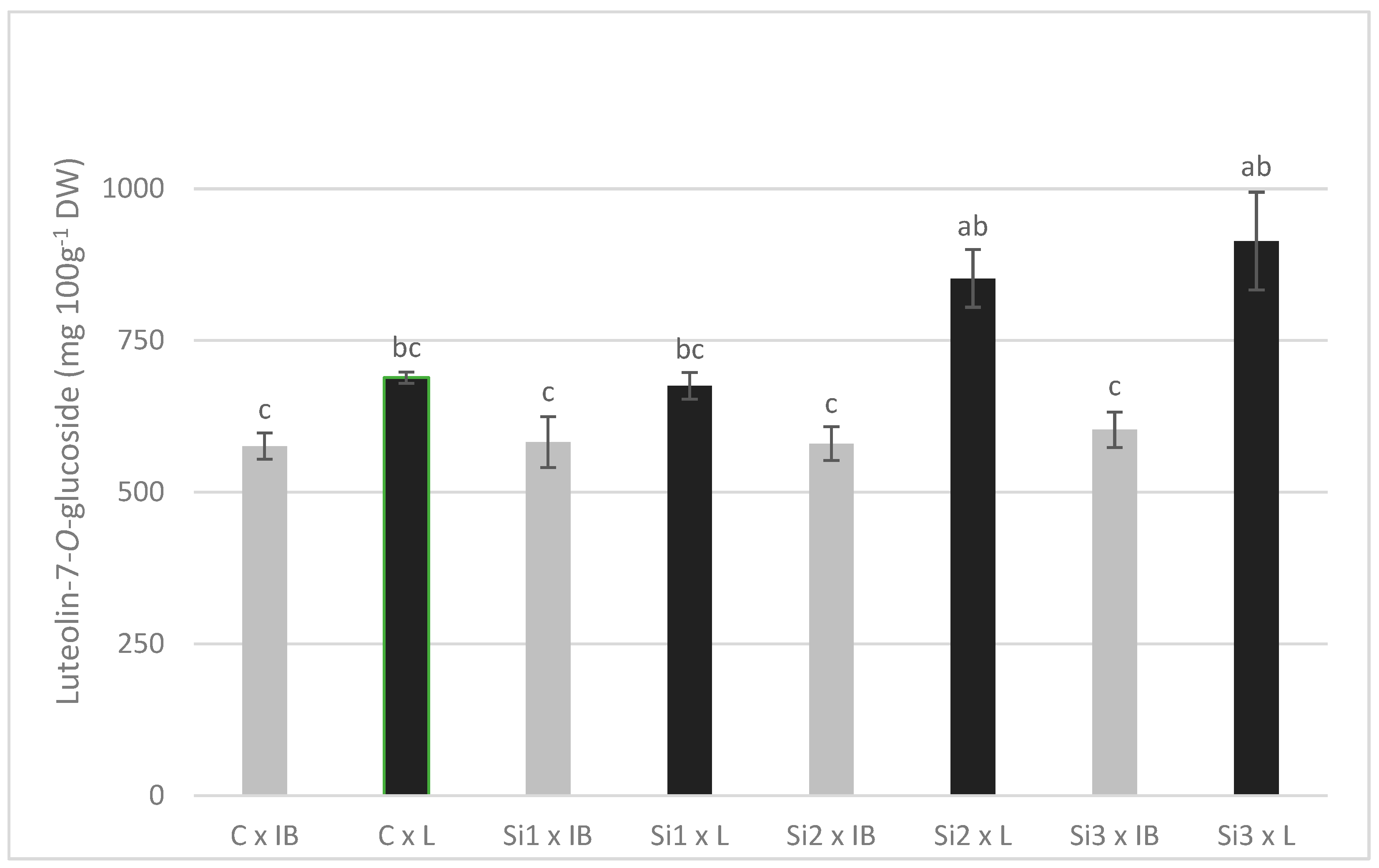
There was a general trend of an increase in the content of phenolics in the leaf with the increase in silicon levels applied. Significant differences between Si3 treatment control and/or Si1 treatment were recorded for rutin, luteolin-7, apigenin-7, luteolin-4, tyrosol, and oleuropein. Ole-aglycone was the only polyphenol that showed a decrease in concentration with the increase in Si-levels, with significant differences between control and Si2 treatment (luteolin concentrations also decreased but were not significant). Two cultivars used in this study showed contrasting concentrations of phenolic compounds—the Leccino cultivar had significantly higher concentrations of luteolin-7, apigenin-7, luteolin-4, luteolin, tyrosol, verbascoside, and ole-aglycone, while the Istarska Bjelica cultivar had higher concentrations of rutin and oleanolic acid. Luteolin, verbascoside, and oleanolic acid were the only polyphenols affected solely by the cultivar; Leccino had higher values for both (Table 1). Oleuropein was one of four polyphenols unaffected by cultivar and, at the same time, the only one significantly affected by Si treatment. There were no significant interactions of the main effects, except for luteolin-7. Here, the large difference between cultivars stands out, as an increasing level of foliar applied Si caused an increase in luteolin-7 content in the Leccino cultivar but not in Istarska Bjelica, where it was unchanged (Figure 1).
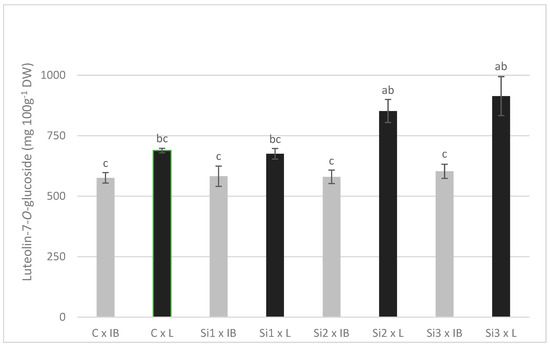
Figure 1.
The interaction effects of Si foliar treatment and cultivar on the content of luteolin-7-O-glucoside in olive leaves. Different lowercase letters represent statistically significant differences between mean values at p ≤ 0.05 obtained by a two-way ANOVA and Tukey’s test.
In terms of abundance per polyphenolic species, the most abundant compounds were oleuropein, luteolin-7, and oleanolic acid, followed by verbascoside and rutin with similar concentrations.
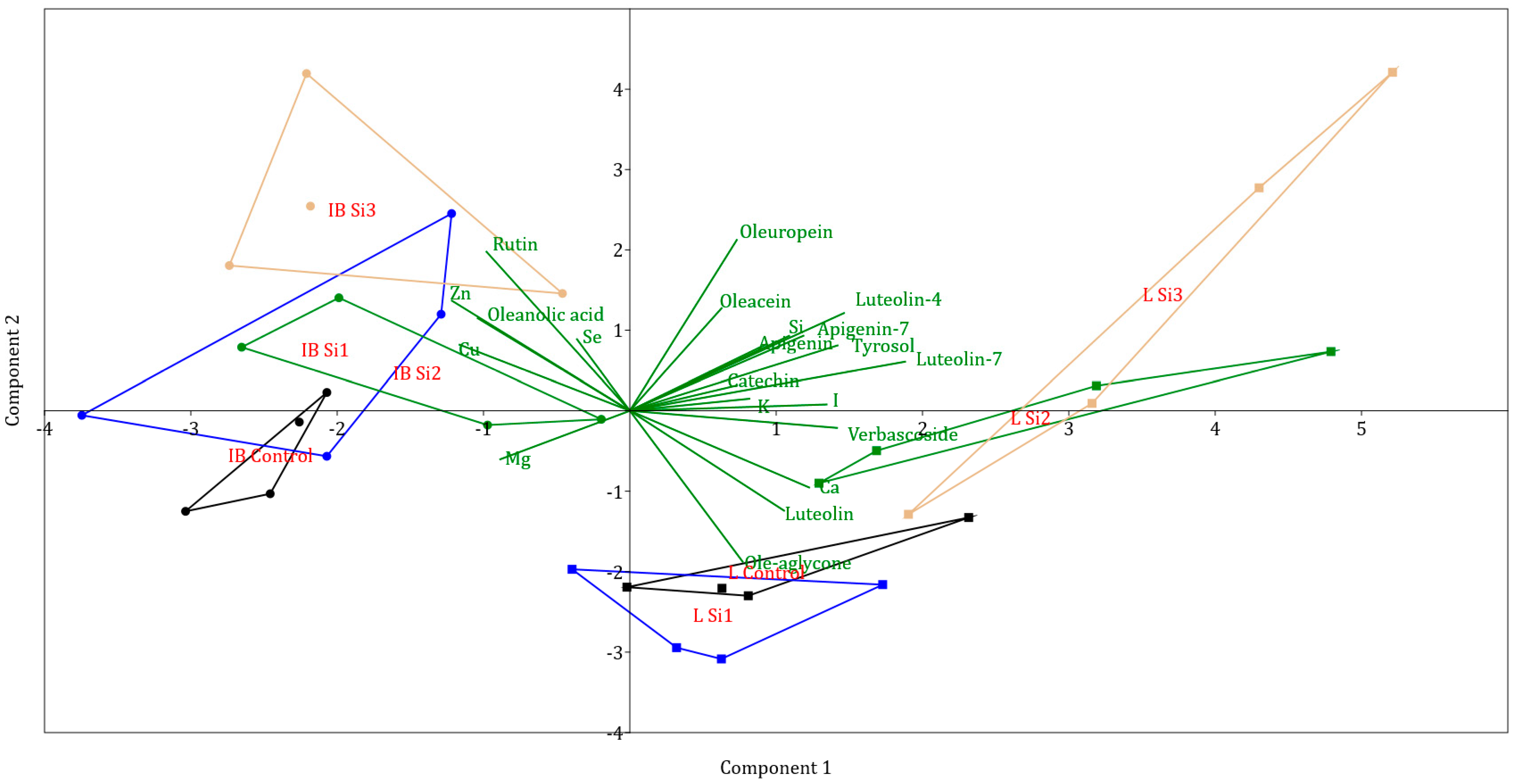
Other polyphenols were present in much smaller concentrations. PCA analysis for investigated polyphenols has clearly separated the Leccino and Istarska Bjelica genotypes. From Figure 2, it can be noticed that the Leccino cultivar was more affected by Si application and was more spread out in the cartesian plane, while Istarska Bjelica was more rigid in response to Si treatment. The separation of treatments per genotype can also be observed from the graph, as control treatments of both cultivars are separated from Si2 and Si3 treatments. The highest correlations with PC1 were for luteolin-7, luteolin-4, and verbascoside, while the highest correlations with PC2 were for ole-aglycone, rutin, oleuropein, and oleacein (Table 2). Due to the characteristic separation of points on the PCA graph, values correlating with PC1 separate points by genotype, while values correlating with PC2 separate points based on Si treatments.
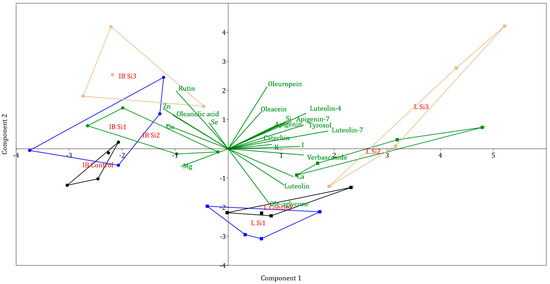
Figure 2.
Separation of cultivars and treatments investigated by principal component analysis (different coloured lines represent convex hulls, which are the smallest polygons that contain all points of a group IB—Istarska Bjelica, L—Leccino, Control, Si1, Si2, Si3 (silicon treatments)).

Table 2.
Parameters of principal component analysis (PCA) for two olive cultivars (IB, L) under different levels of Si foliar application (Si1, Si2, Si3) based on polyphenol and mineral content.
3.2. Effect of Si Foliar Biostimulant Application on Elemental Concentration in Olive Leaf
The effects of Si treatment on elemental leaf concentration were not as straightforward as for phenolic concentrations and significant interaction effects between silicon application and cultivar. Elements significantly affected by Si-foliar application were copper, zinc, and silicon (Table 3).

Table 3.
Elemental concentrations in leaves of two olive cultivars (IB, L) under four different Si foliar fertilisation levels (C, Si1, Si2, Si3).
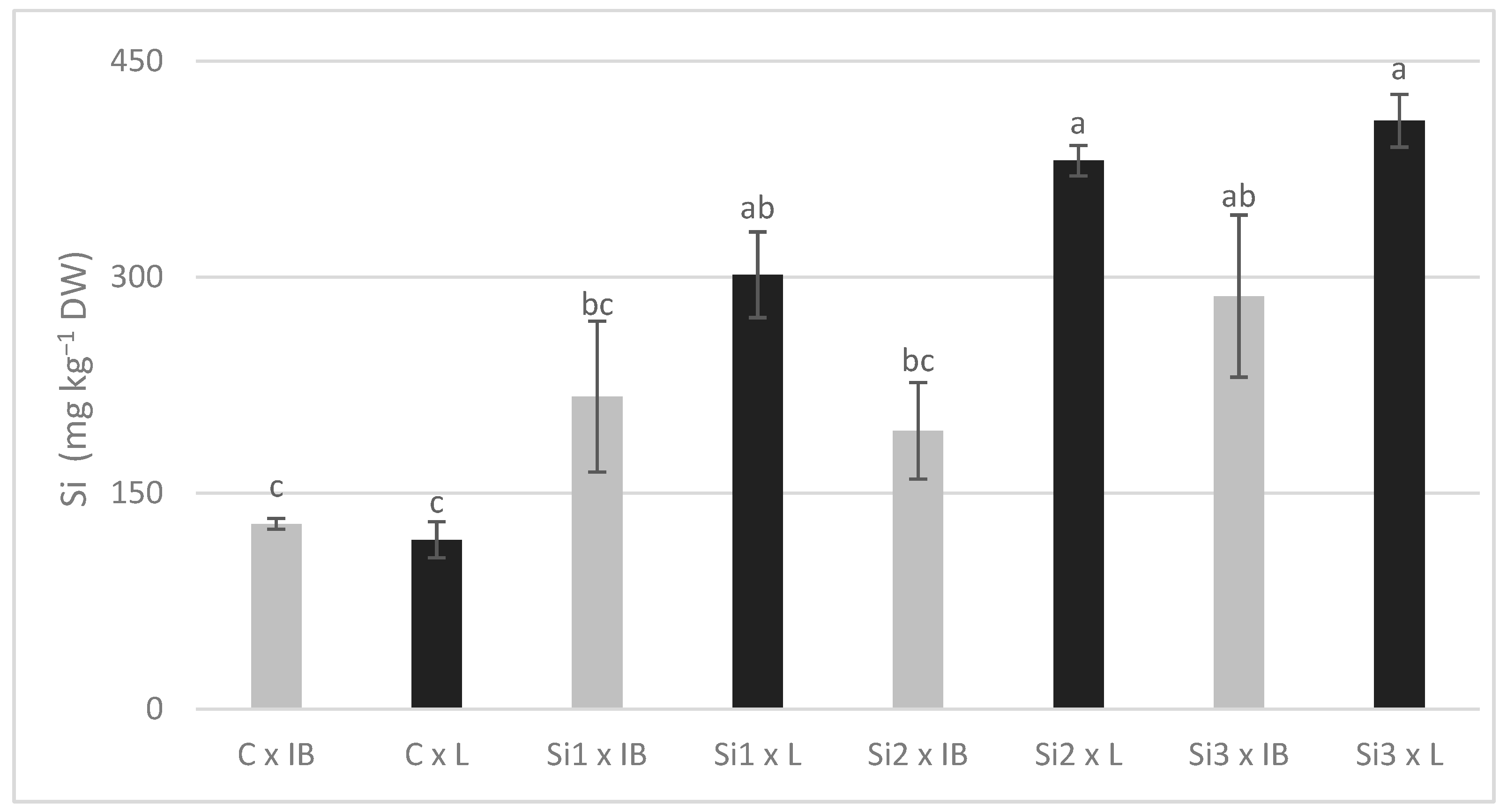
The increase in Si content was the most straightforward. Si concentrations in all treatments were higher than in the control, and the Leccino cultivar had significantly more Si. Due to the significance of the interaction effect, it can be said that Si-foliar application affected Si content differently in these two cultivars (Figure 3).
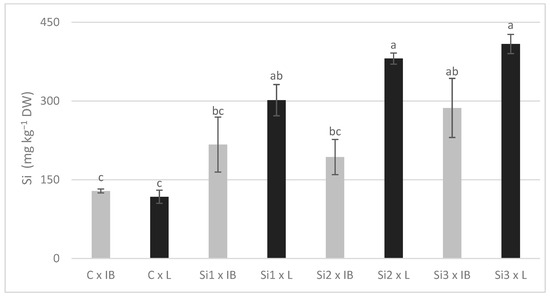
Figure 3.
The interaction effects of Si foliar treatment and cultivar on Si content in olive leaves. Different lowercase letters represent statistically significant differences between mean values at p ≤ 0.05 obtained by a two-way ANOVA and Tukey’s test.
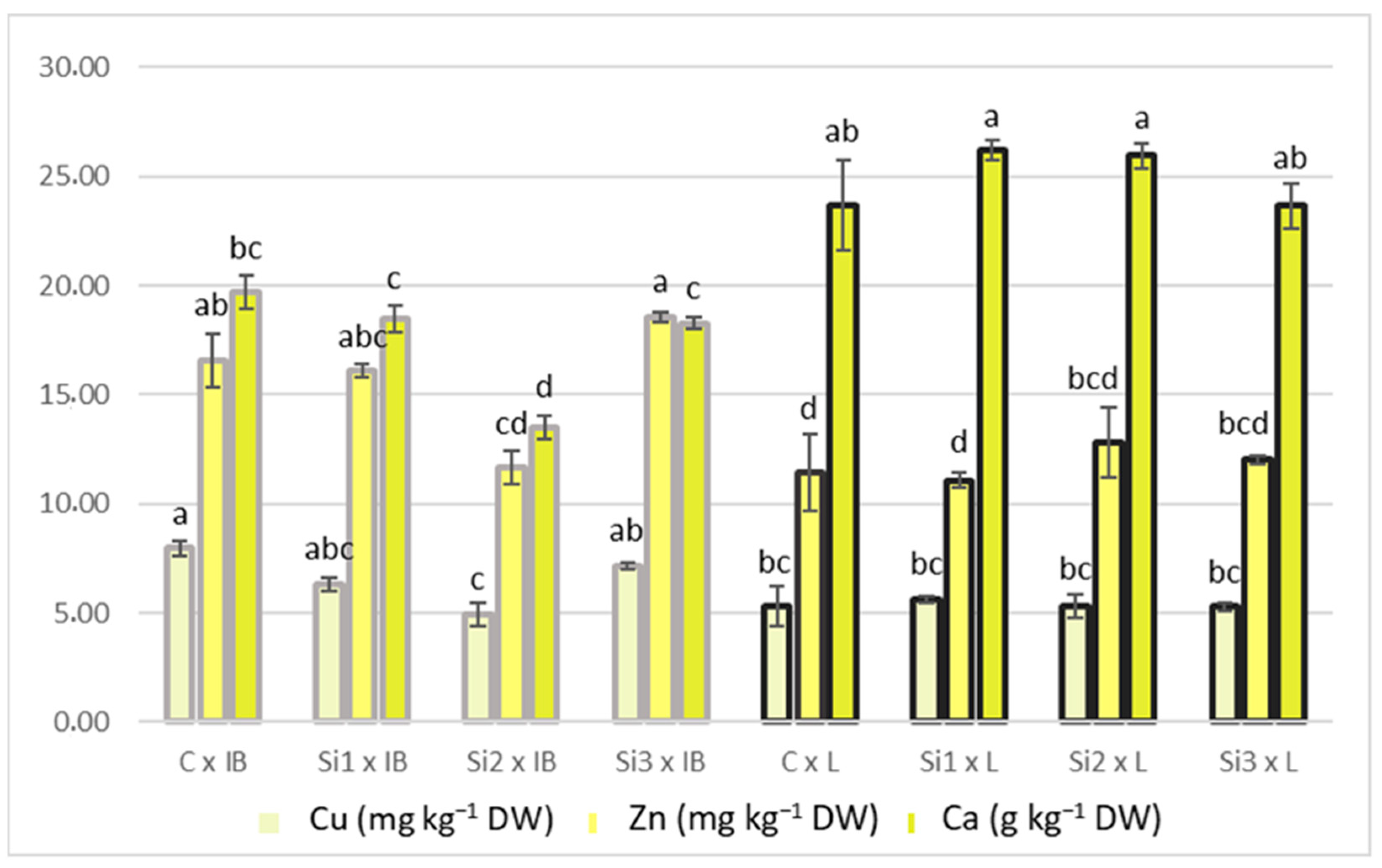
Leccino showed a constant increase with the increase in Si dosage, while in Istarska Bjelica, Si content decreased in Si2 treatment before increasing again at the final Si concentration. In addition, Si content was not decreased in Si2 treatment (Si2: 259.35 ± 32.15, Si1: 287.11 ± 39.01), as shown in Table 3. The copper and zinc response to the foliar application of Si, regarding it as a main effect, was similar for both elements and did not have a clear trend. Differences between the two cultivars were significant, with Istarska Bjelica having higher values for both, whereas the opposite was recorded for Ca. Since the interaction between the two main effects was significant, they are presented in Figure 4.
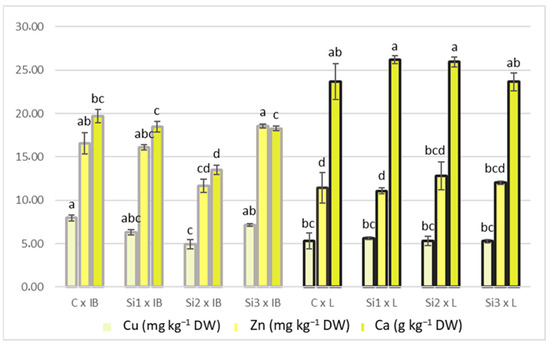
Figure 4.
Interaction of Si foliar treatment and cultivar on Cu, Zn, and Ca content in olive leaves. Different lowercase letters represent statistically significant differences between mean values, for each variable (Cu, Zn, Ca), at p ≤ 0.05 obtained by a two-way ANOVA and Tukey’s test.
Generally, the Leccino cultivar showed stability in Cu and Zn contents across increasing Si levels, and Istarska Bjelica showed a slight decrease in Si2 treatment. The same can be applied to Ca concentrations. For iodine content, the only difference was between cultivars, where Leccino had a significantly higher iodine content. The highest positive correlation with PC1 was recorded for I, Si, and Ca, while the highest negative correlation was recorded for Cu and Zn (Table 2). For mineral content, the only correlation higher than 0.5 for PC2 was recorded for Zn.
3.3. Effect of Extracted Polyphenols from Si3 Treatment on Cancer Cell Line Growth
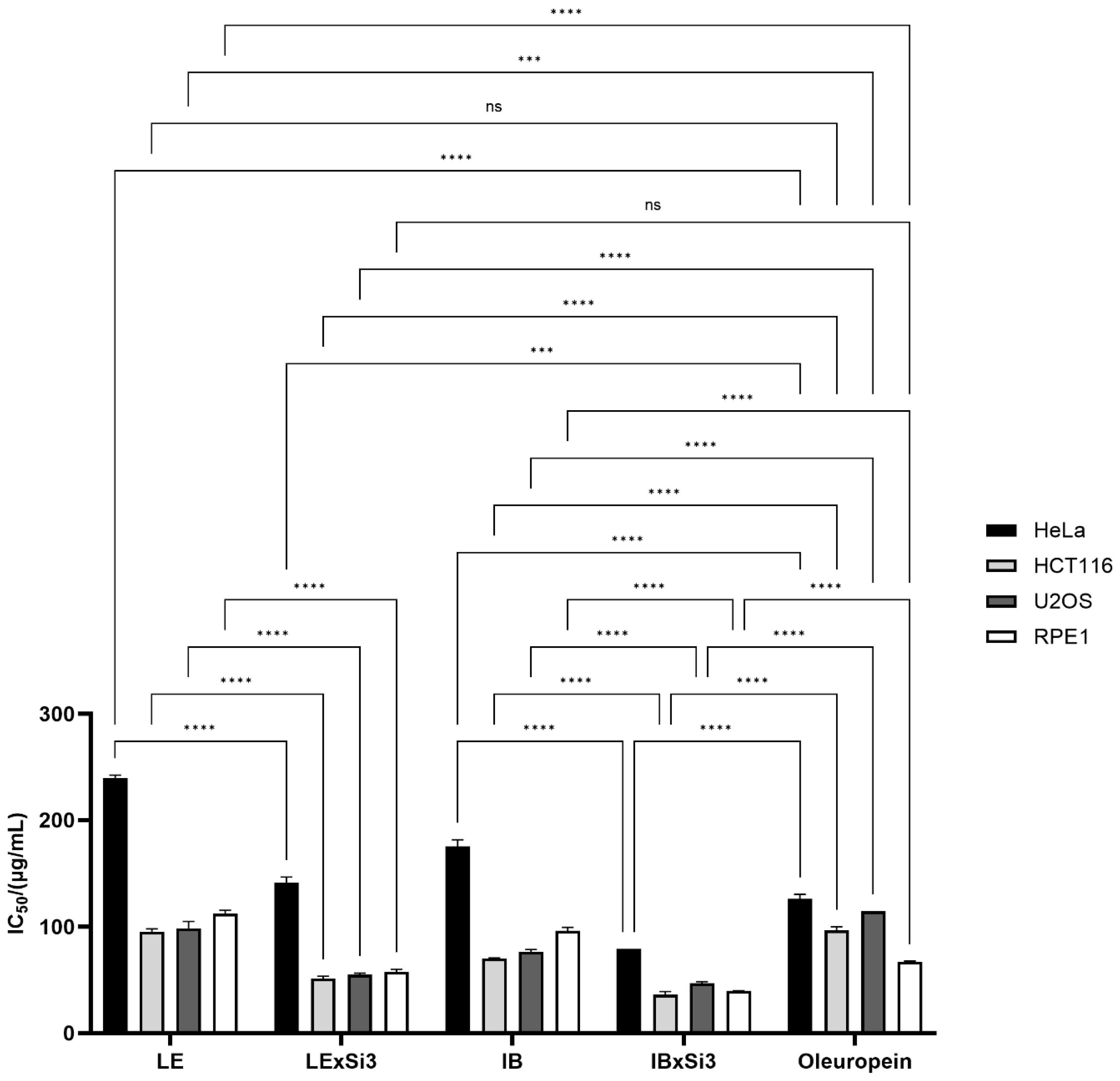
Extracted polyphenols, from both Leccino and Istarska Bjelica cultivars, showed high cytotoxic activity in all three tested cancer cell lines. In general, all cancer cells showed higher growth inhibition when treated with polyphenols from plants with Si treatment than from those without Si treatment. This growth inhibition was significant for all three cancer cell lines (Figure 5). Significant differences were recorded between extracts from non-Si treated plants, as well as between extracts from Si treated plants. Differences were also clear between genotypes from either Si treated or non-treated plants. The most resistant cell line was HeLa, with IC50 values of 239.689 µg/mL for Leccino, 141.433 µg/mL for Leccino with Si, 175.611 µg/mL for Istarska Bjelica, and 79.034 µg/mL for Istarska Bjelica with Si. The HCT116 cell line proved to be the most sensitive to all tested extracts, especially to the plant extracts treated with Si (IC50 values of 95.497 µg/mL for LE, 51.446 µg/mL for LExSi3, 70.142 µg/mL for IB, and 36.075 µg/mL for IBxSI3). The highest growth inhibition was observed for polyphenol extract with Si treatment from the Istarska Bjelica cultivar in all tested cell lines. This result is interesting because the Istarska Bjelica cultivar generally has lower polyphenol concentrations and lower concentrations of accumulated silicon. A healthy cell line, human retinal pigment epithelial-1 (RPE1), showed very similar growth inhibition to the HCT116 and U2OS cell lines, although there were significant differences between the polyphenols isolated from Si-treated and untreated plants.
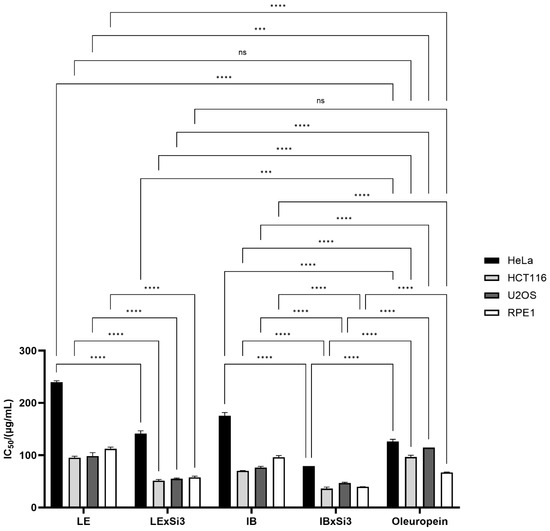
Figure 5.
Cytotoxic activity of extracted polyphenols from non-Si treated Leccino and Istarska Bjelica plants (LE and IB), Si-treated Leccino and Istarska Bjelica plants (LExSi3 and IBxSi3), and oleuropein on HeLa, HCT116, U2OS, and RPE1 cell lines as assessed by the MTS cell proliferation assay. IC50 values are means of three independent experiments, and SE values are labelled as error bars. Statistically significant differences are marked with *** p < 0.001 and **** p < 0.0001.
Since the polyphenols isolated from the plants treated with Si had a significantly higher oleuropein content, its cytotoxic effect was also tested to determine whether it was responsible for the excellent cytotoxic activity. Oleuropein showed significant cytotoxic activity towards the HeLa cell line, comparable with polyphenols from Si-treated Leccino plants but significantly weaker than those extracted from Istarska Bjelica with Si. In the HCT116 and U2OS cell lines, oleuropein inhibited cell growth to the same extent as the LE control but had a weaker cytotoxic effect than the extracts of the IB control and the Si-treated plants. Oleuropein showed slightly lower cytotoxicity on the healthy cell line RPE1 compared to extracts from Si-treated plants but slightly higher compared to extracts from non-Si-treated plants. In summary, oleuropein has better cytotoxic activity than polyphenols extracted from non-Si-treated plants but weaker activity compared to polyphenols from Si-treated plants (Figure 5). Those findings strongly indicate that oleuropein undoubtedly has a significant impact but that other polyphenols (especially in Si-treated plants) are also responsible for the exceptional biological activity.
4. Discussion
OL extract has been historically used as a folk remedy against fevers, malaria, and other diseases [31]. The medicinal properties of olive leaves are usually attributed to polyphenols as secondary metabolites. Many studies have shown that these OL compounds have various benefits, such as antiproliferative and cytotoxic effects on cancer cells, as well as radio-protective properties, anti-fungal activity, and cardioprotective effects [32]. Besides human health benefits, polyphenols in olive leaves serve a role in plant defence mechanisms against biotic and abiotic stressors such as Phytophthora sp. [31] and Verticillium dahliae Kleb. [33], heavy metal salts, UV-irradiation, and temperature [1,34]. The content of polyphenols in leaves tends to change; it is affected by genetic, agronomic, and environmental factors [35]. Differences in polyphenol content in different olive cultivars have been investigated in leaves [36,37,38] and oil [39,40], and changes in phenolic content due to environmental stress have also been addressed [41,42].
Plant tolerance and/or resistance to different kinds of stress could be attributed to nutritional status. Silicon is the second most abundant element in Earth’s crust but still a non-essential element for plant growth. However, it is considered beneficial for plants, as it has a role in pest and disease control and enhances the deposition of phenolic compounds at infection sites [43]. Research on the relationship between silicon and polyphenol content has mainly focused on plants under biotic or abiotic stress factors. Zhang et al. [44] showed that Si alone did not affect phenolics status in rice plants, while in plants inoculated with Rhizoctonia solani, Si application significantly increased phenolic concentrations. Similarly, drought-stressed wheat supplied with Si showed an increase in total phenolics and flavonoids [20]. Schaller et al. [45] showed that silicon supplementation affected the phenolic content of grasses depending on tissue function (photosynthesis or stabilisation), where increased phenolic content was recorded in grass culm. Our results indicate that, in olive, Si foliar application causes a significant increase in the content of several polyphenols in leaves, namely oleuropein, tyrosol, luteolin, apigenin-7, rutin, etc. The variability of polyphenols in olives due to genotype differences has been widely researched. Among factors such as minimum and maximum temperature, humidity, sunshine hours, and mineral nutrition, olive variety was found to be the most influential on polyphenol content [46]. It was shown by Mujić et al. [36] that Istarska Bjelica had the lowest content of polyphenols out of three autochthonic cultivars from Istria (the other two being Buža and Rosinjola). Our results showed that Istarska Bjelica had significantly lower values of some of the detected polyphenols, e.g., 60% difference for ole-aglycone, 36% for rutin, 33% for luteolin, and 30% for tyrosol, when compared to the Leccino cultivar. However, oleanolic acid levels, as an important triterpene in OL, showed 47% higher values in Istarska Bjelica. There was no significant difference in oleuropein content between cultivars used in this research, which is consistent with previously reported results in the harvest period for the two cultivars studied [37]. The clear separation of genotypes in the PCA (Figure 2) shows the differences in polyphenolic content (as well as in mineral composition) between them, but a much closer grouping of points for Istarska Bjelica shows that this cultivar is less sensitive to external factors regarding polyphenolic production. In our previous research, the same observation has been reported [37].
Silicon leaf concentrations in the control of this experiment were at 123.26 (±6.36 SE) mg kg−1, which is in accordance with levels reported by Nascimento-Silva et al. for the Arbequina and Picual cultivars [22]. Furthermore, the application of foliar Si significantly increased the Si content in leaves, which is in agreement with recent findings [47]. According to Therios [48], other minerals were at optimum levels. For some elements, such as calcium and iodine, genotype differences were more pronounced than the effect of Si foliar application. The concentration of copper in Istarska Bjelica leaves was found to be higher than that in the Leccino cultivar, as previously shown by Pasković et al. [37]. The mineral content variability of different genotypes has been previously confirmed in various studies [37,38,49].
Oleuropein, as a major polyphenolic constituent in olive leaves, is known to have various pharmacological functions [50]. Human cervical cancer cells (HeLa) were shown to be sensitive to oleuropein as it stopped their cell cycle at the G2/M phase in oleuropein concentrations of 150–200 µM and induced apoptosis through mitochondrial apoptotic cascade [51]. OL extract (OLE), rich in polyphenols, successfully reduced the viability of HeLa cells with a decrease in cyclin D1 gene expression and an increase in p21 expression, suggesting its involvement in the intrinsic apoptosis pathway [52]. Here, extracts from both cultivars were effective in inhibiting the growth of HeLa cells with an IC50 that differs between the cultivars and the Si treatment. Two of the other most abundant polyphenols in olive leaves in our research were luteolin and oleanolic acid, and both have been shown to inhibit cancer cell growth. Luteolin has been shown to induce autophagy in U2OS cells by sensitising doxorubicin (DOX)-mediated autophagy signalling. Luteolin alone also led to a cell death mechanism, but less efficiently [53]. A study by Pandurangan and Ganapasam [54] showed a significant cytotoxic effect of luteolin on HCT-15 adenocarcinoma cells with increased ROS production, leading to apoptosis and DNA fragmentation. Oleanolic acid, which was the second most abundant polyphenolic species in this research, was shown to cause dose-dependent HCT116 colon cancer cell death and inhibited tumour growth in colon cancer xenograft mice [55]. Oleanolic acid was found to be cytotoxic to several cancer cell lines, MCF-7, DU145 (prostate cancer) and U87 (glioblastoma). It increased the gene expression of p53, cytochrome c, Bax, PARP1, and Cas-3, indicating its ability to activate apoptosis (Kim et al., 2018) [56]. OL extract with a high terpenoid content and a high concentration of oleuropein showed a dose-dependent inhibition of HCT116 cell growth with IC50 values of 97.06 µg/mL, according to P. Xie et al. 2021 [57]. The highest inhibitory effect of extracts from Istarska Bjelica is surprising, as most polyphenols in the leaves of this cultivar had lower concentrations. However, the higher concentrations of rutin and oleanolic acid, which have known anti-cancer properties [58,59], could explain this phenomenon. The inhibition of HeLa, HCT116, and U2OS cell growth in the present study was concentration-dependent, as Si3 treatment showed higher inhibition rates and significantly higher concentrations of various polyphenols (including the most abundant ones). These results suggest that foliar application of Si could be used to increase the production of certain polyphenols in olive leaves, improving the already high inhibitory effect of OL extracts on cancer cells. Further research could investigate the synergistic effects of OL extracts with systemic therapies.
5. Conclusions
This was the first study on the cytotoxic activity of polyphenol extracts from leaves of the Istarska Bjelica and Leccino cultivars after treatment with Si. Silicon treatment significantly increased the amount of oleuropein, the most abundant phenolic compound, and enhanced the cytotoxic effect on all cell lines tested. Interestingly, the extracts isolated from the Si-treated plants showed better cytotoxic activity than the most abundant compound, oleuropein, suggesting that other polyphenols present in the extracts also contribute to the biological activity. The results of the present study emphasise the importance of further research to investigate the full mechanism of action of these polyphenol-rich extracts and open up the possibility of application in pharmacology.
Silicon fertilisation can have a positive impact on the overall production and quality of olive oil by changing the composition of certain phenolic compounds. However, further research is needed to establish the general impact of silicon on olives and olive oil production/quality, as well as its effect on cancerous cell death. This research will help to solidify the economic importance and rationale for the use of silicon fertilisation in orchards.
Author Contributions
Conceptualization I.P. and B.S.; validation and formal analysis M.P.P., Ž.F., P.Ž., N.M., N.V., S.R., I.N. and B.S.; investigation Š.M.; resources I.P. and S.G.B.; data curation I.P., M.F., Ž.F., N.T., I.L. and N.D.; writing—original draft preparation I.P., M.F. and B.S.; writing—review and editing I.P., M.F., M.P.P., N.T., Š.M, I.L., Ž.F., P.Ž., N.M., S.G.B., N.V., S.R., I.N., N.D. and B.S.; visualisation M.F., B.S. and M.P.P.; supervision I.P. and B.S.; project administration I.P. and M.P.P.; funding acquisition I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Croatian Science Foundation (CSF) under the project no. HRZZ-UIP-2017-05-8464 (PhytoFarmOL) and WEAVE HRZZ IP-2022-10-8305 & ARIS project N4-0346 (PROGRESS). In addition, the work of doctoral student Marija Polić Pasković (M.P.P.) was supported in part by the “Young researchers career development project—training of doctoral students” program under the CSF project HRZZ-DOK-2021-02-5517.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting our findings and analyses are contained in the article itself. Readers can access this data by referring to the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean Diet and Cancer: Epidemiological Evidence and Mechanism of Selected Aspects. BMC Surg. 2013, 13, S14. [Google Scholar] [CrossRef]
- Fares, R.; Bazzi, S.; Baydoun, S.E.; Abdel-Massih, R.M. The Antioxidant and Anti-proliferative Activity of the Lebanese Olea europaea Extract. Plant Foods Hum. Nutr. 2011, 66, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a Phenolic Compound of Virgin Olive Oil, Activates AMP-activated Protein kinase to Inhibit Carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Zang, H.; Yin, Z.; Guan, P.; Yu, C.; Shan, A.; Feng, X. Dietary Pterostilbene Exerts Potential Protective Effects by Regulating Lipid Metabolism and Enhancing Antioxidant Capacity on Liver in Broilers. J. Anim. Physiol. Anim. Nutr. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Yu, H.; Wang, S.; Sun, J.; Chai, X.; Sun, X.; Qi, X.; Zhang, R.; Jiao, Y.; Li, Z.; et al. Dietary Rutin Alleviated the Damage by Cold Stress on Inflammation Reaction, Tight Junction Protein and Intestinal Microbial Flora in the Mice Intestine. J. Nutr. Biochem. 2024, 130, 109658. [Google Scholar] [CrossRef]
- Peragón, J.; Rufino-Palomares, E.E.; Muñoz-Espada, I.; Reyes-Zurita, F.J.; Lupiáñez, J.A.A. New HPLC-MS Method for Measuring Maslinic Acid and Oleanolic Acid in HT29 and HepG2 Human Cancer Cells. Int. J. Mol. Sci. 2015, 16, 21681. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Antioxidant Oleuropein: Exceeding the Expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major Phenolic Compounds in Olive Oil: Metabolism and Health Effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant Polyphenol Content, Soil Fertilization and Agricultural Management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Plant Polyphenols-Biofortified Foods as a Novel Tool for the Prevention of Human Gut Diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Soldo, B.; Talhaoui, N.; Palčić, I.; Brkljača, M.; Koprivnjak, O.; Majetić Germek, V.; Ban, D.; Klanjac, J.; Franić, M.; et al. Boron Foliar Application Enhances Oleuropein Level and Modulates Volatile Compound Composition in Olive Leaves. Sci. Hortic. 2019, 257, 108688. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Niemeyer, E.D. Effects of Nitrogen Fertilization on the Phenolic Composition and Antioxidant Properties of Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.B.S.; Leal-Costa, M.V.; Coutinho, M.A.S.; Moreira, N.S.; Lage, C.L.S.; Barbi, N.S.; Costa, S.S.; Tavares, E.S. Increased Antioxidant Activity and Changes in Phenolic Profile of Kalanchoe pinnata (Lamarck) Persoon (Crassulaceae) Specimens Grown under Supplemental Blue Light. Photochem. Photobiol. 2013, 89, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Tekaya, M.; Mechri, B.; Bchir, A.; Attia, F.; Cheheb, H.; Daassa, M.; Hammami, M. Effect of Nutrient-based Fertilisers of Olive Trees on Olive Oil Quality. J. Sci. Food Agric. 2013, 93, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.Q.; Rodrigues, M.Â.; Moutinho-Pereira, J.M.; Correia, C.M.; Arrobas, M. Olive Tree Response to Applied Phosphorus in Field and Pot Experiments. Sci. Hortic. 2018, 234, 236–244. [Google Scholar] [CrossRef]
- Roussos, P.A.; Gasparatos, D.; Kechrologou, K.; Katsenos, P.; Bouchagier, P. Impact of Organic Fertilization on Soil Properties, Plant Physiology and Yield in Two Newly Planted Olive (Olea europaea L.) Cultivars under Mediterranean Conditions. Sci. Hortic. 2017, 220, 11–19. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon Application Alleviates Drought Stress in Wheat Through Transcriptional Regulation of Multiple Antioxidant Defense Pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Hassan, I.F.; Ajaj, R.; Gaballah, M.S.; Ogbaga, C.C.; Kalaji, H.M.; Hatterman-Valenti, H.M.; Alam-Eldein, S.M. Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Nascimento-Silva, K.; Benlloch-González, M.; Fernández-Escobar, R. Silicon Nutrition in Young Olive Plants: Effect of Dose, Application Method, and Cultivar. HortScience 2022, 57, 1534–1539. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic Variation in the Silicon Composition of Plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Martos-García, I.; Fernández-Escobar, R.; Benlloch-González, M. Silicon is a Non-essential Element but Promotes Growth in Olive Plants. Sci. Hortic. 2024, 323, 112541. [Google Scholar] [CrossRef]
- Marcelić, Š.; Vidović, N.; Pasković, I.; Lukić, M.; Jukić Špika, M.; Palčić, I.; LukiĆ, I.; Petek, M.; Pecina, M.; Herak Custić, M.; et al. Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality. Horticulturae 2022, 8, 203. [Google Scholar] [CrossRef]
- Zakraoui, M.; Hannachi, H.; Pasković, I.; Vidović, N.; Polić Pasković, M.; Palčić, I.; Major, N.; Goreta Ban, S.; Hamrouni, L. Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods 2023, 12, 2565. [Google Scholar] [CrossRef]
- Stateras, D.C.; Moustakas, N.K. Seasonal Changes of Macro- and Micro-nutrients Concentration in Olive Leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Puizina, J.; Nazlić, M.; Maravić, A.; Ljubenkov, I.; Soldo, B.; Vuko, E.; Bajić, D. Phytochemical Characterization and Screening of Antioxidant, Antimicrobial and Antiproliferative Properties of Allium × cornutum Clementi and Two Varieties of Allium cepa L. Peel Extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef]
- Statistica, Version 14.1.0.8.; TIBCO Software Inc.: Palo Alto, CA, USA, 2017.
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and Bioactive Properties of Olive (Olea europaea L.) Leaves. Eur. Food Res. Technol. 2016, 243, 89–99. [Google Scholar] [CrossRef]
- González-Báide, A.; Gómez, P. Dysfunctionality of the Xylem in Olea europaea L. Plants Associated with the Infection Process by Verticillium dahliae Kleb. Role of Phenolic Compounds in Plant Defense Mechanism. Artic. J. Agric. Food Chem. 2007, 55, 3373–3377. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Liber, Z.; Montemurro, C.; Miazzi, M.M.; Ljubenkov, I.; Soldo, B.; Žanetić, M.; Vitanović, E.; Politeo, O.; Škevin, D. Quantitatively Unraveling Hierarchy of Factors Impacting Virgin Olive Oil Phenolic Profile and Oxidative Stability. Antioxidants 2022, 11, 594. [Google Scholar] [CrossRef]
- Talhaoui, N. Analytical, Agronomic, and Biological Evaluation of Phenolic Compounds in Olea europaea Products and By-Products. Ph.D. Thesis, University of Granada, Faculty of Sciences, Granada, Spain, 8 January 2016. [Google Scholar]
- Mujić, I.; Živković, J.; Nikolić, G.; Vidović, S.; Trutić, N.; Kosić, U.; Jokić, S.; Ruznić, A. Phenolic Compounds in Olive Leaf Extract as a Source of Useful Antioxidants. Croat. J. Food Technol. Biotehnol. Nutr. 2011, 6, 129–133. [Google Scholar]
- Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Polić Pasković, M.; Vidović, N.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Goreta Ban, S.; Kos, T.; Čoga, L.; Tomljanović, T.; Simonić-Kocijan, S.; et al. Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia. Horticulturae 2023, 9, 594. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Majetić, V.; Brkić Bubola, K.; Kosić, U. Variability of Phenolic and Volatile Compounds in Virgin Olive Oil from Leccino and Istarska Bjelica Cultivars in Relation to Their Fruit Mixtures. Food Technol. Biotechnol. 2012, 50, 216–221. [Google Scholar]
- Baiano, A.; Terracone, C.; Viggiani, I.; Del Nobile, M.A. Effects of Cultivars and Location on Quality, Phenolic Content and Antioxidant Activity of Extra-Virgin Olive Oils. J. Am. Oil Chem. Soc. 2013, 90, 103–111. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. The Response of Phenylalanine Ammonia-lyase, Polyphenol oxidase and Phenols to Cold Stress in the Olive Tree (Olea europaea L. cv. Picual). J. Sci. Food Agric. 2009, 89, 1565–1573. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of Water Deficit on Leaf Phenolic Composition, Gas Exchange, Oxidative Damage and Antioxidant Activity of Four Greek Olive (Olea europaea L.) Cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, R. Olive Nutritional Status and Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cui, Y.; Ding, X.; Dai, Q. Stimulation of Phenolic Metabolism by Silicon Contributes to Rice Resistance to Sheath Blight. J. Plant Nutr. Soil Sci. 2013, 176, 118–124. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Dudel, E.G. Silicon Availability Changes Structural Carbon Ratio and Phenol Content of Grasses. Environ. Exp. Bot. 2012, 77, 283–287. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Alonso, G.L.; Salinas, M.R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf along an Agronomic Cycle. Agronomy 2021, 11, 2447. [Google Scholar] [CrossRef]
- Ashour, H.A.; Mahmoud, A.W.M. Response of Jatropha integerrima Plants Irrigated with Different Levels of Saline Water to Nano Silicon and Gypsum. J. Agric. Stud. 2017, 5, 136–160. [Google Scholar] [CrossRef]
- Therios, I. Mineral nutrition of the olive. In Olives, 1st ed.; Atheron, J., Rees, A., Eds.; Cabi: Wallingford, UK, 2008; pp. 179–209. [Google Scholar]
- Cavalheiro, C.V.; Picoloto, R.S.; Cichoski, A.J.; Wagner, R.; Menezes, C.R.; Zepka, L.Q.; Da Croce, D.M.; Barin, J.S. Olive Leaves Offer More Than Phenolic Compounds—Fatty Acids and Mineral Composition of Varieties from Southern Brazil. Ind. Crops Prod. 2015, 71, 122–127. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Yao, J.; Wu, J.; Yang, X.; Yang, J.; Zhang, Y.; Du, L. Oleuropein Induced Apoptosis in HeLa Cells via a Mitochondrial Apoptotic Cascade Associated with Activation of the c-Jun NH2-terminal kinase. J. Pharmacol. Sci. 2014, 125, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Vizza, D.; Lupinacci, S.; Toteda, G.; Puoci, F.; Ortensia, I.P.; De Bartolo, A.; Lofaro, D.; Scrivano, L.; Bonofiglio, R.; La Russa, A.; et al. An Olive Leaf Extract Rich in Polyphenols Promotes Apoptosis in Cervical Cancer Cells by Upregulating p21Cip/WAF1 Gene Expression. Nutr. Cancer 2019, 71, 320–333. [Google Scholar] [CrossRef]
- Zhang, X.; Reinsmoen, N.L. Impact of Non-human Leukocyte Antigen-specific Antibodies in Kidney and Heart Transplantation. Front. Immunol. 2017, 8, 434. [Google Scholar] [CrossRef][Green Version]
- Panduranga, A.K.; Ganapasam, S.; Kumar, A. Cytotoxic Effect of Luteolin on Human Colorectal Cancer Cell Line (HCT-15): Crucial Involvement of Reactive Oxygen Species. Middle East J. Cancer 2013, 4, 175–180. [Google Scholar]
- Potočnjak, I.; Šimić, L.; Vukelić, I.; Batičić, L.; Domitrović, R. Oleanolic Acid Induces HCT116 Colon Cancer Cell Death Through the p38/FOXO3a/Sirt6 Pathway. Chem. Biol. Interact. 2022, 363, 110010. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Jo, H.J.; Lee, K.J.; Choi, J.W.; An, J.H. Oleanolic acid induces p53-dependent apoptosis via the ERK/JNK/AKT pathway in cancer cell lines in prostatic cancer xenografts in mice. Oncotarget 2018, 9, 26370–26386. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic Acid and its Synthetic Derivatives for the Prevention and Therapy of Cancer: Preclinical and Clinical Evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).