Abstract
This study investigated the effects of irrigation with a fully inorganic nutrient solution (control; NNNN) and an organic instead of an inorganic nutrient solution (OIINS) at the flowering–fruit setting (ONNN), fruit expanding (NONN), color turning (NNON), and harvest (NNNO) stages of the first spike on the type and content of tomato fruit volatiles to provide a theoretical basis for tomato aroma improvement and high-quality cultivation. Compared with the control (NNNN), the results showed that all OIINS-related treatments decreased the number of fruit volatiles and increased the relative content of common volatile compounds, characteristic effect compounds, aldehydes, and cis-3-hexenal. In particular, the relative order of performance of the OIINS-related treatments was NNNO > NNON > ONNN > NONN in terms of the relative content of characteristic compounds. For all treatments, the relative cis-3-hexenal and trans-2-hexenal percentages were 20.99–51.49% and 20.22–27.81%, respectively. Moreover, hexanal was only detected in tomato fruits under the NNNN and NNNO treatments. The effects of irrigation with OIINS on tomato fruit volatiles were related to the fruit developmental stage. At the mature stage, the organic nutrient solution was conducive to the accumulation of characteristic compounds and improved the fruit aroma quality.
1. Introduction
Tomatoes (solanum lycopersicum L.) are rich in nutrients and have a unique flavor, making them popular among consumers as vegetables and fruits [1,2,3]. Tomatoes are one of the most widely cultivated vegetables in the world. China has the highest total tomato production in the world, with annual production of more than 67 million tons, sufficient to meet the population’s daily needs [4]. In recent years, as individuals’ economic status has improved, dietary preferences have shifted towards quality rather than quantity. Consumers now prefer flavorful tomatoes and are willing to pay higher prices [5,6]. Therefore, cultivating high-flavor-quality tomatoes is crucial in meeting the market demand and improving the economic benefits of tomato production.
The aroma produced by food is composed of a variety of volatile aromatic compounds and is detected when the volatiles enter the nasal cavity and are perceived by the olfactory system [7,8]. The aroma is an important component of the tomato flavor quality and, along with sugars and acids, plays a key role in the acceptability of tomato fruits by consumers [9,10,11]. However, over the past few decades, tomato cultivation has focused on high yields, disease resistance, and an extended shelf life, resulting in a notable reduction in the characteristic aroma of the fruits and the flavor quality. Thus, fruit volatile compounds have become a key research focus in terms of enhancing tomato flavor. In recent years, the effects of the genotype [11,12,13] and production management [14,15,16,17] on the volatile compounds of tomato fruit have been studied, and nutrient management has been shown to play a significant role in determining the composition of volatile compounds [18,19,20].
Organic nutrient solutions are abundant in essential mineral nutrients such as nitrogen (N), phosphorus (P), and potassium (K), as well as soluble organic compounds such as small-molecule organic acids, free amino acids, sugars, beneficial microorganisms, and their byproducts. This combination provides organic nutrient solutions with excellent nutritional value and biocontrol properties [21]. Organic nutrient solutions, with their fluid characteristics, can be applied using spraying equipment or directly through microirrigation systems such as drip irrigation. Compared to traditional solid organic fertilizers, organic liquid fertilizers can more easily achieve labor-saving, precise, and efficient management based on crop growth and development [22]. Thus, organic nutrient solutions are considered novel fertilizer sources that meet the requirements of modern fertigation and have considerable application prospects [23,24]. Many studies have been conducted on the effects of the formulation, irrigation concentration, and irrigation scheduling (irrigation volume and frequency) of organic nutrient solutions on crop growth, yields, and fruit flavor quality [20,22,25]. The synthesis and metabolism of substances in fruits vary at different developmental stages; therefore, the influence of organic nutrient solutions on the flavor substances in fruits may be related to the fruit development stage. However, studies on the effects of organic nutrient solutions on tomato fruit volatiles based on the fruit developmental stage are scarce.
This study aimed to explore an approach to improving tomato fruit volatiles through organic instead of inorganic nutrient solutions using precision fertilization. Therefore, the effects of irrigation with a fully inorganic nutrient solution (control; NNNN) and an organic rather than inorganic nutrient solution (OIINS) on the type and content of tomato fruit volatiles during the flowering–fruit setting (ONNN), fruit expanding (NONN), color turning (NNON), and harvest (NNNO) stages of the first spike were investigated.
2. Materials and Methods
2.1. Experimental Materials
The tomato variety used in the experiment was “Jingcai 6” (Beijing Modern Farmer Seedling Technology Co., Ltd., Beijing, China). The cultivation substrate was coconut bran bricks (Qingdao Ranmei Trading Co., Ltd., Qingdao, China) with dimensions of 20 cm (length) × 15 cm (width) × 10 cm (height). The coconut bran bricks were composed of 30% coconut chunks (10–20 mm) and 70% coconut bran (0–6 mm) with EC < 1.00 mS cm−1 and pH 5.8–6.8 and wrapped with a polyethylene film on six sides. The cultivation tank measured 25 × 20 × 15 cm, with a drainage channel and an outlet at the bottom (Figure 1Ⅱ).
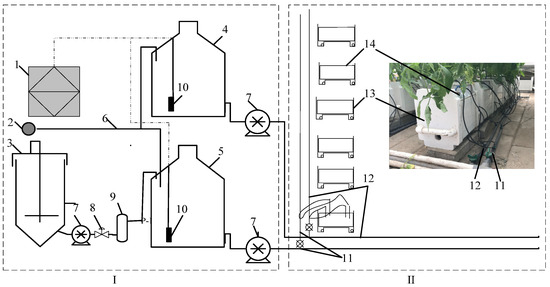
Figure 1.
Schematic diagram of automatic fertigation applicator and irrigation system for organic and inorganic nutrient solutions. (Ⅰ) The automatic fertigation applicator for organic and inorganic nutrient solutions; (Ⅱ) The irrigation system and the cultivation system. 1. Controller; 2. Water source; 3. Fermentation tank; 4. Inorganic nutrient solution bucket; 5. Organic liquid fertilizer bucket; 6. Clean water pipe; 7. Self-priming pump; 8. Solenoid valve; 9. Filter pump; 10. Conductivity sensor; 11. Organic liquid fertilizer irrigation pipes; 12. Inorganic nutrient solution irrigation pipes; 13. Cultivation tank; 14. Substrate (coconut bran brick).
The preparation of the organic liquid fertilizers and irrigation with the organic and inorganic nutrient solutions were controlled using an automatic fertigation applicator developed by the Intelligent Equipment Research Center of the Beijing Academy of Agriculture and Forestry Sciences [24]. The preparation and irrigation of the organic and inorganic nutrient solutions were independent (Figure 1). The formula used to prepare the organic liquid fertilizer was the original one, and the specific details are listed in Table 1. The parameters (oxygen supply 20 min·30 min−1, stirring cycle 10 min·20 min−1) were set in the automatic fertigation applicator system to regulate the fermentation of the organic liquid fertilizer. After 10 d of fermentation, the organic liquid fertilizer was obtained through filtration and stored in a storage tank for later use. The Yamazaki tomato nutrient solution formula (1987) was used as the inorganic nutrient solution. The environment in the equipment room with the installed automatic fertigation applicator (Figure 1Ⅰ) could be controlled to ensure consistent environmental parameters for the fermentation of the organic liquid fertilizer. Previous research has shown that the nutrient elements in organic liquid fertilizers remain stable within 10 days of storage. Therefore, this study strictly controlled the use period of the organic liquid fertilizer to minimize differences in nutrient content, ensuring a consistent concentration (EC) between different batches.

Table 1.
Organic nutrient solution formula per 100 kg of water.
2.2. Experimental Design
The experiment was conducted in the solar greenhouse of the Beijing Academy of Agriculture and Forestry Sciences (39°94′35.28″ N; 116°28′66.02″ E; altitude 50 m) from June to September 2020. The study included five treatments: fully inorganic nutrient solution irrigation (control) and OIINS irrigation at the flowering–fruit setting, fruit expansion, color turning, and harvest stages of the first truss, denoted as NNNN, ONNN, NONN, NNON, and NNNO, respectively. The irrigation scheduling and concentrations of the organic and inorganic nutrient solutions were consistent. The irrigation scheduling adopted a cumulative light radiation method [26]. The irrigation concentration was adjusted based on the growth stages of tomato: 1.62 mS/cm at vegetative growth, 2.15 mS/cm at flowering–fruit setting, and 3.58 mS/cm at the fruit expansion to harvest stages. The irrigation concentrations of the organic and inorganic nutrient solutions are listed in Table 2.

Table 2.
Nutrient concentrations of organic and inorganic nutrient solutions (mg/L).
Tomatoes with five leaves were planted at a density of 4.76 plants/m2. Each treatment was repeated thrice, arranged randomly, and irrigated independently. The cultivation management was consistent for all treatments, with three fruit trusses topped and 3–4 fruits left on each truss.
2.3. Determination of Fruit Development Process
The development of the first and second trusses of the tomatoes was observed and recorded every 2 d. When 60% of the tomato plants have flowered, the fruit diameter is ≥1.5 cm and begins to show a color break or ripening; thus, it is considered to enter the respective stages of flowering–fruit setting, fruit expansion, color turning, or harvesting. The time of entry into each stage of fruit development and the time at which all the fruits were harvested are shown in Table 3. The development process of the second truss fruit lagged behind that of the first truss by 5–9 d.

Table 3.
Development of tomato fruits.
2.4. Determination of Aromatic Volatile Compounds
At the harvesting stage, five fruits of the second truss, with the same size and a fully red color, were randomly selected from the replicates of each treatment and quickly transported to the laboratory to determine the aromatic volatiles.
The aromatic volatiles in the tomatoes were extracted and analyzed using solid-phase microextraction–gas chromatography–mass spectroscopy (SPME-GC-MS) [27]. The solid-phase microextraction needle (50/30 μm DVB/CVR on PDMS, 2 cm) was aged at 250 °C for 2 h in the gas chromatography inlet. Five tomatoes were homogenized using a tissue homogenizer to obtain the pulp. Then, 8.0 g of the homogenate was placed into a 15 mL sample vial and sealed. An aged extraction needle was inserted into the headspace of the sample vial and incubated at 40 °C for 45 min. Subsequently, the extraction needle was inserted into the gas chromatography–mass spectrometry (Shimadzu GC-MS-QP2010) instrument for desorption at 250 °C for 1 min and then analyzed via GC-MS. The GC phase featured a DB-5MS elastic quartz capillary column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness), high-purity He (99.999%) as the carrier gas, and a flow rate of 1.0 mL min−1. The sample inlet temperature was 250 °C with a heating program as follows: hold at 60 °C for 2 min and then increase at 8 °C min−1 to 220 °C for 20 min. The MS phase used electronic ionization with an ionization voltage of 70 eV. The process featured full scanning with a scanning quality range of 30–550 amu.
The aromatic volatile compounds in the tomato fruits were identified by the GC-MS retention time of the chemical compound and retention indices (RI) and searched using the National Institute of Standards and Technology Database 2014 and the related literature [28]. The retention indices were calculated using a normal alkane mixed label, soluble in methanol and ranging between C-7 and C-40.
2.5. Determination of Soluble Solids, Soluble Sugars, and Titratable Acidity
The soluble solid content was determined using a handheld refractometer (PR–32 (0–32%), ATAGO Co., Ltd., Tokyo, Japan). The soluble sugar content was measured using the anthrone method. The titratable acidity was measured by the alkaline titration of the sample using 0.1 M NaOH (titration to pH 8.1) and was expressed as 0.067 g/mmol of malic acid. The sugar–acid ratio is the ratio of soluble solids to titratable acidity.
2.6. Statistical Analysis
Data processing and charting were performed using Microsoft Office Excel (Microsoft Excel 2019; Microsoft, Redmond, WA, USA). Data analysis involved a one-way analysis of variance (ANOVA) followed by Duncan’s test (SPSS version 21.0; IBM Corporation, Armonk, NY, USA), with the significance level set at p < 0.05.
3. Results and Analysis
3.1. Effects of OIINS Irrigation on the Composition of Tomato Fruit Volatile Compounds
The compounds, along with their chemical classes, retention indices, and relative content, are listed in Table A1 in Appendix A. A total of 66 volatile compounds were identified in the second truss of tomato fruits. They could be categorized into different classes based on their chemical structures, including 22 aldehydes, 17 alcohols, 7 esters, 7 ketones, 5 hydrocarbons, and 8 other volatile compounds. Moreover, 41 compounds were detected in the NNNN treatment, whereas 36, 37, 34, and 36 compounds were identified in the OIINS-related treatments (Figure 2A). Irrigation with OIINS decreased the number of volatile fruits. Trans-2-hexenal had the highest relative content in the NNNN treatment, followed by cis-3-hexenal, whereas the reverse was observed for the other four treatments.
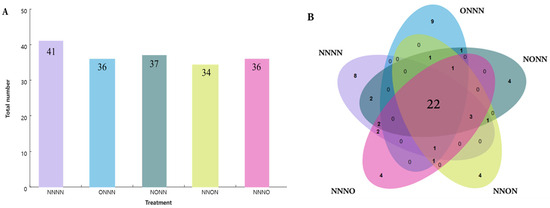
Figure 2.
The number of volatile compounds in the different treatments. (A) The total number of volatile compounds detected during each treatment. (B) A Venn diagram showing that all treatments contained 22 common volatile compounds.
Of these, a total of 22 volatile compounds were detected in each treatment, known as common volatile compounds, including eight aldehydes, five alcohols, four esters, three ketones, and two other types of compounds (Figure 2B). The total relative content of the common volatile compounds in the different treatments was 69.85%, 86.47%, 87.03%, 88.56%, and 74.23%, respectively, and they could be considered the main volatile substances in this variety of tomatoes (Table A1 in Appendix A). The OIINS-related treatments increased the relative content of the common volatile compounds in the fruits, but there were differences among the treatments.
3.2. Effect of OIINS Irrigation on Quantity and Content of Chemical Classes of Tomato Fruit Volatile Compounds
The quantity and relative content of the chemical classes of the tomato fruit volatile compounds under different treatments are shown in Figure 3 and Figure 4, respectively. The most abundant class was aldehydes, followed by alcohols, esters, and ketones. However, the OIINS-related treatments decreased the number of aldehydes, alcohols (except ONNN and NNNO), and ketones (except for NONN). The highest relative content among the five treatments was for aldehydes, ranging from 72.63% to 78.90%, making them the main volatile compounds in the tomato fruits. The OIINS-related treatments increased the relative aldehyde content in the following order: NNON > ONNN > NNNO > NONN > NNNN. This may be attributed to trans-3-hexenal, cis-2-hexenal, and hexanal, which had total content of 76.44% (NNON), 72.22% (ONNN), 71.71%(NONN), 69.36% (NNNO), and 66.87% (NNNN) (Table A1). The relative content of esters, ketones, alcohols, and hydrocarbons was the highest in the NNNO, NNON, NONN, and ONNN treatments, respectively.
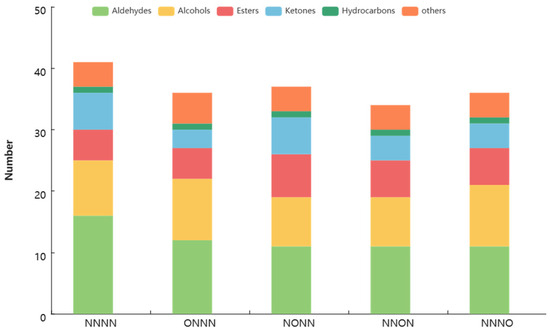
Figure 3.
Quantities of chemical classes of tomato fruit volatile compounds under different treatments.
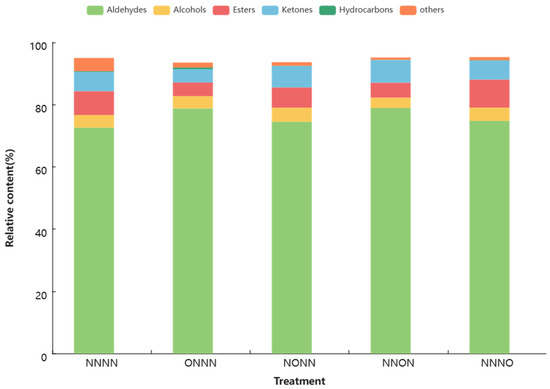
Figure 4.
Relative content of chemical classes of tomato fruit volatile compounds under different treatments.
3.3. Effect of OIINS Irrigation on Characteristic Aroma Compounds of Tomato Fruit
Table 4 presents the characteristic aromatic compounds, with their odor descriptions and relative content, that have been suggested to be important tomato aroma contributors [12,29,30]. Nine characteristic aromatic compounds were identified. Among these, cis-3-hexenal, trans-2-hexenal, trans-2-heptenal, methyl salicylate, 1-penten-3-one, and 3-methyl-1-butanol were detected in all treatments. Hexanal was only detected in the NNNN and NNNO treatments; 3-methylbutanol in the ONNN, NNON, and NNNO treatments; and 2-isobutylthiazole only in the ONNN treatment. In the NNNN- and OIINS-related treatments, the number of characteristic effect compounds was seven, eight, six, seven, and eight, and the relative content was 77.52%, 79.59%, 77.96%, 81.49%, and 82.52%, respectively. The OIINS-related treatments increased the total relative content of these compounds. cis-3-hexenal, trans-2-hexenal, trans-2-heptenal, and hexanal have green odors. cis-3-hexenal, which is associated with bitterness [7], had significantly higher content in the OIINS-related treatments (51.49%, 43.82%, 49.49%, and 29.1%) than in the NNNN treatment (20.99%). The content of trans-2-hexenal significantly decreased in the ONNN treatment, whereas hexanal, with a sweet taste [20], had significantly lower content in the NNNO treatment than in the NNNN treatment. Methyl salicylate has a wintergreen odor, with the highest and lowest content in the NNNO and NNON treatments, respectively. Furthermore, 1-penten-3-one and 6-methyl-5-hepten-2-one have fruity and floral odors. Moreover, 6-methyl-5-hepten-2-one has been linked to the tomato flavor, overall satisfaction, and taste decay. Meanwhile, 3-methyl-1-butanol has an earthy and musty odor. The relative content of these three compounds showed significant variations across the different treatments. Therefore, OIINS irrigation affected the type and relative content of the characteristic aroma compounds, with variations depending on the stage of fruit development.

Table 4.
Characteristic aroma compounds and their relative content in tomato fruits under the five treatments.
3.4. Effect of OIINS Irrigation on Soluble Solids, Soluble Sugars, and Titratable Acidity of Tomato Fruits
The sugar and acid content of tomato fruits, closely related to their sweetness and acidity, are common indicators of the fruit flavor quality. As shown in Figure 5, the soluble sugar content of the tomato fruits was reduced by OIINS irrigation. Furthermore, the NNON and NNNO treatments reached a significant level, with no clear differences. The ONNN treatment had the lowest organic acid content, followed by the NONN treatment, both reaching a significant level, with no significant differences among the other three treatments (p < 0.05). The soluble solid content was ranked as NNNN > NNON > NNNO > NONN > ONNN, with a significant difference observed between the NNNN- and OIINS-related treatments. The fruit sugar–acid ratio was significantly higher in the ONNN treatment than in the other treatments, followed by the NONN treatment. The NNON and NNNO treatments had significantly lower values than the NNNN treatment, with no significant differences.
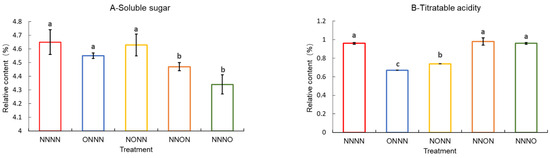
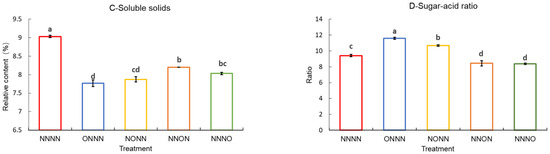
Figure 5.
Soluble sugars, titratable acidity, soluble solids, and sugar–acid ratio in tomato fruits supplied with different nutrient solutions. (A) Soluble sugars; (B) titratable acidity; (C) soluble solids; (D) sugar–acid ratio. Vertical bars represent the standard error of the mean (n = 3). Different letters indicate significant differences between the treatments (Duncan’s test, p < 0.05).
4. Discussion
More than 400 volatile compounds have been identified in tomatoes and categorized into aldehydes, ketones, alcohols, esters, and hydrocarbons based on their chemical structures [11,29]. In this study, 66 volatile compounds were detected in the tomato fruits across the five treatments, of which 22 were common volatile compounds, accounting for 53.66–64.71% in quantity and 69.85–88.56% in relative content. Irrigation with OIINS increased the content of common volatile compounds, aldehydes, and characteristic aroma compounds. This indicates that the genotype plays a key role in determining the volatile compound composition in tomato fruits, and OIINS irrigation significantly impacts the types and content of volatile compounds. Previous studies have shown that organic fertilizers are more effective than chemical fertilizers in enhancing the variety and quantity of volatile compounds in fruits [16,19,20,32]. In this study, the quantity of volatile compounds and aldehydes decreased significantly under the OIINS-related treatments, in contrast to the results of previous studies. This difference may be attributed to the type of organic fertilizer, application method, nutrient characteristics, and crop residue.
Tomato fruit development can be classified into two stages: early and mature. The early fruit development stage includes the flowering–fruit setting and fruit expanding stages, characterized by cell division and enlargement, which determine the fruit size and structure, whereas the maturity stage involves fruit color turning and the formation of metabolic products, which play a key role in quality development [33]. In this experiment, under the ONNN, NONN, and NNON treatments with OIINS irrigation, the second spike occurred in the early fruit development stage, whereas, under the NNNO treatment with OIINS irrigation, they were in the fruit maturity stage. During the early fruit developmental stage with OIINS irrigation, there was higher accumulation of trans-3-hexenal in the fruit, whereas hexanal was not detected. The synthesis of methyl salicylate and anti-2-hexenal (ONNN) was affected. At the fruit maturity stage with OIINS irrigation, there was higher accumulation of anti-2-hexenal and methyl salicylate in the fruits, with a slight increase and decrease in the relative content of trans-3-hexenal and hexanal, respectively. The total relative content of the fruit characteristic aroma compounds was in the following order: NNNO > NNON > ONNN > NONN > NNNN. This suggests that OIINS irrigation has a stage-specific impact on the volatile compounds in fruits. Irrigation during the early fruit development stage significantly influenced the trans-3-hexenal and hexanal levels, whereas irrigation during the fruit maturity stage promoted the accumulation of characteristic aromatic compounds in the tomato fruits. Trans-3-hexenal and hexanal are key volatile compounds in tomato fruits, formed from linoleic acid and linolenic acid precursors, respectively, and catalyzed by lipoxygenase C (TomloxC) and 13-hydroperoxide lyase (13-HPL) [31,34]. Their synthesis initiates post-cell division. Linoleic acid is the primary substrate for LOX enzyme activity in tomato fruits, followed by linolenic acid [35]. Ma et al. [20] suggested that organic liquid fertilizers might affect the content of volatile compounds in tomato fruits by regulating the substrate concentration and enzyme activity. In this study, the relative content of trans-3-hexenal in the ONNN, NONN, and NNON treatments was more than double that in the NNNN treatment, with no detection of hexanal. It has been suggested that OIINS irrigation during early fruit development may influence the levels of linoleic and linolenic acids. Further in-depth research in conjunction with fatty acid metabolism is needed.
The aroma of tomato fruits is mainly determined by the type and content of characteristic aroma compounds. Trans-3-hexenal and cis-2-hexenal, which contribute significantly to the tomato aroma, and hexanal, cis-2-heptenal, and methyl salicylate collectively impart a green and fresh fragrance to tomato fruits. Furthermore, 1-penten-3-one and 6-methyl-5-hepten-2-one have fruity and floral aromas [31]. Buttery et al. [30] proposed that a combination of trans-3-hexenal, cis-2-hexenal, hexanal, trans-3-hexenol, 3-methylbutanol, 1-penten-3-one, 6-methyl-5-hepten-2-one, beta-ionone, methyl salicylate, and 2-isobutylthiazole at suitable concentrations can create an aromatic flavor in freshly ripe tomatoes. In this study, OIINS irrigation increased the total relative content of the characteristic aroma compounds in the tomato fruits. In total, eight out of ten characteristic aroma compounds mentioned in Buttery’s study [30] were found when OIINS irrigation was used during the fruit maturity stage. This suggests that OIINS irrigation is beneficial in enhancing the characteristic aroma and improving the aroma quality of tomato fruits, with a more pronounced effect during the fruit maturity stage.
5. Conclusions
In this study, the effect of OIINS irrigation on the type and content of volatile compounds in tomato fruits was analyzed. The results showed that OIINS irrigation affected the volatile compounds in the tomato fruits. Compared to total inorganic nutrient solution irrigation, OIINS irrigation increased the total relative content of volatile compounds, aldehydes, and characteristic aromatic compounds. OIINS irrigation during the early fruit development stage significantly increased the (Z)-3-hexenal content, but hexanal was not detected. At the fruit maturity stage, OIINS irrigation resulted in the accumulation of more characteristic aromatic compounds. Thus, OIINS irrigation is more conducive to enhancing the characteristic aroma of tomato fruits and improving the fruit aroma quality, with better effects when applied during the fruit color turning and ripening stages.
Author Contributions
Y.L. and X.H. contributed equally to this study. Conceptualization, Y.L. and W.G.; Methodology, Y.L., W.G. and S.L.; Software, Y.L. and Q.Z.; Validation, Y.L., S.L. and J.X.; Formal analysis, Y.L., X.H., S.L. and W.G.; Investigation, Y.L., S.L., R.S., X.H. and W.G.; Resources, Y.L., R.S. and W.G.; Data curation, Y.L., X.H., S.L., R.S., Q.Z. and T.L.; Writing—original draft preparation, Y.L., X.H., S.L., T.L. and J.X.; Writing—review and editing, W.G., Y.L., X.H., S.L. and J.X.; Visualization, Y.L., X.H. and J.X.; Supervision, Y.L., W.G., S.L., X.H. and Q.Z.; Project administration, Y.L. and W.G.; Funding acquisition, W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ningxia Hui Autonomous Region Key Research and Development Program No. 2023BCF01047, Ningxia Academy of Agriculture and Forestry Sciences No. DW-X-2023001, and Beijing Academy of Agriculture and Forestry Sciences Innovation Capacity Building Special Project No. KJCX20210422.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We thank Li Feng from the Institute of Agricultural Economy and Information Technology, Ningxia Academy of Agriculture and Forestry Sciences, for his help with the research process of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Composition and relative content of volatile compounds in tomato fruits under five treatments.
Table A1.
Composition and relative content of volatile compounds in tomato fruits under five treatments.
| Serial Number | Volatile Substances | RI | Relative Content of Each Component (%) | ||||
|---|---|---|---|---|---|---|---|
| NNNN | ONNN | NONN | NNON | NNNO | |||
| Aldehydes | |||||||
| 1 | (Z)-3-Hexenal | 802 | 20.99 ± 1.13 | 51.49 ± 1.37 | 43.82 ± 3.26 | 49.49 ± 2.85 | 29.1 ± 0.68 |
| 2 | (E)-2-Hexenal | 855 | 26.81 ± 0.53 | 20.22 ± 2.88 | 26.08 ± 1.8 | 26.95 ± 2.27 | 27.81 ± 1.63 |
| 3 | (E)-2-Heptenal | 967 | 1.37 ± 0.09 | 0.93 ± 0.01 | 1.13 ± 0.01 | 0.71 ± 0.01 | 0.81 ± 0.014 |
| 4 | (E,E)-2,4-Heptadienal | 1001 | 0.47 ± 0.02 | 0.33 ± 0.02 | 0.39 ± 0.02 | 0.26 ± 0.016 | 0.31 ± 0.02 |
| 5 | Octanal | 1007 | 0.11 ± 0.02 | 0.09 ± 0.01 | 0.07 ± 0 | 0.05 ± 0 | 0.07 ± 0 |
| 6 | (E)-2-Octenal | 1061 | 1.37 ± 0.05 | 0.52 ± 0.03 | 1.07 ± 0.006 | 0.22 ± 0.01 | 0.32 ± 0.009 |
| 7 | Nonanal | 1108 | 0.16 ± 0.02 | 0.13 ± 0 | 0.21 ± 0.01 | 0.1 ± 0.01 | 0.18 ± 0.017 |
| 8 | (E)-2-Pentenal | 768 | 0.77 ± 0.04 | 0.69 ± 0.03 | 0.74 ± 0.016 | 0.47 ± 0.017 | 0.74 ± 0.024 |
| 9 | (E)-3,7-Dimethyl-2,6-Octadienal | 1261 | ─ | 0.12 ± 0.01 | 0.13 ± 0.004 | 0.12 ± 0.009 | ─ |
| 10 | (E,E)-2,4-Decadienal | 1285 | 0.36 ± 0.03 | 0.29 ± 0.011 | ─ | 0.09 ± 0.01 | |
| 11 | Decanal | 1205 | 0.04 ± 0 | ─ | ─ | ─ | 0.05 ± 0 |
| 12 | Hexanal | 803 | 19.06 ± 0.08 | ─ | ─ | ─ | 15.31 ± 0.23 |
| 13 | Pentanal | 730 | ─ | 2.04 ± 0.021 | 0.57 ± 0.034 | ─ | ─ |
| 14 | 3,7-Dimethyl-2,6-Octadienal | 1261 | 0.11 ± 0.02 | ─ | ─ | ─ | ─ |
| 15 | (E,E)-2,4-Dodecadienal | 1382 | 0.22 ± 0.01 | ─ | ─ | ─ | ─ |
| 16 | Heptanal | 906 | 0.13 ± 0.02 | ─ | ─ | ─ | ─ |
| 17 | 2-Undecenal | 1265 | 0.08 ± 0.01 | ─ | ─ | ─ | ─ |
| 18 | Benzaldehyde | 976 | 0.56 ± 0.01 | ─ | ─ | ─ | ─ |
| 19 | 2,4-Decadienal | 1285 | ─ | 0.1 ± 0.02 | ─ | ─ | ─ |
| 20 | 3,4-Dihydro-2H-Pyran-2-Carboxaldehyde | 973 | ─ | 2.13 ± 0.02 | ─ | ─ | ─ |
| 21 | (E,E)-2,4-Hexadienal | 731 | ─ | ─ | ─ | 0.4 ± 0.006 | ─ |
| 22 | 2-Hydroxy-Benzaldehyde | 1067 | ─ | ─ | ─ | 0.13 ± 0 | ─ |
| Alcohols | |||||||
| 23 | Methyl Alcohol | 469 | 1.69 ± 0.04 | 1.81 ± 0.04 | 2.1 ± 0.22 | 1.51 ± 0.022 | 1.92 ± 0.03 |
| 24 | 2-Methyl-1-Butanol | 756 | 0.09 ± 0 | 0.59 ± 0.04 | 0.32 ± 0.009 | 0.6 ± 0.02 | 0.25 ± 0.003 |
| 25 | 1-Pentanol | 776 | 0.79 ± 0.01 | 0.32 ± 0.01 | 0.58 ± 0.029 | 0.16 ± 0.006 | 0.27 ± 0.012 |
| 26 | 1-Octen-3-ol | 992 | 0.32 ± 0.01 | 0.26 ± 0.004 | 0.29 ± 0.03 | 0.23 ± 0.004 | 0.21 ± 0.01 |
| 27 | 6-Methyl-5-Hepten-2-ol | 1006 | 0.09 ± 0.01 | 0.23 ± 0.02 | 0.14 ± 0.008 | 0.17 ± 0.011 | 0.18 ± 0.006 |
| 28 | 1-Hexanol | 871 | 0.35 ± 0.02 | ─ | 0.41 ± 0.006 | 0.51 ± 0.04 | 0.38 ± 0.02 |
| 29 | 1-Heptanol | 981 | 0.32 ± 0.03 | ─ | 0.4 ± 0.025 | ─ | 0.38 ± 0.006 |
| 30 | 3-Methyl-1-Butanol | 753 | ─ | 0.12 ± 0.01 | ─ | 0.15 ± 0.006 | 0.13 ± 0.006 |
| 31 | 1-Octanol | 1091 | 0.23 ± 0.02 | ─ | 0.28 ± 0.015 | ─ | ─ |
| 32 | (Z)-3-Decen-1-ol | 1071 | 0.17 ± 0.03 | ─ | ─ | ─ | ─ |
| 33 | (Z)-4-Methyl-Cyclohexanol | 1008 | ─ | 0.13 ± 0.015 | ─ | ─ | ─ |
| 34 | (2Z)-3-Pentyl-2,4-Pentadiene-1-ol | 949 | ─ | 0.26 ± 0.015 | ─ | ─ | ─ |
| 35 | (E)-2-Hepten-1-ol | 1092 | ─ | 0.15 ± 0 | ─ | ─ | ─ |
| 36 | (Z)-3-Octen-1-ol | 1018 | ─ | 0.08 ± 0.01 | ─ | ─ | ─ |
| 37 | 3,4-Dimethylpent-2-en-1-ol | 1117 | ─ | ─ | ─ | 0.04 ± 0 | ─ |
| 38 | (E)-4-Methyl-Cyclohexanol | 731 | ─ | ─ | ─ | ─ | 0.46 ± 0.006 |
| 39 | 3-Methyl-Cyclohexanol | 1018 | ─ | ─ | ─ | ─ | 0.09 ± 0 |
| Esters | |||||||
| 40 | Acetic acid, 1-methylethyl ester | 712 | 0.09 ± 0.01 | 0.16 ± 0.006 | 0.19 ± 0.024 | 0.05 ± 0 | 0.19 ± 0.002 |
| 41 | 2-Methyl-butanol acetate | 880 | 0.07 ± 0.01 | 0.33 ± 0.023 | 0.3 ± 0.02 | 0.41 ± 0.011 | 0.35 ± 0.005 |
| 42 | Methyl salicylate | 1223 | 5.74 ± 0.03 | 2.27 ± 0.012 | 3.43 ± 0.05 | 1.14 ± 0.012 | 6.16 ± 0.05 |
| 43 | Methyl 2-carbonyl-hexanoate | 1081 | 1.28 ± 0.02 | 1.25 ± 0.01 | 1.57 ± 0.03 | 2.23 ± 0.01 | 1.25 ± 0.04 |
| 44 | Ethyl (E)-2-pentenoate | 1054 | 0.47 ± 0.03 | ─ | 0.63 ± 0.006 | 0.67 ± 0.006 | 0.79 ± 0.03 |
| 45 | Acetic acid, 2-methylpropyl ester | 780 | ─ | 0.37 ± 0.021 | 0.26 ± 0.013 | 0.3 ± 0.01 | 0.28 ± 0.004 |
| 46 | Butyric acid, 4-pentadecyl ester | 876 | ─ | ─ | 0.13 ± 0.006 | ─ | ─ |
| Ketones | |||||||
| 47 | 1-Penten-3-one | 724 | 2.15 ± 0.04 | 2.19 ± 0.031 | 2.09 ± 0.016 | 1.71 ± 0.015 | 2 ± 0.23 |
| 48 | 6-Methyl-5-Hepten-2-one | 997 | 1.39 ± 0.03 | 1.64 ± 0.004 | 1.41 ± 0.02 | 1.34 ± 0.01 | 1.2 ± 0.07 |
| 49 | 6,10-Dimethyl-5,9-Undecadien-2-one | 1441 | 0.44 ± 0.03 | 0.47 ± 0.028 | 0.35 ± 0.013 | 0.54 ± 0.016 | 0.33 ± 0.008 |
| 50 | 7-Oxabicyclo [2.2.1] hept-5-en-2-one | 1102 | 0.19 ± 0 | ─ | 0.19 ± 0.025 | ─ | ─ |
| 51 | 5-Ethyl-2(5H)-Furanone | 972 | 1.72 ± 0.01 | ─ | 2.63 ± 0.02 | 3.73 ± 0.035 | 2.52 ± 0.065 |
| 52 | 1-Octen-3-one | 989 | 0.35 ± 0.01 | ─ | ─ | ─ | ─ |
| 53 | 3-Oxatricyclo [4.2.0.0(2,4)] octan-7-one | 1076 | ─ | ─ | 0.16 ± 0.015 | ─ | ─ |
| Hydrocarbons | |||||||
| 54 | 2-Octene | 881 | ─ | 0.5 ± 0.006 | ─ | ─ | ─ |
| 55 | (E, Z)-2,4-Dodecadiene | 1372 | ─ | ─ | 0.14 ± 0.01 | ─ | ─ |
| 56 | 2-Methyl-2,4-hexadiene | 818 | ─ | ─ | ─ | 0.02 | ─ |
| 57 | 1-Ethyl-1-methyl-cyclopentane | 967 | ─ | ─ | ─ | ─ | 0.14 ± 0.01 |
| 58 | 2-Undecyne | 1059 | 0.19 ± 0.03 | ─ | ─ | ─ | ─ |
| Others | |||||||
| 59 | 2-Amylfuran | 993 | 0.21 ± 0.02 | 0.2 ± 0.01 | 0.18 ± 0.002 | 0.1 ± 0.01 | 0.14 ± 0.005 |
| 60 | 2-Methoxy-phenol | 1109 | 3.45 ± 0.01 | 0.31 ± 0.002 | 0.61 ± 0.02 | 0.23 ± 0 | 0.51 ± 0.02 |
| 61 | 3-Allyl-6-methoxyphenol | 1330 | 0.47 ± 0.03 | 0.15 ± 0.015 | ─ | 0.25 ± 0.015 | 0.18 ± 0.006 |
| 62 | Amyl-doxycycline | 669 | 0.11 ± 0.01 | ─ | 0.21 ± 0.006 | 0.16 ± 0.015 | ─ |
| 63 | 1-Nitro-hexane | 908 | ─ | 0.2 ± 0.031 | ─ | ─ | ─ |
| 64 | Eugenol | 1331 | ─ | ─ | 0.15 ± 0.006 | ─ | ─ |
| 65 | 2-Isobutylthiazole | 1054 | ─ | 0.73 ± 0.015 | ─ | ─ | ─ |
| 66 | Hexyl-Oxirane | 908 | ─ | ─ | ─ | ─ | 0.18 ± 0.015 |
| 67 | Total relative content | 94.99 ± 0.48 | 93.51 ± 1.75 | 93.66 ± 1.15 | 95.15 ± 5.24 | 95.29 ± 1.33 | |
Note: “─” indicates that the compound was not detected or did not exist. The retention indices were calculated using a normal alkane mixed label, soluble in methanol and ranging from C-7 to C-40. Values are presented as the mean ± standard deviation (n = 3).
References
- Kumar, M.; Chandran, D. Valorization potential of tomato (Solanum lycopersicum L.) seed: Nutraceutical quality, food properties, safety aspects, and application as a health promoting ingredient in foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Paola, P.; Fiorella, S. Investigating physicochemical, volatile, and sensory parameters playing a positive or negative role in tomato preference. Food Res. Int. 2013, 50, 409–419. [Google Scholar]
- Perveen, R.; Suleria, H.A.R. Tomato (Solanum lycopersicum) carotenoids and lycopene chemistry; metabolism, absorption, nutrition, and allied health claims: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products. 2021. Annual Data. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 January 2024).
- Pang, X.L.; Sun, Y. Advances and perspectives in research on the volatile flavor quality of agricultural products. Sci. Agric. Sin. 2019, 52, 3192–3198, (In Chinese with an English Abstract). [Google Scholar]
- Klee, H. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.A.; Scott, J.W. Relationship between sensory and instrumental analysis for tomato flavor. J. Am. Soc. Hortic. 1998, 123, 906–915. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. Genetics of fruit flavor preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Malundo, T.M.M.; Shewfelt, R.L. Flavor quality of fresh tomato (Lycopersicon esculentum Mill.) as affected by the sugar and acid levels. Postharvest Biol. Technol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Goodner, K. Interaction of volatiles, sugars, and acids on perception of tomato aroma and flavor descriptors. J. Food Sci. 2008, 73 (Suppl. S6), 294–307. [Google Scholar] [CrossRef]
- El, H.M.; Zhang, F. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wang, X. Comparison of volatile components and aroma profiles between peel and flesh of tomatoes (Solanum lycopersicum). Food Sci. 2021, 42, 249–262. [Google Scholar]
- Stefano, C.; Mauro, C. Effects of single or combined water deficit and aphid attack on tomato Effects of single or combined water deficit and aphid attack on tomato. Environ. Exp. Bot. 2018, 153, 54–62. [Google Scholar]
- Xu, W.N.; Zhang, X. Effects of boron on the volatile components of tomato fruit of “Jinpeng No.1”. Food Sci. 2016, 37, 149–155, (In Chinese with an English Abstract). [Google Scholar]
- Wei, S.H.; Xiao, X.M. Effects of supplemental illumination in different periods on the quality and volatile compounds of tomato fruit in a solar greenhouse. Trans. Chin. Soc. Agric. Eng. 2020, 36, 188–196, (In Chinese with an English Abstract). [Google Scholar]
- Lee, J.H.J.; Jayaprakasha, G.K. Metabolomic studies of volatiles from tomatoes grown in net-house and open-field conditions. Food Chem. 2019, 275, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Paolo, B.; Giulia, M. Impact of drying techniques, seasonal variation, and organic growth on flavor compound profiles in two Italian tomato varieties. Food Chem. 2019, 298, 125062. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, L.; Sun, Y.; Zou, G.; Hang, S. Substitution of 1/2 chemical N input with swine manure and straw increased the nutritional quality, species, and content of volatile substances in tomato fruit. J. Plant Nutr. Fertil. 2020, 26, 1106–1116, (In Chinese with an English Abstract). [Google Scholar]
- Ma, L.; Zhang, J. Effects of different organic nutrient solution formulations and supplements on tomato fruit quality and aromatic volatile compounds. Arch. Agron. Soil. Sci. 2020, 66, 563–575. [Google Scholar]
- Xing, M.Y.; Li, X.W. Changes in the chemical characteristics of water-extracted organic matter from vermicomposting of sewage sludge and cow dung. J. Hazard. Mater. 2012, 205–206, 24–31. [Google Scholar] [CrossRef]
- Li, Y.L.; Guo, W. Irrigation scheduling based on moisture and EC sensors in organic culture of cucumber. Trans. Chin. Soc. Agric. Mach. 2017, 48, 263–270, (In Chinese with an English Abstract). [Google Scholar]
- Antony, R.; Willoughby, A. Molecular insights on dissolved organic matter transformation by supraglacial microbial communities. Environ. Sci. Technol. 2017, 51, 4328–4337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Li, Y.K. Design and testing of integrated water and fertilizer system in organic cultivation. Trans. Chin. Soc. Agric. Mach. 2016, 47, 273–279, (In Chinese with an English Abstract). [Google Scholar]
- Fan, B.H.; Ma, L.L. Effects of application frequency of organic fertilizer extract and irrigation amount on the content of aromatic substances and cellulose in melon fruits. J. Plant Nutr. Fertil. 2020, 26, 773–782. [Google Scholar]
- Alberto, L.; Harm, B. Evaluation of irrigation scheduling of hydroponic tomatoes in Navarra, Spain. Irrig. Drain. 2003, 52, 177–188. [Google Scholar]
- Tang, X.W.; Liu, M.C. Influence of regulated deficit irrigation on sensory quality and flavor components of tomatoes. Plant Nutr. Fertil. Sci. 2010, 16, 970–977, (In Chinese with an English Abstract). [Google Scholar]
- Pérez-Marín, J.; Issa-Issa, H.; Clemente-Villalba, J.; García-Garví, J.M.; Hernández, F.; Carbonell-Barrachina, Á.A.; Calín-Sánchez, Á.; Noguera-Artiaga, L. Physicochemical, Volatile, and Sensory Characterization of Promising Cherry Tomato (Solanum lycopersicum L.) Cultivars: Fresh Market Aptitudes of Pear and Round Fruits. Agronomy 2021, 11, 618. [Google Scholar] [CrossRef]
- Wang, L.; Qian, C.L. Difference in volatile composition between the pericarp tissue and inner tissue of tomato (Solanum lycopersicum) fruit. J. Food Process Preserv. 2017, 42, e13387. [Google Scholar] [CrossRef]
- Buttery, R. Quantitative and sensory aspects of flavor of tomato and other vegetables and fruits. In Flavor Science: Sensible Principles and Techniques; Acree, T.E., Teranishi, R., Eds.; ACS: Washington, DC, USA, 1993; pp. 259–286. [Google Scholar]
- Wang, L.; Baldwin, E.A. Recent advances in aromatic volatile research in tomato fruit: Metabolism and regulation. Food Bioproc. Tech. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- Li, W.; Lu, X. Effect of organic nutrient solution on flavor of ripe cherry tomato fruit: Transcriptome and metabolomic analyses. Environ. Exp. Bot. 2022, 194, 104721. [Google Scholar] [CrossRef]
- Zhang, T.P.; Yang, X.H. Advances in the molecular and physiological mechanisms of early development of tomato fruits. Chin. Bull. Bot. 2018, 53, 856–866, (In Chinese with an English Abstract). [Google Scholar]
- Liu, M.C.; Hao, J. Advances in studies of aroma components in tomato fruits. Sci. Agric. Sin. 2008, 41, 1444–1451, (In Chinese with an English Abstract). [Google Scholar]
- José, L.; Yury, M.T. Volatile landscape of expanded tomatoes. J. Exp. Bot. 2014, 16, 4613–4623. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).