Cellular Basis of Adjuvant Role of n-3 Polyunsaturated Fatty Acids in Cancer Therapy: Molecular Insights and Therapeutic Potential against Human Melanoma
Abstract
1. Introduction
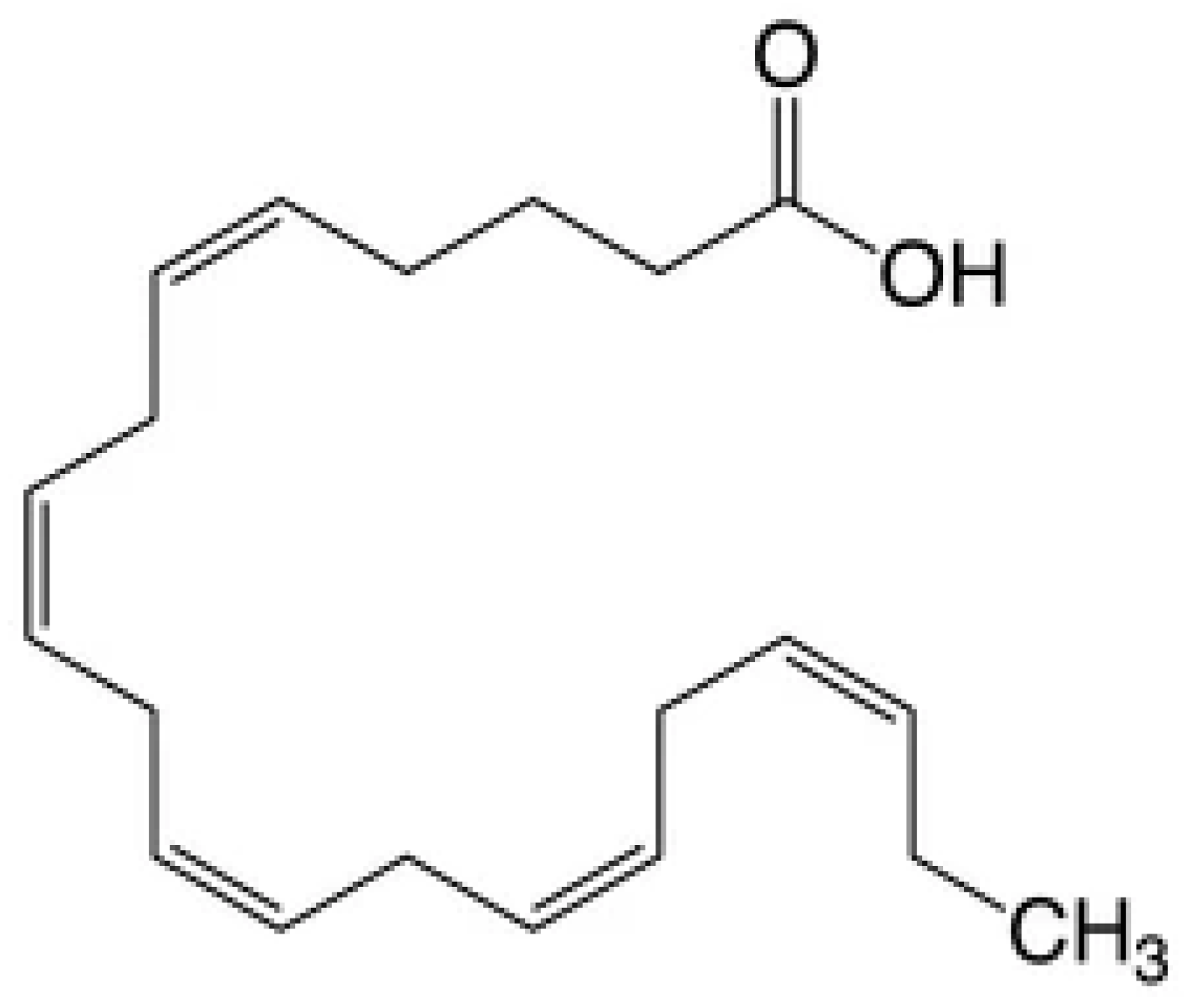
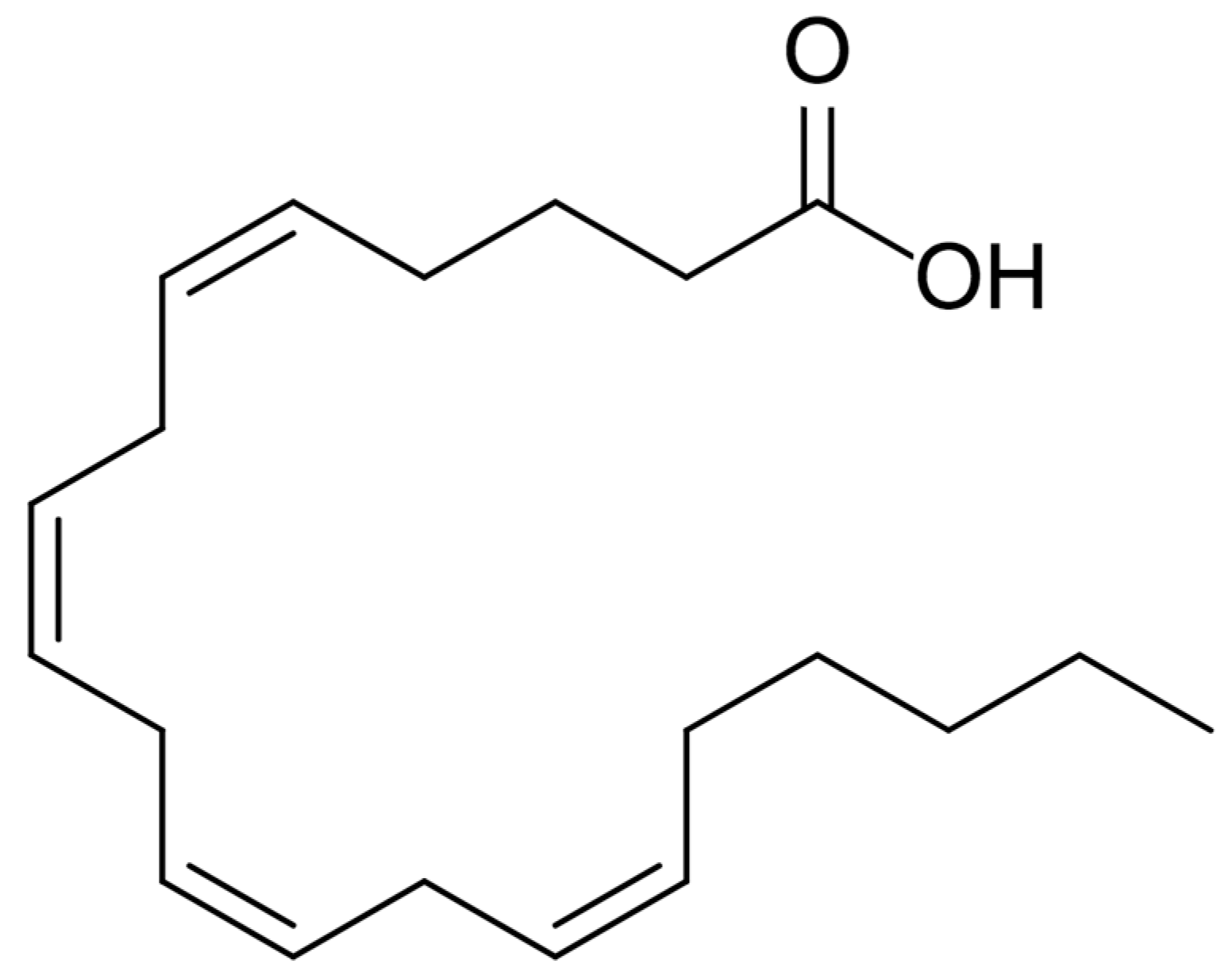
2. Omega-3 Polyunsaturated Fatty Acids
2.1. Diet
2.2. Biological Functions
2.3. Clinical Benefits
2.4. Cancer Research
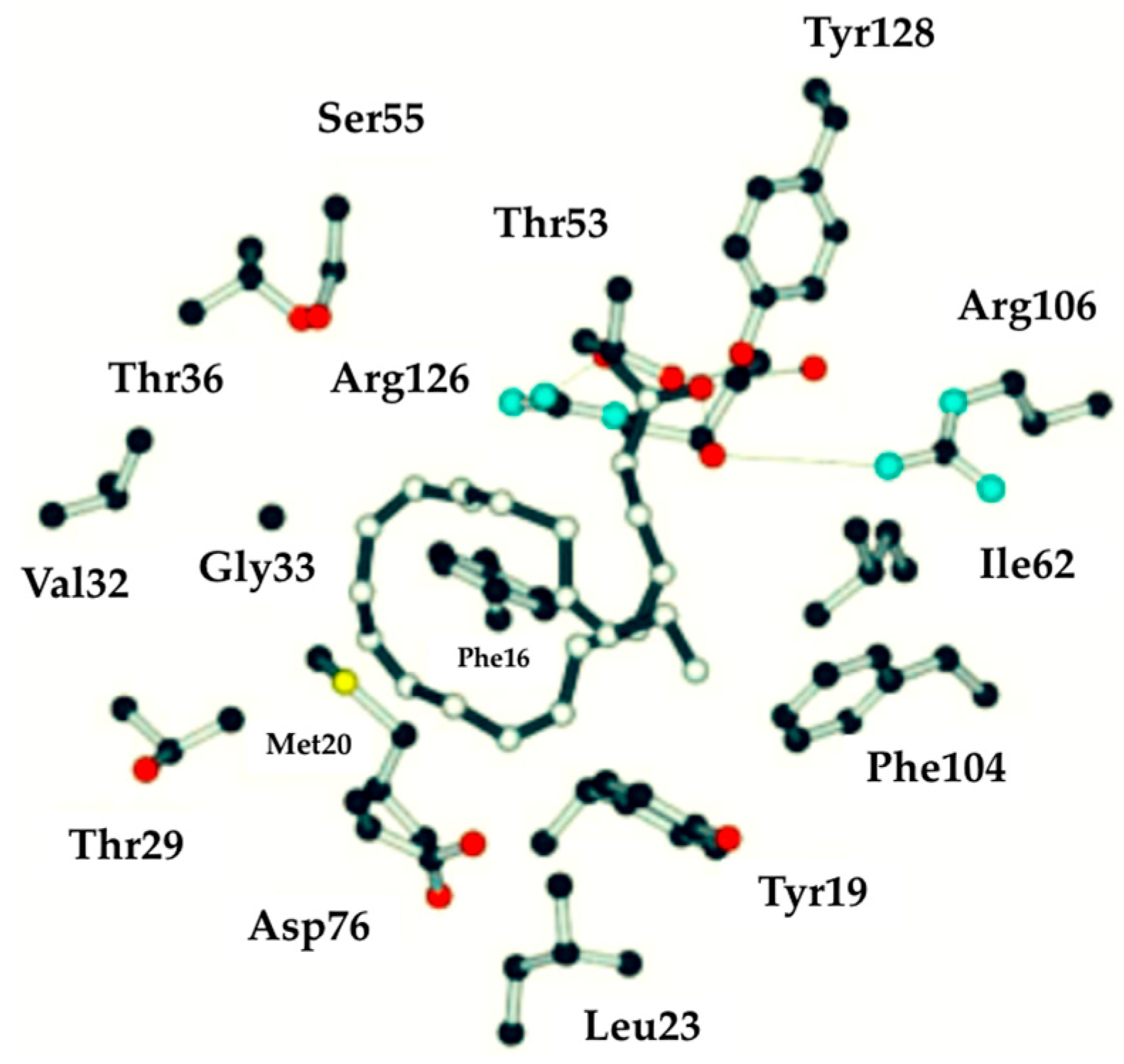
2.5. Characteristics of n-3 PUFA In Silico
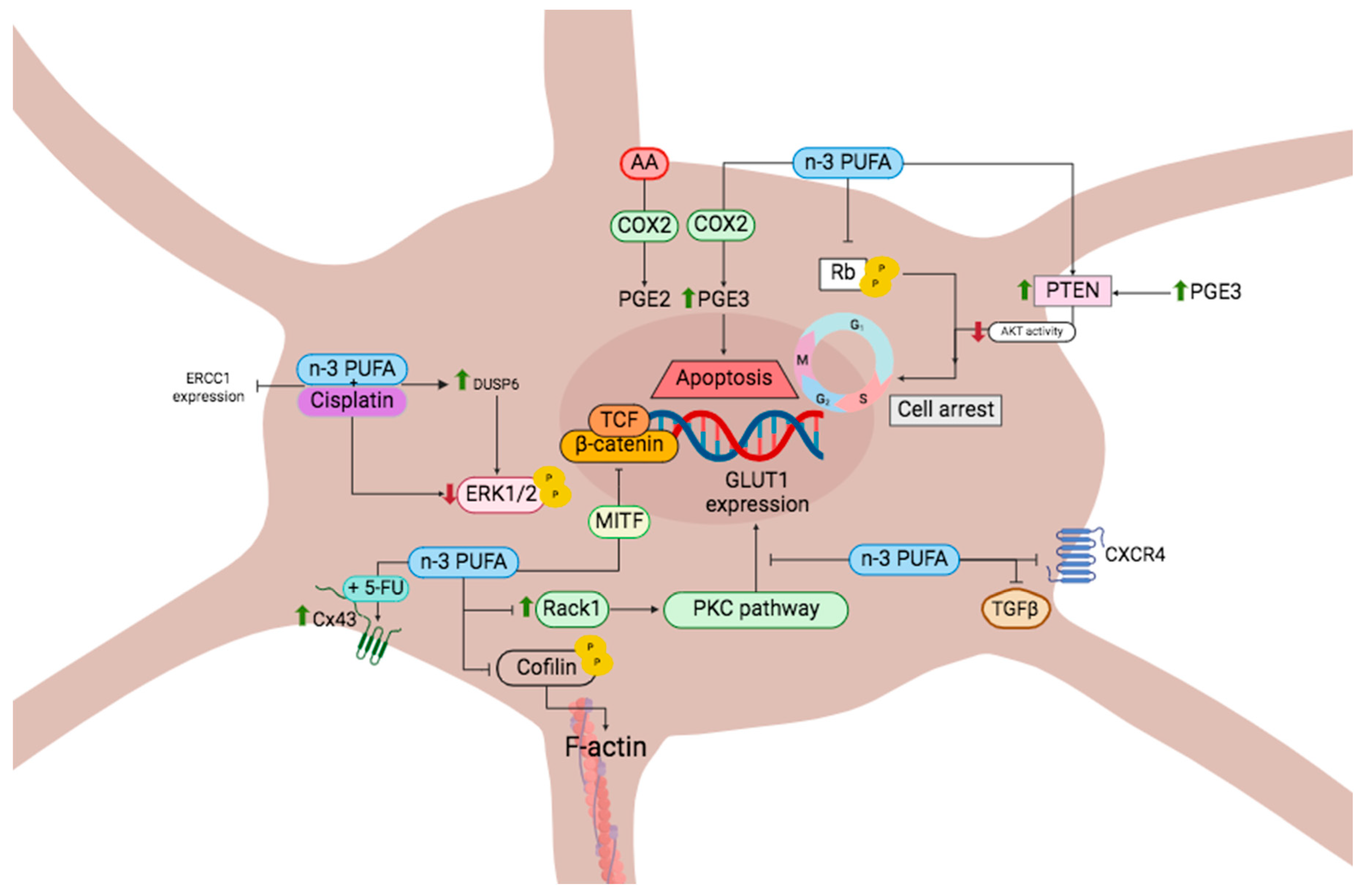
3. Molecular Mechanisms of Omega-3 Fatty Acids in Cancer
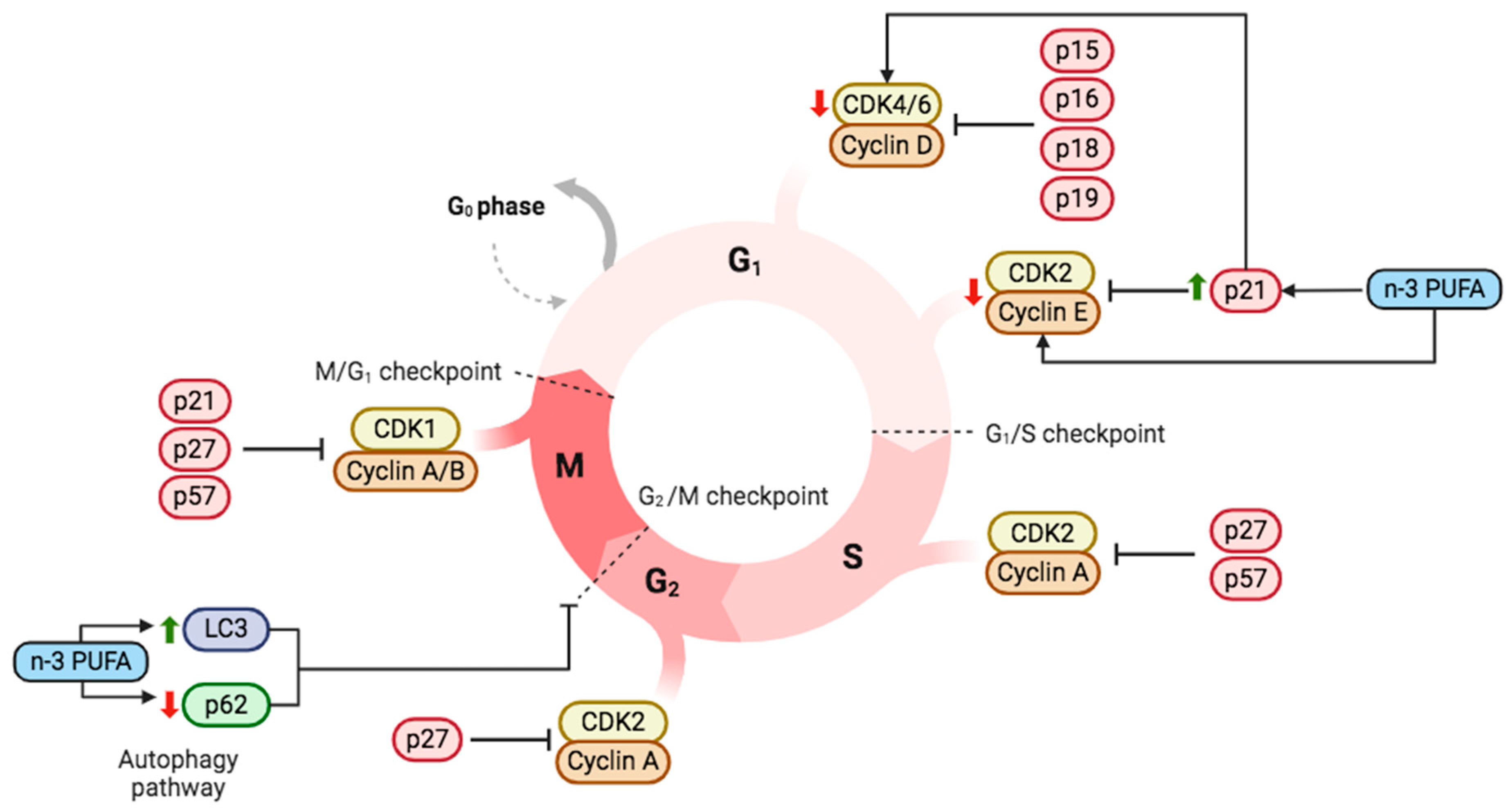
3.1. Cell Cycle and Cell Death in Cancer Mediated by n-3 PUFA
3.2. Cell Cycle and Cell Death in Melanoma Mediated by n-3 PUFA
3.3. DHA and Autophagy in Crosstalk with Cell Cycle Modulation
DHA and Autophagy in Crosstalk with Melanoma Cell Cycle Modulation
3.4. n-3 PUFA Inhibits Angiogenesis
3.5. Regulation of Cancer Metastasis through n-3 PUFA
Regulation of Melanoma Metastasis through n-3 PUFA
3.6. Oxidative Stress and n-3 PUFA
3.7. Inflammation and Immunomodulation
3.8. Nutrigenomics
3.9. Multidrug-Resistant Cancer
4. Interactions of n-3 PUFA with Other Antineoplastic Drugs
5. Studies and Mechanism about n-3 PUFAs and Melanoma
5.1. In Vitro Studies
5.2. In Vivo Studies
5.3. Discrepancy in Results and Approaches to Studies in Humans
| Model | Dose | Results | Reference |
|---|---|---|---|
| In vitro (metastatic B16F10 murine melanoma cells and metastatic WM266-4 human melanoma cells) | CDDP (1, 2.5, and 5 μmol/L), DHA or EPA (10–30 μmol/L) alone or in combination at different time points (24–72 h) | - Growth-inhibitory effect was enhanced with DHA + CDDP - Inhibit melanoma cell migration was enhanced with DHA + CDDP - Both DHA and EPA + CDDP revert ERCC1 expression. - Reduction of pERK1/2 was observed in both cell lines in a DHA dose-dependent manner - DHA or EPA, given alone significantly increased DUSP6 expression with respect to control cells | [128] |
| In vitro (70 W human melanoma cell line that metastasizes to the brain in nude mice) | 50 μM each for 24 h of AA, EPA or DHA | - TNF-α upregulates the expression of both COX-2 mRNA and PGE2 - PGE3 incubation significantly decreased invasive values - DHA and EPA decreased COX-2 mRNA expression - EPA or DHA decreased the cell invasion, whereas AA caused invasion to increase by 2.4 times - EPA and DHA downregulated both COX-2 mRNA and protein expression, with a subsequent decrease in invasion. | [73] |
| In vitro (WM115 and WM266-4 human melanoma, and B16-F10 murine melanoma cell lines) | 10–30 μM of DHA at 24 and 48 h | - Significantly suppressed the anchorage independent colony growth - Inhibits melanoma cell growth, migration and invasion - Inhibits the expression of several matrix metalloproteinases (MMP-2, MMP-13, and MT1-MMP) - These effects are related to the β-catenin increased nuclear expression and PKA-dependent phosphorylation, as well as to the increased expression of MITF. | [150] |
| In vitro (A375, A2058, and G361 human melanoma cells) | AA, EPA and DHA (50 mM and 100 mM) | - Decrease of proliferation, associated by oxidative protein and DNA damage was observed for EPA and DHA (50 mM and 100 mM) in A375, A2058, and G361 cells - In C32 (amelanotic melanoma), EPA and DHA inhibited cell proliferation at 100 mM only and the effect of DHA was more pronounced, whereas AA did not show antiproliferative action in this cell line. | [59] |
| In vitro (human skin melanoma A-375 cells) | NSAIDs, at doses 10–480 μM, was incubated simultaneously with the melanoma cells and 160 μM of DHA for 72 h | - 50 and 100 μM of celecoxib reduced proliferation of the melanoma cells at 72-h incubations by 34% and 82.7%, respectively. - Indomethacin inhibited the cell proliferation by about 40% at 240 and 480 μM - Aspirin and piroxicam do not exhibited cytostatic or cytotoxic effect on the cancer cells. - Both celecoxib and indomethacin, starting from 20 μM, exhibited additive effects on the DHA-induced growth inhibition. - Aspirin enhanced the DHA-induced growth inhibition by 42.8% at 480 μM as well as piroxicam by 15.9–66.4% at 40–240 μM. | [149] |
| In vitro (Primary WM115 and WM266-4 metastatic melanoma cell line) | 10–30 μM DHA, EPA, oleic acid or linoleic acid for 72 h | - The constitutive expression and mRNA-expression of COX-2 protein was inhibited in a dose-dependent manner by 10–30 μM DHA - Degradation of COX-2 mRNA was accelerated by the presence of 30 μM DHA in cells whose mRNA transcription was inhibited by 10 l g/mL actinomycin D. - DHA reduced the expression of cytosolic HuR (stabilizer of COX-2 mRNA) in a concentration- and time-dependent manner - DHA and EPA inhibited the growth and apoptosis of both the cell lines and the effect was concentration dependent - DHA reduced the expression of total β-catenin in both the cell lines studied - DHA and EPA induce apoptosis in melanoma cells through caspase-3 pathway by increasing Bax proteins and decreasing Bcl-2 proteins, therefore, augmenting the Bax/Bcl-2 ratio. - The pro-apoptotic effect was observed for DHA and EPA and not observed with the other fatty acids oleic acid. | [148] |
| In vitro (B16-F10 mouse melanoma cells) | DHA (1–25 μmol/L) for 3 days | - Inhibits melanogenesis in cells through increasing tyrosinase degradation. | [187] |
| In vitro (Melanoma cell lines [B16F10, Colo679, G361, HOMM, and HTMM]) | Cells were cultured with 50 μM of PA or EPA on culture for 24 h or 25 μM of DHA for 24 h | - EPA suppresses membrane-associated Tyr and Tyrp1 - PA induces formation of F-actin, while EPA decreases F-actin levels in melanoma cells - EPA and DHA suppressed gene expression of Glut-1 - PA increases and EPA decreases the level of Rab27a in B16F10 cells - DHA delayed the cell cycle of melanoma - DHA interacts with the receptor for activated C kinase 1, leading to the repression of melanoma cell proliferation through the inhibition of PKC signaling. | [171] |
| In vivo (Fat-1 C57BL/6 transgenic mice with implantation of B16 melanoma cells) | 50 μM AA, 50 μM AA plus 50 μM indomethacin, 50 μM EPA, and 50 μM EPA plus 50 μM indomethacin | - Inhibition of melanoma formation and growth in Fat-1 transgenic mice - Upregulation of PTEN in the tumor and surrounding tissues of Fat-1 mice - AA (50 μM) did not affect cell growth - EPA (50 μM) exhibited an inhibitory effect on the growth of melanoma cells. This growth-inhibitory effect could be blocked by the presence of 50 μM indomethacin | [161] |
| In vivo (Fat-1 C57BL/6 transgenic mice with implantation of B16-F10 melanoma cells) | Their diets (per 100 g) consisted of 4.5 g sucrose, 18.6 g casein, 8.6 g cellulose, 50 g wheat starch, 0.3 g DL-methionine, 7 g mineral mix, 1 g vitamin mix and 10 g safflower oil. A group underwent 100 IU/kg VitE via oral gavage for 3 weeks treatment | - No significant difference in the levels of PGE2 - The protein expression levels of E-cadherin was upregulated and N-cadherin were downregulated in the tumor tissues of fat-1 mice - Fat-1 mouse tumor tissues exhibited a decrease in NF-κB and STAT3 than those from WT mice - After VitE supplementation, no significant difference in the levels of MDA was observed between fat-1 and WT mice, however, fat-1 mice exhibited decreased tumor growth compared with WT-mice - Fat-1 tumor tissues exhibited a marked decrease in the expression levels of β-catenin and c-Myc compared with those from WT mice | [162] |
| In vivo (Fat-1 C57BL/6 transgenic mice with implantation of B16-F0 melanoma cells) | 400 pg/mL 18-HEPE (a n-3 EPA-derived lipid mediator) in cells for 48 h and then 100 pg and 1 μg of 18-HEPE to each mouse through i.v. and intraperitoneal (i.p.) injection every other day, respectively. | - Pulmonary metastasis of melanoma B16-F0 cells is significantly reduced in Fat-1 transgenic mice - 18-HEPE directly repressed CXCR4 expression of B16-F0 cells - Higher lung TGFβ expression in WT mice than Fat-1 mice - Pulmonary MPO activity was higher in WT mice than in Fat-1 mice as well as inflammatory cytokines | [89] |
| In vivo (C57BL/6 mice with implantation of B16-F10 melanoma cells) | Daily intragastrically administered with 50 μL algal oil for four weeks prior to tumor cell injection. The control group were daily intragastrically administered with equivoluminal safflower oil (deficient in n-3 PUFAs but high in n-6 PUFA) | - Algal oil treatment inhibited lung metastases of B16-F10 melanoma cells - Algal oil treatment produced significant increases in n-3 PUFA levels (EPA, DPA and DHA) and the corresponding decreases in AA in lung tumor tissues - The endogenous n-6/n-3 PUFA ratio in lung tumor tissues was significantly lower - Algal oil treatment induced autophagy (measured by ↑ LC3-II protein and ↓ p62) through inactivation of mTOR and p38 MAPK and activation of JNK MAPK in lung tumor tissues from the mice treated with algal oil - Significant decrease in cleaved IL-1β and slight decrease in pro-IL-1β proteins levels in lung tumor tissues from the algal oil-treated mice. | [65] |
| In vitro/In vivo (B16-F10 murine melanoma cells & C57BL/6 mice with B16-F10 incoulation) | Cells with EPA (0–100 μM) for 24 h and then EPA-treated, non-treated, or transfected cells were exposed to 0–40 μM of 5-FU under for 48 h. Groups of tumor-bearing mice were orally administrated with EPA (25 mg/kg) at day 7 followed by 5-FU (40 mg/kg) treatment on days 9, 11, and 13, or with either treatment alone. | - Treatment of cells with 0, 50, 100 μM of EPA induced a dose-dependent increase in Cx43 levels compared to controls. - The levels of intercellular communication through gap junctions exhibited a correlation with the increased expression of Cx43 induced by EPA in melanoma cells. - EPA increased the susceptibility of cells to 5-FU. - EPA in combination with 5-FU enhanced the antitumor activity | [129] |
| In vitro/In vivo (human melanoma cells [A2058, A375, SK-Mel 3]/murine subcutaneous xenograft model of human melanoma) | Cells with DHA at 25–250 μM for 144 h, GW9508 at 25–150 μM and TAK-875 at 0–0.4 μM for 72 h. Animal study group were undergo to a 20:1 ratio of DHA:AA diet for 3 weeks prior to tumor cell inoculation and continued throughout the study period. Animals with established tumors underwent with once daily oral TAK-875 at 100 mg/kg. | - DHA had a profound, selective inhibitory effect on the growth of all human melanoma cell lines - GW9508 and TAK-875 had a profound inhibitory effect on the growth of all human melanoma cell lines - Growth of human melanoma in vivo is inhibited by a diet rich in DHA. - Tumors were smaller in weight and smaller in volume in the TAK-875 animal group | [170] |
| In vivo (C57Bl/6 mice with implantation of a highly metastatic F10-SR melanoma cells) | 5% maize oil diet for 1 week, and then was switched to the 5% fish oil diet for 5 weeks. | - Cells reproduced a lower number of metastatic colonies in the lungs of mice fed with fish oil diet - Reduced lung colonization of melanoma cells in animals fed a fish oil diet is associated with an increased apoptotic activity - There was a reduction of von Willebrand factor immunoreactivity in pulmonary colonies of cells grown in fish oil-fed animals which indicates a decrease of angiogenesis effects. | [91] |
| Human (Observational study) | Not applicable | - No association between the consumption of PUFAs and melanoma risk - Only VitE from food and zinc from food and supplements were found to be associated with melanoma | [177] |
| Human (Observational study) | Not applicable | - EPA-DHA intake showed to have a substantial protective association between dietary PCB exposure and risk of melanoma | [176] |
| Human (Observational study) | Not applicable | - Patients who have high consumption of meat, fish, and fats, leading to relatively elevated levels of omega-3 and omega-6 fatty acids, are at a higher risk of being diagnosed with thick melanomas rather than thin ones. | [175] |
| Human Phase 1 Clinical Trial (DHA-paclitaxel in resistant solid tumor malignancies) | DHA-paclitaxel 200 mg/m2, dose increased from 100 mg/m2 per cohort to 600 mg/m2 (200–300–400–500–600 mg/m2), weekly infusion at i.v. of 2 h. | - Administration of DHA-paclitaxel for 3 weeks is well tolerated, providing consistent prodrug exposure and its metabolite exposure, while minimizing the dose-limiting side effects of the drug. | [178] |
5.4. Safety and Side Effects of n-3 PUFA
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.G.; Moore, C.C. Nonmelanoma Skin Cancer. Facial Plast. Surg. Clin. N. Am. 2019, 27, 1–13. [Google Scholar] [CrossRef]
- Serman, N.; Vranic, S.; Glibo, M.; Serman, L.; Bukvic Mokos, Z. Genetic Risk Factors in Melanoma Etiopathogenesis and the Role of Genetic Counseling: A Concise Review. Bosn. J. Basic Med. Sci. 2022, 22, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Gastman, B.R.; Neumeister, M.W. A Melanoma Update. Clin. Plast. Surg. 2021, 48, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Z.; Barber, B.; Farr, A.M.; Ivanov, B.; Novich, M. Overall Survival in Patients with Metastatic Melanoma. Curr. Med. Res. Opin. 2015, 31, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Garutti, M.; Bergnach, M.; Polesel, J.; Palmero, L.; Pizzichetta, M.A.; Puglisi, F. BRAF and MEK Inhibitors and Their Toxicities: A Meta-Analysis. Cancers 2022, 15, 141. [Google Scholar] [CrossRef]
- Eroglu, Z.; Ribas, A. Combination Therapy with BRAF and MEK Inhibitors for Melanoma: Latest Evidence and Place in Therapy. Ther. Adv. Med. Oncol. 2016, 8, 48–56. [Google Scholar] [CrossRef]
- Priantti, J.N.; Vilbert, M.; Madeira, T.; Moraes, F.C.A.; Hein, E.C.K.; Saeed, A.; Cavalcante, L. Efficacy and Safety of Rechallenge with BRAF/MEK Inhibitors in Advanced Melanoma Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3754. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, L.; Saldías-Fuentes, C.; Carrasco, K.; Halpern, A.C.; Mao, J.J.; Navarrete-Dechent, C. Complementary and Alternative Therapies in Skin Cancer a Literature Review of Biologically Active Compounds. Dermatol. Ther. 2022, 35, e15842. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O. Bioactive Metabolites of Docosahexaenoic Acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Akonjuen, B.M.; Onuh, J.O.; Aryee, A.N.A. Bioactive Fatty Acids from Non-Conventional Lipid Sources and Their Potential Application in Functional Food Development. Food Sci. Nutr. 2023, 11, 5689–5700. [Google Scholar] [CrossRef] [PubMed]
- PubChem Doconexent. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445580 (accessed on 16 May 2024).
- Wu, D.; He, Y. Potential of Spectroscopic Techniques and Chemometric Analysis for Rapid Measurement of Docosahexaenoic Acid and Eicosapentaenoic Acid in Algal Oil. Food Chem. 2014, 158, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation: The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers—An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Spooner, M.H.; Jump, D.B. Nonalcoholic Fatty Liver Disease and Omega-3 Fatty Acids: Mechanisms and Clinical Use. Annu. Rev. Nutr. 2023, 43, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 Supplementation Improves Cognition and Modifies Brain Activation in Young Adults. Hum. Psychopharmacol. 2014, 29, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega-3 Polyunsaturated Fatty Acids and Cancer: Lessons Learned from Clinical Trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; A Yates, A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2002; ISBN 978-0-309-08525-0. [Google Scholar]
- von Schacky, C. Omega-3 Fatty Acids in Pregnancy-The Case for a Target Omega-3 Index. Nutrients 2020, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Mason, R.P.; Libby, P.; Steg, P.G.; Bhatt, D.L. Do Patients Benefit from Omega-3 Fatty Acids? Cardiovasc. Res. 2024, 119, 2884–2901. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Sirtori, C.R.; Carugo, S.; Calder, P.C.; Corsini, A. Omega-3 and Cardiovascular Prevention—Is This Still a Choice? Pharmacol. Res. 2022, 182, 106342. [Google Scholar] [CrossRef]
- Welty, F.K. Omega-3 Fatty Acids and Cognitive Function. Curr. Opin. Lipidol. 2023, 34, 12–21. [Google Scholar] [CrossRef]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.R.; Hamilton, D.L. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The Role of Omega-3 in the Prevention and Treatment of Sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Cordingley, D.M.; Cornish, S.M. Omega-3 Fatty Acids for the Management of Osteoarthritis: A Narrative Review. Nutrients 2022, 14, 3362. [Google Scholar] [CrossRef] [PubMed]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef] [PubMed]
- Elinder, F.; Liin, S. Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.G.J.; Verhoeckx, K.C.M.; Plastina, P.; Wortelboer, H.M.; Meijerink, J.; Witkamp, R.F. Docosahexaenoic Acid and Eicosapentaenoic Acid Are Converted by 3T3-L1 Adipocytes to N-Acyl Ethanolamines with Anti-Inflammatory Properties. Biochim. Biophys. Acta 2010, 1801, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-Inflammatory ω-3 Endocannabinoid Epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, E6034–E6043. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Schnutgen, F.; Scapin, G.; Borchers, T.; Xhong, N.; Lim, K.; Godbout, R.; Spener, F.; Sacchettini, J.C. Crystal Structure and Thermodynamic Analysis of Human Brain Fatty Acid-Binding Protein. J. Biol. Chem. 2000, 275, 27045–27054. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.T.; Yusuf, M.; Bachti, H.H.; Diantini, A.; Zainuddin, A. Computational Model of Doxorubicin Conjugate with Docosahexaenoic Acid and Integrin Avβ3 Ligand for Anticancer. J. Appl. Pharm. Sci. 2018, 8, 001–006. [Google Scholar] [CrossRef][Green Version]
- Ahmed, A.K.K.; Elkazzaz, M. Natural Phytochemicals, Phenformin, and Docosahexaenoic Acid (DHA) as a Novel Inhibitors of IL-6 and ACE2 Receptors, a Therapeutic Strategy for Targeting COVID-19 Cell Entry and Cytokine Storm. An Insilico Approach. Sci. Prepr. 2021. [Google Scholar] [CrossRef]
- Ramya, S.; Soorya, C.; Pushpalatha, G.G.L.; Aruna, D.; Loganathan, T.; Balamurugan, S.; Abraham, G.; Ponrathy, T.; Kandeepan, C.; Jayakumararaj, R. Artificial Intelligence and Machine Learning Approach Based In-Silico ADME-Tox and Pharmacokinetic Profile of α-Linolenic Acid from Catharanthus roseus (L.) G. Don. J. Drug Deliv. Ther. 2022, 12, 96–109. [Google Scholar] [CrossRef]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, H.; Li, S.; Huang, L.; Wang, X.; Li, Y.; Liu, Y.; Xiong, P.; Yang, Q.; Tian, K.; et al. The ω-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid Enhances NK-Cell Antitumor Effector Functions. Cancer Immunol. Res. 2024, OF1–OF15. [Google Scholar] [CrossRef] [PubMed]
- Maralbashi, S.; Aslan, C.; Kahroba, H.; Asadi, M.; Soltani-Zangbar, M.S.; Haghnavaz, N.; Jadidi, F.; Salari, F.; Kazemi, T. Docosahexaenoic Acid (DHA) Impairs Hypoxia-Induced Cellular and Exosomal Overexpression of Immune-Checkpoints and Immunomodulatory Molecules in Different Subtypes of Breast Cancer Cells. BMC Nutr. 2024, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sui, C.; Meng, F.; Ma, P.; Jiang, Y. The Omega-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid Inhibits Proliferation and Progression of Non-Small Cell Lung Cancer Cells through the Reactive Oxygen Species-Mediated Inactivation of the PI3K/Akt Pathway. Lipids Health Dis. 2017, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Hayat, F.; Andrews, J.F.; Migaud, M.E.; Gassman, N.R. Dihydroxyacetone Exposure Alters NAD(P)H and Induces Mitochondrial Stress and Autophagy in HEK293T Cells. Chem. Res. Toxicol. 2019, 32, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Wu, T.; Lim, K. Omega-3 Polyunsaturated Fatty Acids and Cancer. Anticancer Agents Med. Chem. 2013, 13, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Song, E.A.; Kim, H. Docosahexaenoic Acid Induces Oxidative DNA Damage and Apoptosis, and Enhances the Chemosensitivity of Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar] [CrossRef]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting Cell-Cycle Machinery in Cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell Cycle, CDKs and Cancer: A Changing Paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Wei, Z.; Li, D.; Zhu, L.; Yang, L.; Chen, C.; Bai, C.; Li, G. Omega 3 Polyunsaturated Fatty Acids Inhibit Cell Proliferation by Regulating Cell Cycle in Fad3b Transgenic Mouse Embryonic Stem Cells. Lipids Health Dis. 2018, 17, 210. [Google Scholar] [CrossRef] [PubMed]
- So, W.W.; Liu, W.N.; Leung, K.N. Omega-3 Polyunsaturated Fatty Acids Trigger Cell Cycle Arrest and Induce Apoptosis in Human Neuroblastoma LA-N-1 Cells. Nutrients 2015, 7, 6956–6973. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Meng, X.; Han, J.; Zhang, Z.; Wang, B.; Bai, X.; Zhang, X. Anti-Cancer Activity of DHA on Gastric Cancer—An in Vitro and In Vivo Study. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2013, 34, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, C.H.; Størvold, G.L.; Bremseth, H.; Follestad, T.; Sand, K.; Mack, M.; Olsen, K.S.; Lundemo, A.G.; Iversen, J.G.; Krokan, H.E.; et al. DHA Induces ER Stress and Growth Arrest in Human Colon Cancer Cells: Associations with Cholesterol and Calcium Homeostasis. J. Lipid Res. 2008, 49, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Kleizen, B.; Braakman, I. Protein Folding and Quality Control in the Endoplasmic Reticulum. Curr. Opin. Cell Biol. 2004, 16, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cartwright, C.; Chan, D.; Ding, J.; Felix, E.; Pan, Y.; Pang, J.; Rhea, P.; Block, K.; Fischer, S.M.; et al. Anticancer Activity of Fish Oils against Human Lung Cancer Is Associated with Changes in Formation of PGE2 and PGE3 and Alteration of Akt Phosphorylation. Mol. Carcinog. 2014, 53, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, A.; Wilczok, A.; Chodurek, E.; Gruchlik, A.; Dzierzewicz, Z. Polyunsaturated Fatty Acids Inhibit Melanoma Cell Growth in Vitro. Acta Pol. Pharm. 2013, 70, 365–369. [Google Scholar] [PubMed]
- Ziegler, D.V.; Huber, K.; Fajas, L. The Intricate Interplay between Cell Cycle Regulators and Autophagy in Cancer. Cancers 2021, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Lei, Y.-H.; Yao, N.; Wang, C.-R.; Hu, N.; Ye, W.-C.; Zhang, D.-M.; Chen, Z.-S. Autophagy and Multidrug Resistance in Cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef]
- Ma, Q.; Liao, H.; Xu, L.; Li, Q.; Zou, J.; Sun, R.; Xiao, D.; Liu, C.; Pu, W.; Cheng, J.; et al. Autophagy-Dependent Cell Cycle Arrest in Esophageal Cancer Cells Exposed to Dihydroartemisinin. Chin. Med. 2020, 15, 37. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Shi, X.; Li, S.; Tang, P.M.-K.; Li, Z.; Li, H.; Wei, C. Antimalarial Dihydroartemisinin Triggers Autophagy within HeLa Cells of Human Cervical Cancer through Bcl-2 Phosphorylation at Ser70. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 52, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Teng, M.; Liu, J. Dihydroartemisinin Inhibits Activation of the AIM2 Inflammasome Pathway and NF-κB/HIF-1α/VEGF Pathway by Inducing Autophagy in A431 Human Cutaneous Squamous Cell Carcinoma Cells. Int. J. Med. Sci. 2021, 18, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.-H.; Wang, F.; Fan, C.-L.; Zhang, X.-H.; Zhao, J.-S.; Zhang, J.-J.; Yang, Y.; Xi, Y.; Zou, Z.-Q.; Bu, S.-Z. Algal Oil Rich in N-3 Polyunsaturated Fatty Acids Suppresses B16F10 Melanoma Lung Metastasis by Autophagy Induction. Food Funct. 2018, 9, 6179–6186. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.; Mann, C.; Metcalfe, M.; Webb, M.; Pollard, C.; Spencer, D.; Berry, D.; Steward, W.; Dennison, A. The Effect of Omega-3 FAs on Tumour Angiogenesis and Their Therapeutic Potential. Eur. J. Cancer Oxf. Engl. 2009, 45, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. CMLS 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, J.; Lyu, F.; Panigrahy, D.; Ferrara, K.W.; Hammock, B.; Zhang, G. ω-3 Polyunsaturated Fatty Acids-Derived Lipid Metabolites on Angiogenesis, Inflammation and Cancer. Prostaglandins Other Lipid Mediat. 2014, 113, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, Y.; Guo, T.; Chen, Q.; Li, Y.; Su, H.; Chen, X.; Zhao, X.; Guo, Q.; Qi, J. Effect of polyunsaturated fatty acids ω-3 and ω-6 on angiogenesis formation in human gastric cancer. J. Gastrointest. Surg. 2017, 20, 84–89. [Google Scholar]
- Cui, J.; Shan, K.; Yang, Q.; Qi, Y.; Qu, H.; Li, J.; Wang, R.; Jia, L.; Chen, W.; Feng, N.; et al. Prostaglandin E3 Attenuates Macrophage-associated Inflammation and Prostate Tumour Growth by Modulating Polarization. J. Cell. Mol. Med. 2021, 25, 5586–5601. [Google Scholar] [CrossRef]
- Denkins, Y.; Kempf, D.; Ferniz, M.; Nileshwar, S.; Marchetti, D. Role of Omega-3 Polyunsaturated Fatty Acids on Cyclooxygenase-2 Metabolism in Brain-Metastatic Melanoma. J. Lipid Res. 2005, 46, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Groeger, A.L.; Cipollina, C.; Cole, M.P.; Woodcock, S.R.; Bonacci, G.; Rudolph, T.K.; Rudolph, V.; Freeman, B.A.; Schopfer, F.J. Cyclooxygenase-2 Generates Anti-Inflammatory Mediators from Omega-3 Fatty Acids. Nat. Chem. Biol. 2010, 6, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Stahl, A.; Chen, J.; Seaward, M.R.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Connor, K.M.; Aderman, C.M.; Liclican, E.; et al. 5-Lipoxygenase Metabolite 4-HDHA Is a Mediator of the Antiangiogenic Effect of ω-3 Polyunsaturated Fatty Acids. Sci. Transl. Med. 2011, 3, 69ra12. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Liu, A. The Role of the Tissue Omega-6/Omega-3 Fatty Acid Ratio in Regulating Tumor Angiogenesis. Cancer Metastasis Rev. 2013, 32, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Majidpoor, J.; Mortezaee, K. Steps in Metastasis: An Updated Review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, L.-Y.; Lai, S.; He, Y. Dihydroartemisinin Inhibits the Migration of Esophageal Cancer Cells by Inducing Autophagy. Oncol. Lett. 2020, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Khadge, S.; Thiele, G.M.; Sharp, J.G.; McGuire, T.R.; Klassen, L.W.; Black, P.N.; DiRusso, C.C.; Cook, L.; Talmadge, J.E. Long-Chain Omega-3 Polyunsaturated Fatty Acids Decrease Mammary Tumor Growth, Multiorgan Metastasis and Enhance Survival. Clin. Exp. Metastasis 2018, 35, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Kakkassery, V.; Wirtz, C.; Schargus, M.; Grisanti, S.; Tura, A.; Ranjbar, M.; Dick, H.B.; Reinehr, S.; Joachim, S.C. Epidermal Growth Factor Is Increased in Conjunctival Malignant Melanoma. Vivo Athens Greece 2021, 35, 3603–3612. [Google Scholar] [CrossRef]
- Turk, H.F.; Barhoumi, R.; Chapkin, R.S. Alteration of EGFR Spatiotemporal Dynamics Suppresses Signal Transduction. PLoS ONE 2012, 7, e39682. [Google Scholar] [CrossRef]
- Rogers, K.R.; Kikawa, K.D.; Mouradian, M.; Hernandez, K.; McKinnon, K.M.; Ahwah, S.M.; Pardini, R.S. Docosahexaenoic Acid Alters Epidermal Growth Factor Receptor-Related Signaling by Disrupting Its Lipid Raft Association. Carcinogenesis 2010, 31, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.; Baek, W.; Seo, Y.; Kim, J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells 2022, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast Cancer Exosomes Contribute to Pre-Metastatic Niche Formation and Promote Bone Metastasis of Tumor Cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Guan, J.; Yuan, Z.-C.; Lin, X.; Wu, Z.-J.; Liu, B.; He, J.-L. Expression and Predictive Value of miR-489 and miR-21 in Melanoma Metastasis. World J. Clin. Cases 2019, 7, 2930–2941. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Ghosh-Choudhury, T.; Dey, N.; Choudhury, G.G.; Ghosh-Choudhury, N. miR-21 Is Targeted by Omega-3 Polyunsaturated Fatty Acid to Regulate Breast Tumor CSF-1 Expression. Carcinogenesis 2012, 33, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.; Dumont, A.; Derangère, V.; Rébé, C.; de Rosny, C.; Causse, S.; Thomas, C.; Apetoh, L.; Hichami, A.; Ghiringhelli, F.; et al. Inhibition of Colon Cancer Growth by Docosahexaenoic Acid Involves Autocrine Production of TNFα. Oncogene 2016, 35, 4611–4622. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.-Y.; Arita, M.; Kim, K.; Li, X.; Zhang, H.; Kang, J.X. An Omega-3 Polyunsaturated Fatty Acid Derivative, 18-HEPE, Protects against CXCR4-Associated Melanoma Metastasis. Carcinogenesis 2018, 39, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Jie, Z. MiR-21 Promotes the Invasion and Metastasis of Gastric Cancer Cells by Activating Epithelial-Mesenchymal Transition. Eur. Surg. Res. 2019, 60, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Kerstin, N.; Calorini, L.; Mugnai, G.; Ruggieri, S. An Enhanced Apoptosis and a Reduced Angiogenesis Are Associated with the Inhibition of Lung Colonisation in Animals Fed an N-3 Polyunsaturated Fatty Acid-Rich Diet Injected with a Highly Metastatic Murine Melanoma Line. Br. J. Nutr. 2009, 101, 688–693. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. CXCL12/CXCR4/CXCR7 Chemokine Axis and Cancer Progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Aguayo, R.; Ramos, C.; Puentes, Á.; Gajardo, A.; Panieri, E.; Rojas-Solé, C.; Lillo-Moya, J.; Saso, L. Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Molecules 2021, 26, 5702. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Crescente, M.; Menke, L.; Chan, M.V.; Armstrong, P.C.; Warner, T.D. Eicosanoids in Platelets and the Effect of Their Modulation by Aspirin in the Cardiovascular System (and Beyond). Br. J. Pharmacol. 2019, 176, 988–999. [Google Scholar] [CrossRef]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478–495. [Google Scholar] [CrossRef]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef]
- Yum, H.-W.; Kim, S.H.; Kang, J.X.; Surh, Y.-J. Amelioration of UVB-Induced Oxidative Stress and Inflammation in Fat-1 Transgenic Mouse Skin. Biochem. Biophys. Res. Commun. 2018, 502, 1–8. [Google Scholar] [CrossRef]
- Cezar, T.L.C.; Martinez, R.M.; da Rocha, C.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with Maresin 1, a Docosahexaenoic Acid-Derived pro-Resolution Lipid, Protects Skin from Inflammation and Oxidative Stress Caused by UVB Irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef] [PubMed]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish Oil Omega-3 Polyunsaturated Fatty Acids Attenuate Oxidative Stress-Induced DNA Damage in Vascular Endothelial Cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, Y.; Ou, T.; Key, C.-C.C.; Tong, S.H.; Sequeira, R.C.; Nelson, J.M.; Nie, Y.; Wang, Z.; Boudyguina, E.; et al. Dietary PUFAs Attenuate NLRP3 Inflammasome Activation via Enhancing Macrophage Autophagy. J. Lipid Res. 2017, 58, 1808–1821. [Google Scholar] [CrossRef] [PubMed]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The Influence of Diet on Anti-Cancer Immune Responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Mohd Amin, M.C.I.; Yuen, N.P.; Zulfakar, M.H. Immunomodulatory Effectiveness of Fish Oil and Omega-3 Fatty Acids in Human Non-Melanoma Skin Carcinoma Cells. J. Oleo Sci. 2016, 65, 217–224. [Google Scholar] [CrossRef]
- Song, M.; Nishihara, R.; Cao, Y.; Chun, E.; Qian, Z.R.; Mima, K.; Inamura, K.; Masugi, Y.; Nowak, J.A.; Nosho, K.; et al. Marine ω-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer According to Tumor-Infiltrating T Cells. JAMA Oncol. 2016, 2, 1197–1206. [Google Scholar] [CrossRef]
- Kiana, A.K.; Bonetti, G.; Donato, K.; Kaftalli, J.; Herbst, K.L.; Stuppia, L.; Fioretti, F.; Nodari, S.; Perrone, M.; Chiurazzi, P.; et al. Polymorphisms, Diet and Nutrigenomics. J. Prev. Med. Hyg. 2022, 63, E125–E141. [Google Scholar] [CrossRef]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty Acids, Epigenetic Mechanisms and Chronic Diseases: A Systematic Review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The Multi-Factorial Nature of Clinical Multidrug Resistance in Cancer. Drug Resist. Updat. 2019, 46, 100645. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R.; Colomer, R. Exogenous Supplementation with Omega-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid (DHA; 22:6n-3) Synergistically Enhances Taxane Cytotoxicity and Downregulates Her-2/Neu (c-erbB-2) Oncogene Expression in Human Breast Cancer Cells. Eur. J. Cancer Prev. 2005, 14, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, A.; Kałucka, M.; Chodurek, E.; Wilczok, A. DHA but Not AA Enhances Cisplatin Cytotoxicity in Ovarian Cancer Cells. Nutr. Cancer 2018, 70, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, L.; Goupille, C.; Blanc, C.; Pinault, M.; Domingo, I.; Guimaraes, C.; Bougnoux, P.; Chevalier, S.; Mahéo, K. Long Chain N-3 Polyunsaturated Fatty Acids Increase the Efficacy of Docetaxel in Mammary Cancer Cells by Downregulating Akt and PKCε/δ-Induced ERK Pathways. Biochim. Biophys. Acta 2016, 1861, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Sturlan, S.; Baumgartner, M.; Roth, E.; Bachleitner-Hofmann, T. Docosahexaenoic Acid Enhances Arsenic Trioxide-Mediated Apoptosis in Arsenic Trioxide-Resistant HL-60 Cells. Blood 2003, 101, 4990–4997. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, M.; Gleissman, H.; Ponthan, F.; Castro, J.; Kogner, P.; Johnsen, J.I. Neuroblastoma Cell Death in Response to Docosahexaenoic Acid: Sensitization to Chemotherapy and Arsenic-Induced Oxidative Stress. Int. J. Cancer 2006, 118, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Corsetto, P.A.; Colombo, I.; Kopecka, J.; Rizzo, A.M.; Riganti, C. ω-3 Long Chain Polyunsaturated Fatty Acids as Sensitizing Agents and Multidrug Resistance Revertants in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2770. [Google Scholar] [CrossRef]
- Vibet, S.; Goupille, C.; Bougnoux, P.; Steghens, J.-P.; Goré, J.; Mahéo, K. Sensitization by Docosahexaenoic Acid (DHA) of Breast Cancer Cells to Anthracyclines through Loss of Glutathione Peroxidase (GPx1) Response. Free Radic. Biol. Med. 2008, 44, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting Multidrug Resistance in Cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Kodedová, M.; Sychrová, H. Changes in the Sterol Composition of the Plasma Membrane Affect Membrane Potential, Salt Tolerance and the Activity of Multidrug Resistance Pumps in Saccharomyces Cerevisiae. PLoS ONE 2015, 10, e0139306. [Google Scholar] [CrossRef]
- Zarubica, A.; Trompier, D.; Chimini, G. ABCA1, from Pathology to Membrane Function. Pflugers Arch. 2007, 453, 569–579. [Google Scholar] [CrossRef]
- Klappe, K.; Hummel, I.; Hoekstra, D.; Kok, J.W. Lipid Dependence of ABC Transporter Localization and Function. Chem. Phys. Lipids 2009, 161, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Thangapandian, S.; Kapoor, K.; Tajkhorshid, E. Probing Cholesterol Binding and Translocation in P-Glycoprotein. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183090. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, G.; Corsetto, P.A.; Campia, I.; Montorfano, G.; Kopecka, J.; Castella, B.; Gazzano, E.; Ghigo, D.; Rizzo, A.M.; Riganti, C. Omega 3 Fatty Acids Chemosensitize Multidrug Resistant Colon Cancer Cells by Down-Regulating Cholesterol Synthesis and Altering Detergent Resistant Membranes Composition. Mol. Cancer 2013, 12, 137. [Google Scholar] [CrossRef]
- Ottes Vasconcelos, R.; Serini, S.; de Souza Votto, A.P.; Santos Trindade, G.; Fanali, C.; Sgambato, A.; Calviello, G. Combination of ω-3 Fatty Acids and Cisplatin as a Potential Alternative Strategy for Personalized Therapy of Metastatic Melanoma: An in-Vitro Study. Melanoma Res. 2019, 29, 270. [Google Scholar] [CrossRef]
- Yang, C.-J.; Kuo, C.-T.; Wu, L.-H.; Chen, M.-C.; Pangilinan, C.R.; Phacharapiyangkul, N.; Liu, W.; Chen, Y.-H.; Lee, C.-H. Eicosapentaenoic Acids Enhance Chemosensitivity through Connexin 43 Upregulation in Murine Melanoma Models. Int. J. Med. Sci. 2019, 16, 636–643. [Google Scholar] [CrossRef]
- Crovella, S.; Ouhtit, A.; Rahman, S.M.; Rahman, M.M. Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line. Nutrients 2023, 15, 1658. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Kulkoyluoglu Cotul, E.; Chen, H.; Smith, A.; Libring, S.; Solorio, L.; Wendt, M.K. Transglutaminase-2 Mediates Acquisition of Neratinib Resistance in Metastatic Breast Cancer. Mol. Biomed. 2022, 3, 19. [Google Scholar] [CrossRef]
- Wu, C.-P.; Hung, C.-Y.; Murakami, M.; Wu, Y.-S.; Lin, C.-L.; Huang, Y.-H.; Hung, T.-H.; Yu, J.-S.; Ambudkar, S.V. P-Glycoprotein Mediates Resistance to the Anaplastic Lymphoma Kinase Inhiitor Ensartinib in Cancer Cells. Cancers 2022, 14, 2341. [Google Scholar] [CrossRef]
- Herman, J.F.; Mangala, L.S.; Mehta, K. Implications of Increased Tissue Transglutaminase (TG2) Expression in Drug-Resistant Breast Cancer (MCF-7) Cells. Oncogene 2006, 25, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA Is a More Potent Inhibitor of Breast Cancer Metastasis to Bone and Related Osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Fodil, M.; Blanckaert, V.; Ulmann, L.; Mimouni, V.; Chénais, B. Contribution of N-3 Long-Chain Polyunsaturated Fatty Acids to the Prevention of Breast Cancer Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 7936. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, S.G.; Monte, M.G.; Polegato, B.F.; Barbisan, L.F.; Romualdo, G.R. Protective Effects of Omega-3 Supplementation against Doxorubicin-Induced Deleterious Effects on the Liver and Kidneys of Rats. Molecules 2023, 28, 3004. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Khedr, R.; Ibrahim, A.M. Omega 3 Fatty Acids Can Reduce Early Doxorubicin-Induced Cardiotoxicity in Children with Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2022, 69, e29496. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.; Abdelbaset, M.; Hassan, A.; Sharaf, O.; Mahmoud, S.; Hegazy, R. Omega-3 Fatty Acids Ameliorate Doxorubicin-Induced Cardiorenal Toxicity: In-Vivo Regulation of Oxidative Stress, Apoptosis and Renal Nox4, and in-Vitro Preservation of the Cytotoxic Efficacy. PLoS ONE 2020, 15, e0242175. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Thorne, R.F.; Zhang, X.D.; Hersey, P. Regulation of Docetaxel-Induced Apoptosis of Human Melanoma Cells by Different Isoforms of Protein Kinase C. Mol. Cancer Res. MCR 2007, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Díaz Bessone, M.I.; Berardi, D.E.; Campodónico, P.B.; Todaro, L.B.; Lothstein, L.; Bal de Kier Joffé, E.D.; Urtreger, A.J. Involvement of PKC Delta (PKCδ) in the Resistance against Different Doxorubicin Analogs. Breast Cancer Res. Treat. 2011, 126, 577–587. [Google Scholar] [CrossRef]
- Goupille, C.; Vibet, S.; Frank, P.G.; Mahéo, K. EPA and DHA Fatty Acids Induce a Remodeling of Tumor Vasculature and Potentiate Docetaxel Activity. Int. J. Mol. Sci. 2020, 21, 4965. [Google Scholar] [CrossRef]
- Albino, A.P.; Juan, G.; Traganos, F.; Reinhart, L.; Connolly, J.; Rose, D.P.; Darzynkiewicz, Z. Cell Cycle Arrest and Apoptosis of Melanoma Cells by Docosahexaenoic Acid: Association with Decreased pRb Phosphorylation. Cancer Res. 2000, 60, 4139–4145. [Google Scholar]
- Yang, L.; Ritchie, A.-M.; Melton, D.W. Disruption of DNA Repair in Cancer Cells by Ubiquitination of a Destabilising Dimerization Domain of Nucleotide Excision Repair Protein ERCC1. Oncotarget 2017, 8, 55246–55264. [Google Scholar] [CrossRef]
- Zhou, P.; Qin, J.; Li, Y.; Li, G.; Wang, Y.; Zhang, N.; Chen, P.; Li, C. Combination Therapy of PKCζ and COX-2 Inhibitors Synergistically Suppress Melanoma Metastasis. J. Exp. Clin. Cancer Res. 2017, 36, 115. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, C.; Lentini, A.; Provenzano, B.; Gismondi, A.; Rossi, S.; Beninati, S. Similar Antineoplastic Effects of Nimesulide, a Selective COX-2 Inhibitor, and Prostaglandin E1 on B16-F10 Murine Melanoma Cells. Melanoma Res. 2010, 20, 273–279. [Google Scholar] [CrossRef]
- de Souza do Nascimento, J.; Carlos, R.; Delgado-Azañero, W.; Mosqueda Taylor, A.; de Almeida, O.P.; Romañach, M.J.; de Andrade, B.A.B. Immunohistochemical Expression of Cyclooxygenase-2 (COX-2) in Oral Nevi and Melanoma. J. Oral Pathol. Med. 2016, 45, 440–443. [Google Scholar] [CrossRef]
- Jafarian, A.H.; Mohamadian Roshan, N.; Gharib, M.; Moshirahmadi, V.; Tasbandi, A.; Ayatollahi, A.A.; Ayatollahi, H. Evaluation of Cyclooxygenase-2 Expression in Association with Clinical-Pathological Factors in Malignant Melanoma. Iran. J. Pathol. 2019, 14, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Fasano, E.; Piccioni, E.; Monego, G.; Cittadini, A.R.M.; Celleno, L.; Ranelletti, F.O.; Calviello, G. DHA Induces Apoptosis and Differentiation in Human Melanoma Cells in Vitro: Involvement of HuR-Mediated COX-2 mRNA Stabilization and β-Catenin Nuclear Translocation. Carcinogenesis 2012, 33, 164–173. [Google Scholar] [CrossRef]
- Chiu, L.C.M.; Tong, K.F.; Ooi, V.E.C. Cytostatic and Cytotoxic Effects of Cyclooxygenase Inhibitors and Their Synergy with Docosahexaenoic Acid on the Growth of Human Skin Melanoma A-375 Cells. Biomed. Pharmacother. 2005, 59 (Suppl. S2), S293–S297. [Google Scholar] [CrossRef]
- Serini, S.; Zinzi, A.; Ottes Vasconcelos, R.; Fasano, E.; Riillo, M.G.; Celleno, L.; Trombino, S.; Cassano, R.; Calviello, G. Role of β-Catenin Signaling in the Anti-Invasive Effect of the Omega-3 Fatty Acid DHA in Human Melanoma Cells. J. Dermatol. Sci. 2016, 84, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wellbrock, C.; Arozarena, I. Microphthalmia-Associated Transcription Factor in Melanoma Development and MAP-Kinase Pathway Targeted Therapy. Pigment Cell Melanoma Res. 2015, 28, 390–406. [Google Scholar] [CrossRef]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Bella, V.D.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional Roles of Matrix Metalloproteinases and Their Inhibitors in Melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Danbara, N.; Yuri, T.; Tsujita-Kyutoku, M.; Sato, M.; Senzaki, H.; Takada, H.; Hada, T.; Miyazawa, T.; Okazaki, K.; Tsubura, A. Conjugated Docosahexaenoic Acid Is a Potent Inducer of Cell Cycle Arrest and Apoptosis and Inhibits Growth of Colo 201 Human Colon Cancer Cells. Nutr. Cancer 2004, 50, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Di Nicuolo, F.; Serini, S.; Piccioni, E.; Boninsegna, A.; Maggiano, N.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic Acid Enhances the Susceptibility of Human Colorectal Cancer Cells to 5-Fluorouracil. Cancer Chemother. Pharmacol. 2005, 55, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative Stress in Melanogenesis and Melanoma Development. Contemp. Oncol. Poznan Pol. 2022, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Indra, A.K. Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies. Cancers 2023, 15, 3038. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meyskens, F.L.; Estrela, J.M. Oxidative Stress and Antioxidants in the Pathophysiology of Malignant Melanoma. Biol. Chem. 2019, 400, 589–612. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, Z.; Mohajeri, Z.; Dana, N.; Haghjooy Javanmard, S. The Dual Role of Nrf2 in Melanoma: A Systematic Review. BMC Mol. Cell Biol. 2023, 24, 5. [Google Scholar] [CrossRef]
- Xia, S.; Lu, Y.; Wang, J.; He, C.; Hong, S.; Serhan, C.N.; Kang, J.X. Melanoma Growth Is Reduced in Fat-1 Transgenic Mice: Impact of Omega-6/Omega-3 Essential Fatty Acids. Proc. Natl. Acad. Sci. USA 2006, 103, 12499–12504. [Google Scholar] [CrossRef]
- Yin, X.; Yu, X.-W.; Zhu, P.; Zhang, Y.-M.; Zhang, X.-H.; Wang, F.; Zhang, J.-J.; Yan, W.; Xi, Y.; Wan, J.-B.; et al. Endogenously Synthesized N-3 Fatty Acids in Fat-1 Transgenic Mice Prevent Melanoma Progression by Increasing E-Cadherin Expression and Inhibiting β-Catenin Signaling. Mol. Med. Rep. 2016, 14, 3476–3484. [Google Scholar] [CrossRef]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.A.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, M.; Rahimi, A.; Faramarzi, F.; Alizadeh-Navaei, R.; Rafiei, A. Overexpression of Chemokine Receptor CXCR4 Predicts Lymph Node Metastatic Risk in Patients with Melanoma: A Systematic Review and Meta-Analysis. Cytokine 2021, 148, 155691. [Google Scholar] [CrossRef] [PubMed]
- Siauciunaite, R.; Foulkes, N.S.; Calabrò, V.; Vallone, D. Evolution Shapes the Gene Expression Response to Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 3040. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Lai, C.-H.; Chen, M.-C.; Liu, C.-F.; Kuan, Y.-D.; Lin, S.-T.; Lee, C.-H. Salmonella Enhance Chemosensitivity in Tumor through Connexin 43 Upregulation. Int. J. Cancer 2013, 133, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-J.; Chang, M.-Y.; Chang, W.-W.; Wang, W.-K.; Liu, C.-F.; Lin, S.-T.; Lee, C.-H. Resveratrol Enhances Chemosensitivity in Mouse Melanoma Model Through Connexin 43 Upregulation. Environ. Toxicol. 2015, 30, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Im, D.-S. Functions of Omega-3 Fatty Acids and FFA4 (GPR120) in Macrophages. Eur. J. Pharmacol. 2016, 785, 36–43. [Google Scholar] [CrossRef]
- Nehra, D.; Pan, A.H.; Le, H.D.; Fallon, E.M.; Carlson, S.J.; Kalish, B.T.; Puder, M. DHA, G-Protein Coupled Receptors and Melanoma: Is GPR40 a Potential Therapeutic Target? J. Surg. Res. 2014, 188, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Hakozaki, M.; Uemura, A.; Yamashita, T. Effect of Fatty Acids on Melanogenesis and Tumor Cell Growth in Melanoma Cells. J. Lipid Res. 2019, 60, 1491–1502. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Tursun, M.; Hai, Y.; Tursun, H.; Mamtimin, B.; Hasim, A. Receptor for Activated C Kinase 1 Promotes Cervical Cancer Lymph Node Metastasis via the Glycolysis-dependent AKT/mTOR Signaling. Int. J. Oncol. 2022, 61, 83. [Google Scholar] [CrossRef]
- Otake, S.; Kobayashi, M.; Narumi, K.; Sasaki, S.; Kikutani, Y.; Furugen, A.; Watanabe, M.; Takahashi, N.; Ogura, J.; Yamaguchi, H.; et al. Regulation of the Expression and Activity of Glucose and Lactic Acid Metabolism-Related Genes by Protein Kinase C in Skeletal Muscle Cells. Biol. Pharm. Bull. 2013, 36, 1435–1439. [Google Scholar] [CrossRef]
- Salem, M.L.; Kishihara, K.; Abe, K.; Matsuzaki, G.; Nomoto, K. N-3 Polyunsaturated Fatty Acids Accentuate B16 Melanoma Growth and Metastasis through Suppression of Tumoricidal Function of T Cells and Macrophages. Anticancer Res. 2000, 20, 3195–3203. [Google Scholar]
- Mahamat-Saleh, Y.; Hughes, M.C.B.; Miura, K.; Malt, M.K.; von Schuckmann, L.; Khosrotehrani, K.; Smithers, B.M.; Green, A.C. Patterns of Omega-3 and Omega-6 Fatty Acid Dietary Intake and Melanoma Thickness at Diagnosis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Donat-Vargas, C.; Berglund, M.; Glynn, A.; Wolk, A.; Åkesson, A. Dietary Polychlorinated Biphenyls, Long-Chain n-3 Polyunsaturated Fatty Acids and Incidence of Malignant Melanoma. Eur. J. Cancer 2017, 72, 137–143. [Google Scholar] [CrossRef]
- Kirkpatrick, C.S.; White, E.; Lee, J.A. Case-Control Study of Malignant Melanoma in Washington State. II. Diet, Alcohol, and Obesity. Am. J. Epidemiol. 1994, 139, 869–880. [Google Scholar] [CrossRef]
- Fracasso, P.M.; Picus, J.; Wildi, J.D.; Goodner, S.A.; Creekmore, A.N.; Gao, F.; Govindan, R.; Ellis, M.J.; Tan, B.R.; Linette, G.P.; et al. Phase 1 and Pharmacokinetic Study of Weekly Docosahexaenoic Acid-Paclitaxel, Taxoprexin, in Resistant Solid Tumor Malignancies. Cancer Chemother. Pharmacol. 2009, 63, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; O’Donnell, A.; Scurr, M.; Reade, S.; Cole, C.; Judson, I.; Greystoke, A.; Twelves, C.; Kaye, S. Phase I/II Study of DHA-Paclitaxel in Combination with Carboplatin in Patients with Advanced Malignant Solid Tumours. Br. J. Cancer 2004, 91, 1651–1655. [Google Scholar] [CrossRef]
- Wolff, A.C.; Donehower, R.C.; Carducci, M.K.; Carducci, M.A.; Brahmer, J.R.; Zabelina, Y.; Bradley, M.O.; Anthony, F.H.; Swindell, C.S.; Witman, P.A.; et al. Phase I Study of Docosahexaenoic Acid-Paclitaxel: A Taxane-Fatty Acid Conjugate with a Unique Pharmacology and Toxicity Profile. Clin. Cancer Res. 2003, 9, 3589–3597. [Google Scholar] [PubMed]
- Yang, Y.; Xia, Y.; Zhang, B.; Li, D.; Yan, J.; Yang, J.; Sun, J.; Cao, H.; Wang, Y.; Zhang, F. Effects of Different N-6/n-3 Polyunsaturated Fatty Acids Ratios on Lipid Metabolism in Patients with Hyperlipidemia: A Randomized Controlled Clinical Trial. Front. Nutr. 2023, 10, 1166702. [Google Scholar] [CrossRef]
- van Elst, K.; Brouwers, J.F.; Merkens, J.E.; Broekhoven, M.H.; Birtoli, B.; Helms, J.B.; Kas, M.J.H. Chronic Dietary Changes in N-6/n-3 Polyunsaturated Fatty Acid Ratios Cause Developmental Delay and Reduce Social Interest in Mice. Eur. Neuropsychopharmacol. 2019, 29, 16–31. [Google Scholar] [CrossRef]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The Imbalance between N-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A Comprehensive Review and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.-J.; Lee, J.; Lee, J.K.; Byun, J.; Kim, I.; Ha, J.-H. Lowering N-6/n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in Ob/Ob Mice. Int. J. Mol. Sci. 2022, 23, 6384. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine N-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- GISSI-Prevenzione Investigators. Dietary Supplementation with N-3 Polyunsaturated Fatty Acids and Vitamin E after Myocardial Infarction: Results of the GISSI-Prevenzione Trial. Gruppo Italiano per Lo Studio Della Sopravvivenza Nell’Infarto Miocardico. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Balcos, M.C.; Kim, S.Y.; Jeong, H.; Yun, H.; Baek, K.J.; Kwon, N.S.; Park, K.; Kim, D. Docosahexaenoic Acid Inhibits Melanin Synthesis in Murine Melanoma Cells in Vitro through Increasing Tyrosinase Degradation. Acta Pharmacol. Sin. 2014, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Krämer, H.J.; Stevens, J.; Grimminger, F.; Seeger, W. Fish Oil Fatty Acids and Human Platelets: Dose-Dependent Decrease in Dienoic and Increase in Trienoic Thromboxane Generation. Biochem. Pharmacol. 1996, 52, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Javierre, C.; Vidal, J.; Segura, R.; Lizarraga, M.A.; Medina, J.; Ventura, J.L. The Effect of Supplementation with N-3 Fatty Acids on the Physical Performance in Subjects with Spinal Cord Injury. J. Physiol. Biochem. 2006, 62, 271–279. [Google Scholar] [CrossRef]
- Lien, E.L. Toxicology and Safety of DHA. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 125–132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Solé, C.; Torres-Herrera, B.; Gelerstein-Claro, S.; Medina-Pérez, D.; Gómez-Venegas, H.; Alzolay-Sepúlveda, J.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Cellular Basis of Adjuvant Role of n-3 Polyunsaturated Fatty Acids in Cancer Therapy: Molecular Insights and Therapeutic Potential against Human Melanoma. Appl. Sci. 2024, 14, 4548. https://doi.org/10.3390/app14114548
Rojas-Solé C, Torres-Herrera B, Gelerstein-Claro S, Medina-Pérez D, Gómez-Venegas H, Alzolay-Sepúlveda J, Chichiarelli S, Saso L, Rodrigo R. Cellular Basis of Adjuvant Role of n-3 Polyunsaturated Fatty Acids in Cancer Therapy: Molecular Insights and Therapeutic Potential against Human Melanoma. Applied Sciences. 2024; 14(11):4548. https://doi.org/10.3390/app14114548
Chicago/Turabian StyleRojas-Solé, Catalina, Benjamín Torres-Herrera, Santiago Gelerstein-Claro, Diego Medina-Pérez, Haziel Gómez-Venegas, Javier Alzolay-Sepúlveda, Silvia Chichiarelli, Luciano Saso, and Ramón Rodrigo. 2024. "Cellular Basis of Adjuvant Role of n-3 Polyunsaturated Fatty Acids in Cancer Therapy: Molecular Insights and Therapeutic Potential against Human Melanoma" Applied Sciences 14, no. 11: 4548. https://doi.org/10.3390/app14114548
APA StyleRojas-Solé, C., Torres-Herrera, B., Gelerstein-Claro, S., Medina-Pérez, D., Gómez-Venegas, H., Alzolay-Sepúlveda, J., Chichiarelli, S., Saso, L., & Rodrigo, R. (2024). Cellular Basis of Adjuvant Role of n-3 Polyunsaturated Fatty Acids in Cancer Therapy: Molecular Insights and Therapeutic Potential against Human Melanoma. Applied Sciences, 14(11), 4548. https://doi.org/10.3390/app14114548









