Component Effects in Binary Droplet Impact Behaviors on the Heated Plate: Comparison Study of Ethanol/Propanol and Ethanol/Water Droplets and Observation of Novel Bubble Shrinkage Phenomenon
Abstract
Featured Application
Abstract
1. Introduction
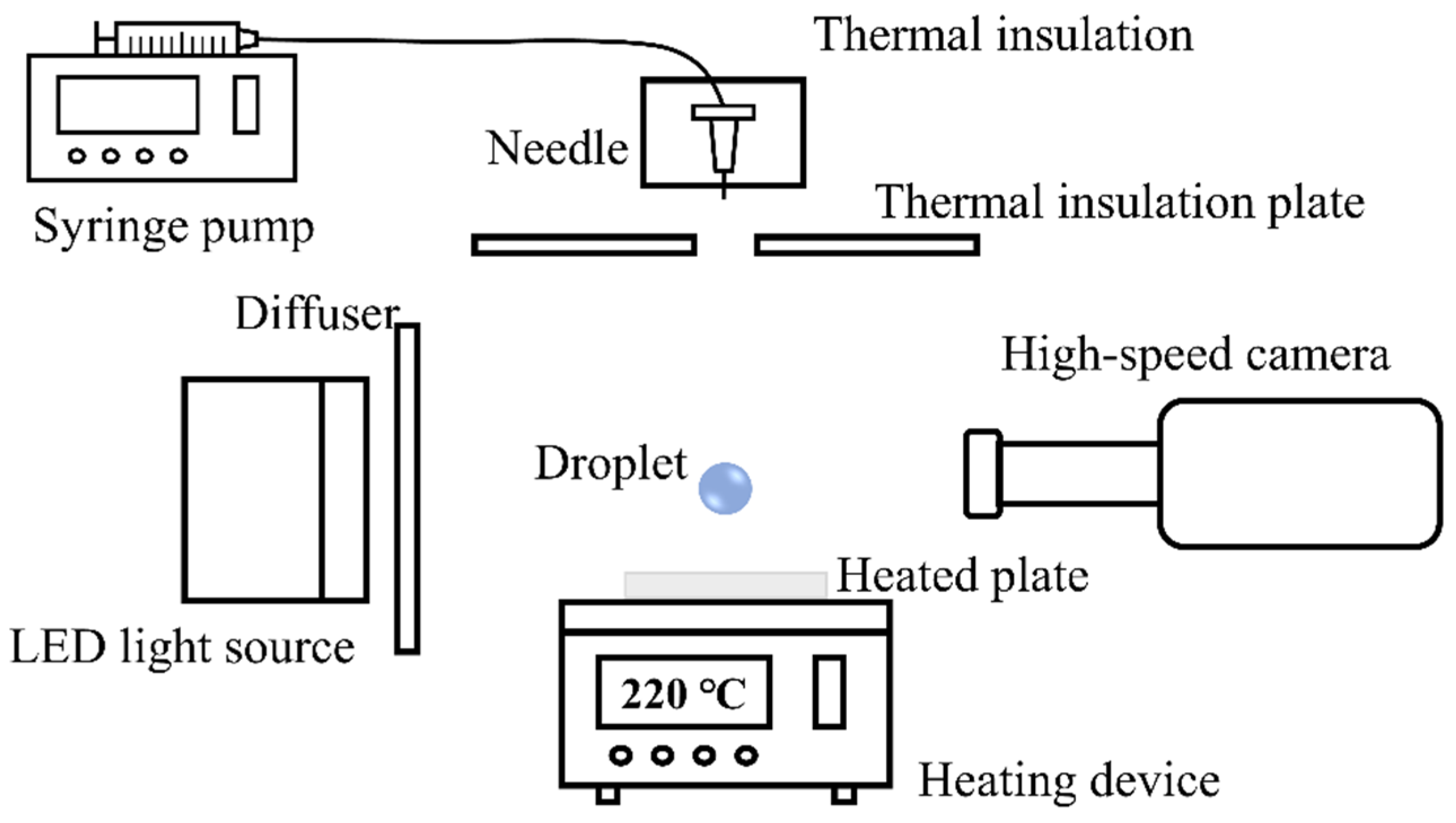
2. Materials and Methods
3. Results and Discussion
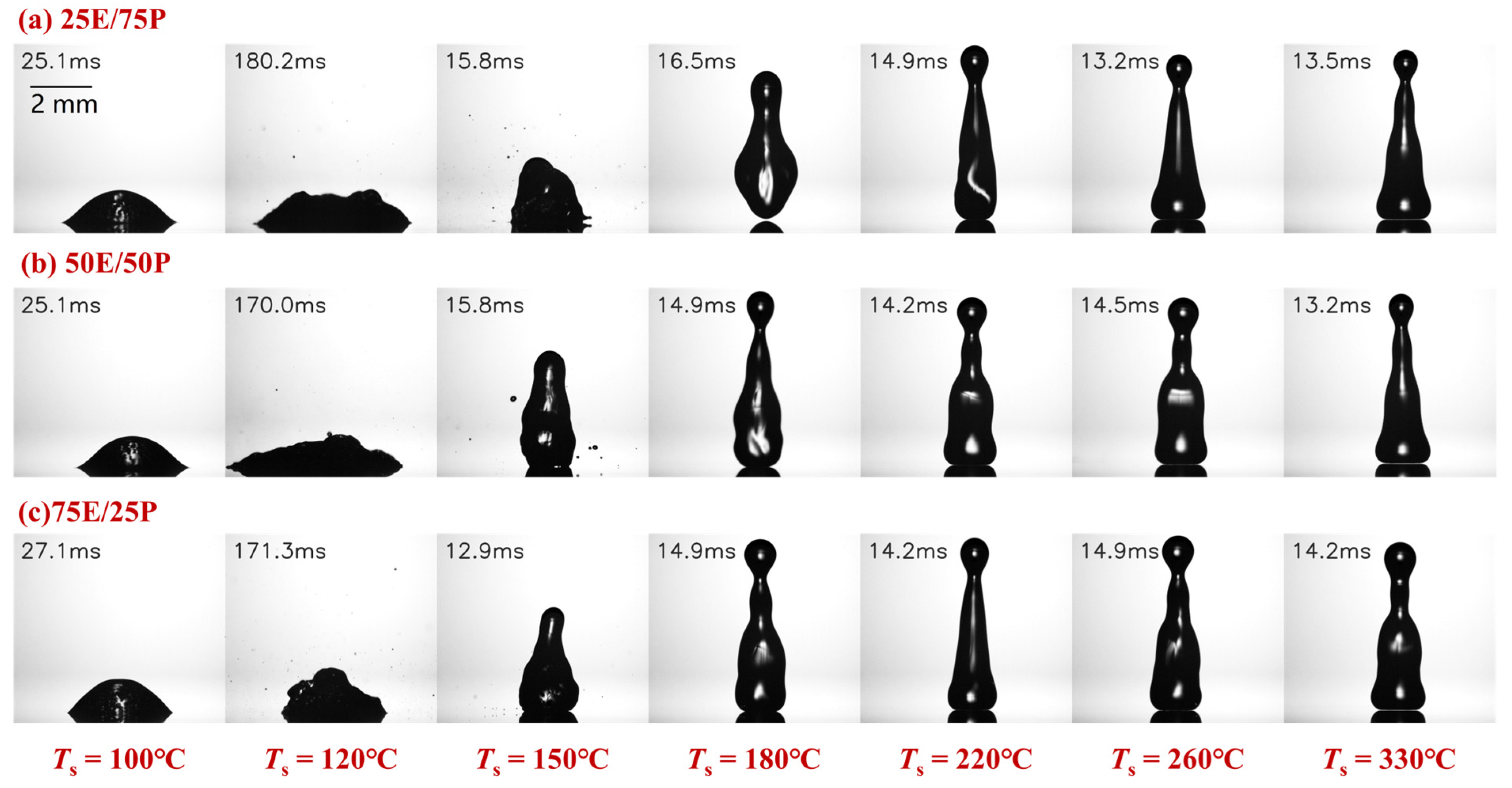
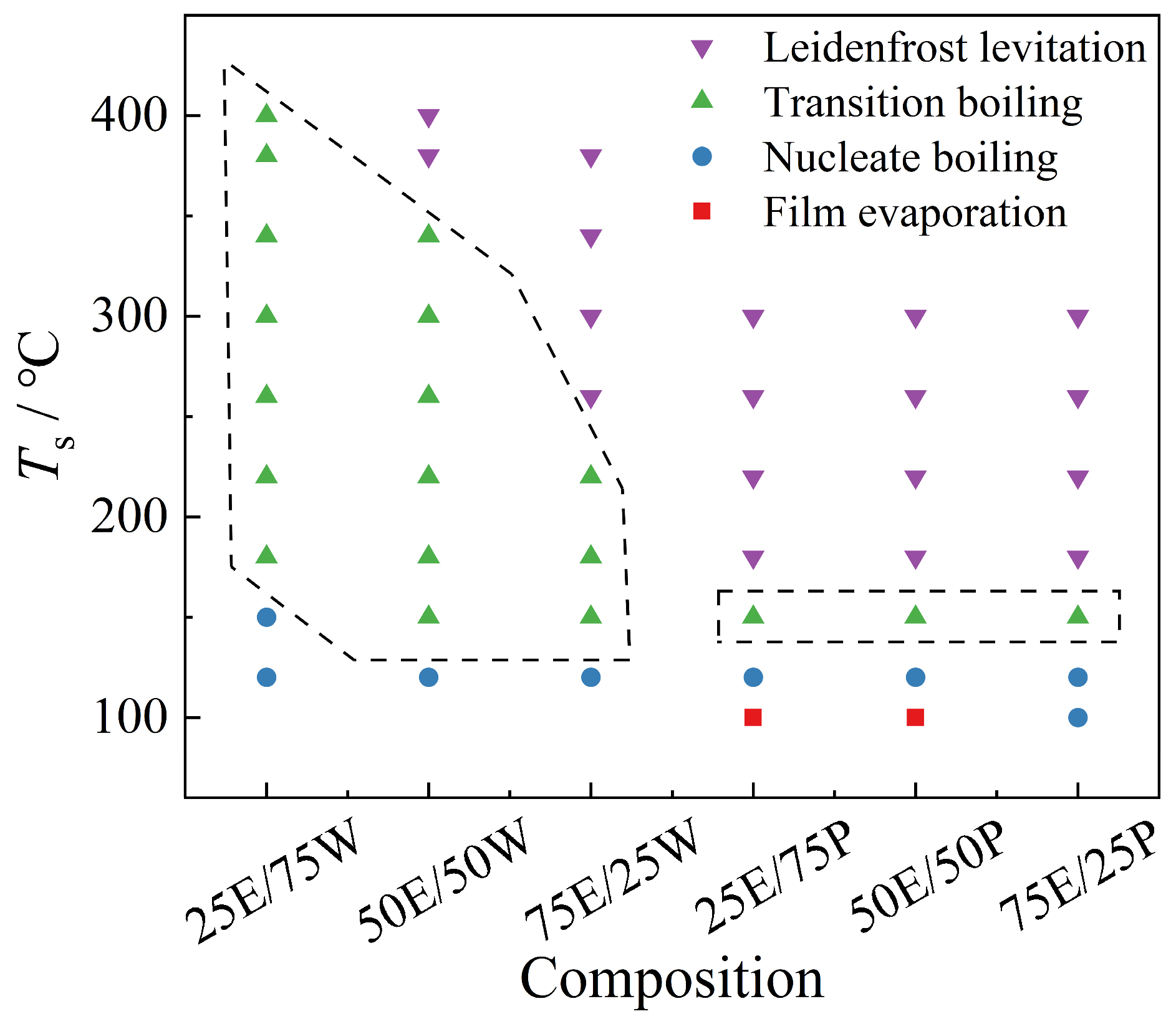
3.1. Morphology and Regime Map of Different Impact Behaviors
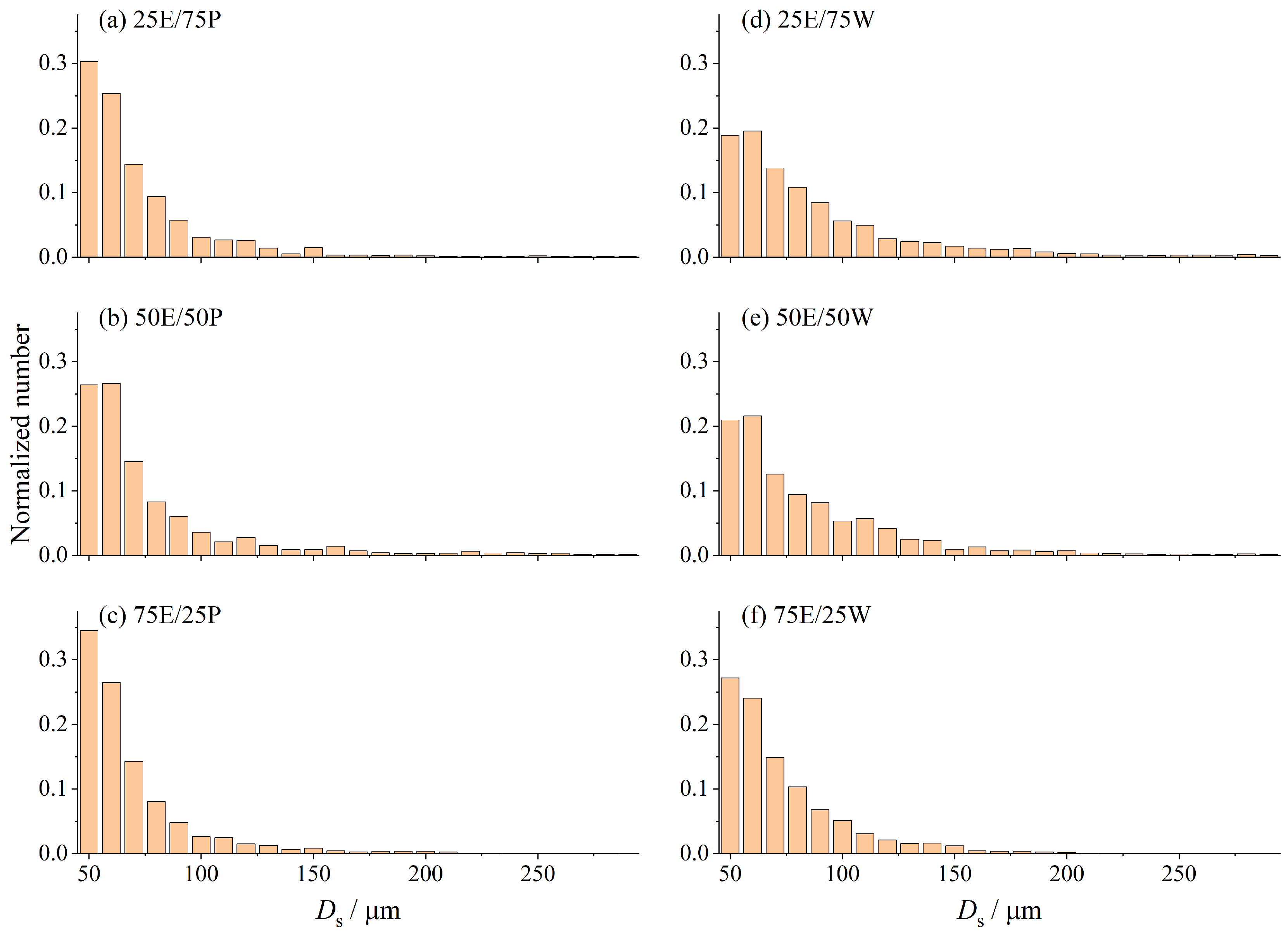
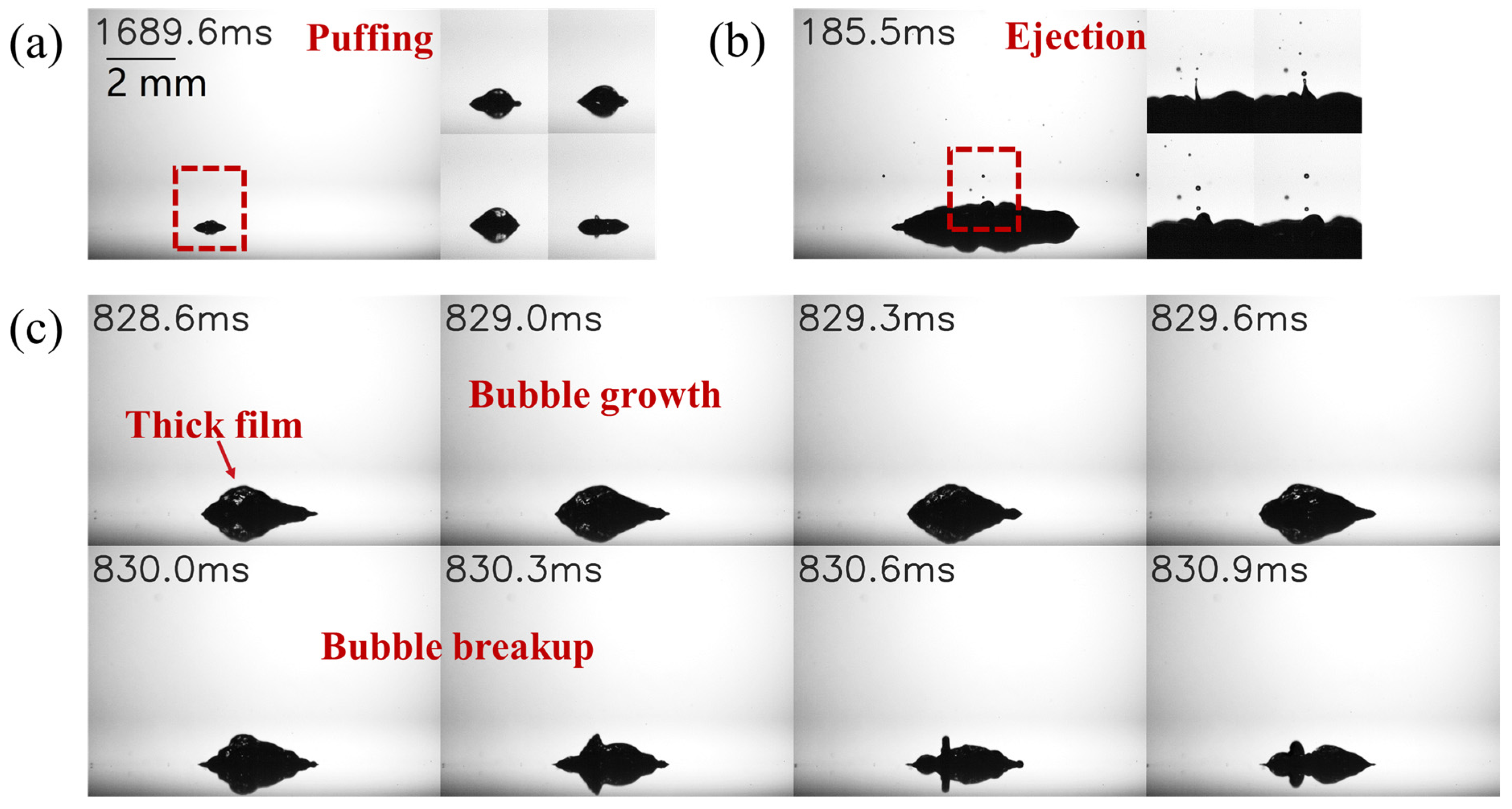
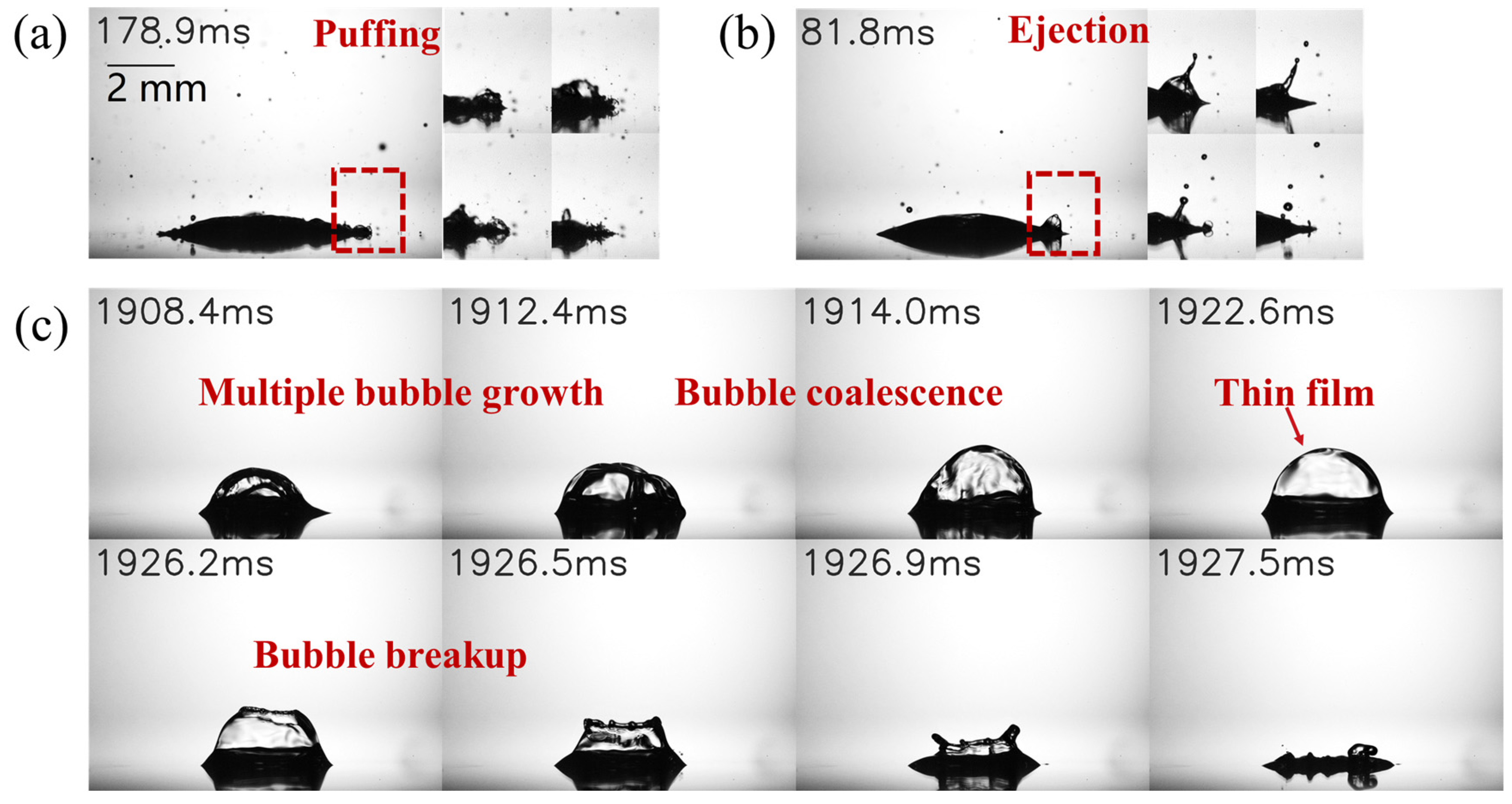
3.2. Characteristics of Satellite Droplet Formation and Bubble Dynamics
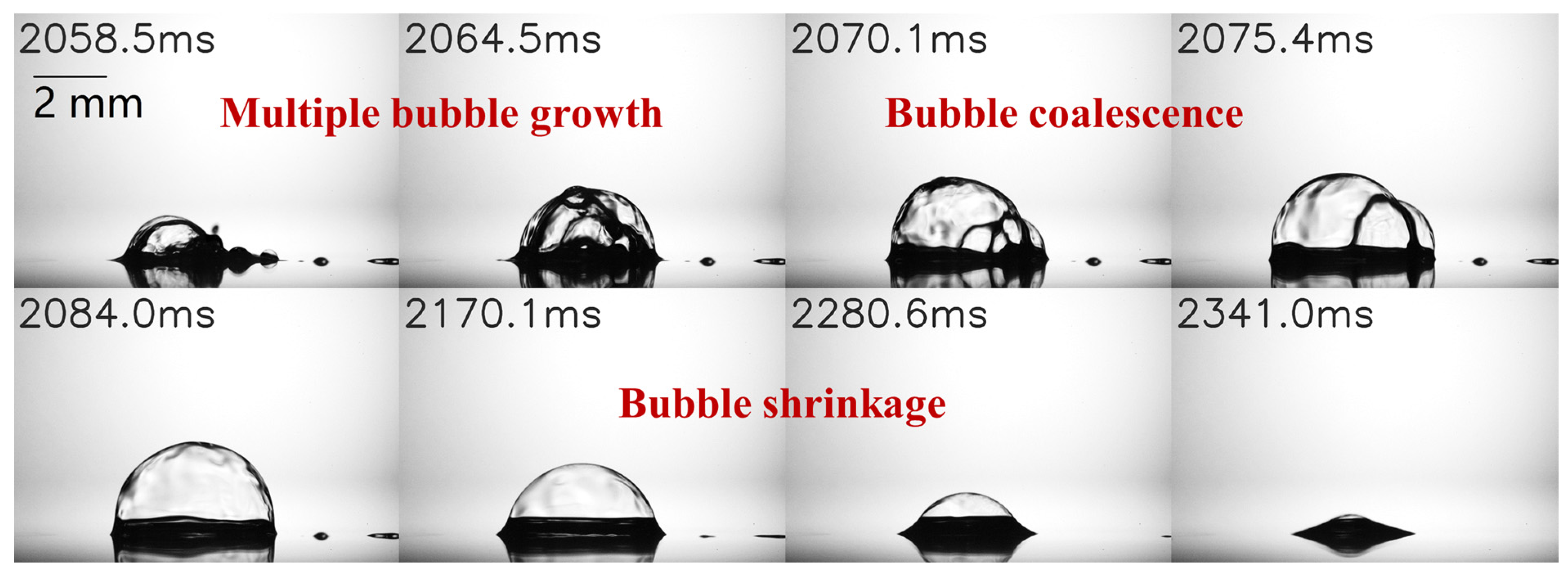
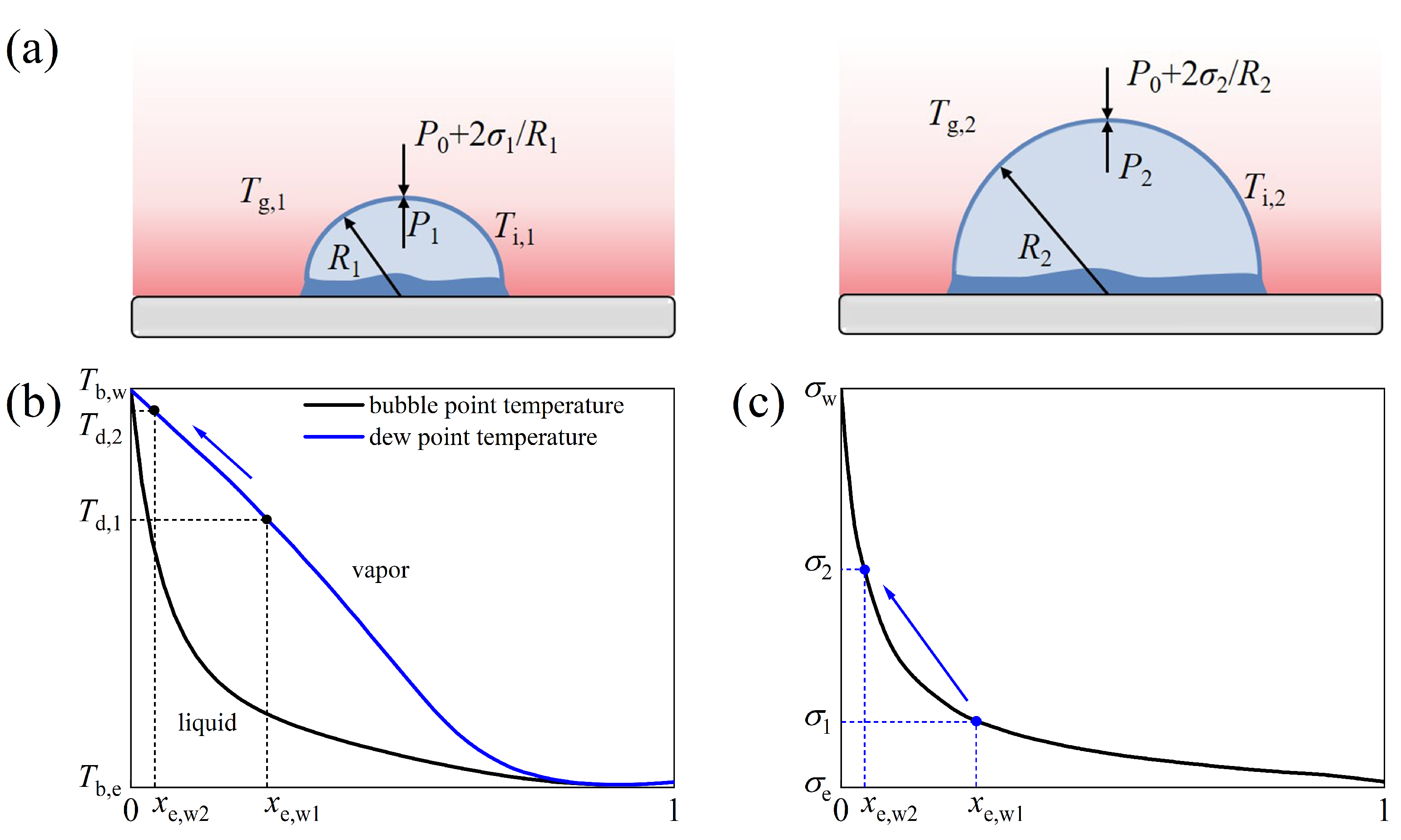
3.3. Observation and Preliminary Analysis of Novel Bubble Shrinkage Phenomenon
4. Conclusions
- Compared to ethanol/propanol binary droplets, the impact behavior of ethanol/water binary droplets exhibits greater diversity. The transition boiling regime for ethanol/water binary droplets is significantly extended and thus elevates the LFP temperature. However, the LFP temperature of the ethanol/propanol binary droplet exhibits minimal variation with increasing ethanol content. Ethanol/water binary droplets can significantly reduce the droplet residence time within a certain temperature range, but this reduction effect decreases with increasing ethanol content.
- During the secondary atomization process, ethanol/water binary droplets undergo a unique breakup of the parent droplet, leading to the formation of several fragment droplets with large diameters (Ds > 0.3 mm). Both types of binary droplets generate satellite droplets with smaller diameters (Ds < 0.3 mm) through mechanisms of puffing and ejection. Puffing tends to produce smaller satellite droplets and is more likely to occur in ethanol/propanol binary droplets. Conversely, ejection produces satellite droplets with larger diameters; this is more likely to occur in ethanol/water binary droplets. The distribution of the satellite droplet diameter for ethanol/water binary droplets is relatively wider, and the satellite droplet diameter decreases as the ethanol content increases. The satellite droplet diameter of the ethanol/propanol binary droplet is relatively small and undergoes minimal changes with an increase in ethanol content. This discrepancy is mainly attributed to the higher surface tension of the ethanol/water binary droplets.
- A novel large bubble shrinkage phenomenon is observed during the later stages of droplet evaporation when a 25E/75W binary droplet impacts on a heated wall at Ts = 120 °C. Different from the previously reported bubble shrinkage phenomenon in n-butanol/PODE4 binary droplet combustion [41,42], this work identifies a combination of three factors leading to bubble shrinkage. As the ethanol content decreases, there is an increase in the surface tension and saturation temperature of the binary mixture. Moreover, the ambient gas temperature above the bubble surface decreases with bubble growth. This causes an imbalance in the pressure inside and outside the bubble, which causes the bubble to shrink.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclatures
| ρl | Liquid density | [kg/m3] |
| cp,l | Specific heat of liquid | [kJ/(kg°C)] |
| cp,g | Specific heat of gas | [kJ/(kg°C)] |
| hfg | Liquid latent heat | [kJ/kg] |
| Tb | Liquid boiling point | [°C] |
| Tsat | Liquid saturation temperature | [°C] |
| Ts | Surface temperature | [°C] |
| TL | Leidenfrost temperature | [°C] |
| ΔT | Superheat of the droplet with respect to the wall | [°C] |
| ΔTbp | Boiling point difference | [°C] |
| Tg | Temperature of ambient gas | [°C] |
| Ti | Temperature of bubble interface | [°C] |
| D0 | Initial droplet diameter | [mm] |
| R0 | Initial droplet radius | [mm] |
| Ds | Diameter of secondary droplet | [μm] |
| V0 | Initial droplet velocity | [m/s] |
| tr | Residence time | [ms] |
| μ | Liquid viscosity | [mPa s] |
| σ | Surface tension | [mN] |
| R | Radius of curvature of the bubble | [mm] |
| P | Internal pressure of bubble | [atm] |
| P0 | Ambient pressure | [atm] |
| Φ | Content ratio | [%/%] |
| yi | Mass fraction of component i | |
| xi | Mole fraction of component i | |
| xi,j | Mole fraction of component i in the mixture of i and j | |
| θL | Dimensionless Leidenfrost temperature | |
| θb | Dimensionless boiling point |
References
- Josserand, C.; Thoroddsen, S.T. Drop impact on a solid surface. Annu. Rev. Fluid Mech. 2016, 48, 365–391. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop impact dynamics: Splashing, spreading, receding, bouncing…. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Moghtadernejad, S.; Lee, C.; Jadidi, M. An Introduction of Droplet Impact Dynamics to Engineering Students. Fluids 2020, 5, 107. [Google Scholar] [CrossRef]
- Ko, Y.S.; Chung, S.H. An experiment on the breakup of impinging droplets on a hot surface. Exp. Fluids 1996, 21, 118–123. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of drop impact on heated walls. Int. J. Heat Mass Transf. 2017, 106, 103–126. [Google Scholar] [CrossRef]
- Qin, M.; Guo, Y.; Tang, C.; Zhang, P.; Huang, Z. Spreading and bouncing of liquid alkane droplets upon impacting on a heated surface. Int. J. Heat Mass Transf. 2020, 159, 120076. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.E. Dynamic Leidenfrost behaviors of different fluid drops on superheated surface: Scaling for vapor film thickness. Phys. Fluids 2019, 31, 101702. [Google Scholar] [CrossRef]
- Cai, C.; Liu, H.; Chen, H.; Si, C. Alcohol-induced elevation in the dynamic Leidenfrost point temperature for water droplet impact. Int. J. Heat Mass Transf. 2023, 215, 124483. [Google Scholar] [CrossRef]
- Cai, C.; Si, C.; Liu, H.; Yin, H. Influence of alcohol additive and surface temperature on impact and spreading characteristics of a single water droplet. Int. J. Heat Mass Transf. 2021, 180, 121795. [Google Scholar] [CrossRef]
- Kwon, D.; Kang, D.; Yeom, E. Impact and boiling characteristics of an impinging ethanol drop on a heated Al alloy surface. Int. J. Heat Mass Transf. 2021, 169, 120927. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Chen, R. Cooling effect of droplet impacting on heated solid surface. Int. J. Heat Mass Transf. 2022, 183, 122070. [Google Scholar] [CrossRef]
- Staat, H.J.J.; Tran, T.; Geerdink, B.; Riboux, G.; Sun, C.; Gordillo, J.M.; Lohse, D. Phase diagram for droplet impact on superheated surfaces. J. Fluid Mech. 2015, 779, R3. [Google Scholar] [CrossRef]
- Peta, K.; Bartkowiak, T.; Rybicki, M.; Galek, P.; Mendak, M.; Wieczorowski, M.; Brown, C.A. Scale-dependent wetting behavior of bioinspired lubricants on electrical discharge machined Ti6Al4V surfaces. Tribol. Int. 2024, 194, 109562. [Google Scholar] [CrossRef]
- Kim, S.H.; Jiang, Y.; Kim, H. Droplet impact and LFP on wettability and nanostructured surface. Exp. Therm. Fluid Sci. 2018, 99, 85–93. [Google Scholar] [CrossRef]
- Saneie, N.; Kulkarni, V.; Fezzaa, K.; Patankar, N.A.; Anand, S. Boiling Transitions During Droplet Contact on Superheated Nano/Micro-Structured Surfaces. ACS Appl. Mater. Interfaces 2022, 14, 15774–15783. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Qiu, L.; Jin, J.; Sun, L.; Duan, F. Unique lift-off of droplet impact on high temperature nanotube surfaces. Appl. Phys. Lett. 2017, 111, 091605. [Google Scholar] [CrossRef]
- Van Limbeek, M.A.J.; Ramírez-Soto, O.; Prosperetti, A.; Lohse, D. How ambient conditions affect the Leidenfrost temperature. Soft Matter 2021, 17, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, R.; Zhang, X.; Xie, B.; Luo, X. Explosive bouncing on heated silicon surfaces under low ambient pressure. Soft Matter 2019, 15, 4320–4325. [Google Scholar] [CrossRef] [PubMed]
- Chausalkar, A.; Kweon, C.-B.M.; Kong, S.-C.; Michael, J.B. Leidenfrost behavior in drop-wall impacts at combustor-relevant ambient pressures. Int. J. Heat Mass Transf. 2020, 153, 119571. [Google Scholar] [CrossRef]
- Ithnin, A.M.; Yahya, W.J.; Ahmad, M.A.; Ramlan, N.A.; Abdul Kadir, H.; Sidik, N.A.C.; Koga, T. Emulsifier-free Water-in-Diesel emulsion fuel: Its stability behaviour, engine performance and exhaust emission. Fuel 2018, 215, 454–462. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Rastogi, P.; Krishnasamy, A.; Basavaraj, M.G.; Kaisare, N.; Aidhen, I.S. Synthesis and characterization of emulsion fuels –Implications to spray and engine studies. Prog. Energy Combust. Sci. 2024, 101, 101133. [Google Scholar] [CrossRef]
- Vellaiyan, S. Recent advancements in water emulsion fuel to explore efficient and cleaner production from various biodiesels: A retrospective review. Renew. Sustain. Energy Rev. 2023, 187, 113704. [Google Scholar] [CrossRef]
- Cai, C.; Mudawar, I. Review of the dynamic Leidenfrost point temperature for droplet impact on a heated solid surface. Int. J. Heat Mass Transf. 2023, 217, 124639. [Google Scholar] [CrossRef]
- Chen, P.; Toubal, M.; Carlier, J.; Harmand, S.; Nongaillard, B.; Bigerelle, M. Evaporation of Binary Sessile Drops: Infrared and Acoustic Methods To Track Alcohol Concentration at the Interface and on the Surface. Langmuir 2016, 32, 9836–9845. [Google Scholar] [CrossRef] [PubMed]
- Ouenzerfi, S.; Harmand, S.; Schiffler, J. Leidenfrost Self-Rewetting Drops. J. Phys. Chem. B 2018, 122, 4922–4930. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.M.; Kim, N.I. Breakup characteristics of a single-droplet of water-in-oil emulsion impinging on a hot surface. Fuel 2021, 291, 120191. [Google Scholar] [CrossRef]
- Piskunov, M.; Breitenbach, J.; Schmidt, J.B.; Strizhak, P.; Tropea, C.; Roisman, I.V. Secondary atomization of water-in-oil emulsion drops impinging on a heated surface in the film boiling regime. Int. J. Heat Mass Transf. 2021, 165, 120672. [Google Scholar] [CrossRef]
- Cen, C.; Wu, H.; Lee, C.-f.; Fan, L.; Liu, F. Experimental investigation on the sputtering and micro-explosion of emulsion fuel droplets during impact on a heated surface. Int. J. Heat Mass Transf. 2019, 132, 130–137. [Google Scholar] [CrossRef]
- Chausalkar, A.; Kong, S.-C.; Michael, J.B. Multicomponent drop breakup during impact with heated walls. Int. J. Heat Mass Transf. 2019, 141, 685–695. [Google Scholar] [CrossRef]
- Chausalkar, A.; Kweon, C.-B.M.; Michael, J.B. Multi-component fuel drop-wall interactions at high ambient pressures. Fuel 2021, 283, 119071. [Google Scholar] [CrossRef]
- Cai, C.; Chen, H.; Liu, H.; Si, C. Effect of iso-propanol additive on the impact dynamics of a Leidenfrost water droplet. Appl. Therm. Eng. 2023, 234, 121326. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, Y.; Hou, Y.; Feng, H.; Sun, L. Unique dynamics of water-ethanol binary droplets impacting onto a superheated surface with nanotubes. Int. J. Heat Mass Transf. 2021, 164, 120571. [Google Scholar] [CrossRef]
- Sen, U.; Roy, T.; Ganguly, R.; Angeloni, L.A.; Schroeder, W.A.; Megaridis, C.M. Explosive behavior during binary-droplet impact on superheated substrates. Int. J. Heat Mass Transf. 2020, 154, 119658. [Google Scholar] [CrossRef]
- NIST. Standard Reference Database-REFPROP, Version 8.0; NIST: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. The Properties of Gases and Liquids; Mcgraw-Hill: New York, NY, USA, 2001; Volume 5. [Google Scholar]
- Gmehling, J. (Ed.) Ethanol-Water Mixture Surface Tension: Datasheet from “Dortmund Data Bank (DDB)—Thermophysical Properties Edition 2014” in Springer Materials; Springer: Berlin/Heidelberg, Germany; DDBST GmbH: Oldenburg, Germany, 2010; Available online: https://materials.springer.com/thermophysical/docs/msft_c11c174 (accessed on 20 January 2023).
- Mundo, C.; Sommerfeld, M.; Tropea, C. Droplet-wall collisions: Experimental studies of the deformation and breakup process. Int. J. Multiph. Flow 1995, 21, 151–173. [Google Scholar] [CrossRef]
- Yang, X.; Huang, B.; Zhang, Y.; Lian, T.; Luo, L.; Li, Y. Characterizing boiling behaviors in water/ethanol binary droplet impact on a heated plate. Exp. Therm. Fluid Sci. 2024, 156, 111224. [Google Scholar] [CrossRef]
- Pacheco-Vázquez, F.; Ledesma-Alonso, R.; Palacio-Rangel, J.L.; Moreau, F. Triple Leidenfrost Effect: Preventing Coalescence of Drops on a Hot Plate. Phys. Rev. Lett. 2021, 127, 204501. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, X.; Zhang, Y.; Zhang, H.; Li, W.; Li, Y. Characterizing combustion and atomization of PODEn and ethanol/PODEn binary droplets. Fuel 2023, 341, 127672. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.; Zhang, Y.; Zhang, H.; Li, W.; Li, Y. Characterizing bubble dynamics in combustion and atomization of alcohol/PODE4 and alcohol/n-undecane binary droplets. Fuel 2023, 352, 129029. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Y.; Yang, X.; Zhang, H.; Li, W.; Li, Y. Novel bubble shrinkage phenomenon in n-butanol/PODE4 binary droplet combustion. Combust. Flame 2024, 259, 113125. [Google Scholar] [CrossRef]


| Properties | Ethanol | Propanol | Water |
|---|---|---|---|
| ρl [kg/m3] | 785.92 | 799.8 | 997.07 |
| cp,l [kJ/(kg°C)] | 2.439 | 2.678 | 4.184 |
| hfg [kJ/kg] | 922.79 | 765.68 | 2435.12 |
| Tb [°C] | 78.31 | 97.15 | 100.02 |
| σ [mN] | 21.7 | 26.3 | 72.1 |
| μ [mPa s] | 1.077 | 1.89 | 0.9125 |
| Term | *1 25E/75P | 50E/50P | 75E/25P | 25E/75W | 50E/50W | 75E/25W |
|---|---|---|---|---|---|---|
| ΔTbp [°C] | 21.71 | 18.84 | ||||
| Φ | 25%/75% | 50%/50% | 75%/25% | 25%/75% | 50%/50% | 75%/25% |
| ρl [kg/m3] | 796.33 | 792.86 | 789.39 | 944.28 | 891.50 | 838.71 |
| cp,l [kJ/(kg°C)] | 2.619 | 2.560 | 2.500 | 3.821 | 3.415 | 2.958 |
| hfg [kJ/kg] | 804.44 | 843.55 | 882.99 | 2120.44 | 1768.50 | 1372.26 |
| *2 Tsat [°C] | 90.9 | 85.58 | 81.53 | 86.6 | 82.3 | 79.9 |
| σ [mN] | 24.9 | 23.7 | 22.6 | 39.5 | 30.2 | 25.5 |
| D0 [mm] | 2.20 | 2.21 | 2.20 | 2.34 | 2.27 | 2.24 |
| V0 [m/s] | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| We | 34.4 | 36.2 | 37.6 | 27.4 | 32.8 | 36.1 |
| Bo | 0.379 | 0.400 | 0.413 | 0.321 | 0.373 | 0.404 |
| Properties | Ethanol | Propanol | Water |
|---|---|---|---|
| Tb [°C] | 78.31 | 97.15 | 100.02 |
| cp,g [kJ/(kg°C)] | 1.7174 | 1.7225 | 2.08 |
| hfg [kJ/kg] | 849.91 | 670.211 | 2256.40 |
| *TL [°C] | 162.8 | 179.5 | 217.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Huang, B.; Zhang, Y.; Li, Y. Component Effects in Binary Droplet Impact Behaviors on the Heated Plate: Comparison Study of Ethanol/Propanol and Ethanol/Water Droplets and Observation of Novel Bubble Shrinkage Phenomenon. Appl. Sci. 2024, 14, 4459. https://doi.org/10.3390/app14114459
Yang X, Huang B, Zhang Y, Li Y. Component Effects in Binary Droplet Impact Behaviors on the Heated Plate: Comparison Study of Ethanol/Propanol and Ethanol/Water Droplets and Observation of Novel Bubble Shrinkage Phenomenon. Applied Sciences. 2024; 14(11):4459. https://doi.org/10.3390/app14114459
Chicago/Turabian StyleYang, Xiaoyuan, Bingyao Huang, Yi Zhang, and Yuyang Li. 2024. "Component Effects in Binary Droplet Impact Behaviors on the Heated Plate: Comparison Study of Ethanol/Propanol and Ethanol/Water Droplets and Observation of Novel Bubble Shrinkage Phenomenon" Applied Sciences 14, no. 11: 4459. https://doi.org/10.3390/app14114459
APA StyleYang, X., Huang, B., Zhang, Y., & Li, Y. (2024). Component Effects in Binary Droplet Impact Behaviors on the Heated Plate: Comparison Study of Ethanol/Propanol and Ethanol/Water Droplets and Observation of Novel Bubble Shrinkage Phenomenon. Applied Sciences, 14(11), 4459. https://doi.org/10.3390/app14114459






