Abstract
In this study, we demonstrate the direct preparation of dihalo-γ-lactams featuring two distinct halogens from dichloroamides using a novel atom exchange radical cyclization (AERC) procedure. This method integrates the established atom transfer radical cyclization (ATRC) with halogen exchange in solution. The technique operates under mild conditions and requires small amounts of metallic copper, serving as both a supplemental activator and reducing agent.
1. Introduction
Thanks to the reactivity of open-shell organic compounds, radical synthetic methodologies often require a low energy input. Atom transfer radical techniques provide the additional benefit of a high atom economy, achieved by terminating the generated radical intermediates with halogen atoms, thereby preserving the initially present functionality. These properties can be exploited to reach a high efficiency and sustainability in the synthesis of new molecular or polymeric targets.
The described reactivity is typically controlled using a catalytic amount of a transition metal complex, capable to oversee the generation and termination of the intermediate radical species, resulting in high product selectivities.
Among the various atom transfer radical techniques, atom transfer radical polymerization (ATRP) stands out as the most widely used today [1]. ATRP enables the construction of polymeric materials with precise control, i.e., narrow polydispersity, and halogen-capped chain ends. In addition, other atom transfer radical techniques applied to the synthesis of low molecular weight targets are also well known. These include atom transfer radical addition (ATRA) and atom transfer radical cyclization (ATRC) processes [2], which provide an environmental-friendly route to industrially appealing building blocks or pharmaceuticals.
A classic example is the transition metal-catalyzed ATRC of N-allyl-2,2-dichloroamides, which enables access to functionalized specialty chemicals [3]. The process starts with the reversible interaction between the substrate (e.g., 1 of Figure 1) and the metal catalyst, composed of a transition metal salt (e.g., CuCl) and a nitrogen polydentate ligand (L, e.g., tris(2-pyridylmethyl)amine, TPMA).
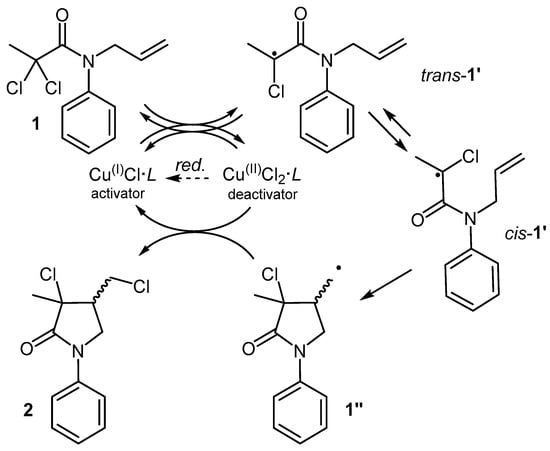
Figure 1.
Mechanism for the transition metal catalyzed ATRC of 1.
Through a monoelectronic halogen transfer, the active catalyst Cu(I)Cl·L is oxidized to Cu(II)Cl2·L, generating the radical 1′. This species can be considered electrophilic, since the radical-bearing carbon is surrounded by electron-withdrawing groups (EWGs). Featuring a low-lying SOMO, its interaction with the electron-rich alkenyl moiety is anticipated to be rapid, particularly when an appropriate auxiliary group on the nitrogen atom (e.g., Ph) induces a favorable conformational push from trans-1′ to cis-1′ [4], leading to a fast conversion towards the cyclic radical 1″. The absence of EWGs near the radical center in 1″ renders it nucleophilic, thus slowing down its intermolecular addition to other substrate molecules. This allows the use of less solvent while maintaining a high productivity and a high selectivity towards the cyclic product, even when working at molar concentrations. Typically, 1″ exhibits a high affinity for the oxidized form of the metal catalyst, which acts as a persistent radical [5]. This interaction not only leads to the conversion of 1″ into product 2, but also lets the regeneration of the metal catalyst in its active form. This irreversible regeneration step suggests that the radical cycle may be sustained by a very low amount of catalyst but, in practice, inevitable termination events lead to the progressive build-up of the oxidized form of the catalyst. Thus, to achieve complete conversion, higher amounts of metal catalysts are employed, typically around 10 mol%.
To further improve reaction control and sustainability, a proper reducing agent (“red.” in Figure 1) can be added to reactivate the spent catalyst. Among the various alternatives, the activators regenerated by electron transfer (ARGET) process, which employs non-radical reducing agents, is well developed and can work with very low metal loads [6]. Thus, the ARGET-ATRC of easily assembled dichloroamides offers an effective and sustainable route to dichloro-γ-lactams. These compounds represent advanced intermediates found within various valuable targets, such as anti-hypertensives [7], psychotropic agents [8], proteolysis inhibitors [9], antimuscarinic agents [10], and herbicides [11,12].
The numerous functional groups within the backbone of dichloro-γ-lactams readily explain their extensive synthetic flexibility. An example of this is the well-known elimination/substitution sequence (solid arrow in the upper Figure 2), which provides access to maleimides or their related hydrolysis products, i.e., maleic anhydrides [13]. The notable acidity of the hydrogen atom in position 4 of these structures (A, Figure 2) make them highly susceptible to first undergo elimination of hydrogen halide, a feature that was exploited for the preparation of unsaturated pyrrolidin-2-ones (B) [14].
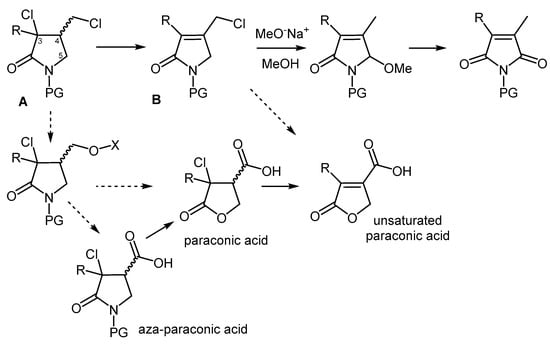
Figure 2.
Some synthetic applications of dichloro-γ-lactams.
In addition to the described transformations, the selective substitution of the exocyclic halogen atom of A holds significant potential. Specifically, the use of oxygen nucleophiles shows promise for generating higher oxidation state structures, including paraconic acids, aza-paraconic acids, and their unsaturated counterparts (dashed arrows in the lower Figure 2), which represent valuable bioactive targets.
In an attempt to implement such a transformation on 2 (Figure 1), our initial approach was to evaluate several oxidative substitution protocols suitable for neutral or low-basicity environments, aiming to circumvent the described 3,4 elimination. Unfortunately, potentially suitable methods such as the Kornblum [15], H2O2/EtOH-based [16,17], H2O2/AlCl3-based [18], or trimethylamine N-oxide-based systems [19], all failed to yield a clean exocyclic substitution in our experiments.
The replacement of the exocyclic chlorine atom with a more efficient leaving group such as iodine or the more atom-economical bromine was anticipated to yield improved outcomes. A bibliographic search revealed that achieving a dihalo-γ-pyrrolidinone with a more activated halide on the exocyclic side could be accomplished through various synthetic avenues: (i) an ATRC of a chlorobromoamide, (ii) a selective halogen exchange process on the exocyclic chlorine atom [20], or (iii) proper trapping of the cyclic radical 1″ (Figure 1). The first approach is hindered by the non-trivial preparation of the dihaloamide [14], while the second option may warrant further investigation. However, among the three methods, the last one shows the highest potential in terms of sustainability, due to its ability to exploit the intrinsic reactivity of 1″. An already known approach involved the addition of a Kharasch radical trap [21], but its application is hampered by the use of toxic tetrahalomethanes.
Therefore, the aim of this study is to evaluate the feasibility of leveraging the innate affinity of 1″ towards the metal complex catalyst, along with the option to modify the composition of the latter through some inorganic halides added in solution, according to some pertinent studies related to ATRP [22]. This should enable an overall atom exchange radical cyclization (AERC) process.
2. Materials and Methods
2.1. General
Reagents and solvents were reagent-grade products and were used without further purification. For the catalytic systems we used: CuCl2 (≥97%), TPMA (98%), and ascorbic acid (>99.5%) from Merck, and Na2CO3 from Carlo Erba (>99.5%). The starting amides 1 and 3 were prepared from dichloroacyl chlorides, through condensation with alkyl, allyl amines (obtained through N-alkylation [23]). 2,2-Dichloropropanoyl chloride was prepared from commercial propanoyl chloride, as described in ref. [24]. Dichlorolactam 2 [25] and trichlorolactam 5 [26] are known compounds. Silica Gel 60 from Merck (40–63 µm) was employed for flash chromatography purifications.
Elemental analyses (EA) were performed with a Thermo Scientific FLASH 2000 organic elemental analyzer. GC-MS spectra were acquired with a ‘HP G1800C GCD System Series II’ (Agilent Technologies Italy, Cernusco sul Naviglio, Italy). IR spectra were acquired with a FTIR-4700LE spectrometer (Jasco Europe, Cremella, Italy).
The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 600 spectrometer (Billerica, MA, USA). All the mono- and bi-dimensional experiments were performed with standard pulses programs. The 1H NMR and 13C NMR signals attribution was based on 1H,1H-DQF-COSY, 1H,13C-EditedHSQC, and 1H,13C-HMBC experiments. The structural assignment of compounds 3 and 6 were determined by homonuclear nuclear Overhauser enhancement NMR correlation techniques. Carbon signals were detectable and assigned only to major products.
2.2. ARGET-ATRC towards 2
In an oven-dried Schlenk tube (previously rinsed with 10% aqueous NH4OH and water in sequence), ascorbic acid (0.2 mmol, 35.2 mg), Na2CO3 (0.4 mmol, 42.4 mg), and amide 1 (8 mmol, 2.065 g) were accurately weighted. After three cycles of vacuum/argon (around 10 min, each), ethyl acetate (AcOEt, 3 mL) was added. Once the substrate had fully dissolved, a 0.04 mol/L solution of CuCl2/TPMA in absolute ethanol (EtOH, 1 mL) was introduced under an argon atmosphere. The reaction mixture was heated at 35 °C and stirred at 700 rpm for 8 h. The reaction was then quenched with water (8 mL), acidified with 10% aqueous HCl and extracted with CH2Cl2 (3 × 6 mL). The combined organic layers were concentrated to dryness at the rotary evaporator, and the crude product was purified by flash chromatography on silica gel, eluting with a petroleum ether (bp 40–60 °C)/diethyl ether (Et2O) gradient (from 100/0 to 0/100). This gave the pyrrolidinone 2 as a colorless oil (94%, 1.941 g), and as an inseparable mixture of cis/trans diastereomers (87:13).
2.3. SARA-AERC towards 3 or 6
Preparation of 3. In an oven-dried Schlenk tube (previously rinsed with 10% aqueous NH4OH and water in sequence), Na2CO3 (0.2 mmol, 21 mg), NaBr (4 mmol, 0.412 g), amide 1 (4 mmol, 1.032 g), and metallic copper wire (length 40 mm, diameter 1 mm, 99.9%) were inserted. After three cycles of vacuum/argon (around 10 min, each), AcOEt (2 mL), EtOH (96 vol%, 1 mL), and TPMA (1 mL of 0.04 mol/L solution in absolute ethanol) were added under an argon atmosphere. The Schlenk tube was heated at 37 °C and stirred at 700 rpm for 16 h. The reaction was then quenched with water (6 mL), acidified with 10% aqueous HCl (1 mL) and extracted with CH2Cl2 (3 × 5 mL). The combined organic layers were concentrated to dryness at the rotary evaporator, giving the crude product 3 as a brownish solid (95%, 3.8 mmol, 1.150 g), which resulted contaminated by a small amount (~5% GC) of ATRC dichlorolactam 2, and a small amount (~5% GC) of isomeric dibromolactam 3b (see Figure 3 and Figure S1). A purer version of 3 was obtained through (unoptimized) recrystallization from a mixture of CH2Cl2:toluene:Et2O in a 65:15:20 ratio, giving a beige solid with an 82% yield, as an inseparable mixture of cis/trans diastereomers (97:3).
Compound 3: (cis-isomer, 97%): 1H NMR (600 MHz, CDCl3): δ = 1.86 (s, 3 H, CH3), 2.75 (m, 1 H, CH), 3.65 (pq, J = 9.4 Hz, 2 H, CHHBr, CHHNCO), 3.71 (pdd, J = 4.74, 10.4 Hz, 1 H, CHHBr), 4.02 (pdd, J = 6.9, 9.9 Hz, 1 H, CHHNCO), 7.21 (pt, J = 7.4 Hz, 1 H, p-ArH), 7.40 (pt, J = 7.6 Hz, 2 H, m-ArH), 7.65 (pd, J = 8.1 Hz, 2H, o-ArH) ppm. 13C NMR (150 MHz, CDCl3): δ = 25.0 (CH3), 29.2 (CH2Br), 47.3 (CH), 50.6 (CH2NCO), 120.3 (o-ArC), 125.7 (p-ArC), 129.2 (m-ArC), 138.7 (N-ArC), 170.0 (NCO) ppm. (trans-isomer, 3%): 1H NMR (600 MHz, CDCl3): δ = 1.72 (s, 3 H, CH3), 3.15 (m, 1 H, CH), 3.36 (t, J = 10.4 Hz, 1 H, CHHBr), 3.69 (m, 1 H, CHHNCO), 4.04 (m, 1 H, CHHBr), 4.18 (pdd, J = 6.8, 10.1 Hz, 1 H, CHHNCO), 7.21 (pt, J = 7.4 Hz, 1 H, p-ArH), 7.40 (pt, J = 7.6 Hz, 2 H, m-ArH), 7.65 (pd, J = 8.1 Hz, 2H, o-ArH) ppm.
- EA found: C 47.7 H 4.2 N 4.8; calcd for (C12H13NOClBr): C 47.63 H 4.33 N 4.63
- MS molecular peak [3]+ isotopic pattern: calcd m/z = 300.9 (76.8%); 301.9 (10.4%); 302.9 (100.0%); 303.9 (13.5%); 304.9 (24.9%); 305.9 (3.3%); 306.9 (0.3%). Found m/z = 300.9 (76.2%); 301.9 (10.6%); 302.9 (100.0%); 303.9 (13.9%); 304.9 (25.2%); 305.9 (3.0%).
Preparation of 6. In an oven-dried Schlenk tube (previously rinsed with 10% aqueous NH4OH and water in sequence), Na2CO3 (0.2 mmol, 21 mg), NaBr (4 mmol, 0.412 g), amide 4 (4 mmol, 1.312 g), and a piece of copper wire (length 40 mm, 1 mm diameter, 99.9%) were inserted. After three cycles of vacuum/argon (around 10 min, each), AcOEt (3 mL), EtOH (96 vol%, 1.5 mL), and TPMA (1 mL of 0.04 mol/L solution in absolute ethanol) were added under an argon atmosphere. The Schlenk tube was heated at 37 °C and stirred at 700 rpm for 16 h. The reaction was then quenched with water (6 mL), acidified with 10% aqueous HCl (1 mL) and extracted with CH2Cl2 (3 × 5 mL). The combined organic layers were concentrated to dryness at the rotary evaporator, giving the crude product 6 as a brownish solid (96%, 1.260 g), which resulted contaminated by a small amount (~9% GC) of trichlorolactam 5 (see also Figure S2). A purer version of 6 was obtained through (unoptimized) recrystallization from a mixture of CH2Cl2:petroleum ether in 1:2 ratio, giving a yellowish solid with a 78% yield.
Compound 6: 1H NMR (600 MHz, CDCl3): δ = 3.06 (q, J = 9.3 Hz, 1 H, CHHNCO), 3.10 (m, 1 H, CH), 3.43–3.53 (m, 2 H, CHHNCO, CHHBr), 3.78 (dd, J = 3.9, 10.4 Hz, 1 H, CHHBr), 4.43 (d, J = 14.6 Hz, 1 H, CHHPh), 4.65 (d, J = 14.6 Hz, 1 H, CHHPh), 7.24 (pd, J = 7.5 Hz, 2 H, o-ArH), 7.31–7.39 (m, 3 H, m,p-ArH) ppm. 13C NMR (150 MHz, CDCl3): δ = 27.8 (CH2Br), 48.0 (CH2Ph), 48.3 (CH2NCO), 51.9 (CH), 84.5 (CCl2), 128.4 (o-ArC), 128.5 (p-ArC), 129.2 (m-ArC), 166.3 (NCO) ppm.
- EA found: C 42.9 H 3.7 N 4.2; calcd for (C12H12NOCl2Br): C 42.76 H 3.59 N 4.16
- MS molecular peak [6]+ isotopic pattern: calcd m/z = 334.9 (61.6%); 335.9 (8.4%); 336.9 (100.0%); 337.9 (13.5%); 338.9 (45.7%); 339.9 (6.1%); 340.9 (6.6%); 341.9 (0.9%); 342.9 (0.1%). Found m/z = 334.9 (60.9%); 335.9 (8.7%); 336.9 (100.0%); 337.9 (13.8%); 338.9 (45.2%); 339.9 (6.3%); 340.9 (6.9%).
3. Results and Discussion
The ratio between bromide and chloride concentrations in solution was expected to reflect the prevalent form of the radical deactivator metal complex, as a result of the fast halide exchange processes [22]. A higher concentration of Cu(II)Br·L would potentially be associated with a dominant AERC, whereas an abundance of Cu(II)Cl·L was expected to predominantly favour ATRC. The development of the AERC should, therefore, be based on a cyclization process requiring a minimal starting amount of metal chlorides.
The ARGET-ATRC of dichloroamide 1 (Figure 1) [25], that can work with only 0.01 mol of CuCl2 for each mol of substrate (see Section 2.2), was thus considered a suitable starting point. The inclusion of increasing amounts of NaBr within the reaction mixture was then expected to lead to the formation of increasing amounts of brominated compounds, as depicted in Figure 3.
The desired product (3) may arise from the entrapment of the transient radical 1″ by Cu(II)Br·L., i.e., through the AERC mechanism. Conversely, the same metal complex may promote the formation of the constitutional isomeric lactam 3a via a radical substitution at the 3 position of the ATRC product 2 [6]. An analogous substitution mechanism may also apply to the formation of dibromide 3b from the AERC product 3. However, the formation of 3a and 3b was expected to be less likely due to the low operating temperature (37 °C) and the higher C–Cl bond dissociation energy (83 kcal/mol), compared to that of the C–Br bond (77 kcal/mol) [27].
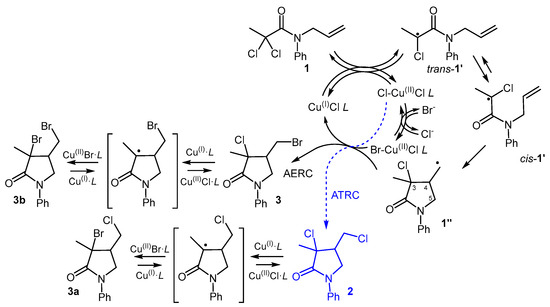
Figure 3.
Competition between ATRC (ending in blue drawing) and AERC of 1. Possible further substitution products are also described.
When a moderate amount of NaBr (0.25 mol for each mol of 1) was included into the described ARGET-ATRC reaction mixture, a new product was formed, together with the standard product 2. This new compound, which exhibited higher retention in GC-MS (r.t. ~13.95 min) compared to 2 (GC r.t. ~12.90 min), was characterized by a MS pattern compatible with a bromochlorolactam. Further characterization through multinuclear NMR spectroscopy, after careful chromatographic fractionation, confirmed the identity of compound 3. The limited effectiveness of the radical substitution mechanisms that produce 3a or 3b under AERC conditions was then confirmed. This was further confirmed by heating a purified sample of compound 2 at 50 °C for 24 h in the presence of 10 mol% of the Cu(II)Br2·TPMA metal complex. Under these conditions, 2 converted to another compound, characterized by a molecular ion very near to that of lactam 3 but showing a different GC retention time (~13.85 min) and mass fragmentation (Figure 4). It was thus assigned to 3a.
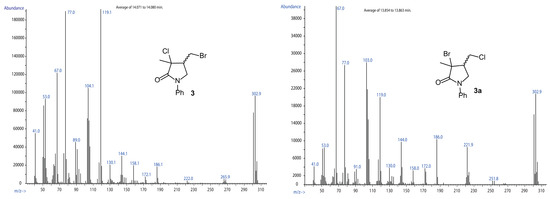
Figure 4.
MS characterization of 3 and 3a.
As the operativity of the AERC mechanism was demonstrated, further experimentation was directed towards increasing the production of 3. Therefore, a set of ATRC on 1 were set up, incorporating increased amounts of NaBr. Unfortunately, the GC yield of 3 did not correlate linearly with the amount of added bromide. This unexpected behavior was attributed to the chemical complexity of the system. Indeed, the ARGET-ATRC involves a multitude of chemical species, such as Na2CO3, ascorbic acid, and the metal complex, whose mutual interactions and relative solubilities can be altered by the added salts.
To simplify the composition of the reaction mixture, a supplemental activator reducing agent (SARA) process was considered. In the SARA radical mechanism, copper comproportionation is assumed to dominate over disproportionation [28], making it possible to utilize Cu0 as both a reducing agent and a metal source for the catalytic complex. This eliminates the need for the addition of the copper salt and the reductant. Furthermore, provided that the metal possesses adequate surface area, the reaction rate can be controlled by adjusting the amount of nitrogen ligand, which determines the concentration of the formed catalytic complex. As a result, in the SARA process, some species required in the ARGET process (such as CuCl2 and ascorbic acid) can be replaced by a piece of copper wire. A small amount of carbonate (~5 mol%) was also maintained [29], due to the ability of the EtOH/Na2CO3 couple to function as a reducing agent [30].
The SARA ATRC was successfully applied to 1, resulting in a clean and complete conversion into the expected dichloro-γ-lactam 2 within 16 h at 37 °C (entry 1, Table 1). Subsequently, one mole of bromide salt for each mole of substrate was added to achieve partial AERC. A marked effect of the metal cation used, whether lithium (entry 2), sodium (entry 3), or potassium (entry 4), on the formation of different proportions of bromolactam 3 and dichlorolactam 2 was observed. Specifically, sodium bromide/carbonate (entry 3) exhibited the best selectivity towards 3, while the potassium salts demonstrated a propensity towards 2.

Table 1.
SARA ATRC/AERC of 1.
Using the sodium salts as the reference, various solvent polarities (i.e., diverse EtOAc/EtOH ratios) were explored. Lower polarities (entry 5) worsened the selectivity towards 3, while a slight improvement was observed when EtOH was increased to 50 vol% (entry 6) or higher (entry 7). Several other interventions aimed at improving the selectivity were investigated, including the following: (i) increasing the amount of bromide (1.5 moles NaBr/moles of 1), (ii) adding an anion transporter (5 mol% of Bu4N+Br−/Et4N+Br−), and (iii) adding of a cation-catching species (5 mol% of 15-crown-5 ether). Unfortunately, none of these interventions yielded improved results compared to those obtained in entry 6.
The greater selectivity observed at higher EtOH concentrations suggested that salt solubility might play a significant role in determining the reaction’s outcome. Accordingly, the addition of a minor amount of a polar co-solvent was evaluated, as shown in Table 2. The addition of some MeCN (entry 1) resulted in a significant improvement in selectivity towards 3, but also in a reduced reaction rate, highlighted by incomplete conversion. In contrast, DMSO (entries 2, 3, 4) [28] brought to high selectivities towards 3, along with quantitative conversions of 1. It is worth noting that the best result was attained including the lowest amount of DMSO (entry 2).

Table 2.
Solvent changes in the AERC of 1.
Lastly, the addition of water in small amounts (entries 5 and 6) led to an almost complete conversion of 1 into 3. Such an addition can be conveniently implemented by using 96 vol% ethanol instead of absolute ethanol. An explanation of the observed effect may involve the more efficient solvation of chloride ions by polar solvents compared to bromide ions.
Another parameter investigated, aimed at assessing possible increments of process productivity, was the solvent volume. We found that halving the volume of solvents had no impact on yield and selectivity, offering the opportunity to use reactors with half the volume. However, further reductions in volume worsened the results. The final procedure (see Section 2) gave raw 3 in a 95% isolated yield. A small amount (~5 mol%) of dibromolactam 3b was also present as a contaminant (see Figure S1), likely deriving from 3 through a copper-promoted radical substitution (Figure 3).
Having demonstrated the facile and regioselective generation of the bromochloro-γ-lactam 3 from the dichloroamide 1, the generality of the method was expanded by considering a second substrate, characterized by a marked structural variation.
Trichloroacetamides are well known reactive substrates for atom transfer radical processes, owing to the ease of formation of radical intermediates through halogen extraction [31]. However, in the absence of an effective catalytic cycle, the same radical intermediates are involved in uncontrolled processes, eroding the selectivity and synthetic appeal of the method. For instance, hydrogen transfer from the solvent may also occur, resulting in de-halogenated byproducts.
Moreover, if the metal complex halogen exchange is not fast enough, the high reaction rate of trichloroacetamides should favour the formation of the “standard” ATRC product (5, Figure 5) instead of the AERC one (6).
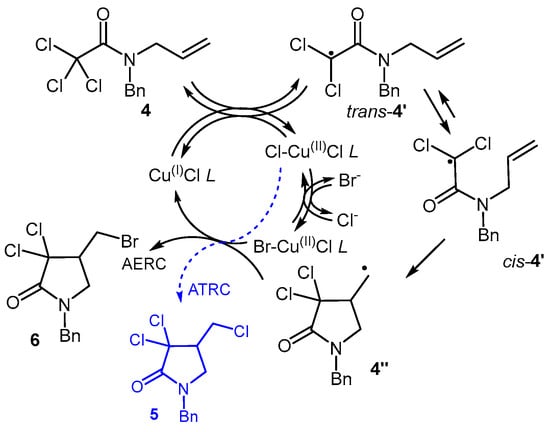
Figure 5.
ATRC (ending in blue drawing) and AERC of trichloroacetamide 4.
The technique was thus put to the test with trichloro compound 4, prompting a re-evaluation of the operating conditions.
Following the same approach used earlier, several cyclization experiments were conducted on 4, as shown in Table 3.

Table 3.
SARA ATRC/AERC of 4.
Proceeding in a similar manner, various solvent proportions were tested (entries 1–4), revealing that a EtOAc:EtOH ratio of 1:1 (entry 3) works best, likewise to what was observed for 1. Keeping this solvent system, its total volume was varied (entries 5 and 6), and the inclusion of small amounts of water was considered as well. These investigations resulted in high selectivities towards 6, especially when 1.5 mL of solvent mixture and 15 µL of water were employed for each mmol of substrate (entry 5). Under these conditions (see also Section 2), raw 6 was obtained in a 96% isolated yield. A minor amount (~ 15%, based on H NMR) of trichlorolactam 5 was also present as a contaminant (see Figure S2), formed through ATRC of substrate 4 (Figure 5).
The obtained bromochloro-γ-lactams (3 and 6) can be further purified by crystallization (see Section 2).
4. Conclusions
Through the AERC methodology developed herein, we have demonstrated the facile and regioselective preparation of dihalo-γ-lactams containing two distinct halogens from dichloroamides. This method, which integrates the established SARA ATRC with halogen exchange in solution, operates under mild conditions and requires low amounts of metallic copper, serving as both a supplemental activator and the reducing agent. Starting from readily available substrates, the efficiencies and selectivities observed in the studied cases suggest significant potential for the sustainable preparation of novel, valuable halogenated synthetic targets. This approach shows clear advantages over the use of Kharasch radical traps [21], as well as an industrial appeal [11,12].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14114357/s1, Figure S1: 1H and 13C NMR of 3 (raw reaction mixture, after extraction), featuring small amounts of byproducts: Br, Br isomer (6%) and ATRC product 2 (6%); Figure S2: 1H and 13C NMR of 6 (raw reaction mixture, after extraction), contaminated by minor amounts of ATRC product 5 (15%).
Author Contributions
Conceptualization, F.R.; methodology, V.A.; validation, A.S., C.F. (Claudio Fontanesi) and C.F. (Camilla Ferrari); investigation, V.A. and B.A.; resources, F.R.; data curation, V.A.; writing—original draft preparation, F.R.; writing—review and editing, A.S., C.F. (Camilla Ferrari) and N.B.; visualization, V.A.; supervision, F.R.; project administration, F.R. and C.F. (Claudio Fontanesi); funding acquisition, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Pintauer, T. Catalyst Regeneration in Transition-Metal-Mediated Atom-Transfer Radical Addition (ATRA) and Cyclization (ATRC) Reactions. Eur. J. Inorg. Chem. 2010, 2010, 2449–2460. [Google Scholar] [CrossRef]
- Clark, A.J. Copper Catalyzed Atom Transfer Radical Cyclization Reactions. Eur. J. Org. Chem. 2016, 2016, 2231–2243. [Google Scholar] [CrossRef]
- Clark, A.J.; Cornia, A.; Felluga, F.; Gennaro, A.; Ghelfi, F.; Isse, A.A.; Menziani, M.C.; Muniz-Miranda, F.; Roncaglia, F.; Spinelli, D. Arylsulfonyl Groups: The Best Cyclization Auxiliaries for the Preparation of ATRC γ-Lactams Can Be Acidolytically Removed. Eur. J. Org. Chem. 2014, 2014, 6734–6745. [Google Scholar] [CrossRef]
- Fischer, H. The Persistent Radical Effect: A Principle for Selective Radical Reactions and Living Radical Polymerizations. Chem. Rev. 2001, 101, 3581–3610. [Google Scholar] [CrossRef] [PubMed]
- Casolari, R.; Felluga, F.; Frenna, V.; Ghelfi, F.; Pagnoni, U.M.; Parsons, A.F.; Spinelli, D. A Green Way to γ-Lactams through a Copper Catalyzed ARGET-ATRC in Ethanol and in the Presence of Ascorbic Acid. Tetrahedron 2011, 67, 408–416. [Google Scholar] [CrossRef]
- Bergmann, R.; Gericke, R. Synthesis and Antihypertensive Activity of 4-(1,2-Dihydro-2-Oxo-1-Pyridyl)-2H-1-Benzopyrans and Related Compounds, New Potassium Channel Activators. J. Med. Chem. 1990, 33, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.M.; Young, D.W. Stereospecific Synthesis of 4-Alkylglutamates and 4-Alkylprolines. Tetrahedron Lett. 1994, 35, 7277–7280. [Google Scholar] [CrossRef]
- Corey, E.J.; Li, W.-D.Z. Total Synthesis and Biological Activity of Lactacystin, Omuralide and Analogs. Chem. Pharm. Bull. 1999, 47, 1–10. [Google Scholar] [CrossRef]
- Nilsson, B.M.; Hacksell, U. Base-Catalyzed Cyclization of N-propargylamides to Oxazoles. J. Heterocycl. Chem. 1989, 26, 269–275. [Google Scholar] [CrossRef]
- Oda, K.; Moriyasu, K.; Hayashi, M.; Nishida, M.; Oyamada, M.; Fujiwara, A.; Watanabe, J. 1-(3-Substituted Benzyl)-3-Halogenoalkyl)-2-Pyrrolidinone Derivatives and Herbicides Containing Them. EP0402893 A1, 13 June 1990. pp. 1–34.. [Google Scholar]
- Woolard, F.X. N-Benzyl-4-Alkyl-Pyrrolidinone Herbicides. US5302726, 12 April 1994. pp. 1–5.. [Google Scholar]
- Ghelfi, F.; Pattarozzi, M.; Roncaglia, F.; Parsons, A.; Felluga, F.; Pagnoni, U.; Valentin, E.; Mucci, A.; Bellesia, F. Preparation of the Maleic Anhydride Nucleus from Dichloro γ-Lactams: Focus on the Role of the N-Substituent in the Functional Rearrangement and in the Hydrolytic Steps. Synthesis 2008, 2008, 3131–3141. [Google Scholar] [CrossRef]
- Slough, G.A. (Ph3P)3RuCl2 Catalyzed Equilibration and Elimination of α-Chloro-N-Tosyl-2-Pyrrolidinones: A Unique Route to Unsaturated 2-Pyrrolidinones. Tetrahedron Lett. 1993, 34, 6825–6828. [Google Scholar] [CrossRef]
- Kornblum, N.; Jones, W.J.; Anderson, G.J. A New and Selective Method of Oxidation. The Conversion of Alkyl Halides and Alkyl Tosylates to Aldehydes. J. Am. Chem. Soc. 1959, 81, 4113–4114. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, J.; Shen, Z.; Zhang, Y. Efficient and Convenient Oxidation of Organic Halides to Carbonyl Compounds by H2O2 in Ethanol. Tetrahedron Lett. 2007, 48, 1919–1921. [Google Scholar] [CrossRef]
- Patil, R.D.; Adimurthy, S. Direct and Selective Conversion of Benzyl Bromides to Benzaldehydes with Aqueous H2O2 Without Catalyst. Synth. Commun. 2011, 41, 2712–2718. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, R. Oxidation of Alcohols Using H2O2 as Oxidant Catalyzed by AlCl3. Catal. Commun. 2008, 9, 740–742. [Google Scholar] [CrossRef]
- Zheng, P.; Yan, L.; Ji, X.; Duan, X. A Green Procedure for the Oxidation of Benzyl Halides to Aromatic Aldehydes or Ketones in Aqueous Media. Synth. Commun. 2010, 41, 16–19. [Google Scholar] [CrossRef]
- Smith, M.B. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; Section 10.45; p. 534. [Google Scholar]
- Boivin, J.; Yousfi, M.; Zard, S.Z. A Versatile Radical Based Synthesis of γ-Lactams Using Nickel Powder/Acetic Acid. Tetrahedron Lett. 1994, 35, 5629–5632. [Google Scholar] [CrossRef]
- Peng, C.-H.; Kong, J.; Seeliger, F.; Matyjaszewski, K. Mechanism of Halogen Exchange in ATRP. Macromolecules 2011, 44, 7546–7557. [Google Scholar] [CrossRef]
- Ince, J.; Ross, T.M.; Shipman, M.; Slawin, A.M.Z.; Ennis, D.S. Observations on the Synthesis of Functionalised Methyleneaziridines. Tetrahedron 1996, 52, 7037–7044. [Google Scholar] [CrossRef]
- Bellesia, F.; D’Anna, F.; Felluga, F.; Frenna, V.; Ghelfi, F.; Parsons, A.; Reverberi, F.; Spinelli, D. Breakthrough in the α-Perchlorination of Acyl Chlorides. Synthesis 2012, 2012, 605–609. [Google Scholar] [CrossRef]
- Bellesia, F.; Clark, A.J.; Felluga, F.; Gennaro, A.; Isse, A.A.; Roncaglia, F.; Ghelfi, F. Efficient and Green Route to γ-Lactams by Copper-Catalysed Reversed Atom Transfer Radical Cyclisation of α-Polychloro-N-allylamides, Using a Low Load of Metal (0.5 mol%). Adv. Synth. Catal. 2013, 355, 1649–1660. [Google Scholar] [CrossRef]
- Benedetti, M.; Forti, L.; Ghelfi, F.; Pagnoni, U.M.; Ronzoni, R. Halogen Atom Transfer Radical Cyclization of N-Allyl-N-Benzyl-2,2-Dihaloamides to 2-Pyrrolidinones, Promoted by Fe0-FeCl3 or CuCl-TMEDA. Tetrahedron 1997, 53, 14031–14042. [Google Scholar] [CrossRef]
- McGivern, W.S.; Derecskei-Kovacs, A.; North, S.W.; Francisco, J.S. Computationally Efficient Methodology to Calculate C−H and C−X (X = F, Cl, and Br) Bond Dissociation Energies in Haloalkanes. J. Phys. Chem. A 2000, 104, 436–442. [Google Scholar] [CrossRef]
- Peng, C.-H.; Zhong, M.; Wang, Y.; Kwak, Y.; Zhang, Y.; Zhu, W.; Tonge, M.; Buback, J.; Park, S.; Krys, P.; et al. Reversible-Deactivation Radical Polymerization in the Presence of Metallic Copper. Activation of Alkyl Halides by Cu0. Macromolecules 2013, 46, 3803–3815. [Google Scholar] [CrossRef]
- Clark, A.J.; Duckmanton, J.N.; Felluga, F.; Gennaro, A.; Ghelfi, F.; Hardiman, J.R.D.; Isse, A.A.; Manferdini, C.; Spinelli, D. Cu0-Promoted Cyclisation of Unsaturated α-Halogeno Amides to Give β-and γ-Lactams. Eur. J. Org. Chem. 2016, 2016, 2479–2491. [Google Scholar] [CrossRef]
- Braidi, N.; Buffagni, M.; Ghelfi, F.; Parenti, F.; Gennaro, A.; Isse, A.A.; Bedogni, E.; Bonifaci, L.; Cavalca, G.; Ferrando, A.; et al. ARGET ATRP of Styrene in EtOAc/EtOH Using Only Na2CO3 to Promote the Copper Catalyst Regeneration. J. Macromol. Sci. A 2021, 58, 376–386. [Google Scholar] [CrossRef]
- Pattarozzi, M.; Roncaglia, F.; Giangiordano, V.; Davoli, P.; Prati, F.; Ghelfi, F. ‘Ligand-Free-Like’ CuCl-Catalyzed Atom Transfer Radical Cyclization of N-Substituted N-Allyl Polychloroamides to γ-Lactams. Synthesis 2010, 2010, 694–700. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).