Development of Polymer-Cored Akaganeite Adsorbent for Phosphate Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Powdered Iron Oxyhydroxide
2.1.1. Materials
2.1.2. Synthesis of Iron Oxyhydroxide Adsorbents
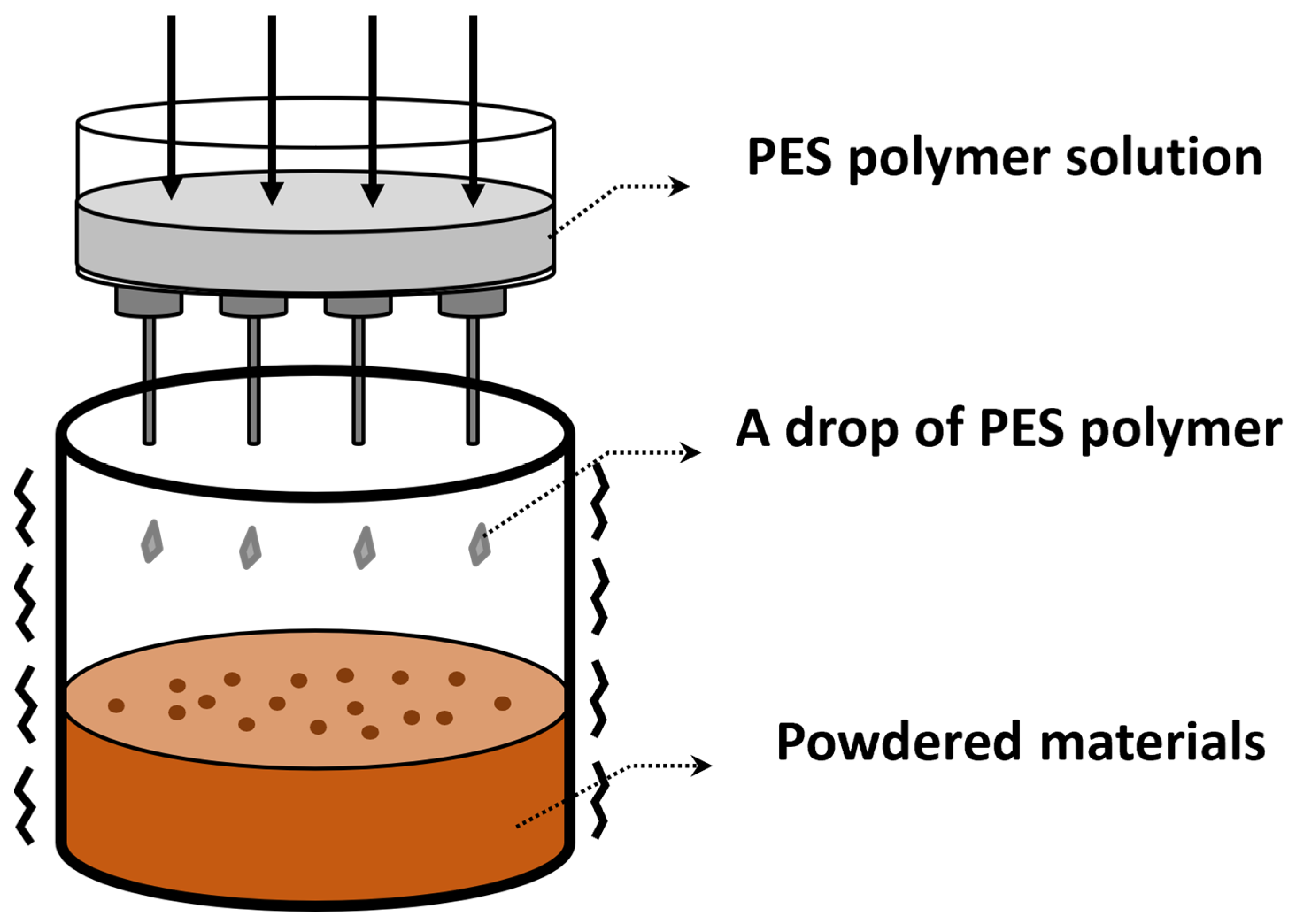
2.2. Granulation of Powdered Adsorbents
2.3. Characterization
2.3.1. X-ray Diffraction (XRD) Analysis
2.3.2. Transmission Electron Microscopy (TEM) Analysis
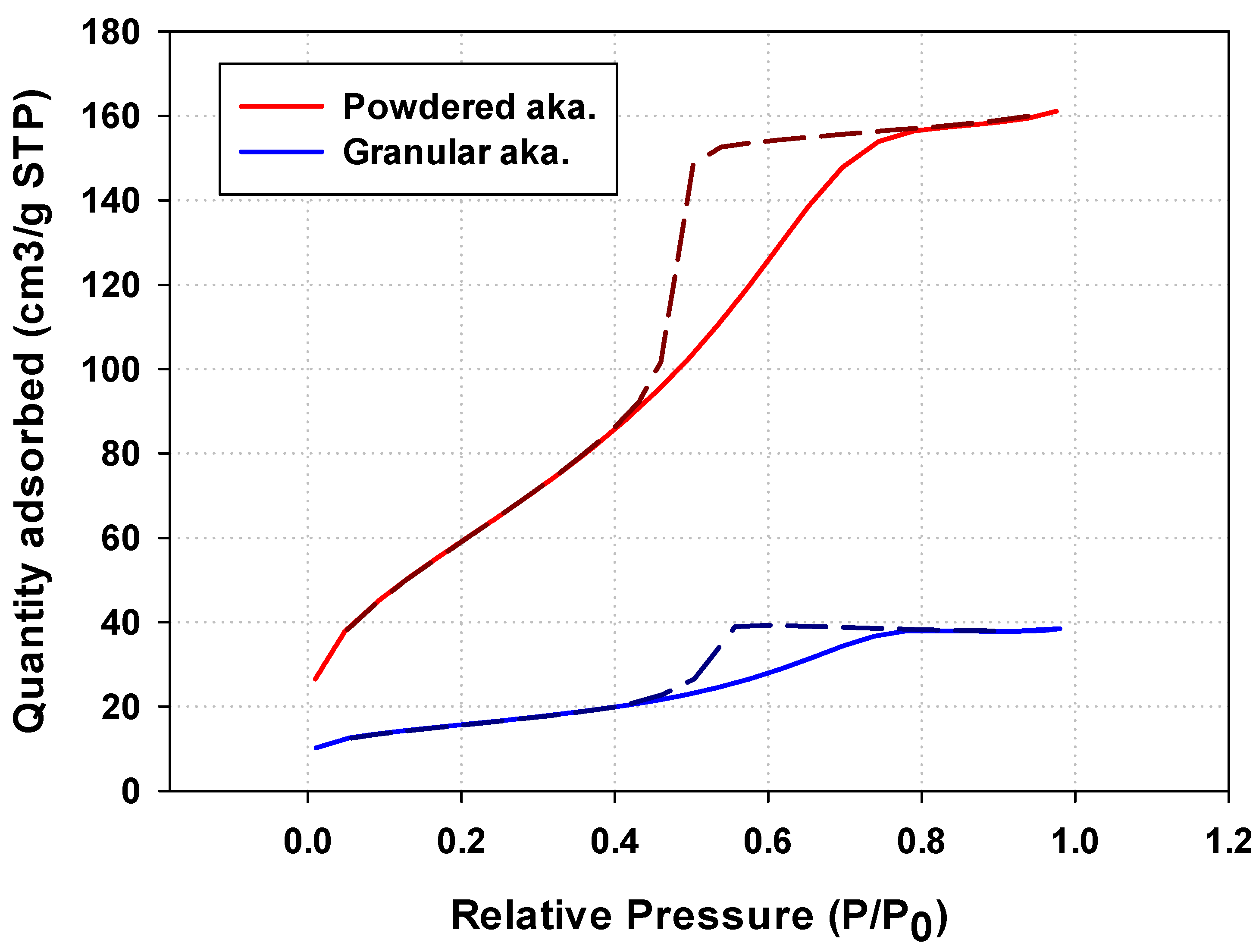
2.3.3. Nitrogen Adsorption–Desorption Measurement
2.3.4. Isoelectric Point Measurement and Zeta Potential Measurement
2.3.5. Scanning Electron Microscopy (SEM) Analysis
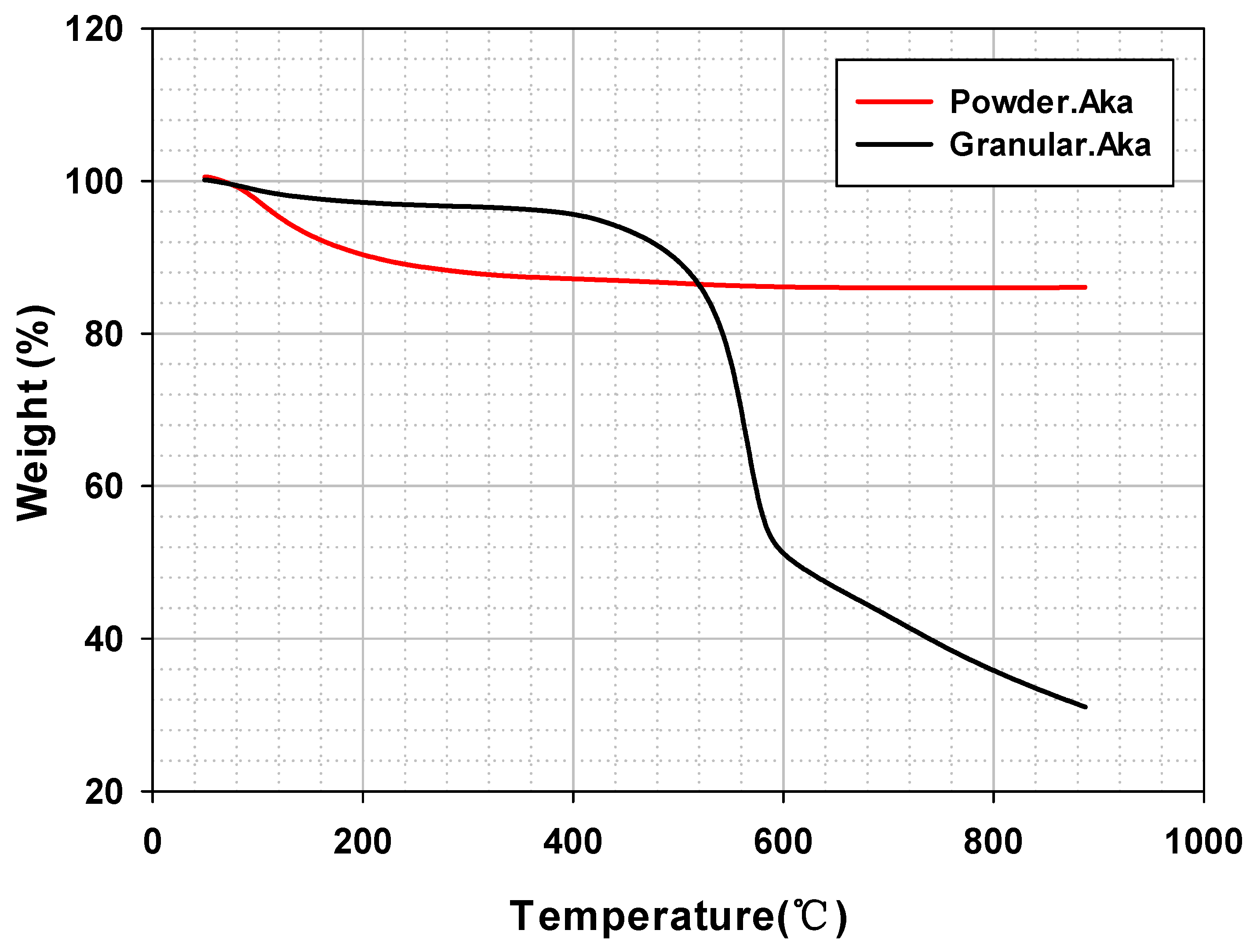
2.3.6. Thermogravimetric Analysis (TGA)
2.4. Adsorption/Desorption Experiments
Batch Experiment
3. Results and Discussion
3.1. Characteristic Analysis of Iron Oxyhydroxide Material
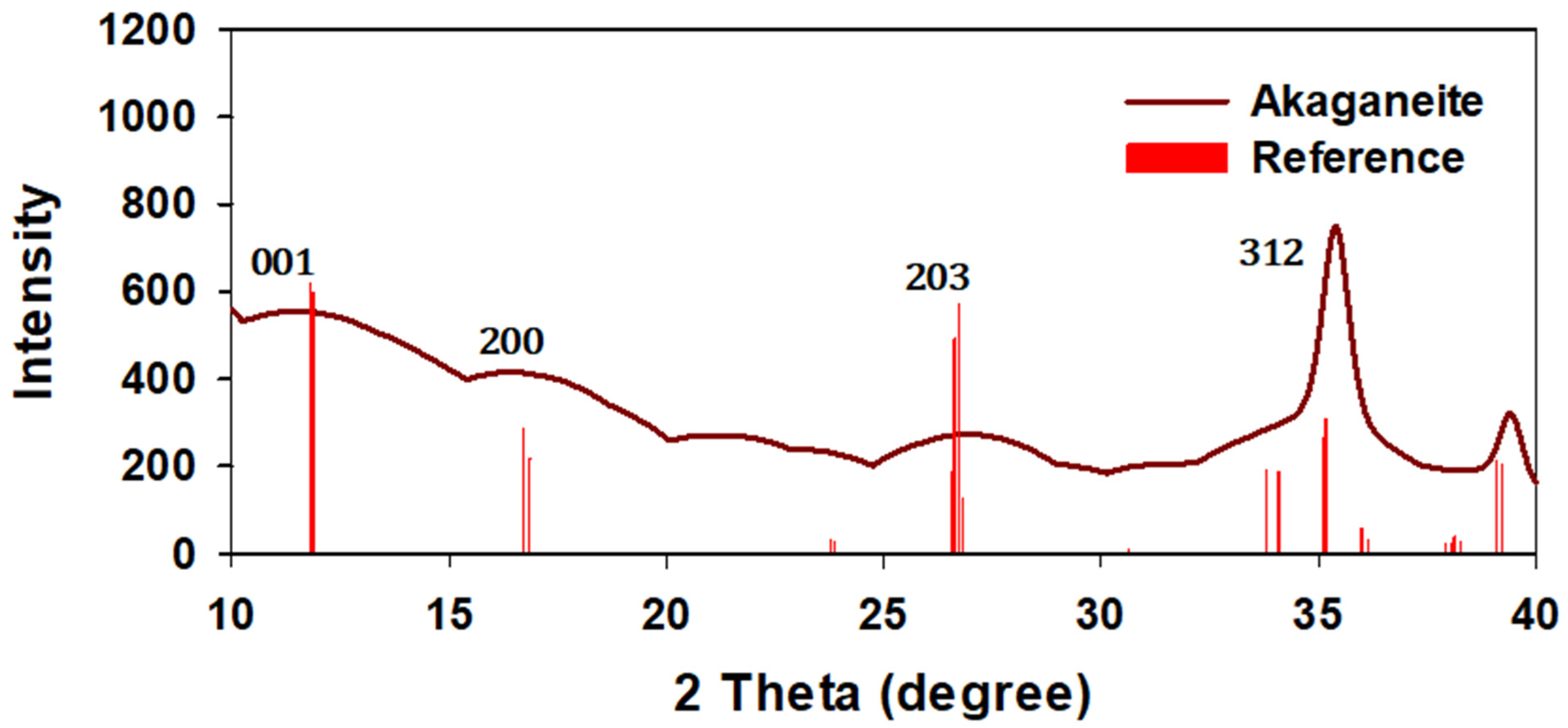
3.1.1. XRD Analysis of Powdered Iron Oxyhydroxide
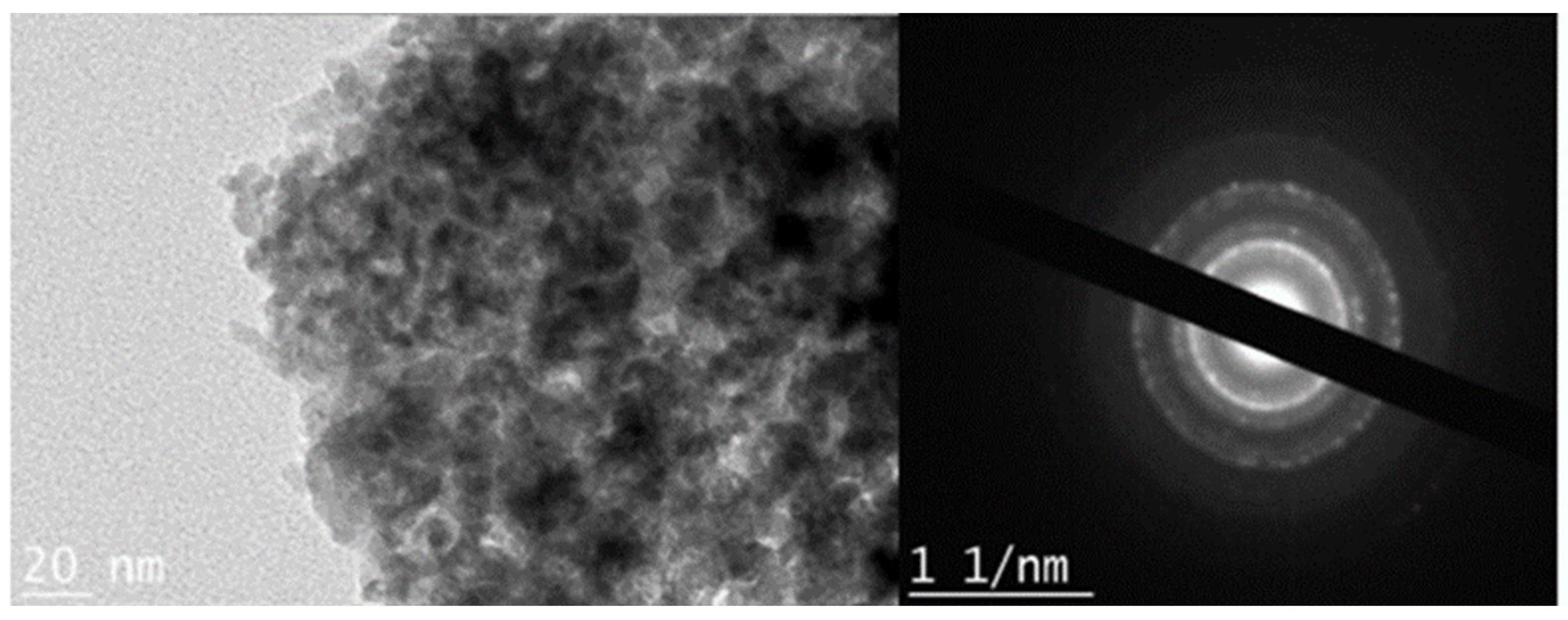
3.1.2. Transmission Electron Microscopy (TEM) Analysis of Powdered Iron Oxyhydroxide
3.1.3. BET Analysis and Zeta-Potential Analysis of Powdered Iron Oxyhydroxide
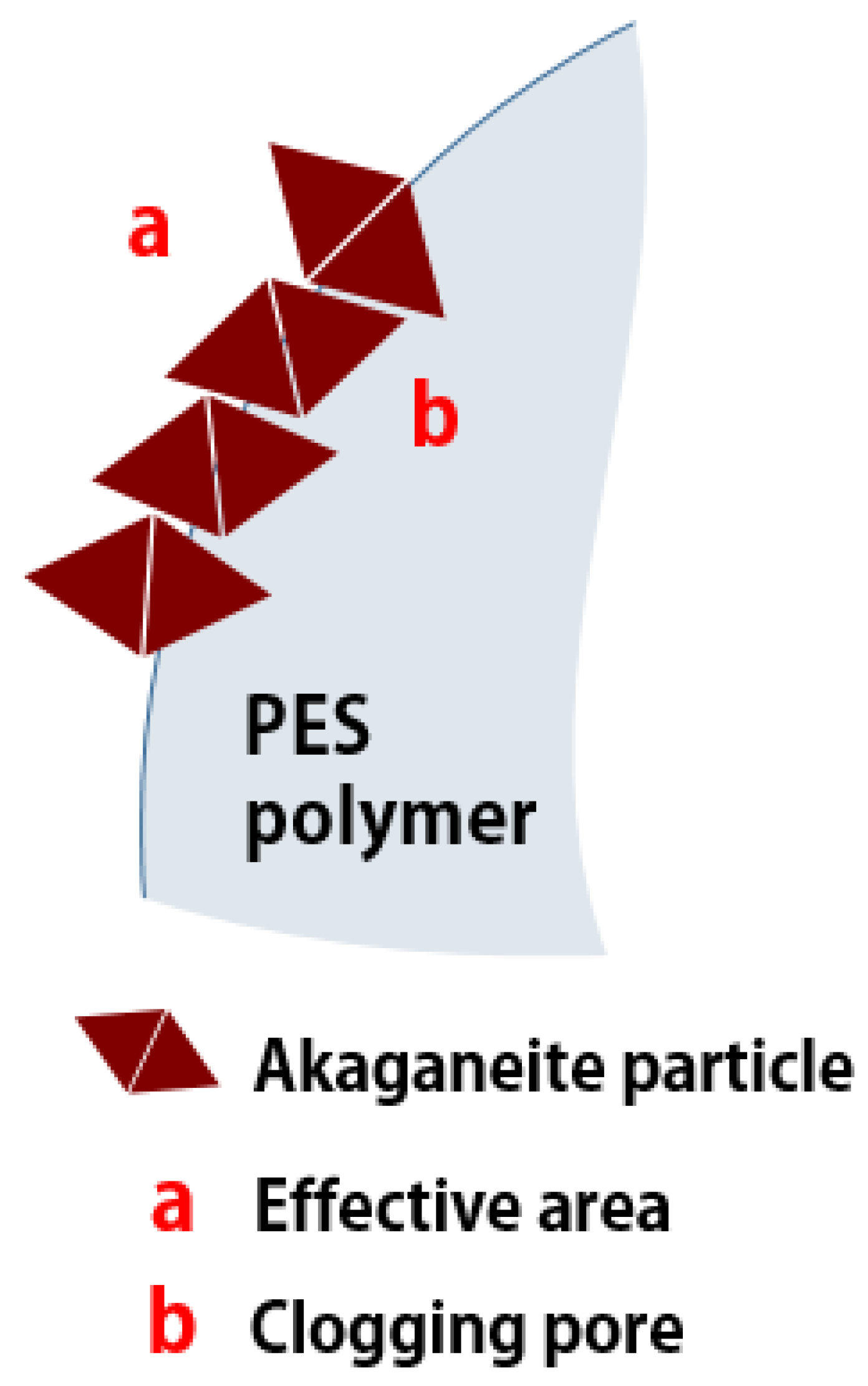
3.2. Granulation of Akaganeite
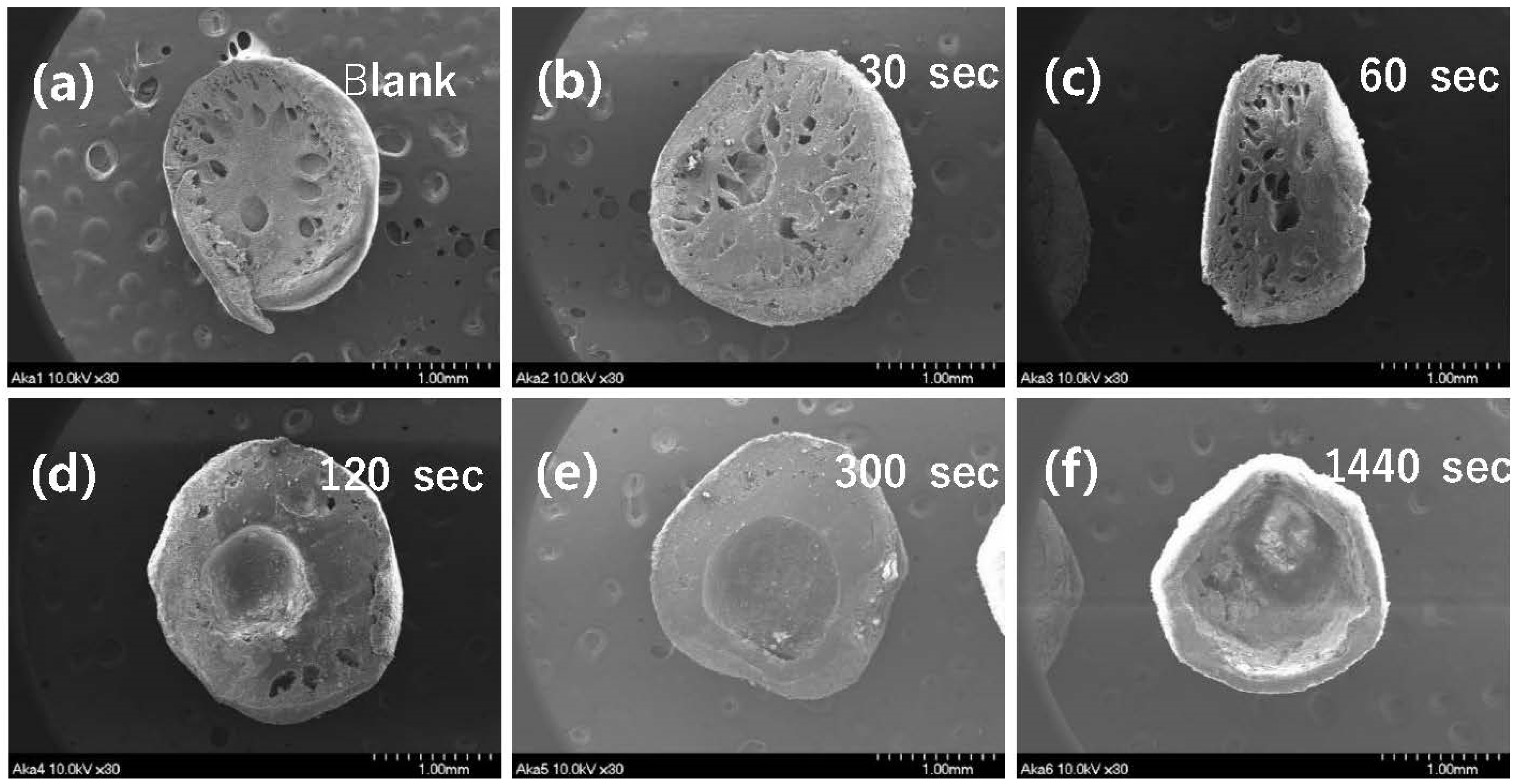
3.2.1. Optimum Granulation Condition for Iron Oxyhydroxide Powder
3.2.2. TGA Analysis of Granular Iron Oxyhydroxide
3.2.3. BET Analysis of Granular Iron Oxyhydroxide
3.3. Adsorption Experiments
3.3.1. Kinetic Adsorption/Desorption Experiment
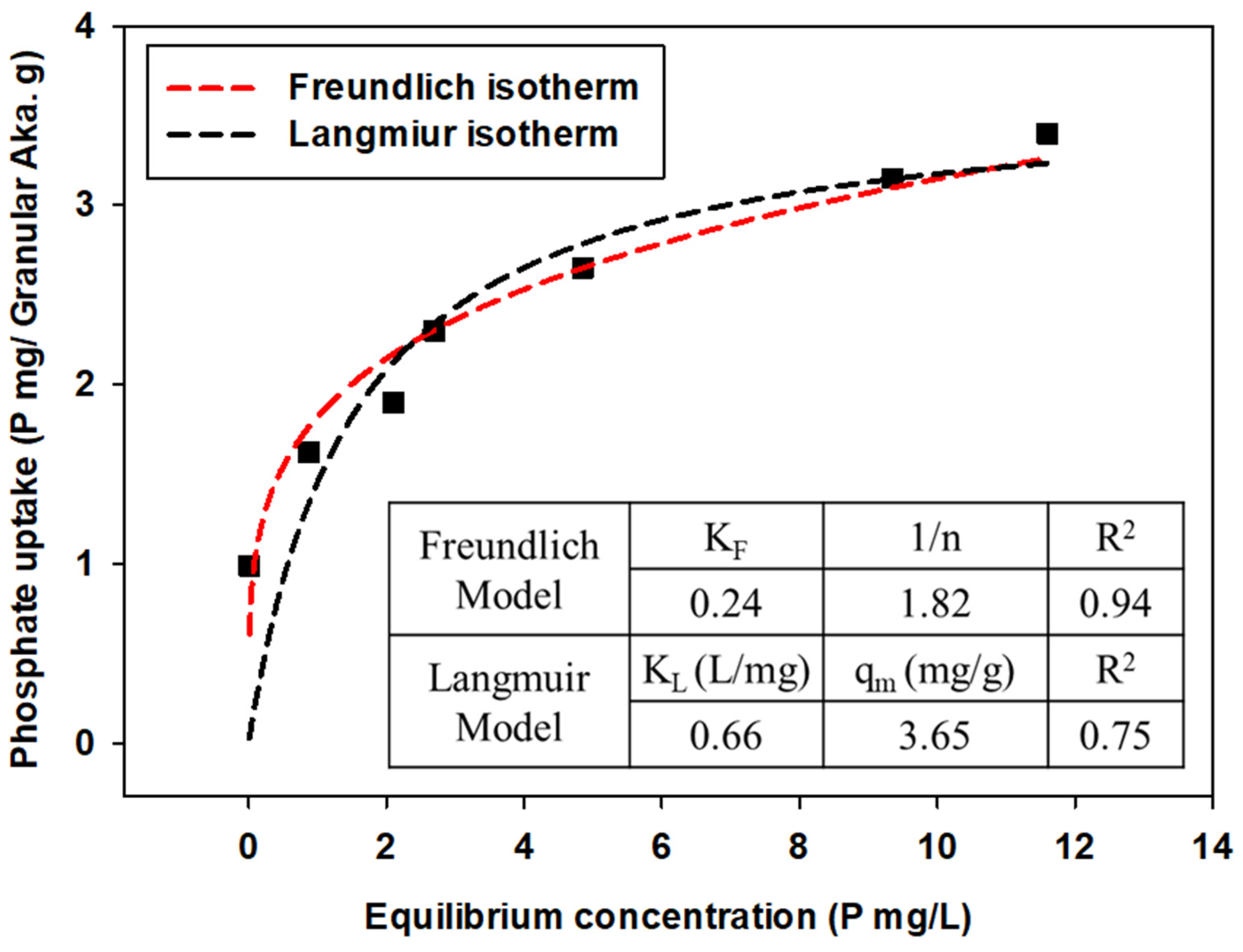
3.3.2. Isothermal Adsorption Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajammal, S.S.; Zlowodzki, M.; Lelwica, A.; Tornetta, P., 3rd; Einhorn, T.A.; Buckley, R.; Leighton, R.; Russell, T.A.; Larsson, S.; Bhandari, M. The use of calcium phosphate bone cement in fracture in treatment: A meta-analysis of randomized trials. J. Bone Jt. Surg. 2008, 90, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Putt, M.S.; Milleman, K.R.; Milleman, J.L.; Ghassemi, A. Comparison of a dual-phase fluoride thoothpaste containing calcium, phosphate, and sodium bicarbonate with a regular fluoride thoothpaste on calculus formation. Compend. Contin. Educ. Dent. 2004, 25, 44–51. [Google Scholar] [PubMed]
- Dal’Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M. Moisturinzing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Ski. Res. Technol. 2006, 12, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, S.; Xiong, H. phosphate adsorption removals by five synthesized isomeric α-, β-, γ-FeOOH. Water Air Soil Pollut. 2022, 233, 454. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Zouboulis, A.; Mitrakas, M. Thermodynamic study of phosphate adsorption and removal from water using iron oxyhydroxides. Water 2022, 14, 1163. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef]
- de Ridder, M.; de Jong, S.; Polchar, J.; Lingemann, S. Risks and Opportunities in the Global Phosphate Rock Market; The Hague Centre for Strategic Studies: Hague, The Netherlands, 2012. [Google Scholar]
- Jasinski, S.M. Mineral Commodity Summaries 2011; U.S. Geological Survey: Reston, VA, USA, 2011.
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Abu-Obaid, S.; Aktij, S.A.; Tabe, S.; Sadrzadeh, M.; Farnood, R.R. Surfactant-modified adsorptive electrospun nanofiber membrane impregnated with akageneite for phosphorus recovery from wastewater. J. Environ. Chem. Eng. 2022, 10, 108786. [Google Scholar] [CrossRef]
- Wilsenach, J.A.; Schuurbiers, C.A.; van Loosdrecht, M.C. Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res. 2007, 41, 458–466. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, T.-J.; Kim, S.-H.; Yang, H.-J. Characteristics of Residual Metals from Phosphorus Removal in Sewage Treatment Plants around Paldang Lake; National Institute of Environmental Research in Korea: Incheon, Republic of Korea, 2012.
- Cheng, W.; Li, J.; Sun, J.; Luo, T.; Marsac, R.; Boily, J.F.; Hanna, K. Nalidixic Acid and Fe (II)/Cu (II) Coadsorption at Goethite and Akaganéite Surfaces. Environ. Sci. Technol. 2023, 57, 15680–15692. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Altundogan, H.S. Cr (VI) removal from aqueous solution by iron (III) hydroxide-loaded sugar beet pulp. Process Biochmistry 2005, 40, 1443–1452. [Google Scholar] [CrossRef]
- Phuengprasop, T.; Sittiwong, J.; Unob, F. Removal of heavy metal ions by iron oxide coated sewage sludge. J. Hazard. Mater. 2011, 186, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-L.; Wang, X.-Y.; Zeng, G.-M.; Chen, L.; Deng, J.-H.; Zhang, X.-R.; Niu, Q.-Y. Copper (II) by pectin-iron oxide magnetic nanocomposite adsorbent. Chem. Eng. J. 2012, 185–186, 100–107. [Google Scholar] [CrossRef]
- Gu, B.; Lian, L.; Dickey, J.; Yin, X.; Dai, S. Reductive precipitation of uranium (VI) by zero-valent iron. Environ. Sci. Technol. 1998, 32, 3366–3373. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible application in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Navrotsky, A.; Mazeina, L.; Majzlan, J. Size-driven structural and thermodynamic complecity in iron oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef]
- Kim, J.; Li, W.; Philipsc, B.L.; Grey, C.P. Phosphate adsorption on the iron oxyhydroxides goethite (α-FeOOH), akaganeite (β-FeOOH), and lepidocrocite (γ-FeOOH): P NMR study. Energy Environ. Sci. 2011, 4, 4298–4305. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Phosphate adsorption on synthetic goethite and akaganeite. J. Colloid Interface Sci. 2006, 298, 602–608. [Google Scholar] [CrossRef]
- Li, N.; Bai, R. Copper adsorption on chitosan-cellulose hydrogel beads: Behaviors and mechanisms. Sep. Purif. Technol. 2005, 42, 237–247. [Google Scholar] [CrossRef]
- Chang, J.; Woo, H.; Ko, M.S.; Lee, J.; Lee, S.; Yun, S.T.; Lee, S. Targeted removal of trichlorophenol in water by oleic acid-coated nanoscale palladium/zero-valent iron alginate beads. J. Hazard. Mater. 2015, 293, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Grisdanurak, N.; Akewaranugulsiri, S.; Futalan, C.; Tsai, W.-C. The study of copper adsorption from aqueous solution using crosslinked chitosan immobilized on bentonite. J. Appl. Polym. Sci. 2012, 125, 132–142. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Odusote, G.; Qu, F.; Priestley, R.D. Core-shell Fe3O4 polydopamine nanoparticles serve multipurpose as drug carrier, catalyst support and carbon adsorbent. ACS Appl. Mater. Interfaces 2013, 5, 9167–9171. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Xu, Z.-L. Effect of TIO2 nanoparticles on the surface morphology performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Song, X. Surface and Bulk Reactivity of Iron Oxyhydroxides: A Molecular Perspective. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2013. [Google Scholar]
- Jemutai-Kimosop, S.; Okello, V.A.; Shikuku, V.O.; Orata, F.; Getenga, Z.M. Synthesis of mesoporous akaganeite functionalized maize cob biochar for adsorptive abatement of carbamazepine: Kinetics, isotherms, and thermodynamics. Clean. Mater. 2022, 5, 100104. [Google Scholar] [CrossRef]
- Klaysom, C.; Moon, S.-H.; Ladewig, B.; Lu, G.Q.; Wang, L. Preparation of porous ion-exchange membranes (IEMs) and their characterizaotns. J. Membr. Sci. 2011, 371, 37–44. [Google Scholar] [CrossRef]
- Wen, S.; Lu, Y.; Dai, J.; Huang, X.; An, S.; Liu, J.; Liu, Z.; Du, Y.; Zhang, Y. Stability of organic matter-iron-phosphate associations during abiotic reduction of iron. J. Hazard. Mater. 2023, 449, 131016. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Estroff, L.A. Role of Akaganeite (β-FeOOH) in the Growth of Hematite (α-Fe2O3) in an inorganic silica hydrogel. Cryst. Growth Des. 2015, 15, 3388–3398. [Google Scholar] [CrossRef]
- Choi, J.; Cha, J.; Lee, J.-K. Synthesis of various magnetite nanoparticles through simple phase transformation and their shape dependent magnetic properties. RSC Adv. 2013, 3, 8365–8371. [Google Scholar] [CrossRef]
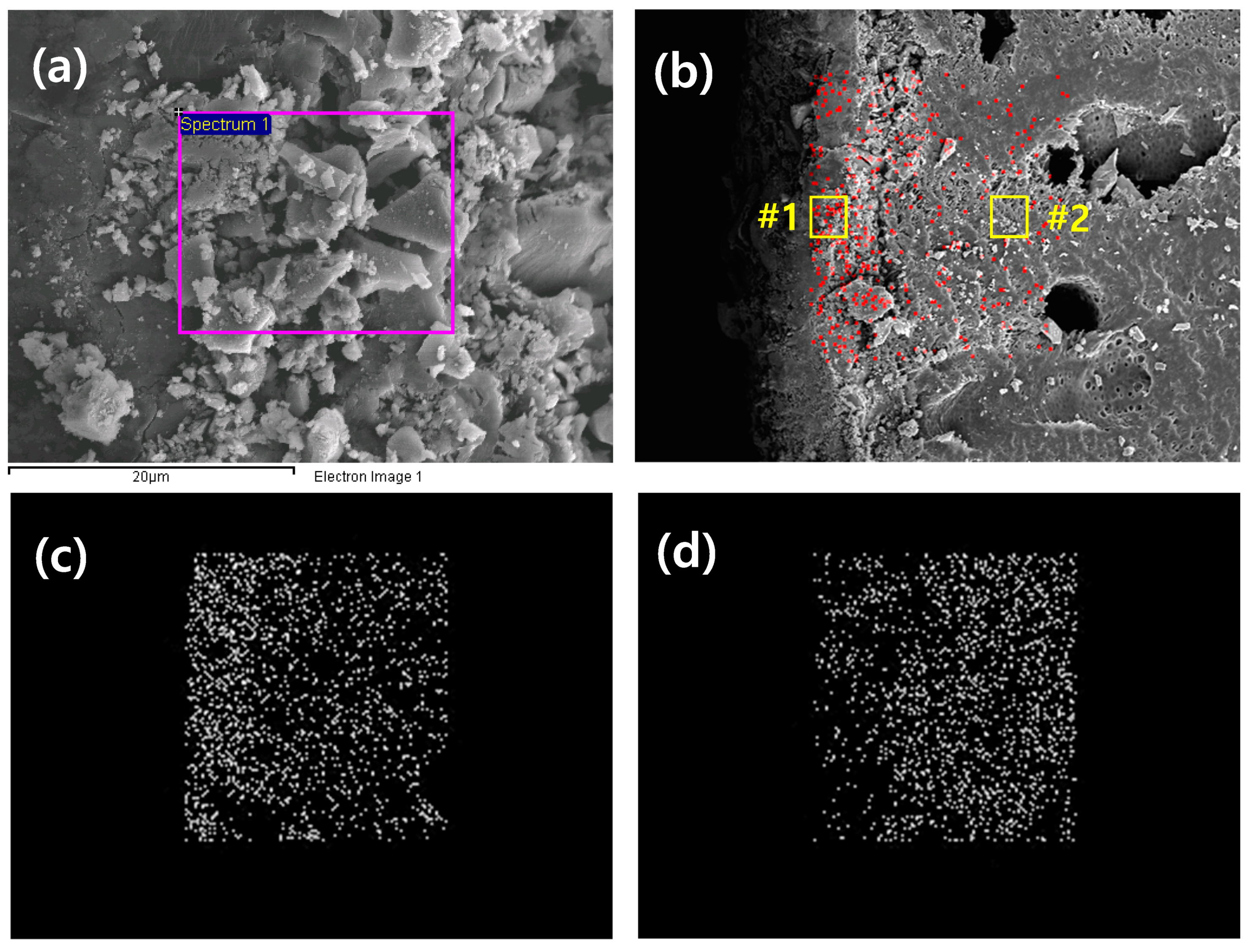


| Only PES | #1 | #2 | |||||
|---|---|---|---|---|---|---|---|
| Element | Weight (%) | Atomic (%) | Element | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) |
| C K | 53.60 | 66.36 | O K | 35.81 | 64.05 | 37.50 | 62.20 |
| O K | 26.03 | 24.20 | S K | 8.03 | 7.16 | 22.97 | 19.01 |
| S K | 20.37 | 9.45 | Fe K | 56.17 | 28.78 | 39.53 | 18.78 |
| Total | 100.00 | Total | 100.00 | 100.00 | |||
| Powdered Akaganeite | Granular Akaganeite | |
|---|---|---|
| BET surface area (m3/g) | 231 | 54 |
| Pore volume (cm3/g) | 0.249 | 0.060 |
| Pore size (nm) | 4.3 | 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.; We, H.; Kim, S.; Oh, S.; Baek, S. Development of Polymer-Cored Akaganeite Adsorbent for Phosphate Adsorption. Appl. Sci. 2024, 14, 146. https://doi.org/10.3390/app14010146
Bae J, We H, Kim S, Oh S, Baek S. Development of Polymer-Cored Akaganeite Adsorbent for Phosphate Adsorption. Applied Sciences. 2024; 14(1):146. https://doi.org/10.3390/app14010146
Chicago/Turabian StyleBae, Jiyeol, Hyobin We, Suho Kim, Sungjik Oh, and Soyoung Baek. 2024. "Development of Polymer-Cored Akaganeite Adsorbent for Phosphate Adsorption" Applied Sciences 14, no. 1: 146. https://doi.org/10.3390/app14010146
APA StyleBae, J., We, H., Kim, S., Oh, S., & Baek, S. (2024). Development of Polymer-Cored Akaganeite Adsorbent for Phosphate Adsorption. Applied Sciences, 14(1), 146. https://doi.org/10.3390/app14010146






