Abstract
It is a challenge to research and develop silicon surfactants with good acid and alkali stability. In the present paper, methylpropenyl polyether modified nonionic silicone surfactant (MPNTS), an alkali-resistant and hydrolysis-resistant silicone surfactant, was synthesized by hydrosilylation of 1,1,1,3,5,5,5-heptamethyl trisiloxane (MDHM) and methylpropenyl polyether (EO(7)). The polyether segments are grafted onto the main chain of organosilicon heptamethyl trisiloxane at a molar ratio of 1:1.05 (n(Si-H):n(C=C)). To evidence product formation, the MPNTS were analyzed by FT-IR and 1H NMR spectroscopy. The surface tension of NPNTS is 18.68 mN/m, and the CMC value is 78 mg/L by a contact angle tester. MPTNS shows hydrolytic stability and maintains that it could keep surface activity after standing for 60 days at pH = 7–10. The compatibility performance analysis shows that MPNTS has good compatibility and synergy with cationic, anionic, and nonionic surfactants, and it reveals the application prospects in daily chemicals, agricultural adjuvants, and other products.
1. Introduction
Silicone surfactants, because of their good surface properties, are widely used in cosmetics [1], household detergents [2], textile dyeing industry [3,4], care products [5], agricultural auxiliaries [6,7], and industrial detergents [8]. The hydrolytic stability of silicone surfactants also plays a very important role in practical applications. Hydrolytic stability can affect not only the role of surfactants in products, but also the effective time of products. Therefore, it is of great significance to develop hydrolysis-resistant silicone surfactants to expend the use of daily chemicals and to resolve industrial problems.
With polysiloxane as the main chain and organic group as the straight chain, the silicone surfactant has excellent wettability and defoaming property [9]. Silicone surfactants can provide lower surface tension (usually 20 mN/m). Similar to traditional hydrocarbon surfactants, straight-chain silicone is hydrophobic. However, due to the joint action of the main chain and the branched chain, the modified silicone surfactant has higher utilization value [10]. Through introducing hydrophilic or lipophilic groups, the performance of branched chain is increased while retaining its defoaming properties. In this way, we can design and synthesize products to meet the needs of industry, which is the development direction of modern silicone surfactants.
Trisiloxane is a new type of surfactant with high surface activity [11]. It has been proved that trisiloxane surfactants can effectively reduce the surface tension of water from 72 to 20 mN/m. The molecular formula of trisiloxane is usually C7H22O2Si3. Dense methyl groups are arranged on the main chain, which makes them have higher surface activity than hydrosiloxane [12,13]. At present, trisiloxane surfactants are mostly used as agricultural auxiliaries [14] because they can effectively increase the ductility of aqueous solutions on highly hydrophobic surfaces [15], such as leaves, plastics, and so on. At the same time, they also maintain product activity and good acid and alkali resistance and have better stability than hydrocarbon siloxane [16].
Polyether trisiloxane is a kind of modified silicone surfactant formed by introducing polyether segment modification with excellent extensibility, so that the hydrophobic surface can be well infiltrated, and the hydrophilic surface can be completely spread [17]. It has a good synergistic effect on some hydrophobic products [18], especially coatings [19], cosmetics [20], toiletries [21], etc. However, the application of polyether trisiloxane still faces many challenges. This is due to the fact that, in the silicone skeleton, Si–O–Si and C–O–C are sensitive to hydrolysis [22], and the polarity of the silicon–oxygen bond is strong [23], which easily reacts with acid, alkali, aqueous solution, and alcohol, resulting in hydrolysis. In fact, there are many causes for the hydrolysis of silicone surfactants. More specifically, previous studies have proved that pH and time are the main factors causing hydrolysis. Knoche et al. [24] studied the hydrolytic stability of SilwetL-77. When SilwetL-77 was in weakly acidic or weakly alkaline solutions, it could be stably stored for 60 days. However, it was hydrolyzed quickly in a strong acidic or alkaline solution. The hydrolysis of trisiloxane in acid or alkaline is caused by molecular rearrangement. Therefore, it is very necessary to find ways to improve their hydrolytic stability.
In this paper, methylpropenyl polyether was introduced to change the group grafted on the main chain of trisiloxane to maximize its hydrophilicity [25], and on this basis, to changing the change in extensibility caused by the long chain segment. There are few studies on its hydrolytic stability, and this present paper focuses on the stability and application performances. We have successfully synthesized an alkali-resistant hydrolyzed methyl allyl polyether trisiloxane (MPNTS) with a simple process, and we systematically tested its surface tension, hydrolysis stability, and compatibility. In order to explore its stability and application, this paper provided a new scheme for the application of silicone surfactants in daily chemical products.
2. Experimental
2.1. Materials
1,1,1,3,5,5,5-heptamethyltrisiloxane (Mn 222, AR ≥ 99.5%, MDHM) was produced in Zhejiang Runhe Organic Silicon New Material Co., Ltd. (Hangzhou, China). Methylpropenyl polyether (Mn 380, EO(7)) was produced in Nanjing Jiuxin Chemical Co., Ltd. (Nanjing, China). Chloroplatinum hexahydrate (H2PtCl·6H2O, S) and isopropanol (AR ≥ 99.5%) were purchased from Aladdin Co. (Shanghai, China) High-purity nitrogen was purchased from Guangzhou Yuejia Gas Co., Ltd. (Guangzhou, China).
2.2. Synthesis of MPNTS
First, the chloroplatinic acid hexahydrate was dissolved in isopropanol to prepare a catalyst solution with 2% platinum content. The catalyst was aged for 15 days in the dark to form stable catalytic performance. An amount of 50 g of MDHM was added into a four-necked flask, and 0.15 g of catalyst was also added into the nitrogen atmosphere. The mixture was elevated to a temperature of 85–90 °C to activate the catalyst for 30 min. Then, 82.83 g methacryloyl polyether was added into the flask, the mixture was elevated to a temperature of 110–115 °C, and it was reacted at 115–118 °C in nitrogen atmosphere for 3 h. A light-yellow liquid methallyl polyether trisiloxane was obtained.
2.3. Characterization
2.3.1. FT-IR
The infrared spectra were measured by the full band infrared spectrum workstation (iS50R, Thermo Filsher, Waltham, MA, USA) in ATR mode at ambient temperature. With air as the collection background, 64 scans were conducted for each test, with a resolution of 0.09 cm−1 and a spectral range of 4000–500 cm−1. An amount of 20 mg sample of MPNTS was added to the test platform in a clean and pollution-free environment to obtain the infrared spectrum. Each reported spectrum is the average of 64 scans.
2.3.2. 1H NMR
The 1H NMR was measured by digital superconducting nuclear magnetic resonance spectrometer (600 MHz, Bruker, Switzerland). An amount of 20.3 mg of MPNTS, 20.6 mg of EO(7), and 20.1 mg MDHM were dissolved in 500 µL of deuterated chloroform to prepare 1H NMR test samples. The prepared samples are all transparent and nonmagnetic solutions, which are then injected into the nuclear magnetic tube and scanned 128 times at atmosphere temperature to obtain the 1H NMR spectra.
2.4. Performance Testing
2.4.1. Surface Tension and CMC Test
The surface tension of methylpropenyl polyether modified nonionic silicone surfactant was measured by a contact angle analyzer (DSA-25, KRUSS, Germany). An amount of 100 mg MPNTS was treated with ultra-pure water to 100 mL to 100 mL test solution for the surface tension and critical micelle degree (CMC). By using the pendant drop method, the disposable microsyringe was tested under the lens, and Young’s-Laplace equation was used to fit the profile of water droplets on the needle for 120 s, and the surface tension was obtained. All the results are the average values of three measurements.
2.4.2. Stability Test
An amount of 100 mg organosilicon was dissolved in ultra-pure water to make 100 mL 0.1% (volume fraction) aqueous solution. The pH values of these solutions were 3, 4, 5, 7, 9, and 10, respectively, and then they stayed at ambient temperature for 1 h, 24 h, 7 d, 15 d, 45 d, and 60 d, respectively. The changes in surface tension of the samples under different pH were tested, and the stability of the samples was evaluated.
2.4.3. Compatibility Test
Silicone was, respectively, mixed with cationic surfactant, anionic surfactant, and nonionic surfactant. An amount of 0.1% (volume fraction) of aqueous solutions was prepared at a mass ratio of 1:1, 1:2, 1:5, 1:10, 1:50, and 1:100, respectively, and the changes of surface tension were measured to evaluate the compatibility of silicone with different surfactants.
3. Results and Discussion
3.1. Synthesis of MPNTS
The synthesis of methylpropenyl polyether modified nonionic silicone surfactant (MPNTS) is shown in Figure 1. MPNTS was produced by hydrosilylation of MDHM and methacrylic polyether (EO(7)) in the presence of chloroplatinic acid catalyst at 118 °C. The Si–H bond on the main chain of MDHM reacts with C=C on EO(7) under the action of catalyst to graft the hydrophilic polyether segment onto the silicone main chain. In order to make sure that the Si-H reaction was complete, excessive allyl polyether was used, and the mole ratio of MDHM to allyl polyether was 1:1.05. Hydrosilylation is easily affected by water in air. Therefore, nitrogen should be introduced before the reaction to avoid hydrolysis. In addition, platinum-catalyzed hydrosilylation of olefins is usually accompanied by side reactions, such as dehydrosilanization, olefin hydrogenation, olefin isomerization, olefin oligomerization, and redistribution of hydrogenated silane. For this reason, the reaction temperature must be controlled below 120 °C to facilitate the silane hydrogen reaction.
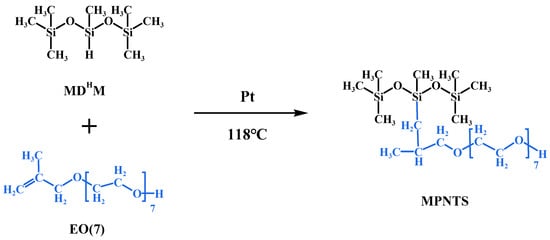
Figure 1.
Synthesis mechanism diagram of MPNTS (MDHM, EO(7), and MPNTS represent 1,1,1,3,5,5,5-heptamethyltrisiloxane, methylpropenyl polyether, and methylpropenyl polyether modified nonionic silicone surfactant, respectively).
3.2. Structural Characterization of MPNTS
3.2.1. FT-IR
The Fourier transform-infrared (FT-IR) spectra of three samples are shown in Figure 2. The peaks at 2150 cm−1 and 916 cm−1 are attributed to the Si–H of heptathyltrisiloxane (Figure 2I). However, they are disappearing in the infrared spectrum of the product (Figure 2III). This proves that, during hydrosilylation, the Si–H of heptathyltrisiloxane fractures in the presence of Pt catalyst and the methyl propenyl polyether grafts onto the main chain of heptathyltrisiloxane. The broad peaks at 1062 cm−1 (Figure 2III) were widened due to the overlap between Si–O–Si and C–O–C. Compared to the IR spectrum of methyl propenyl polyether (Figure 2II), this indicates that the polyether segment has successfully grafted onto the main chain of silicone. The stronger absorption peak at 2870 cm−1 indicates that the amount of –CH2 in the product increases (Figure 2II,III), which also confirms the formation of MPNTS.
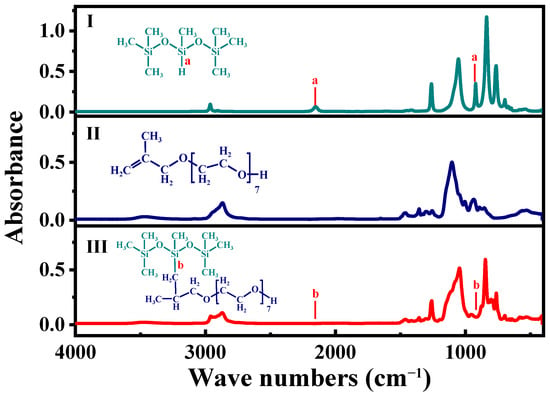
Figure 2.
Infrared spectra of MPNTS (I) 1,1,3,5,5,5-heptathyltrisiloxane, (II) methyl propenyl polyether, and (III) methylpropenyl polyether modified nonionic silicone surfactants, (a,b) represents the characteristic peak of Si–H.
3.2.2. 1H NMR
The 1H NMR spectra of MPNT, 1,1,3,5,5,5-heptathyl trisiloxane and methyl propenyl polyether are shown in Figure 3. In the spectrum of heptamethyltrisiloxane (Figure 3c), The signals corresponding to Si-CH3 are observed at about δ 0.1 ppm, and the signal for the Si–H proton is observed at δ 4.53 ppm. In the methallyl polyether spectrum (Figure 3b), the peak at δ 5.87 ppm is attributed to the C–CH3 protons, and the peaks at δ 5.24 ppm are assigned to C=CH2 protons. The characteristic peak of the Si–H proton and the characteristic peaks of the C=CH2 protons do not appear in the spectrum of MPNTS (Figure 3a), and two new peaks appeared at δ 0.34 ppm and δ 1.50 ppm, which are attributed to the Si–CH2 protons and the Si–C–CH proton, respectively. These proved, again, that the C=C of methyl propenyl polyether has been grafted onto the main chain of heptathyltrisiloxane to form MPNTS.
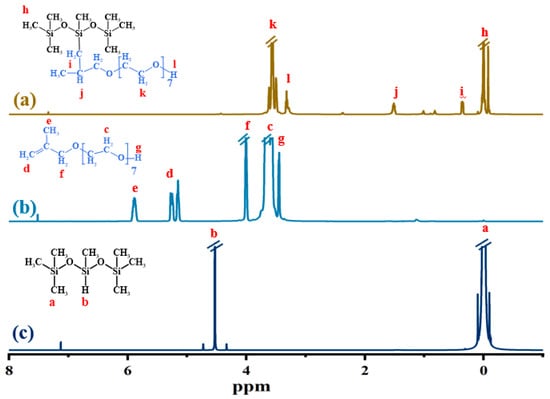
Figure 3.
1H MNR map of MPNTS (a) methylpropenyl polyether modified nonionic silicone surfactants, (b) methylpropenyl polyether, and (c) 1,1,3,5,5,5-heptamethyltrisiloxane. (The letters of the structural formula correspond to the characteristic peaks, representing H elements in different positions).
According to Figure 1, the mole ratio of MDHM to methyl propenyl polyether is 1:1. However, the real mole ratio of MDHM to methyl propenyl polyether in the experiment was 1:1.05. The excess polyether made the active hydrogen of MDHM (Si-H) react completely [26]. According to the performance test results to be described later, the excess polyether hardly affects the surface properties and stability of MPNTS. The precursor of the reaction, heptamethyltrisiloxane, was a hydrophobic substance, so the changes in hydrophobicity can be observed to initially judge the changes in the above structure. Specifically, the whole system was in a clear state before the addition of polyether, but with the addition of polyether, the reaction system would become cloudy. Finally, under the action of the catalyst, the whole system would become a clear and transparent solution again [27]. This is also an easy way to see if the reaction was complete. According to these results, we successfully synthesized our expected target product MPNTS through a simple process, which proved that the synthesis scheme was feasible.
3.3. Surface Properties of MPNTS
Critical micelle concentration (CMC) is an important index to determine the properties of surfactants [28]. It represents the concentration at which the surfactant begins to form a large number of micelles. At the CMC of the surfactant aqueous solution, the hydrophobic groups of the surfactant molecules aggregate to form a hydrophobic region, while the hydrophilic groups outside the hydrophobic groups gather in the water to form a hydrophilic region. Such an arrangement forms a spherical-like structure known as a micelle. The appearance of micelles can vary, depending on their structure and composition. Generally speaking, the outer layer of micelles is the surface layer formed by hydrophilic groups, while the inner layer is the core region formed by the aggregation of hydrophobic groups. According to the morphology of the hydrophobic groups inside the micelles, the shape of the micelles can be spherical, rod-like, plate-like, or other shapes. The appearance and properties of micelles have a significant impact on their applications in various fields. For example, in biology, the structure of micelles is closely related to the formation and function of cell membranes; in the cosmetics industry, and micelles are widely used for skin cleaning and moisturizing purposes. When the solution reaches the critical micelle concentration, the surface tension of the solution decreases to the lowest value [29]. At this time, if the concentration of the surfactant is increased, the surface tension of the solution will no longer decrease, but a large number of micelles will be formed. In this case, the surface tension of the solution is the minimum surface tension that the surfactant can achieve. The smaller the CMC value, the lower the concentration needed for the surfactant to form micelles and the lower the concentration of saturated adsorption on the surface. Therefore, a lower concentration is required to modify surface properties to enable wetting, emulsification, compatibilization, foaming, and other functions. Additionally, the CMC is an important factor in determining the surfactant’s application range.
In this experiment, the surface tension method was adopted, and the CMC value of MPNTS was measured by the linear fitting method. It is considered the inflection point of surface tension with a change in concentration, which refers to the intersection points of the straight lines of surface tension under high and low concentrations. This method was simple, intuitive, and had little error. For NPNTS, it was observed that the surface tension dropped sharply when the concentration was from 1 mg/L to 60 mg/L, as well as when surface tension reduced from 64 mN/m to 20 mN/m. However, the surface tension remained almost unchanged after the concentration was 70 mg/L, and it finally stabilized at 18.68 mN/m (Figure 4). This is because increasing the concentration of the surfactant after the concentration exceeds the CMC can only lead to the formation of more micelles, while the actual surfactant composition in the water is only maintained at the CMC.
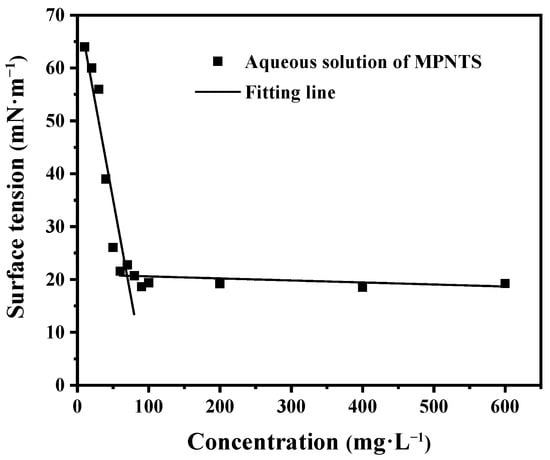
Figure 4.
Effect of concentration of MPNTS on the surface tension of MPNTS aqueous solution.
In order to prove the reliability of the test data, some studies on the determination of CMC were compared. Aula Al Muslim [30], MM Mabrouk [31] et al. used the surface tension method to accurately measure the CMC of surfactants. Based on this, we found, by linear fitting, that the intersection point of surface tension between high and low concentrations of MPNTS was located at 78 mg/L. So, we can confirm that the CMC value of MPNTS is 78 mg/L, and we found that the surface tension at this point was 18.68 mN/m. Since MPNTS has a very low CMC value, a small dosage is sufficient to provide excellent surface properties, and more costs in practical applications will be saved.
3.4. Stable Performance of MPNTS
Hydrolytic stability is the focus of this study. The primary cause for the hydrolysis of trisiloxane is that the Si atom is relatively large and easily polarized. There are 3 d empty orbitals on the atom that can provide bonding conditions. In addition, the polarity Si–O bond is very strong, so it is easy to react with protonic acids, alkali metals, hydroxides, and water, resulting in a decrease in surface activity. Some related studies show that the hydrolysis of trisiloxane is another reason for this phenomenon. For example, Snow et al. [32] believe that the hydrolysis of trisiloxane in acidic or alkaline conditions was due to the rearrangement of the Si–O–Si bond. Therefore, the pH [33] of the aqueous solution is also an important factor affecting its hydrolysis. However, there are relatively few related studies on the hydrolysis of trisiloxane. However, it is an essential consideration for some uses of silicone surfactants. We hope this study can provide a reliable direction for the research field of silicone surfactants.
In order to determine the hydrolytic stability of MPNTS, the surface tensions changes of MPNTS in aqueous solutions at different pH values were measured for different periods of time (Figure 5a). The aqueous solutions of 0.1% (mass fraction) were prepared by adding the same dosage of MPNTS, and the pH values were adjusted to 3, 4, 5, 7, 9, and 10, respectively. The surface tensions of the samples were measured after standing for 3 h, 24 h, 7 d, 15 d, 45 d, and 60 d, respectively. We found that, after standing for 3 h, the surface tension of each aqueous solution at different pH was about 20 mN/m. After 24 h, the surface tension of aqueous solution at pH = 3 began to hydrolyze, and the surface tension rose sharply to 58.75 mN/m. However, it was still stable at other pH values. After standing until the 7th day, the surface tension of the aqueous solution with pH = 3–5 increased, indicating that the aqueous solution had been hydrolyzed. Solutions at other pH values were still stable. After 15 days, the aqueous solutions in acidic conditions had been hydrolyzed nearly completely. After 45–60 days, the aqueous solution at pH = 7–10 was still stable and had not been hydrolyzed, and the surface tension was maintained at 18–20 mN/m.
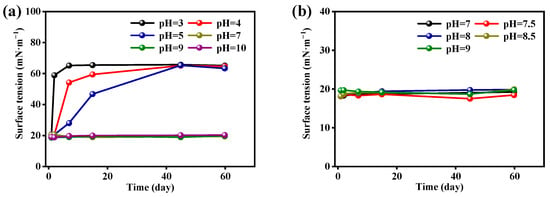
Figure 5.
The effect of time on the surface tension of MPNTS aqueous solutions in different pH values: (a) represents the change in surface tensions of pH = 3, 4, 5, 7, 9, and 10 with time; (b) represents the change in surface tensions of pH = 7, 7.5, 8, 8.5, and 9 with time.
In order to further determine the hydrolytic stability of MPNTS, we prepared MPNTS aqueous solutions with pH = 7, 7.5, 8, 8.5, and 9, and we tested their surface tension changes for 60 days to determine the hydrolytic stability of MPNTS (Figure 5b). The results showed that the surface tensions of MPNTS did not change significantly after standing for 60 days in neutral and alkaline conditions, which proved that MPNTS did not undergo hydrolysis. MPNTS has good stability in neutral and alkaline environments, but it was easily hydrolyzed in acidic conditions. This is due to the Si-O-Si bond in the MPNTS molecule being highly polar, and when it meets H+ or OH− ions, it undergoes molecular rearrangement, resulting in the formation of two new molecules. However, these two molecules exhibit very poor surface properties in aqueous solutions. In acidic aqueous solution, H+ has a strong attack on MPNTS, resulting in the hydrolysis of MPNTS within 24 h. In alkaline and neutral aqueous solutions, the introduction of EO(7) increases the hydrophilicity of MPNTS and its steric hindrance, making it more challenging for water molecules to attack MPNTS. Consequently, the hydrolysis reaction becomes difficult to proceed, and MPNTS can maintain high surface activity in neutral and alkaline environments. The aqueous solution of MPNTS shows good hydrolysis resistance at pH = 7–10, and the product can maintain a longer effective time. In practical application, it can show better stability.
3.5. Compatibility of MPNTS
Compatibility is the basis of the application of surfactants, and the surface properties of ordinary hydrosiloxanes in aqueous solution are limited [34]. In order to improve the use of various surfactants, in the daily preparation of formulations, the form of compounding is often used to integrate various functions of surfactants into one system, so as to achieve a synergistic effect. Surface property of a single surfactant system is often not optimal. In contrast, the adsorption of multiple surfactants in a complex system is considered synergistic, meaning that a small number of adsorbed surfactants can induce significant, ordered changes in oil molecules on the interface. These molecules will attract more surfactants to adsorb. Therefore, two or more surfactants with low affinity can exert better surface properties under the synergistic effect in the same water or oil system. Fainerman V.B. et al. [35,36] discussed these points in detail using anionic, cationic, and nonionic surfactants. This present paper primarily discusses the formation of complex systems of MPNTS and different types of surfactants, and it studies their synergistic properties in the binary system while predicting the solubilization ability of MPNTS.
Compatibility experiments with common cationic surfactants, anionic surfactants, and nonionic surfactants were carried out with MPNTS. The effects of the mass ratio of MPNTS to cationic surfactants on the surface tensions of the aqueous solutions. are shown in Figure 6a. The cationic surfactants used are dodecyl trimethyl ammonium chloride (1231), dodecyl dimethyl benzyl ammonium chloride (1227) and dioctyl dimethyl ammonium chloride (D0821), respectively. The results showed that the surface tensions of 1231, 1227, and D0821 aqueous solutions were 53.13 mN/m, 40.18 mN/m, and 25.31 mN/m, respectively, before cationic surfactants were added to MPNTS. With the addition of MPNTS, the surface tension of the system was significantly reduced, and as the ratio of the two increases, the surface tension decreased. The specific performances were that the surface tension decreases most obviously when the mass ratio of MPNTS to cationic surfactants were at 1:5 and 1:10, showing a strong synergistic effect, and finally the surface tensions of the entire system would be reduced to near the surface tension of aqueous solutions of single component MPNTS. The anionic surfactant was sodium dodecyl benzene sulfonate (LAS). The effects of the mass ratio of MPNTS to anionic surfactant on the surface tensions of the aqueous solutions were shown in Figure 6b. The results showed that the surface tension of the LAS-MPNTS compound system aqueous solutions decreased from 36.20 mN/m of the single component to about 20 mN/m, which further reflected the synergistic effect of MPNTS. The effects of the mass ratio of MPNTS to nonionic surfactant (EN70) on the surface tensions of the aqueous solutions were shown in Figure 6b. The surface tension of nonionic surfactant aqueous solutions was 27.57 mN/m, and the same synergism occurs when MPNTS was added. According to the above results, it can be inferred that most surfactants with surface tension greater than MPNTS (18.68 mN/m) can be well compounded with it and show excellent synergistic effect, thus greatly increasing the surface properties of the system.
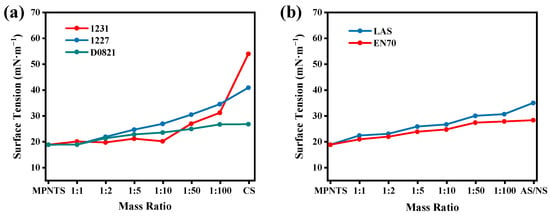
Figure 6.
Surface tension changes of different types of surfactants mixed with MPNTS in different mass ratios: (a) compatibility of MPNTS with cationic surfactants and (b) compatibility of MPNTS with anionic surfactants and nonionic surfactants.
Research indicates that the surfactant system in the binary complex exhibits superior surface activity compared to a single surfactant, with a synergistic effect in reducing surface tension, critical micelle concentration, and mixed micelle formation in the solution under consideration. The synergistic effect of formulation enables the attainment of maximum cleaning, wetting, or solubilizing power with a reduced amount of surfactant, thereby minimizing costs and promoting environmental protection [37]. The above results showed that MPNTS had significant synergistic effect with cationic, anionic. and nonionic surfactants, respectively. Combined with the common functions of all kinds of surfactants, we infer that MPNTS has good compatibility with cationic surfactants and has a good application prospect in printing aids, fungicides, hair conditioners, and other products. Then, it has good compatibility with anionic surfactant and has a good application prospect in detergents, such as detergent and shampoo. Finally, it has good compatibility with nonionic surfactants and has a good application prospect in dispersants, solubilizers, and other products.
4. Conclusions
MPNTS was successfully synthesized by hydrosilylation of 1,1,1,3,5,5,5-heptamethyltrisiloxane and methylpropenyl polyether. The introduction of polyether segments is grafted onto the organosilicon heptamethyltrisiloxane main chain to produce the silicone surfactant MPNTS with hydrolysis stability. MPNTS exhibits both low surface tension (18.68 mN/m) and a critical micelle concentration (CMC) of 78 mg/L, indicating its potency even at small dosages. MPNTS, due to its compatible performance, will have good hydrolysis resistance, and it cannot be hydrolyzed after standing for 60 days under neutral and alkaline conditions. Compatibility performance analysis showed that MPNTS had a good application prospect in daily chemical products. Heptamethyltrisiloxane exhibits good hydrophilicity by introducing hydrophilic groups, which fully demonstrated that the modification of the main chain of siloxane can provide silicone with different properties. This has a lot to do with the introduction of different groups. Future research will focus on introducing different types and quantities of groups to modify silicone and apply MPNTS research to the development of daily chemical products.
Author Contributions
Conceptualization, H.J.; methodology, L.L.; data curation, Y.C.; writing—original draft, Y.C.; writing—review and editing, H.J.; supervision, W.T.; funding acquisition, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was sponsored by the National Natural Science Foundation of China (51972348, 21905313, and 21425627), the National Natural Science Foundation of China-SINOPEC Joint Fund (U1663220), the Guangdong Provincial Key R&D Program (2019B110206002), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01C102) and Guangdong Provincial Key Laboratory of Plant Resources Biorefinery (2021GDKLPRB10).
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sa, L.L.; Rodrigues, R.V.; Alves, V.M.; Gaspar, L.R. Strategies for the evaluation of the eye irritation potential of different types of surfactants and silicones used in cosmetic products. Toxicol. In Vitro 2022, 81, 105351. [Google Scholar] [CrossRef] [PubMed]
- Pukale, D.D.; Bansode, A.S.; Pinjari, D.V.; Kulkarni, R.R.; Sayed, U. Application of Silicone Surfactant Along with Hydrocarbon Surfactants to Textile Washing for the Removal of Different Complex Stains. J. Surfactants Deterg. 2016, 20, 287–295. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Dou, H.; Pei, L. Influence of Ethylene Oxide Content in Nonionic Surfactant to the Hydrolysis of Reactive Dye in Silicone Non-Aqueous Dyeing System. Polymers 2018, 10, 1158. [Google Scholar] [CrossRef]
- Chrusciel, J.J. Modifications of Textile Materials with Functional Silanes, Liquid Silicone Softeners, and Silicone Rubbers—A Review. Polymers 2022, 14, 4382. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Ferreira, V.F.; da Silva, F.d.C.; Freitas, C.S.; Pereira, P.R.; Paschoalin, V.M.F. Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics 2022, 14, 1307. [Google Scholar] [CrossRef]
- Gandov, Z.; Karaibryam, S.; Gandova, V.; Petrova, I. Temperature dependence of surface tension in three eseential oils with application as adjuvants in agriculture. In Proceedings of the 2022 8th International Conference on Energy Efficiency and Agricultural Engineering (EE&AE), Ruse, Bulgaria, 30 June–2 July 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Elencovan, V.; Yahaya, N.; Raoov, M.; Zain, N.N.M. Exploring a novel silicone surfactant-based deep eutectic solvent functionalized magnetic iron particles for the extraction of organophosphorus pesticides in vegetable samples. Food Chem. 2022, 396, 133670. [Google Scholar] [CrossRef]
- Wei, K.S.; Ghani, A.A.A.; Ramle, S.F.M.; Hamid, Z.A.A. Modification on the formulation of silicone based antifoam for industrial surfactant. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2022; p. 050021. [Google Scholar]
- Szymańska, M.; Hoppe, J.; Dutkiewicz, M.; Sobolewski, P.; Palacz, M.; Janus, E.; Zielińska, B.; Drozd, R. Silicone polyether surfactant enhances bacterial cellulose synthesis and water holding capacity. Int. J. Biol. Macromol. 2022, 208, 642–653. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, M.; Feng, S. Synthesis and Solution Behavior of Sulfonate-Based Silicone Surfactants with Specific, Atomically Defined Hydrophobic Tails. Langmuir 2019, 35, 9785–9793. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.B.; Marquardt, D.; Letofsky-Papst, I.; Pabst, G.; Zelisko, P.M. Analysis of Trisiloxane Phosphocholine Bilayers. Langmuir 2017, 33, 4948–4953. [Google Scholar] [CrossRef]
- Pricop, L.; Fortună, M.E.; Popovici, D.; Asandulesa, M.; Racles, C.; Zaltariov, M.F.; Marangoci, N.; Savin, M.; Harabagiu, V. Nickel Complexes of Guanidine Functionalized Trisiloxane. J. Inorg. Organomet. Polym. Mater. 2019, 29, 2024–2034. [Google Scholar] [CrossRef]
- Fuchise, K.; Sato, K.; Igarashi, M. Precise Synthesis of Side-Chain-Functionalized Linear Polysiloxanes by Organocatalytic Ring-Opening Polymerization of Monofunctional Cyclotrisiloxanes. Macromolecules 2021, 54, 5204–5217. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, B.; Bai, Y.; Tai, X.; Wang, G. Effects of butynediol alkoxylate trisiloxane on the surface activity, wetting, and foam properties of polyether trisiloxane. J. Mol. Liq. 2020, 320, 114438. [Google Scholar] [CrossRef]
- Collins, J.K.; Jackson, J.M. Application of a Screening-Level Pollinator Risk Assessment Framework to Trisiloxane Polyether Surfactants. Environ. Toxicol. Chem. 2022, 41, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Tyukanko, V.; Demyanenko, A.; Dyuryagina, A.; Ostrovnoy, K.; Aubakirova, G. Optimizing the Composition of Silicone Enamel to Ensure Maximum Aggregative Stability of Its Suspensions Using Surfactant Obtained from Oil Refining Waste. Polymers 2022, 14, 3819. [Google Scholar] [CrossRef]
- Sankaran, A.; Karakashev, S.I.; Sett, S.; Grozev, N.; Yarin, A.L. On the nature of the superspreaders. Adv. Colloid Interface Sci. 2019, 263, 1–18. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Wang, W.; Tai, X.; Wang, G. Green glucamine-based trisiloxane surfactant: Surface activity, aggregate behavior, and superspreading on hydrophobic surfaces. ACS Sustain. Chem. Eng. 2019, 7, 4390–4398. [Google Scholar] [CrossRef]
- He, S.; Shang, L.; Gao, Y.; Shi, Y.; Tan, F.; Chen, X.; Yue, G. Holistically modulating charge recombination via trisiloxane surface treatment for efficient dye-sensitized solar cells. J. Alloys Compd. 2022, 896, 162864. [Google Scholar] [CrossRef]
- Chaiyabutr, C.; Sukakul, T.; Kumpangsin, T.; Bunyavaree, M.; Charoenpipatsin, N.; Wongdama, S.; Boonchai, W. Ultraviolet filters in sunscreens and cosmetic products—A market survey. Contact Dermat. 2021, 85, 58–68. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Santos, L. Simultaneous determination of synthetic musks and UV-filters in water matrices by dispersive liquid-liquid microextraction followed by gas chromatography tandem mass-spectrometry. J. Chromatogr. A 2019, 1590, 47–57. [Google Scholar] [CrossRef]
- Karasiewicz, J.; Krawczyk, J. Thermodynamic analysis of trisiloxane surfactant adsorption and aggregation processes. Molecules 2020, 25, 5669. [Google Scholar] [CrossRef]
- Kim, D.W.; Noh, S.T. Synthesis and surface-active properties of a trisiloxane-modified oligo (propylene oxide-block-ethylene oxide) wetting agent. J. Appl. Polym. Sci. 2004, 92, 3292–3302. [Google Scholar] [CrossRef]
- Knoche, M.; Tamura, H.; Bukovac, M.J. Performance and stability of the organosilicon surfactant L-77: Effect of pH, concentration, and temperature. J. Agric. Food Chem. 1991, 39, 202–206. [Google Scholar] [CrossRef]
- Nuthalapati, K.; Sheng, Y.-J.; Tsao, H.-K. Abnormal wetting dynamics of Silwet-laden droplets on partially wetting substrates. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129381. [Google Scholar]
- Wang, G.-Y.; Du, Z.-P.; Zhang, W.; Cao, Q.-Y. Synthesis and surface properties of trisiloxane-modified oligo (ethylene oxide). Tenside Surfactants Deterg. 2009, 46, 214–217. [Google Scholar] [CrossRef]
- Qiu, R.; Wu, Z.; Li, S.; Jiang, H.; Wang, Q.; Chen, Y.; Liu, X.; Zhang, L.; Chen, J. Replacing alkyl side chain of non-fullerene acceptor with siloxane-terminated side chain enables lower surface energy towards optimizing bulk-heterojunction morphology and high photovoltaic performance. Sci. China Chem. 2021, 64, 1208–1218. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, B.; Bai, Y.; Tai, X.; Wang, G. Solution properties of mixed system containing butynediol-ethoxylate polysiloxanes and polyether trisiloxane surfactant. Colloid Interface Sci. Commun. 2021, 41, 100367. [Google Scholar] [CrossRef]
- Sagisaka, M.; Endo, T.; Fujita, K.; Umetsu, Y.; Osaki, S.; Narumi, T.; Yoshizawa, A.; Mohamed, A.; Guittard, F.; Hill, C. Very low surface tensions with “Hedgehog” surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127690. [Google Scholar] [CrossRef]
- Muslim, A.A.; Ayyash, D.; Gujral, S.S.; Mekhail, G.M.; Rao, P.P.; Wettig, S.D. Synthesis and characterization of asymmetrical gemini surfactants. Phys. Chem. Chem. Phys. 2017, 19, 1953–1962. [Google Scholar] [CrossRef]
- Mabrouk, M.M.; Hamed, N.A.; Mansour, F.R. Physicochemical and electrochemical methods for determination of critical micelle concentrations of surfactants: A comprehensive review. Mon. Chem. Chem. Mon. 2022, 153, 125–138. [Google Scholar] [CrossRef]
- Snow, S.A.; Fenton, W.N.; Owen, M.J. Synthesis and characterization of zwitterionic silicone sulfobetaine surfactants. Langmuir 1990, 6, 385–391. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Chen, L.; Hao, L.; Chen, H.; Zhou, X. Carboxymethyl chitosan grafted trisiloxane surfactant nanoparticles with pH sensitivity for sustained release of pesticide. Carbohydr. Polym. 2020, 243, 116433. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, H. Synergistic Solubilization of Phenanthrene by Mixed Micelles Composed of Biosurfactants and a Conventional Non-Ionic Surfactant. Molecules 2020, 25, 4327. [Google Scholar] [CrossRef] [PubMed]
- Fainerman, V.B.; Aksenenko, E.V.; Kovalchuk, V.I.; Mucic, N.; Javadi, A.; Liggieri, L.; Ravera, F.; Loglio, G.; Makievski, A.V.; Schneck, E.; et al. New view of the adsorption of surfactants at water/alkane interfaces—Competitive and cooperative effects of surfactant and alkane molecules. Adv. Colloid Interface Sci. 2020, 279, 102143. [Google Scholar] [CrossRef] [PubMed]
- Ananth, R.; Snow, A.W.; Hinnant, K.M.; Giles, S.L.; Farley, J.P. Synergisms between siloxane-polyoxyethylene and alkyl polyglycoside surfactants in foam stability and pool fire extinction. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123686. [Google Scholar] [CrossRef]
- Szumała, P.; Mówińska, A. Perfectly Wetting Mixtures of Surfactants from Renewable Resources: The Interaction and Synergistic Effects on Adsorption and Micellization. J. Surfactants Deterg. 2016, 19, 437–445. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).