New Composite Materials with Cross-Linked Structures Based on Grafted Copolymers of Acrylates on Cod Collagen
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Cod Collagen
2.3. Synthesis of Graft Copolymers under Photocatalysis Conditions
2.4. Analysis of Molecular Weight Characteristics by GPC
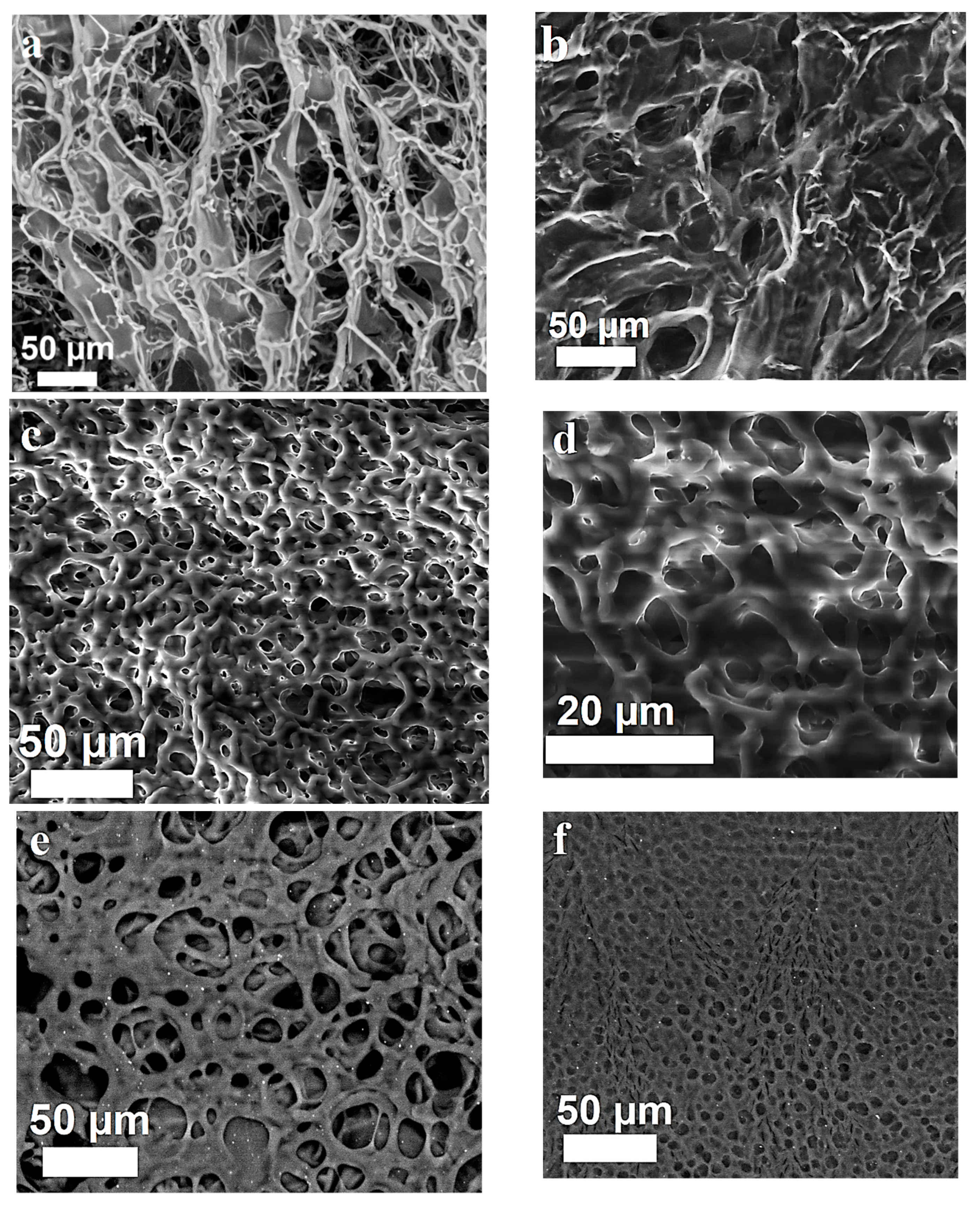
2.5. Scanning Electron Microscopy
2.6. Elemental Analysis of Copolymers
2.7. Freeze Drying
2.8. Tests of Polymer Films for Resistance to the Action of Microscopic Fungi
3. Results and Discussion
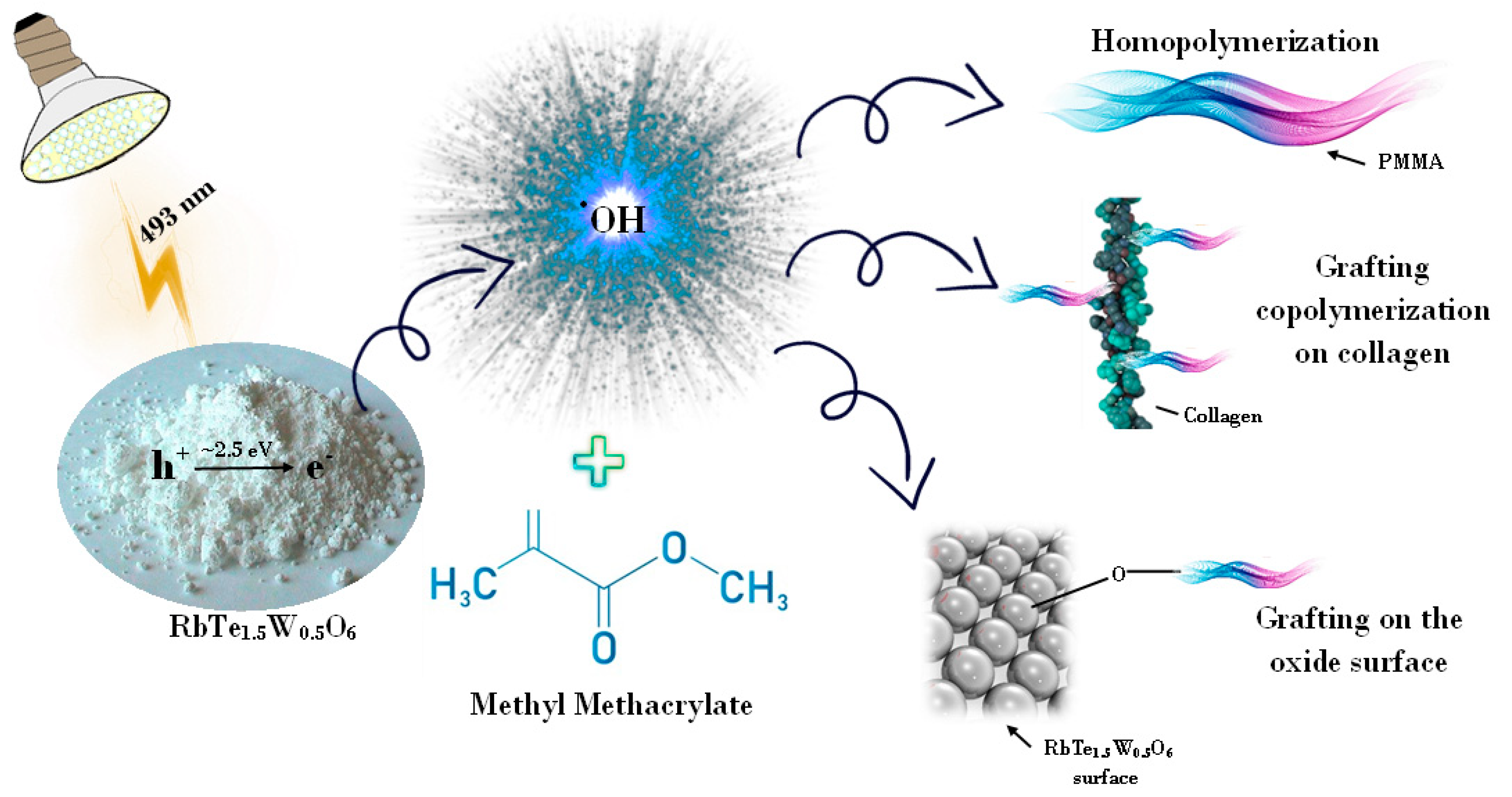
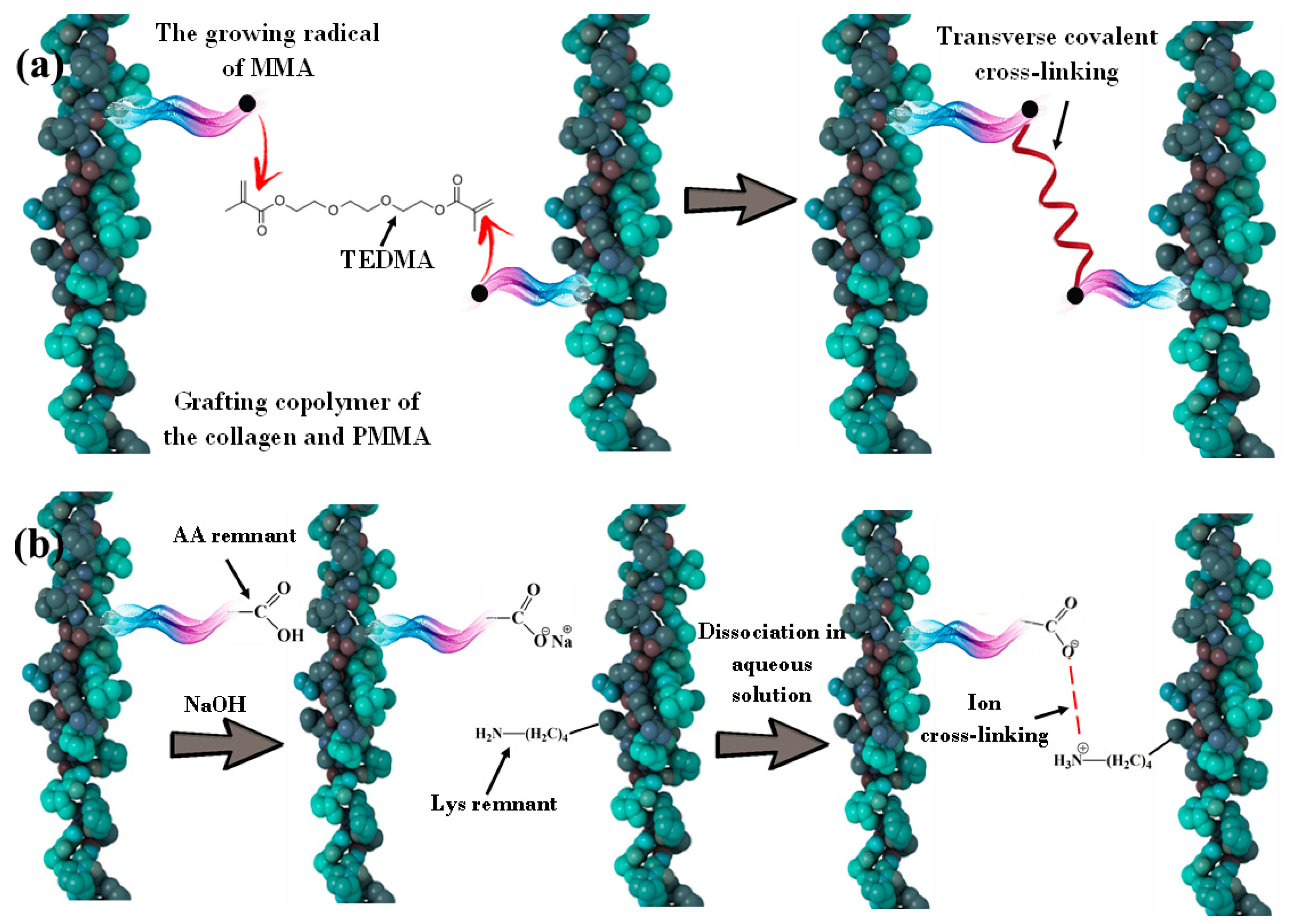
- The irradiated complex oxide generates electron–hole pairs, which leads to different radical formations [37,40]. There is a possibility of several chemical reactions occurring in the mixture. Collagen and MMA grafting are associated with an extremely active hydroxyl radical in the aqueous dispersion (Figure 1).
- The hydroxyl radical forms oxygen- or carbon-centered radicals, which are more stable than the HO• radical in the collagen macromolecule. It is related to separation of the hydrogen atom from the hydroxyl group in the amino acid residue (for example, hydroxyproline) or the hydrocarbon part of the amino acid residue molecule (for example, alanine) [41]. These radicals interact with the monomer and form a grafted synthetic fragment. Simultaneously, there is MMA grafting on the surface of RbTe1.5W0.5O6, and the formation of the MMA polymer is due to the reaction between hydroxyl radical and monomer [10]. Schematically, the reactions that occurred in a mixture of catalyst, MMA, and CC are shown in Figure 1.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gold, M.H.; Sadick, N.S. Optimizing outcomes with polymethylmethacrylate fillers. Cosmet. Dermatol. 2018, 17, 298–304. [Google Scholar] [CrossRef]
- Nishimura, S.; Hokazono, N.; Taki, Y.; Motoda, H.; Morita, Y.; Yamamoto, K.; Higashi, N.; Koga, T. Photocleavable peptide−poly(2-hydroxyethyl methacrylate) hybrid graft copolymer via postpolymerization modification by click chemistry to modulate the cell affinities of 2D and 3D materials. ACS Appl. Mater. Interfaces 2019, 11, 24577–24587. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cheng, S.; Lei, J.; Hang, Y.; Liu, Q.; Wang, H.; Yuan, L. Heparin mimics and fibroblast growth factor-2 fabricated nanogold composite in promoting neural differentiation of mouse embryonic stem cells. J. Biomater. Sci. Polym. Ed. 2020, 31, 1623–1647. [Google Scholar] [CrossRef]
- Qi, D.; Wu, S.; Kuss, M.A.; Shi, W.; Chung, S.; Deegan, P.T.; Kamenskiy, A.; He, Y.; Duan, B. Mechanically robust cryogels with injectability and bioprinting supportability for adipose tissue engineering. Acta Biomater. 2018, 74, 131–142. [Google Scholar] [CrossRef]
- Gamo, R.; Pinedo, F.; Viecente, J.; Naz, E.; Calzado, L.; Ruiz-Genao, D.; Gomez de la Fuente, E.; Alvarez, G.; Arranz, E.; Lopez-Estebaranz, J.-L. Keratoacanthoma-like reaction after a hyaluronic acid and acrylic hydrogel cosmetic filler. Dermatol. Surg. 2008, 34, 954–959. [Google Scholar] [CrossRef]
- Wolfram, D.; Tzankov, A.; Piza-Katzer, H. Surgery for foreign body reactions due to injectable fillers. Dermatology 2006, 213, 300–304. [Google Scholar] [CrossRef]
- Mukhametov, U.F.; Lyulin, S.V.; Borzunov, D.Y.; Gareev, I.F.; Beylerli, O.A.; Yang, G. Alloplastic and implant materials for bone grafting: A literature review. Creat. Surg. Oncol. 2021, 11, 343–353. [Google Scholar] [CrossRef]
- Nashchekina, Y.A.; Lukonina, O.A.; Mikhailova, N.A. Chemical crosslinking agents for collagen: Interaction mechanisms and prospects for use in regenerative medicine. Cytology 2020, 62, 459–472. [Google Scholar] [CrossRef]
- Kadimaliev, D.; Parchaykina, O.; Zamylina, L.; Cousin, E.; Mamin, B.; Mishkin, V.; Marisova, Y. Biodegradable Film. RF Patent No. 2564824 C1, 10 October 2015. [Google Scholar]
- Semenycheva, L.; Chasova, V.; Fukina, D.; Koryagin, A.; Valetova, N.; Suleymanov, E. Synthesis of polymethyl-methacrylate–collagen-graft copolymer using a complex oxide RbTe1.5W0.5O6 photocatalyst. Polym. Sci. Ser. D 2022, 5, 110–117. [Google Scholar] [CrossRef]
- Semenycheva, L.; Uromicheva, M.; Chasova, V.; Fukina, D.; Koryagin, A.; Valetova, N.; Suleymanov, E. Synthesis of grafted polybutylacrylate copolymer on fish collagen using a photocatalyst—Complex oxide RbTe1,5W0,5O6. Proc. Univ. Appl. Chem. Biotechnol. 2022, 12, 97–108. [Google Scholar] [CrossRef]
- Uromicheva, M.A.; Kuznetsova, Y.L.; Valetova, N.B.; Mitin, A.V.; Semenycheva, L.L.; Smirnova, O.V. Synthesis of grafted polybutyl acrylate copolymer on fish collagen. Proc. Univ. Appl. Chem. Biotechnol. 2021, 11, 16–25. [Google Scholar] [CrossRef]
- Chasova, V.; Semenycheva, L.; Egorikhina, M.; Charykova, I.; Linkova, D.; Rubtsova, Y.; Fukina, D.; Koryagin, A.; Valetova, N.; Suleymanov, E. Gelatin as an alternative to cod collagen in hybrid materials for regenerative medicine. Macromol. Res. 2022, 30, 212–221. [Google Scholar] [CrossRef]
- Semenycheva, L.; Chasova, V.; Fukina, D.; Koryagin, A.; Belousov, A.; Valetova, N.; Suleimanov, E. Photocatalytic synthesis of materials for regenerative medicine using complex oxides with β-pyrochlore structure. Life. 2023, 13, 352. [Google Scholar] [CrossRef]
- Chasova, V.; Fukina, D.; Buryakov, A.; Zhizhin, E.; Koroleva, A.; Semenycheva, L.; Suleymanov, E. The effect of transformations of methyl methacrylate during photocatalysis in the presence of RbTe1,5W0,5O6 on the change in the surface of the complex oxide. Proc. Univ. Appl. Chem. Biotechnol. 2022, 12, 208–221. [Google Scholar] [CrossRef]
- Suleymanov, E.; Chasova, V.; Valetova, N.; Semenycheva, L.; Fukina, D.; Koryagin, A.; Smirnov, V.; Smirnova, O. Method of Obtaining Methylmethacrylate Copolymers. RF Patent No. 2777896 C1, 11 August 2022. [Google Scholar]
- Nikitin, I.G.; Baykova, I.E.; Gogova, L.M. Pegylated medicinal preparations: The current state of the problem and prospects. Lechebnoye Delo. 2005, 4, 18–24. [Google Scholar]
- Porfirieva, N.N.; Mustafina, R.I.; Khutoryansky, V.V. Pegylated systems in pharmaceuticals. Polym. Sci. Ser. C 2020, 62, 66–80. [Google Scholar] [CrossRef]
- Martinez, A.P.; Qamar, B.; Marin, A.; Fuerst, T.R.; Muro, S.; Andrianov, A.K. Biodegradable “Scaffold” polyphosphazenes for non-covalent PEGylation of proteins. In Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis; Andrianov, A., Allcock, H., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; pp. 121–141. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Rajulu, A.V.; Ramakrishn, S.; Thomas, S.; Pruncu, C.I. Development of biocomposite films from natural protein sources for food packaging applications: Structural characterization and physicochemical properties. J. Appl. Polym. Sci. 2022, 139, 51665. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, G.; Fu, M.; Chen, C.; Fu, W.; Zhang, Z.; Xia, H.; Stadler, F.J. Designing a multifaceted bio-interface nanofiber tissue-engineered tubular scaffold graft to promote neo-vascularization for urethral regeneration. J. Mater. Chem. B 2020, 8, 1748–1758. [Google Scholar] [CrossRef]
- Zalipsky, S. Active Carbonates of Polyalkylene Oxides for Modification of Polypeptides. US Patent No. 5122614A, 16 June 1992. [Google Scholar]
- Gelli, R.; Mugnaini, G.; Bolognesi, T.; Bonini, M. Cross-linked porous gelatin microparticles with tunable shape, size, and porosity. Langmuir 2021, 37, 12781–12789. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Y.; He, C.; Kang, Y.; Zhou, J. Ionically crosslinked chitosan/poly(acrylic acid) hydrogels with high strength, toughness and antifreezing capability. Carbohyd. Polym. 2020, 242, 116420. [Google Scholar] [CrossRef]
- Fatkhutdinova, N.L.; Vasiliev, A.V.; Bukharova, T.B.; Osidak, E.O.; Starikova, N.V.; Domogatsky, S.P.; Kulakov, D.V.; Goldstein, A.A. Prospects of using collagen hydrogel as a base for cured and activated bone-plastic materials. Stomatologiia 2018, 6, 78–83. [Google Scholar] [CrossRef]
- Park, K.M.; Park, K.D.; Sevastianov, V.I.; Nemetz, E.A.; Vasilets, V.N. In situ crosslinkable hydrogels for engineered cellular microenvironment. Regen. Med. Cell. Technol. 2017, 19, 53–64. [Google Scholar] [CrossRef]
- Wang, G.; Lin, Z.; Jin, S.; Li, M.; Jing, L. Gelatin-derived honeycomb like porous carbon for high mass loading supercapacitors. J. Energy Storage. 2022, 45, 103525. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, B.; Li, D.; He, M.; Han, Q.; Chang, J. Remediation and resource utilization of chromium(III)-containing tannery effluent based on chitosan-sodium alginate hydrogel. Carbohydr. Polym. 2022, 284, 119179. [Google Scholar] [CrossRef]
- Dhingra, K.; Dinda, A.K.; Kottarath, S.K.; Chaudhari, P.K.; Verma, F. Mucoadhesive silver nanoparticle-based local drug delivery system for peri-implantitis management in COVID-19 era. Part 1: Antimicrobial and safety in-vitro analysis. J. Oral Biol. Craniofac. Res. 2022, 12, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Fatima, M.; Khan, A.Q.; Naeem, Z.; Javaid, S.; Sajid, N.; Habib, A. Hydrogels: A novel drug delivery system. J. Biomed. Res. Environ. Sci. 2020, 1, 439–451. [Google Scholar] [CrossRef]
- Saha, R.; Patkar, S.; Maniar, D.; Pillai, M.M.; Tayalia, P. A bilayered skin substitute developed using an eggshell membrane crosslinked gelatin–chitosan cryogel. Biomater. Sci. 2021, 9, 7921–7933. [Google Scholar] [CrossRef] [PubMed]
- Do Livramento Linhares Rodrigues Menezesa, M.; da Rocha Pires, N.; Rodrigues da Cunha, P.L.; de Freitas Rosa, M.; Silva de Souza, B.W.; Pessoa de Andrade Feitosa, J.; de Sá Moreira de Souza Filho, M. Effect of tannic acid as crosslinking agent on fish skin gelatin-silver nanocomposite film. Food Packag. Shelf Life. 2019, 19, 7–15. [Google Scholar] [CrossRef]
- Liguori, A.; Uranga, J.; Panzavolta, S.; Guerrero, P.; de la Caba, K.; Focarete, M.L. Electrospinning of fish gelatin solution containing citric acid: An environmentally friendly approach to prepare crosslinked gelatin fibers. Materials 2019, 12, 2808. [Google Scholar] [CrossRef]
- Moghadas, B.; Solouk, A.; Sadeghi, D. Development of chitosan membrane using non-toxic crosslinkers for potential wound dressing applications. Polym. Bull. 2021, 78, 4919–4929. [Google Scholar] [CrossRef]
- da Silva, M.T.S.; Pinto, J.C. Production of crosslinked gelatin beads in inverse suspension processes. Polym. Eng. Sci. 2018, 59, 519–525. [Google Scholar] [CrossRef]
- Medum. Available online: https://medum.ru/e1521 (accessed on 16 March 2023).
- Fukina, D.G.; Koryagin, A.V.; Koroleva, A.V.; Zhizhin, E.V.; Suleimanov, E.V.; Kirillova, N.I. Photocatalytic properties of β-pyrochlore RbTe1.5W0.5O6 under visible-light irradiation. J. Solid State Chem. 2021, 300, 122235. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Kuznetsova, J.L.; Valetova, N.B.; Geras’kina, E.V.; Tarankova, O.A. Method for Production of Acetic Dispersion of High Molecular Fish Collagen. RF Patent No. 2567171 C1, 10 November 2015. [Google Scholar]
- Kirillov, V.N.; Berenson, V.F.; Krashakov, Y.F.; Skribachilin, V.B.; Semenov, S.A.; Popovkin, B.A.; Ugarova, N.N.; Brovko, L.Y.; Polyakova, A.V.; Ivanova, I.G.; et al. Unified system of corrosion and ageing protection. Polymer materials and their components. Methods of laboratory tests for mould resistance. Gosstandart of the USSR, no. 9.049-91, 1991. Available online: https://allgosts.ru/19/040/gost_9.049-91 (accessed on 1 March 2023).
- Fukina, D.G.; Suleimanov, E.V.; Boryakov, A.V.; Zubkov, S.Y.; Koryagin, A.V.; Volkova, N.S.; Gorshkov, A.P. Structure analysis and electronic properties of ATe4+0.5Te6+1.5−xM6+xO6 (A=Rb, Cs, M6+=Mo,W) solid solutions with β-pyrochlore structure. J. Solid State Chem. 2021, 293, 121787. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Egorikhina, M.N.; Chasova, V.O.; Valetova, N.B.; Fukina, D.G.; Sukhareva, A.A.; Ostrosablin, A.N.; Kobyakova, I.I.; Farafontova, E.A.; Rubtsova, Y.P. An environmentally friendly solution related to the use of fish production waste to manufacture new materials for biomedicine. Examines Mar. Biol. Oceanogr. 2022, 5, 000606. [Google Scholar] [CrossRef]
- Erofeev, V.T.; Smirnov, V.F.; Morozov, E.A. Microbiological Degradation of Materials; Association of Construction Universities: Moscow, Russia, 2008; p. 123. [Google Scholar]
- Eliazyan, G.A.; Markaryan, S.M.; Paronikyan, A.E.; Margaryan, L.Y. Study of biocidal treatment of the leather of medieval book covers by the method of retanning with an aluminum complex stabilized with trimethylolmelem. Proc. NPU Armen. Metall. Mater. Sci. Min. Eng. 2021, 1, 1–82. [Google Scholar]
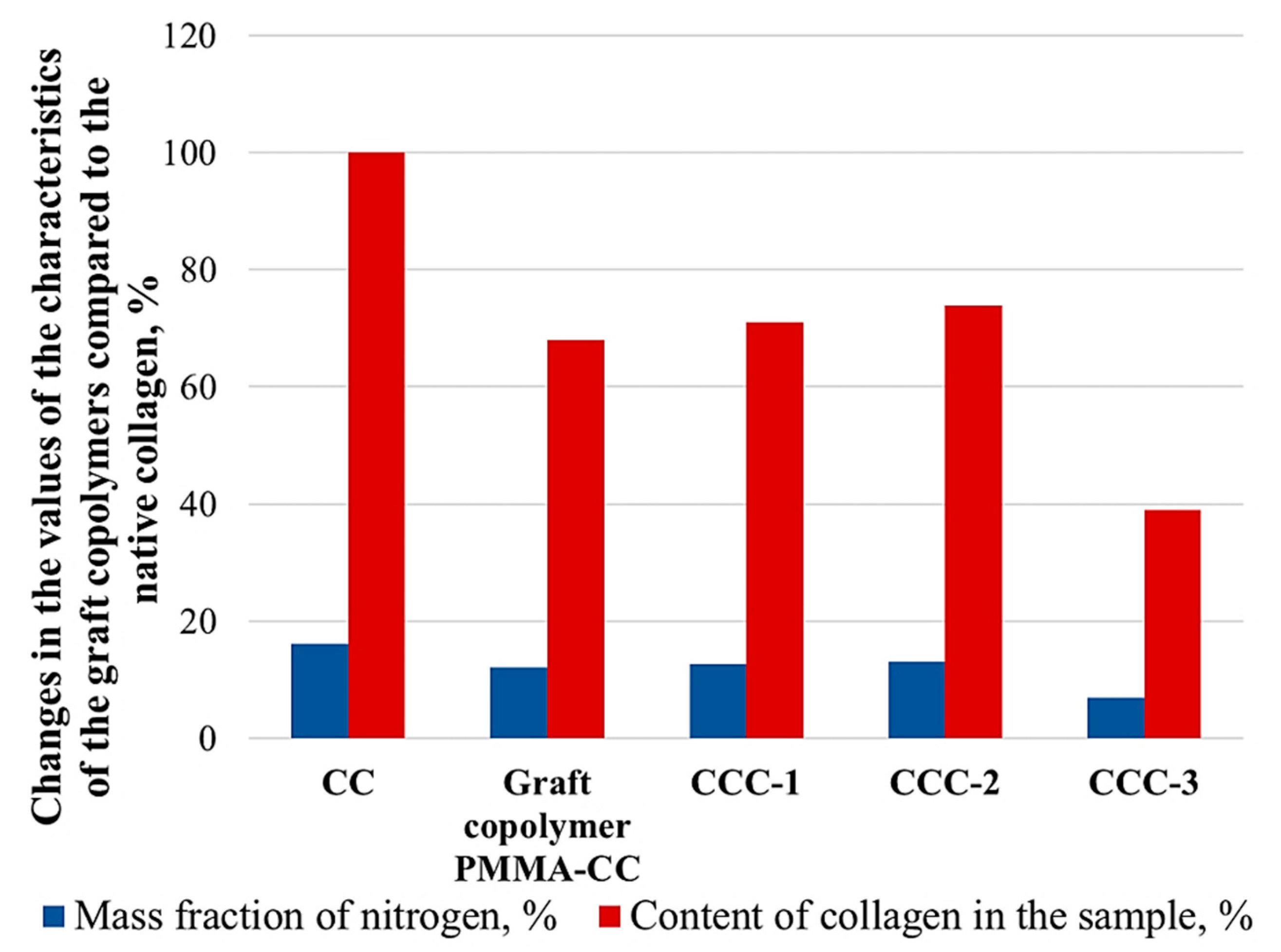


| Sample | Content | Ratio of Components, wt.% | Grafting, % |
|---|---|---|---|
| CCC-1 | CC:MMA:TEDMA:AA:water | 4.35:4.35:0.03:4.34:86.95 | 29 |
| CCC-2 | CC:MMA:TEDMA:AA:water | 8.00:6.00:0.05:6.00:79.95 | 26 |
| CCC-3 | CC:MMA:TEDMA:AA:PEG:water | 7.7:3.80:0.05:3.80:7.7:76.88 | 61 |
| Score | Score Characteristic |
|---|---|
| 0 | The germination of spores and conidia was not observed under a microscope |
| 1 | Germinated spores and slightly developed mycelium were visible under a microscope |
| 2 | A developed mycelium was clearly visible under a microscope; sporulation could be also observed |
| 3 | Mycelium and/or sporulation were barely visible to the naked eye, but clearly visible under a microscope |
| 4 | The development of fungi was clearly visible to the naked eye, covering less than 25% of the test surface |
| 5 | The development of fungi was clearly visible to the naked eye, covering more than 25% of the test surface |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludmila, S.; Victoria, C.; Angelina, S.; Diana, F.; Andrey, K.; Natalia, V.; Olga, S.; Evgeny, S. New Composite Materials with Cross-Linked Structures Based on Grafted Copolymers of Acrylates on Cod Collagen. Appl. Sci. 2023, 13, 5455. https://doi.org/10.3390/app13095455
Ludmila S, Victoria C, Angelina S, Diana F, Andrey K, Natalia V, Olga S, Evgeny S. New Composite Materials with Cross-Linked Structures Based on Grafted Copolymers of Acrylates on Cod Collagen. Applied Sciences. 2023; 13(9):5455. https://doi.org/10.3390/app13095455
Chicago/Turabian StyleLudmila, Semenycheva, Chasova Victoria, Sukhareva Angelina, Fukina Diana, Koryagin Andrey, Valetova Natalia, Smirnova Olga, and Suleimanov Evgeny. 2023. "New Composite Materials with Cross-Linked Structures Based on Grafted Copolymers of Acrylates on Cod Collagen" Applied Sciences 13, no. 9: 5455. https://doi.org/10.3390/app13095455
APA StyleLudmila, S., Victoria, C., Angelina, S., Diana, F., Andrey, K., Natalia, V., Olga, S., & Evgeny, S. (2023). New Composite Materials with Cross-Linked Structures Based on Grafted Copolymers of Acrylates on Cod Collagen. Applied Sciences, 13(9), 5455. https://doi.org/10.3390/app13095455







