Arsenic Contamination in Groundwater, Soil and the Food-Chain: Risk Management in a Densely Populated Area (Versilia Plain, Italy)
Abstract
1. Introduction
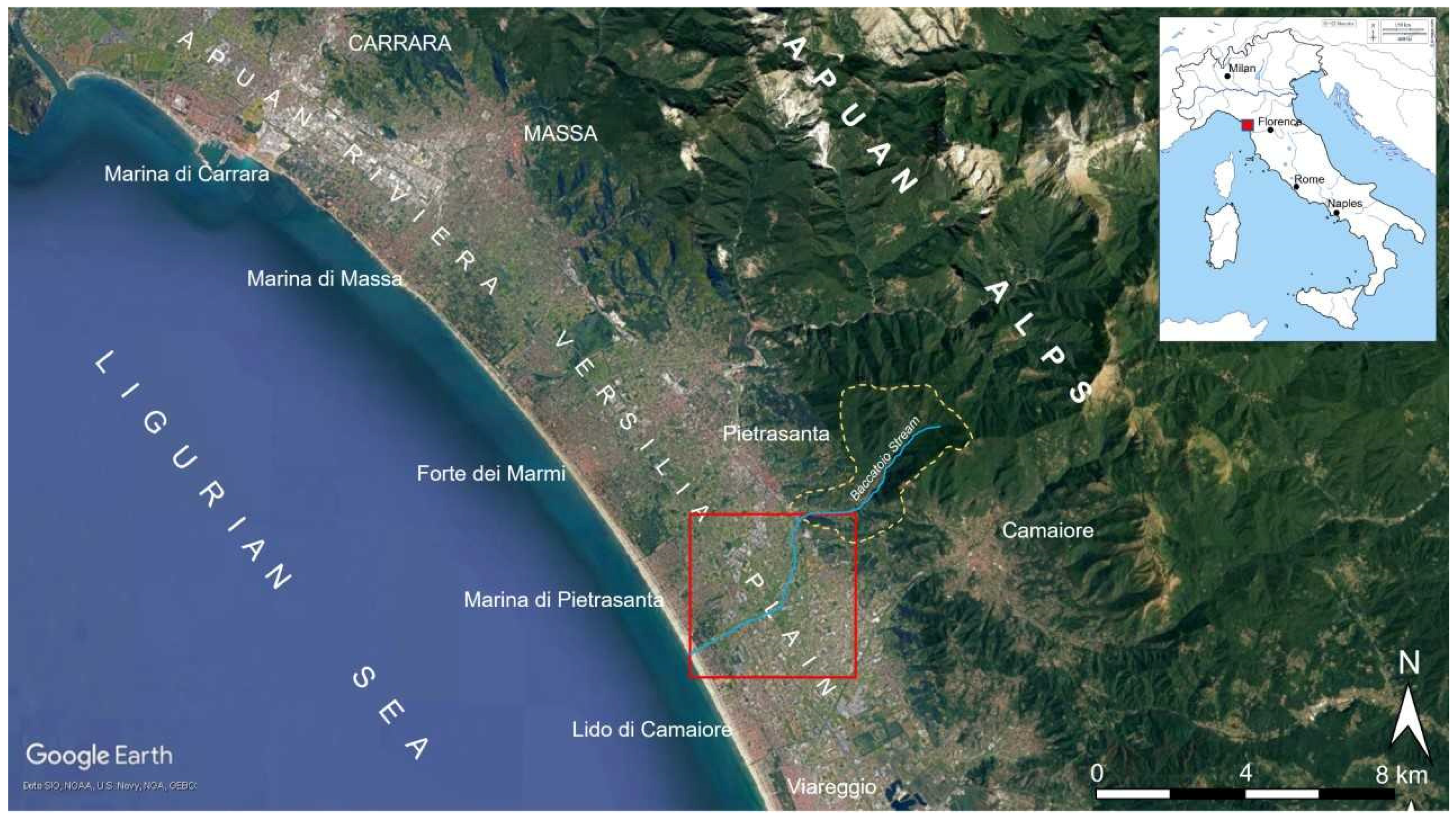
2. Geological and Hydrogeological Setting
3. Materials and Methods
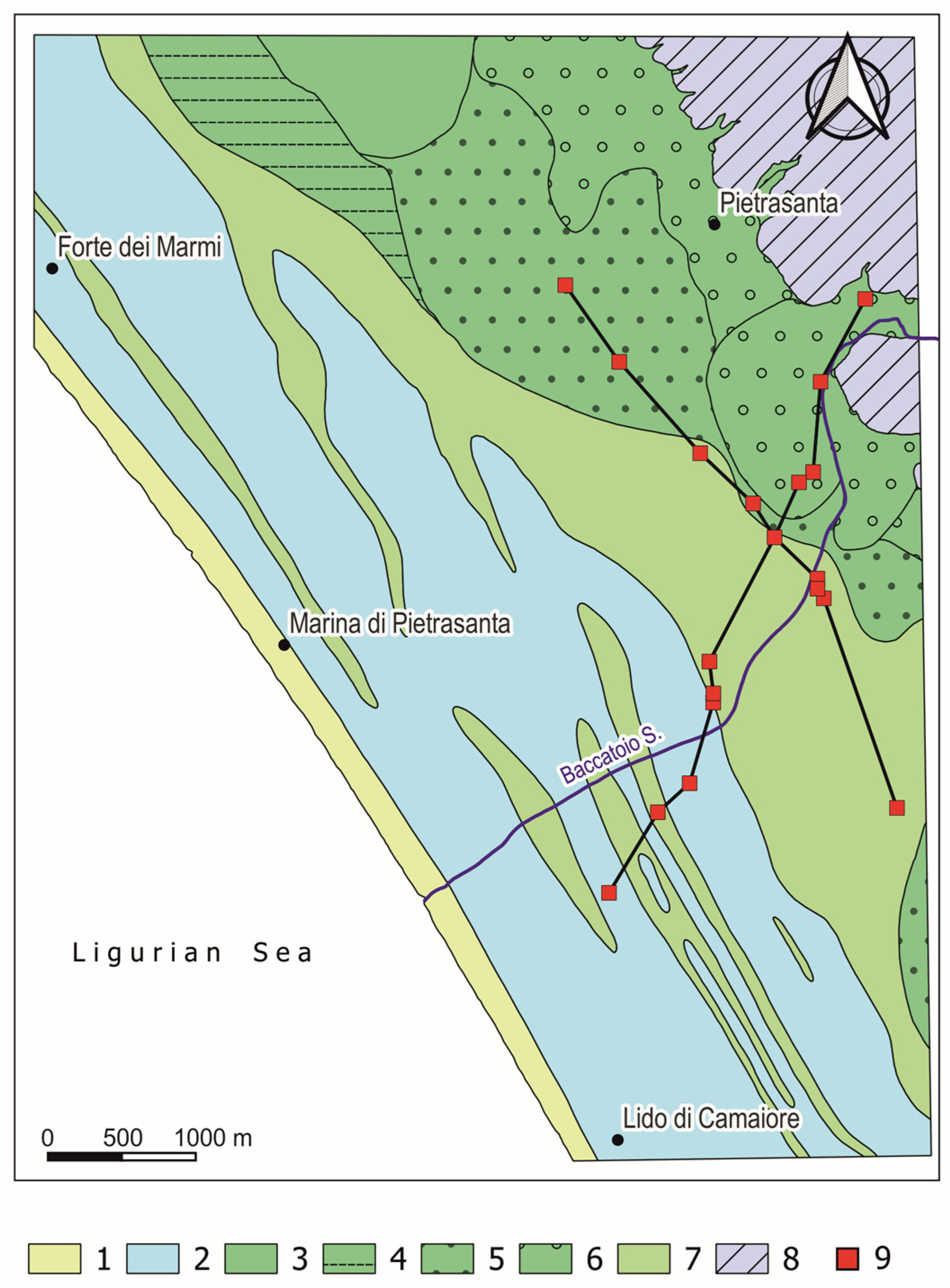
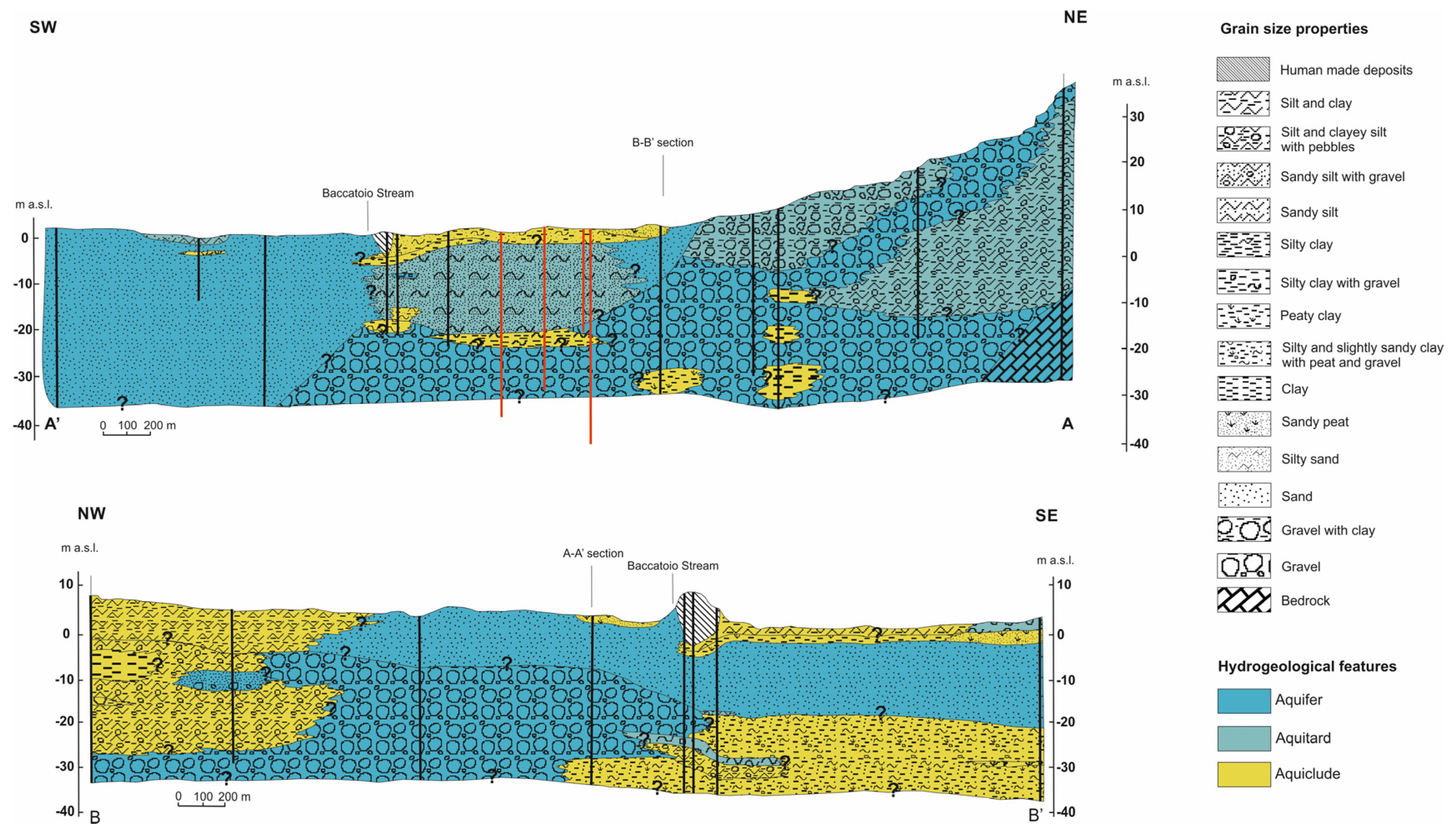
3.1. Hydrostratigraphic Reconstruction
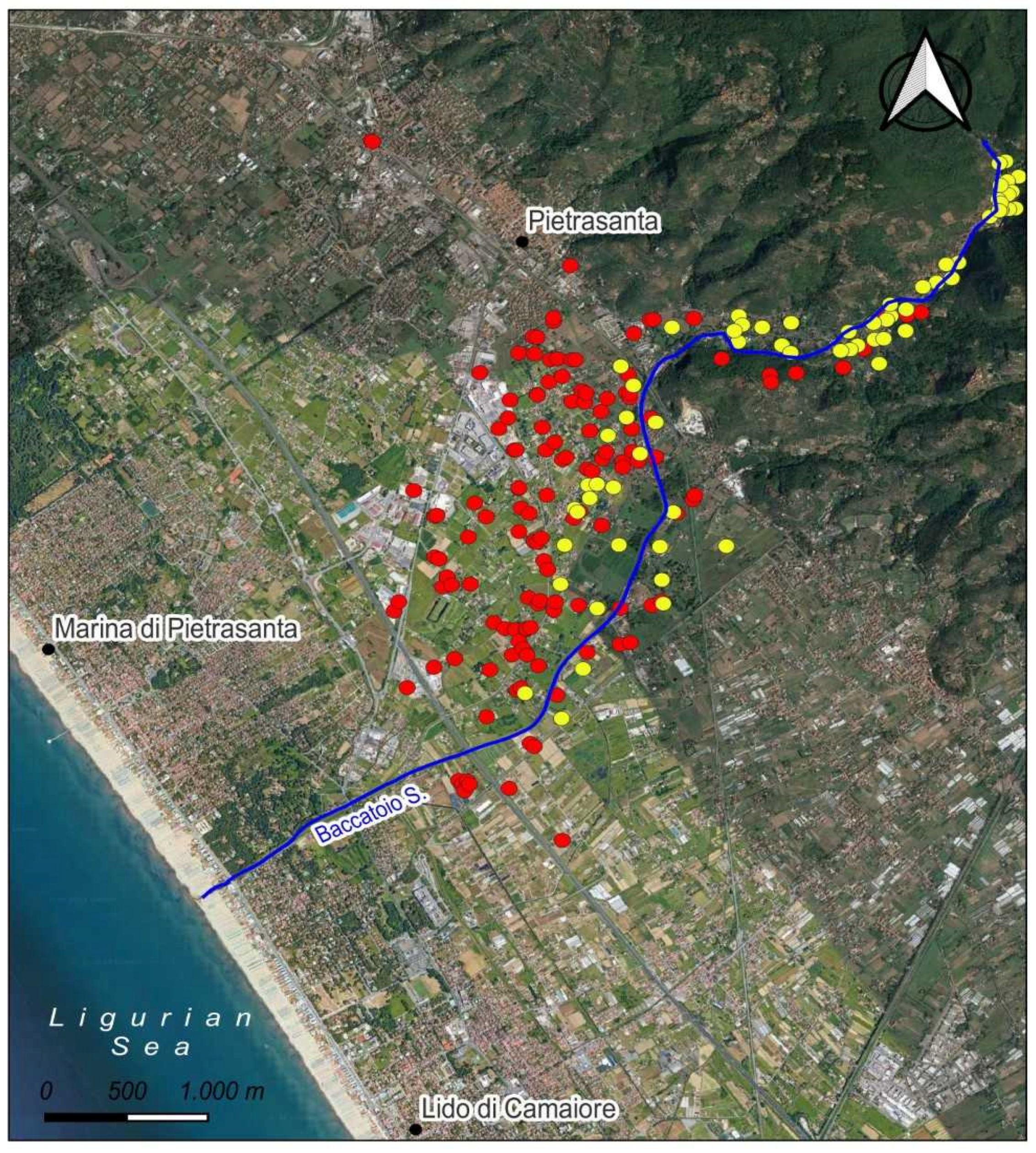
3.2. Water
3.3. Vegetable and Soil
3.4. Risk Analysis
4. Results
4.1. Hydrostratigraphic Structure
- -
- deposits associated with fluvial processes, mainly consisting of coarse material (gravel) in a finer matrix (sand, silt, clay), especially in the fan area, whereas the fine matrix seems to decrease towards the SW;
- -
- deposits resulting from marine processes, mainly sandy with some lenses of finer grain size (silty sands);
- -
- mixed lacustrine and marsh deposits, consisting of mainly clayey and silty sediments, including peaty levels; these deposits can also occur as lenses within other deposits.
- -
- aquifers—mainly gravelly and sandy horizons (sky blue color in sections A-A′ and B-B′);
- -
- aquitards—gravels in silty and silty–clayey matrix; silty sands, sandy peats, sandy silts; gravelly silts (light sky blue color in sections A-A′ and B-B′);
- -
- aquicludes—predominantly clayey and clayey–silty and peaty deposits (yellow color in sections A-A′ and B-B′).
4.2. Water Chemistry
4.3. Soils and Vegetables
4.4. Risk Analysis
5. Discussion
5.1. Source and Fate of Arsenic Contamination
5.2. Soil, Vegetables and Risk Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Enviroin. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Huhes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Hopenhayn, C. Arsenic in drinking water: Impact on human health. Elements 2006, 2, 103–107. [Google Scholar] [CrossRef]
- Ravencroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; RGS-IBG Book Series; Wiley-Blackwell: Hoboken, NJ, USA, 2009; 616p. [Google Scholar]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Majumder, S.; Banik, P. Geographical variation of arsenic distribution in paddy soil, rice and rice-based products: A meta-analytic approach and implications to human health. J. Environ. Manag. 2019, 233, 184–199. [Google Scholar] [CrossRef]
- WHO. Arsenic. In Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1993; Volume 1. [Google Scholar]
- Smedley, P.L. Arsenic in groundwater. In Arsenic in Ground Water; Welch, A.H., Stollenwerk, K.G., Eds.; Geochemistry and Occurrence; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2003; pp. 179–209. [Google Scholar]
- Charlet, L.; Polya, D.A. Arsenic in shallow, reducing groundwaters in southern Asia: An environmental health disaster. Elements 2006, 2, 91–96. [Google Scholar] [CrossRef]
- Mukherjee, A.; Verma, S.; Gupta, S.; Henke, K.R.; Bhattacharya, P. Influence of tectonics, sedimentation and aqueous flow cycles on the origin of global groundwater arsenic: Paradigms from three continents. J. Hydrol. 2014, 518, 284–288. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Ashekuzzaman, S.M.; Jiang, A.; Sharifuzzaman, S.M.; Chowdhury, S.R. Arsenic Contaminated Groundwater and Its Treatment Options in Bangladesh. Int. J. Environ. Res. Public Health 2013, 10, 18–46. [Google Scholar] [CrossRef]
- Abbasnejad, A.; Mirzaie, A.; Derakhshani, R.; Esmaeilzadeh, E. Arsenic in groundwaters of the alluvial aquifer of Bardsir plain, SE Iran. Environ. Earth Sci. 2013, 69, 2549–2557. [Google Scholar] [CrossRef]
- Webster, J.G.; Nordstrom, D.K. Geothermal arsenic. In Arsenic in Ground Water; Welch, A.H., Stollenwerk, K.G., Eds.; Geochemistry and Occurrence; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2003; pp. 101–125. [Google Scholar]
- Bundschuh, J.; Maity, J.P. Geothermal arsenic: Occurrence, mobility and environmental implications. Renew. Sustain. Energy Rev. 2015, 42, 1214–1222. [Google Scholar] [CrossRef]
- Ortega-Guerrero, A. Evaporative concentration of arsenic in groundwater: Health and environmental implications, La Laguna Region, Mexico. Environ. Geochem. Health 2017, 39, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S. Industries using arsenic and arsenic compounds. Appl. Organomet. Chem. 1992, 6, 323–331. [Google Scholar] [CrossRef]
- Han, F.X.; Su, Y.; Monts, D.L.; Plodinec, M.J.; Banin, A.; Triplett, G.E. Assessment of global industrial-age anthropogenic arsenic contamination. Naturwissenschaften 2003, 90, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Barrachina, A.A.; Signes-Pastor, A.J.; Vázquez-Araújo, L.; Sengupta, B. Presence of arsenic in agricultural products from arsenic-endemic areas and strategies to reduce arsenic intake in rural villages. Mol. Nutr. Food Res. 2009, 53, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Abad-Valle, P.; Álvarez-Ayuso, E.; Murciego, A.; Muñoz-Centeno, L.M.; Alonso-Rojo, P.; Villar-Alonso, P. Arsenic distribution in a pasture area impacted by past mining activities. Ecotoxicol. Environ. Saf. 2018, 147, 228–237. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Tun, A.Z.; Chotpantarat, S.; Siriwong, W. Related health risk assessment of exposure to arsenic in some heavy metals in gold mines in Banmauk Township, Myanmar. Sci. Rep. 2021, 11, 22843. [Google Scholar] [CrossRef]
- Park, J.H.; Han, Y.-S.; Ahn, J.S. Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream. Water Res. 2016, 106, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lewińska, K.; Duczmal-Czernikiewicz, A.; Karczewska, A.; Dradrach, A.; Iqbal, M. Arsenic forms in soils of various settings in the historical ore mining and processing site of Radzimowice, Western Sudetes. Minerals 2021, 11, 491. [Google Scholar] [CrossRef]
- Panagiotaras, D.; Nikolopoulos, D. Arsenic occurrence and fate in the environment; a geochemical perspective. J. Earth Sci. Clim. Chang. 2015, 6, 269. [Google Scholar]
- Barral-Fraga, L.; Barral, M.T.; MacNeill, K.L.; Martiñá-Prieto, D.; Morin, S.; Rodríguez-Castro, M.C.; Tuulaikhuu, B.-A.; Guash, H. Biotic and abiotic factors influencing arsenic biogeochemistry and toxicity in fluvial ecosystems: A review. Int. J. Environ. Res. Public Health 2020, 17, 2331. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.; Francesconi, K. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; Wiley and Sons: New York, NY, USA, 1990; 393p. [Google Scholar]
- Stollenwerk, K.G. Geochemical processes controlling transport of arsenic in groundwater: A review of adsorption. In Arsenic in Ground Water; Welch, A.H., Stollenwerk, K.G., Eds.; Geochemistry and Occurrence; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2003; pp. 67–100. [Google Scholar]
- Root, R.A.; Dixit, S.; Campbell, K.M.; Jew, A.D.; Hering, J.G.; O’Day, P.A. Arsenic sequestration by sorption processes in high-iron sediments. Geochim. Cosmochim. Acta 2007, 71, 5782–5803. [Google Scholar] [CrossRef]
- Schacht, L.; Ginder-Vogel, M. Arsenite depletion by manganese oxides: A case study on the limitations of observed first order rate constants. Soil Syst. 2018, 2, 39. [Google Scholar] [CrossRef]
- Jain, A.; Loeppert, R.H. Effects of competing anions on the adsorption or arsenate and arsenite by ferrihydrite. J. Environ. Qual. 2000, 9, 1422–1430. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Effects of natural organic matter on arsenic release from soils and sediments into groundwater. Environ. Geochem. Health 2006, 28, 197–214. [Google Scholar] [CrossRef]
- Williams, P.N.; Zhang, H.; Davison, W.; Meharg, W.D.; Hossain, M.; Norton, G.J.; Brammer, H.; Islam, M.R. Organic matter- solid phase interactions are critical for predicting arsenic release and plant uptake in Bangladesh paddy soils. Environ. Sci. Technol. 2011, 45, 6080–6087. [Google Scholar] [CrossRef]
- Langner, P.; Mikutta, C.; Kretzschmar, R. Arsenic sequestration by organic sulphur in peat. Nat. Geosci. 2012, 5, 66–73. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Song, W.-Y. Arsenic uptake and translocation in plants. Plant Cell Physiol. 2016, 57, 4–13. [Google Scholar] [CrossRef]
- Meena, M.K.; Singh, A.K.; Prasad, L.K.; Islam, A.; Meena, M.D.; Dotaniya, M.L.; Singh, H.; Yadav, B.L. Impact of arsenic-polluted groundwater on soil and produce quality: A food chain study. Environ. Monit. Assess. 2020, 192, 785. [Google Scholar] [CrossRef]
- Luppichini, M.; Noti, V.; Pavone, D.; Bonato, M.; Ghizzani Marcìa, F.; Genovesi, S.; Lemmi, F.; Rosselli, L.; Chiarenza, N.; Colombo, M.; et al. Web mapping and real–virtual itineraries to promote feasible archaeological and environmental tourism in Versilia (Italy). ISPRS Int. J. Geo-Inform. 2022, 11, 460. [Google Scholar] [CrossRef]
- Carmignani, L.; Giglia, G.; Kligfield, R. Structural evolution of the Apuan Alps: An example of continental margin deformation in the northern Apennines, Italy. J. Geol. 1978, 86, 487–504. [Google Scholar] [CrossRef]
- Piccini, L.; Nannoni, A.; Poggetti, E. Hydrodynamics of karst aquifers in metamorphic carbonate rocks: Results from spring monitoring in the Apuan Alps (Tuscany, Italy). Hydrogeol. J. 2023, 31, 241–255. [Google Scholar] [CrossRef]
- Lattanzi, P.; Benvenuti, M.; Costagliola, P.; Tanelli, G. An overview on recent research on the metallogeny of Tuscany, with special reference to Apuane Alps. Mem. Soc. Geol. It. 1994, 48, 613–625. [Google Scholar]
- D’Orazio, M.; Biagioni, C.; Dini, A.; Vezzoni, S. Thallium-rich pyrite ores from Apuan Alps, Tuscany, Italy: Constraints for their origin and environmental concerns. Miner. Deposita 2017, 52, 687–707. [Google Scholar] [CrossRef]
- Perotti, M.; Petrini, R.; D’Orazio, M.; Ghezzi, L.; Giannecchini, R.; Vezzoni, S. Thallium and other potentially toxic elements in the Baccatoio Stream catchment (northern Tuscany, Italy) receiving drainages from abandoned mines. Mine Water Environ. 2018, 37, 431–441. [Google Scholar] [CrossRef]
- Campanella, B.; Onor, M.; D’Ulivo, A.; Giannecchini, R.; D’Orazio, M.; Petrini, R.; Bramanti, E. Human exposure to thallium through tap water: A study from Valdicastello Carducci and Pietrasanta (northern Tuscany, Italy). Sci. Total Environ. 2015, 548–549, 33–42. [Google Scholar] [CrossRef]
- Ghezzi, L.; D’Orazio, M.; Doveri, M.; Lelli, M.; Petrini, R.; Giannecchini, R. Groundwater and potentially toxic elements in a dismissed mining area: Thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). J. Geoch. Expl. 2019, 197, 84–92. [Google Scholar] [CrossRef]
- Antisari, L.V.; Bianchini, G.; Dinelli, E.; Falsone, G.; Gardini, A.; Simoni, A.; Tassinari, R.; Vianello, G. Critical evaluation of an intercalibration project focused on the definition of new multi-element soil reference materials (AMS-MO1 and AMS-ML1), EQA. EQA Int. J. Environ. Qual. 2014, 15, 41–64. [Google Scholar]
- Report E 2081-00; Standard Provisional Guide for Risk-Based Corrective Action. American Society for Testing Materials (ASTM): West Conshohocken, PA, USA, 2000.
- US EPA. Soil Screening Guidance: Technical Background Document; EPA/540/R-95/128; US Environmental Protection Agency, Office of Emergency and Remedial Response: Washington, DC, USA, 1996.
- US EPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; OSWER 9355.4-24; US Environmental Protection Agency, Office of Emergency and Remedial Response: Washington, DC, USA, 2002.
- US EPA. Risk Assessment; US Environmental Protection Agency: Washington, DC, USA, 2022. Available online: https://www.epa.gov/risk (accessed on 27 March 2023).
- US EPA. Human Health Evaluation Manual, Supplemental Guidance: Update of Standard Default Exposure Factors; OSWER 9200.1-120; US Environmental Protection Agency, Office of Solid Waste and Emergency Response: Washington, DC, USA, 2014.
- US EPA. Regional Screening Level (RSLs)—Generic Tables; US Environmental Protection Agency: Washington, DC, USA, 2022. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 27 March 2023).
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef]
- US EPA. Risk Assessment Guidance for Superfund. Volume I. Human Health Evaluation Manual, Supplemental Guidance: Standard Default Exposure Factors; EPA/540/1-89/002; US Environmental Protection Agency, Office of Emergency and Remediation Response: Washington, DC, USA, 1989.
- US EPA. Risk Assessment Guidance for Superfund—Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); U.S.EPA-540-R-070-002; Office of Superfund Remediation and Technology Innovation, US Environmental Protection Agency: Washington, DC, USA, 2009.
- Ghezzi, L.; Arrighi, S.; Giannecchini, R.; Bini, M.; Valerio, M.; Petrini, R. The Legacy of Mercury Contamination from a Past Leather Manufacturer and Health Risk Assessment in an Urban Area (Pisa Municipality, Italy). Sustainability 2022, 14, 4367. [Google Scholar] [CrossRef]
- US EPA. Regional Screening Level (RSLs)—Equations; US Environmental Protection Agency: Washington, DC, USA, 2022. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-equations#res (accessed on 27 March 2023).
- US EPA. Risk Assessment Guidance for Superfund. Volume I. Human Health Evaluation Manual, Part B; EPA/540/R-92/003; US Environmental Protection Agency, Office of Emergency and Remedial Response: Washington, DC, USA, 1991.
- Baroni, C.; Pieruccini, P.; Bini, M.; Coltorti, M.; Fantozzi, P.L.; Guidobaldi, G.; Nannini, D.; Ribolini, A.; Salvatore, M.C. Geomorphological and neotectonic map of the Apuan Alps (Tuscany, Italy). Geogr. Fis. Din. Quat. 2015, 38, 201–227. [Google Scholar]
- Vittori Antisari, L.; Bini, C.; Ferronato, C.; Gherardi, M.; Vianello, G. Translocation of potential toxic elements from soil to black cabbage (Brassica oleracea L.) growing in an abandoned mining district area of the Apuan Alps (Tuscany, Italy). Environ. Geochem. Health 2020, 42, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Battistel, M.; Stolze, L.; Muniruzzaman, M.; Rolle, M. Arsenic release and transport during oxidative dissolution of spatially distributed sulfide minerals. J. Hazard. Mat. 2021, 409, 124651. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, L.; Buccianti, A.; Giannecchini, R.; Guidi, M.; Petrini, R. Geochemistry of mine stream sediments and the contro, of potentially toxic element migration: A case study from the Baccatoio basin (Tuscany, Italy). Mine Water Environ. 2021, 40, 722–735. [Google Scholar]
- Finnegan, P.M.; Chen, W. Arsenic Toxicity: The Effects on Plant Metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic behaviour in soil-plant system: Biogeochemical reactions and chemical speciation influences. In Enhancing Cleanup of Environmental Pollutants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–140. [Google Scholar]
- Patel, K.S.; Pandey, P.K.; Martín-Ramos, P.; Corns, W.T.; Varol, S.; Bhattacharyaf, P.; Zhu, Y. A review on arsenic in the environment: Contamination, mobility, sources, and exposure. RSC Adv. 2023, 13, 8803. [Google Scholar] [CrossRef]
- Austruy, A.; Wanat, N.; Moussard, C.; Vernay, P.; Joussein, E.; Ledoigt, G.; Hitmi, A. Physiological impacts of soil pollution and arsenic uptake in three plant species: Agrostis capillaris, Solanum nigrum and Vicia faba. Ecotoxicol. Environ. Saf. 2013, 90, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Natasha; Shahid, M.; Khalid, S.; Niazi, N.K.; Murtaza, B.; Ahmad, N.; Farooq, A.; Zakir, A.; Imran, M.; Abbas, G. Health risks of arsenic build-up in soil and food crops after wastewater irrigation. Sci. Tot. Environ. 2021, 772, 145266. [Google Scholar] [CrossRef]
- Spognardi, S.; Bravo, I.; Beni, C.; Menegoni, P.; Pietrelli, L.; Papetti, P. Arsenic accumulation in edible vegetables and health risk reduction by groundwater treatment using an adsorption process. Environ. Sci. Pollut. Res. 2019, 26, 32505–32516. [Google Scholar] [CrossRef]
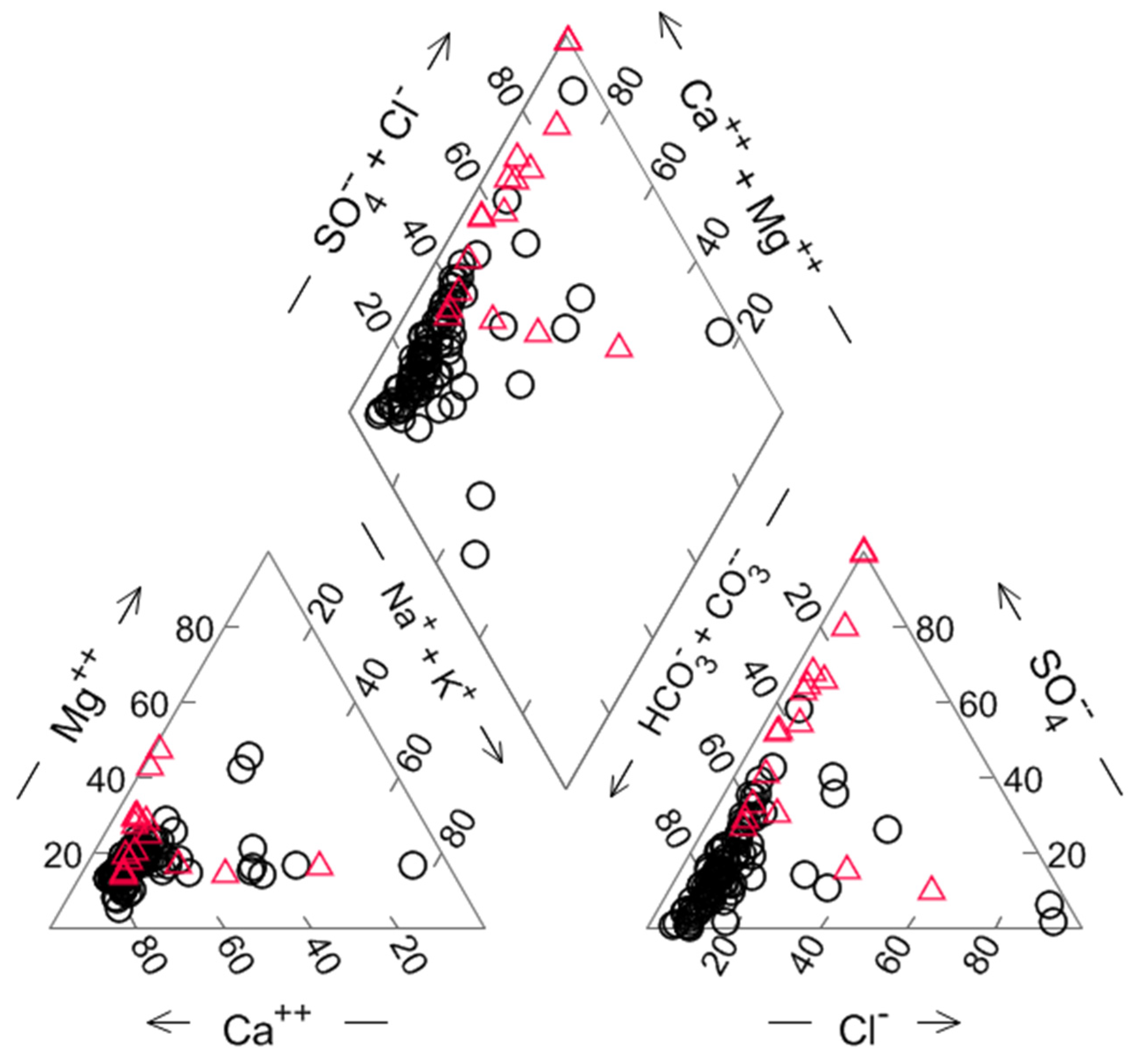


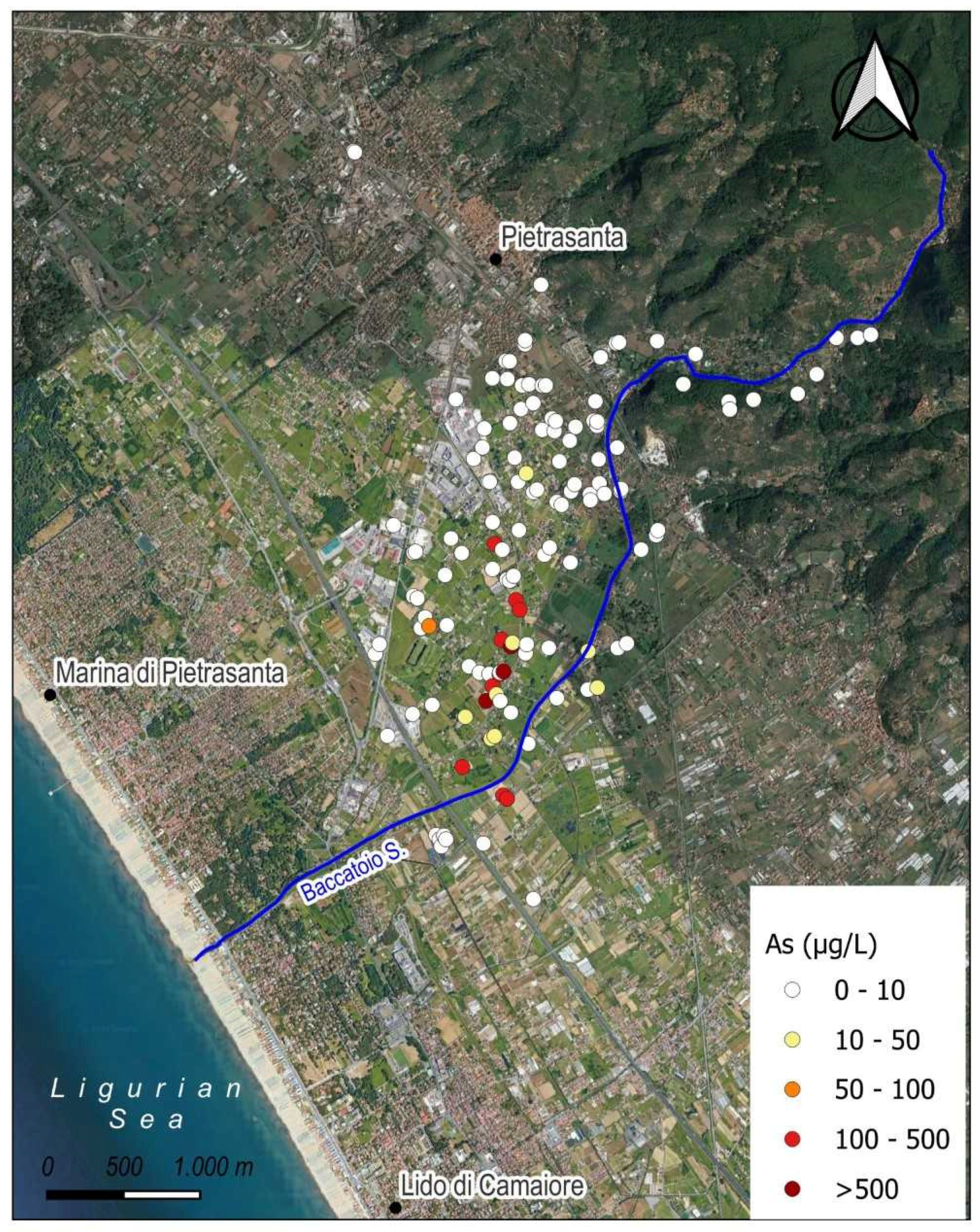
| Sample | Deep (m) | T (°C) | HCO3− (mg/L) | pH | O2 (mg/L) | EC (µS/cm) | Na+ (mg/L) | K+ (mg/L) | Mg2+ (mg/L) | Ca2+ (mg/L) | Cl− (mg/L) | NO3− (mg/L) | SO42− (mg/L) | NH4+ (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W-2 | n.d | 18 | 368 | 6.8 | 3.4 | 637 | 15.5 | 2.6 | 17.4 | 96 | 25.3 | 4.1 | 33 | 0.05 |
| W-3 | 6.0 | 19.1 | 326 | 7.1 | n.d | 609 | 22.9 | 1.9 | 15.8 | 86 | 34 | 5.0 | 40 | 0.06 |
| W-4 | n.d | 17.8 | 226 | 7.0 | n.d | 579 | 20.2 | 1.7 | 14.1 | 81 | 34 | 5.5 | 35 | 0.05 |
| W-5 | 29.0 | 17.2 | 171 | 7.3 | n.d | 7460 | 1313 | 54 | 159 | 126 | 2305 | 5.0 | 219 | 2.40 |
| W-6 | n.d | 18.6 | 338 | 7.1 | n.d | 739 | 20.9 | 4.1 | 20.7 | 112 | 38 | 13.3 | 34 | 0.05 |
| W-7 | 30.0 | 16.8 | 271 | 7.4 | n.d | 520 | 12.5 | 1.9 | 8.4 | 85 | 17.8 | 5.6 | 43 | 0.05 |
| W-8 | 15.0 | 18.5 | 300 | 7.0 | n.d | 646 | 18.1 | 3.6 | 16.9 | 101 | 27.9 | 12.3 | 37 | 0.05 |
| W-9 | 20.0 | 17.3 | 294 | 7.1 | n.d | 687 | 17.4 | 2.4 | 15.4 | 107 | 28.0 | 15.7 | 38 | 0.05 |
| W-10 | 30.0 | 18.5 | 354 | 7.5 | n.d | 641 | 15.5 | 2.6 | 17.4 | 96 | 25.3 | 4.1 | 33 | 0.05 |
| W-11 | 38.0 | 18.1 | 303 | 7.0 | n.d | 638 | 15.8 | 1.7 | 11.7 | 105 | 24.2 | 13.8 | 43 | 0.05 |
| W-12 | 40.0 | 16.9 | 276 | 7.3 | n.d | 624 | 15.2 | 1.6 | 11.9 | 100 | 22.3 | 12.6 | 42 | 0.05 |
| W-13 | 12.0 | 19.3 | 377 | 7.3 | n.d | 1275 | 70 | 7.5 | 23.5 | 159 | 152 | 1.3 | 59 | 0.18 |
| W-14 | n.d | 17.8 | 285 | 7.1 | n.d | 564 | 16.6 | 5.1 | 16.8 | 76 | 19.3 | 0.50 | 27.6 | 0.12 |
| W-15 | n.d | 15.7 | 287 | 7.6 | 1.3 | 365 | 22.0 | 9.6 | 12.9 | 71 | 30 | 1.5 | 20.0 | n.d |
| W-16 | 6.0 | 18.1 | 331 | 8.1 | n.d | 620 | 26.1 | 4.6 | 13.4 | 86 | 15.4 | 1.0 | 22.1 | 0.05 |
| W-17 | 6.0 | 17.2 | 288 | 8.1 | n.d | 664 | 26.4 | 6.0 | 16.0 | 92 | 25.3 | 11.9 | 63 | 0.06 |
| W-18 | n.d | 16.8 | 328 | 8.0 | n.d | 597 | 13.4 | 8.1 | 18.8 | 83 | 16.4 | 6.9 | 35 | 0.05 |
| W-19 | 6.0 | 16.7 | 298 | 7.6 | n.d | 710 | 22.8 | 7.9 | 15.6 | 99 | 33.3 | 21.8 | 37 | 3.10 |
| W-20 | 8.0 | 18.1 | 377 | 7.3 | n.d | 638 | 13.5 | 1.8 | 18.3 | 96 | 23.6 | 0.50 | 20.5 | 0.12 |
| W-21 | 6.0 | 15.9 | 287 | 7.9 | n.d | 673 | 14.9 | 9.0 | 20.1 | 93 | 27.2 | 9.5 | 59 | 0.05 |
| W-22 | 7.0 | 17.9 | 246 | 7.1 | n.d | 456 | 14.6 | 0.8 | 12.3 | 70 | 17.8 | 3.7 | 30 | 0.05 |
| W-23 | 5.0 | 16.7 | 316 | 7.3 | n.d | 664 | 15.7 | 2.2 | 20.0 | 96 | 25.6 | 0.50 | 26.5 | 0.10 |
| W-24 | 8.0 | 17.2 | 598 | 7.7 | n.d | 994 | 26.8 | 4.1 | 28.7 | 150 | 38.8 | 0.60 | 20.4 | 0.92 |
| W-25 | 28.0 | 15.9 | 285 | 7.5 | n.d | 574 | 13.0 | 2.8 | 11.1 | 95 | 19.3 | 9.8 | 46 | 0.05 |
| W-26 | 7.0 | 18.4 | 362 | 7.0 | n.d | 1547 | 149 | 6.8 | 31.1 | 157 | 138 | 2.0 | 264 | 2.60 |
| W-27 | 8.0 | 19.3 | 614 | 7.3 | n.d | 1277 | 66 | 23.0 | 76.6 | 105 | 68 | 1.0 | 114 | 0.76 |
| W-28 | 8.0 | 17.1 | 302 | 7.7 | n.d | 567 | 15.4 | 4.9 | 22.1 | 75 | 20.6 | 0.5 | 27.5 | 0.15 |
| W-29 | 60.0 | 17.7 | 298 | 8.7 | n.d | 558 | 13.1 | 1.3 | 9.7 | 99 | 19.5 | 10.1 | 55 | 0.05 |
| W-30 | 8.0 | 19.2 | 341 | 6.8 | n.d | 1426 | 31 | 7.0 | 51 | 245 | 35 | 1.0 | 440 | 1.20 |
| W-31 | 50.0 | 17.8 | 270 | 7.6 | n.d | 632 | 17.2 | 1.8 | 10.0 | 102 | 31 | 9.7 | 73 | 0.05 |
| W-32 | n.d | 19 | 454 | 7.1 | 7.0 | 757 | 12.4 | 14 | 16.2 | 120 | 20.2 | 12.6 | 17.6 | 0.84 |
| W-33 | 50.0 | 16.9 | 302 | 7.5 | n.d | 594 | 11.3 | 2.0 | 13.9 | 103 | 15.6 | 1.2 | 46 | 0.05 |
| W-34 | 39 | 16.5 | 271 | 7.9 | 2.6 | 387 | 49 | 5.9 | 9.3 | 48 | 35 | 0.36 | 4.0 | n.d |
| W-35 | 28.0 | 15.6 | 316 | 6.8 | n.d | 716 | 15.6 | 2.4 | 15.5 | 118 | 22.7 | 8.2 | 47 | 0.10 |
| W-36 | 12.0 | 18.7 | 306 | 7.6 | n.d | 630 | 30 | 11.8 | 38 | 44 | 25.0 | 0.50 | 46 | 0.62 |
| W-37 | 28.0 | 18.2 | 370 | 7.3 | 7.7 | 600 | 15.6 | 2.4 | 15.5 | 118 | 22.7 | 8.2 | 47 | n.d |
| W-39 | 7.0 | 16.9 | 435 | 7.0 | n.d | 869 | 22.8 | 1.8 | 16.5 | 151 | 22.3 | 19.6 | 48 | 0.10 |
| W-40 | n.d | 19.1 | 297 | 7.3 | n.d | 825 | 67.8 | 16.0 | 23.4 | 79 | 90 | 1.0 | 57 | 0.32 |
| W-41 | 10.0 | 18.7 | 31 | 7.2 | 4.8 | 599 | 17.9 | 4.3 | 24.9 | 127 | 302 | n.d | 7.4 | 1.3 |
| W-42 | 10.0 | 16.7 | 503 | 7.3 | n.d | 859 | 17.9 | 4.3 | 24.9 | 127 | 30 | 1.0 | 7.4 | 1.30 |
| W-43 | 11.0 | 17.2 | 362 | 7.2 | n.d | 720 | 14.4 | 1.7 | 20.3 | 111 | 24.9 | 0.50 | 15.8 | 0.11 |
| W-45 | 39.0 | 16.9 | 270 | 7.4 | n.d | 505 | 13.7 | 1.6 | 12.3 | 74 | 14.8 | 0.50 | 10.5 | 0.49 |
| W-46 | 8.0 | 16.2 | 351 | 7.0 | n.d | 1584 | 70 | 9.0 | 31.9 | 234 | 127 | 1.0 | 303 | 0.94 |
| W-47 | 46 | 14.7 | 251 | 7.7 | 5.0 | 659 | 13 | 6.5 | 12.7 | 87 | 18.51 | n.d | 42 | n.d |
| W-48 | 35 | 15.3 | 302 | 7.0 | 6.4 | 471 | 60 | 2.7 | 11.3 | 39 | 19.7 | 0.85 | 0.25 | n.d |
| W-49 | n.d | 14.3 | 364 | 6.7 | 5.4 | 594 | 17 | 5.9 | 9.1 | 116 | 21.1 | 8.37 | 17.0 | n.d |
| W-51 | 22 | 15.7 | 299 | 7.7 | 1.1 | 363 | 15 | 4.0 | 12.9 | 78 | 13.7 | 0.29 | 12.0 | n.d |
| W-53 | 9.0 | 18.2 | 514 | 7.1 | n.d | 835 | 16.4 | 2.1 | 23.3 | 127 | 31 | 1.0 | 2.4 | 1.70 |
| W-57 | 25 | 15.1 | 256 | 7.6 | 7.5 | 391 | 12 | 1.7 | 10.3 | 106 | 19.1 | 12.2 | 69 | n.d |
| W-59 | 16.0 | 14.2 | 341 | 7.0 | n.d | 741 | 18.3 | 1.4 | 13.5 | 126 | 20.6 | 26.8 | 35 | 0.10 |
| W-62 | 25.0 | 15.8 | 309 | 7.4 | n.d | 627 | 12.3 | 1.4 | 11.1 | 106 | 18.3 | 11.6 | 74 | 0.05 |
| W-67 | 23 | 15.3 | 345 | 6.4 | 2.8 | 640 | 13 | 4.7 | 17.5 | 105 | 23.9 | 10.3 | 63 | n.d |
| W-69 | 20 | 18.1 | 329 | 7.5 | n.d | 630 | 12.3 | 1.5 | 13.7 | 128 | 17.6 | 11.0 | 71 | 0.05 |
| W-71 | 22.0 | 18.2 | 301 | 7.0 | n.d | 684 | 16.0 | 2.0 | 15.2 | 109 | 22.6 | 9.7 | 56 | 0.05 |
| W-81 | 51.0 | 17.4 | 280 | 6.9 | n.d | 683 | 21.2 | 2.6 | 8.9 | 108 | 37 | 22.4 | 64 | 0.10 |
| W-84 | 11.0 | 13.4 | 386 | 7.0 | 7.3 | 619 | 19.4 | 2.0 | 18.7 | 123 | 29.4 | 4.8 | 52 | 4.1 |
| W-87 | 9.0 | 15.7 | 396 | 7.0 | 6.5 | 603 | 12 | 5.0 | 17.3 | 107 | 22.3 | n.d | 10.0 | n.d |
| W-91 | 7.0 | 23 | 413 | 7.0 | 1.3 | 829 | 17.5 | 23.4 | 27.4 | 103 | 19.2 | n.d | 68 | n.d |
| W-95 | 57 | 15.5 | 306 | 6.9 | 4.8 | 672 | 13 | 10.3 | 3.7 | 100 | 20.2 | 18.5 | 26 | n.d |
| W-96 | 12.0 | 15.6 | 312 | 6.7 | 5.4 | 650 | 18 | 4.8 | 8.5 | 117 | 25.5 | 12.6 | 35 | n.d |
| W-98 | 25.0 | 16.1 | 223 | 7.5 | 6.9 | 404 | 11 | 1.5 | 10.1 | 103 | 16.9 | 9.7 | 77 | n.d |
| W-103 | 12.0 | 16.5 | 246 | 6.6 | 2 | 720 | 12 | 4.3 | 16.4 | 107 | 22.8 | 7.9 | 95 | n.d |
| W-110 | 23.0 | 17.9 | 303 | 7.1 | n.d | 628 | 12.4 | 1.2 | 10.5 | 105 | 18.6 | 11.0 | 74 | 0.07 |
| W-111 | 9.0 | 15.9 | 304 | 6.9 | 6.2 | 585 | 11 | 6.0 | 16.5 | 97 | 21.8 | 0.96 | 61 | n.d |
| W-116 | 36 | 15.1 | 212 | 6.9 | 8.4 | 578 | 11.3 | 3.9 | 10.2 | 95 | 17.5 | 7.5 | 75 | n.d |
| W-118 | 21.0 | 18.2 | 231 | 7.1 | n.d | 605 | 12.3 | 2.1 | 10.5 | 104 | 18.8 | 12.2 | 65 | 0.05 |
| W-124 | 9.0 | 15.7 | 541 | 7.3 | 3.8 | 808 | 14.4 | 1.9 | 20.8 | 132 | 20.0 | n.d | 3.9 | 1.0 |
| W-126 | 22.0 | 17.1 | 317 | 7.4 | n.d | 659 | 17.1 | 7.5 | 15.3 | 102 | 21.6 | 1.0 | 43 | 1.5 |
| W-132 | 30.0 | 16.8 | 254 | 7.0 | n.d | 638 | 12.6 | 2.0 | 10.3 | 93 | 20.0 | 8.0 | 81 | 0.05 |
| W-133 | 18.0 | 18.2 | 206 | 7.5 | n.d | 674 | 9.9 | 1.5 | 11.6 | 91 | 16.6 | 4.0 | 103 | 0.05 |
| W-140 | 25.0 | 13.4 | 204 | 7.3 | 7.1 | 859 | 10.6 | 1.5 | 10.9 | 100 | 16.7 | 8.6 | 88 | n.d |
| W-147 | n.d | 15.9 | 231 | 6.9 | 8.5 | 709 | 11.0 | 1.1 | 12.2 | 122 | 16.9 | 8.7 | 131 | 0.8 |
| W-157 | 10.0 | 17.8 | 329 | 7.6 | n.d | 587 | 15.5 | 2.2 | 14 | 117 | 22.9 | 7.2 | 51 | n.d |
| W-160 | 66.0 | 16.6 | 254 | 7.0 | n.d | 520 | 13.3 | 2.3 | 7.0 | 84 | 21.1 | 13.2 | 18.8 | 0.05 |
| W-163 | 66 | 15.5 | 329 | 6.9 | 8.4 | 550 | 15.0 | 3.3 | 5.9 | 101 | 22.8 | 13.4 | 19.0 | n.d |
| W-164 | 66 | 15.8 | 310 | 7.1 | n.d | 578 | 14.5 | 1.9 | 6.1 | 100 | 23.4 | 14.1 | 19.5 | 0.05 |
| W-165 | 8.0 | 13.3 | 260 | 6.9 | 8.3 | 594 | 10.0 | 7.2 | 11.2 | 89 | 21.2 | 6.7 | 76 | n.d |
| W-166 | 67 | 15.4 | 248 | 6.6 | 4.3 | 547 | 16.0 | 4.1 | 13.1 | 77 | 26.2 | 5.0 | 57 | n.d |
| W-167 | 5.0 | 13.6 | 237 | 6.6 | 8.1 | 523 | 11.0 | 3.2 | 12.0 | 94 | 22.7 | 7.3 | 90 | n.d |
| W-168 | 37 | 17.2 | 31 | 5.9 | n.d | 187 | 14.4 | 0.20 | 3.2 | 15 | 24.3 | 11.9 | 20.3 | 0.05 |
| W-169 | 9.0 | 15.3 | 232 | 6.6 | 4 | 583 | 14.0 | 5.5 | 12.9 | 100 | 26.8 | 7.4 | 97 | n.d |
| W-170 | 72 | 16.1 | 305 | 7.2 | n.d | 879 | 19.6 | 1.4 | 24.7 | 138 | 27.3 | 1.8 | 207 | 0.10 |
| W-171 | 8.0 | 15.2 | 246 | 6.6 | 6.6 | 438 | 12.0 | 6.0 | 5.5 | 77 | 18.3 | 10.7 | 22.0 | n.d |
| W-172 | n.d | 15.7 | 234 | 6.9 | 8.2 | 641 | 11.0 | 7.7 | 7.9 | 93 | 21.4 | 18.3 | 53 | n.d |
| Sample | Mn (µg/L) | Zn (µg/L) | Ba (µg/L) | Tl (µg/L) | Fe (µg/L) | As (µg/L) |
|---|---|---|---|---|---|---|
| MCL | 50 | 3000 | 2 | 200 | 10 | |
| W-1 | 4.0 | 574 | 50 | 0.05 | 23 | 1.0 |
| W-2 | 1.0 | 87 | 235 | <0.05 | <15 | 1.7 |
| W-3 | 6.3 | 25 | 106 | 0.05 | 20 | 1.8 |
| W-4 | 1.0 | 25 | 82 | 0.05 | <15 | 1.1 |
| W-5 | 242 | 49 | 24 | 0.05 | 866 | 4.2 |
| W-6 | 1.4 | 16.0 | 93 | 0.05 | <15 | 1.3 |
| W-7 | 9.9 | 826 | 93 | 0.61 | <15 | 1.0 |
| W-8 | 1.0 | 241 | 201 | 0.05 | <15 | 2.1 |
| W-9 | 1.0 | 22 | 267 | 0.05 | <15 | 1.0 |
| W-10 | 1.0 | 87 | 235 | 0.05 | <15 | 1.7 |
| W-11 | 2.1 | 17.0 | 326 | 0.05 | <15 | 2.4 |
| W-12 | 1.1 | 23 | 349 | 0.05 | <15 | 2.7 |
| W-13 | 303 | 159 | 20 | 0.05 | 402 | 1.0 |
| W-14 | 230 | 45 | 203 | 0.05 | 1428 | 59 |
| W-15 | 0.46 | 202 | 0.55 | 0.04 | 366 | 2.6 |
| W-16 | 258 | 148 | 31 | 0.05 | 62 | 1.0 |
| W-17 | 231 | 49 | 2.9 | 0.05 | 45 | 2.1 |
| W-18 | 137 | 15.0 | 2.2 | 0.05 | <15 | 1.0 |
| W-19 | 335 | 11.0 | 8.8 | 0.05 | 31 | 1.4 |
| W-20 | 200 | 22 | 128 | 0.05 | 150 | 1.0 |
| W-21 | 253 | 14.0 | 18.0 | 0.05 | 173 | 3.6 |
| W-22 | 5.3 | 209 | 69 | 0.05 | 33 | 1.0 |
| W-23 | 127 | 14.0 | 290 | 0.05 | 708 | 2.5 |
| W-24 | 99 | 4.3 | 148 | 0.05 | 677 | 1.0 |
| W-25 | 1.0 | 462 | 195 | 0.05 | <15 | 2.6 |
| W-26 | 527 | 223 | 5.3 | 0.05 | 6465 | 102 |
| W-27 | 590 | 189 | 6.6 | 0.05 | 1880 | 24 |
| W-28 | 54 | 88 | 5.0 | 0.05 | 487 | 1.0 |
| W-29 | 1.0 | 5.0 | 244 | 0.05 | <15 | 1.8 |
| W-30 | 898 | 43 | 18.0 | 0.05 | 2585 | 5.7 |
| W-31 | 1.0 | 8.2 | 131 | 0.05 | <15 | 1.2 |
| W-32 | 454 | 539 | 16 | <0.05 | <15 | 1.4 |
| W-33 | 43 | 16.0 | 118 | 0.05 | 48 | 1.5 |
| W-34 | 193 | 38 | 0.61 | 0.06 | 1313 | 1103 |
| W-35 | 7.8 | 62 | 233 | 0.05 | 260 | 2.7 |
| W-36 | 76 | 15.0 | 4.0 | 0.05 | 213 | 2.8 |
| W-37 | 8.9 | 66 | 237 | <0.05 | 255 | 2.6 |
| W-38 | 762 | 123 | 2.6 | <0.05 | 9750 | 27 |
| W-39 | 5.9 | 12.0 | 199 | 0.05 | <15 | 1.0 |
| W-40 | 23 | 238 | 71 | 0.05 | 459 | 247 |
| W-41 | 56 | 36 | 91 | 0.10 | 59 | 1.4 |
| W-42 | 55 | 30 | 80 | 0.10 | 29 | 1.3 |
| W-43 | 159 | 261 | 242 | 0.05 | 2725 | 1.0 |
| W-45 | 495 | 73 | 512 | 0.05 | 4240 | 249 |
| W-46 | 1770 | 40 | 16.0 | 0.05 | 6390 | 13.0 |
| W-47 | 0.47 | 26 | n.d | 0.02 | 2635 | 153 |
| W-48 | 133 | 141 | 1205 | 0.05 | 238 | 107 |
| W-49 | 12.6 | 29 | 58 | 0.04 | 161 | 5.4 |
| W-50 | 12.0 | 60 | 145 | 0.05 | <15 | 1.5 |
| W-51 | 0.45 | 75 | 0.53 | 0.02 | 1673 | 117 |
| W-53 | 105 | 132 | 442 | <0.05 | 1219 | 1.0 |
| W-54 | 63 | 15 | 290 | <0.05 | 68 | 224 |
| W-55 | 207 | 59 | 1802 | <0.05 | 1814 | 1274 |
| W-56 | 25 | 68 | 169 | 0.12 | 181 | 1.0 |
| W-57 | 0.93 | 42 | 111 | 0.05 | 59 | 2.3 |
| W-58 | 439 | 13 | 37 | <0.05 | 4152 | 140 |
| W-59 | 1.0 | 6.4 | 238 | 0.05 | <15 | 1.3 |
| W-60 | 25 | 68 | 169 | 0.12 | 181 | 1.0 |
| W-61 | 2.7 | 335 | 222 | 0.05 | <15 | 1.6 |
| W-62 | 1.0 | 15.0 | 98.0 | 0.05 | <15 | 2.5 |
| W-64 | 638 | 16 | 1755 | <0.05 | 2170 | 798 |
| W-65 | 12 | 60 | 145 | <0.05 | <15 | 1.5 |
| W-66 | 171 | 2.9 | 75 | <0.05 | 2570 | 32 |
| W-67 | 0.76 | 62 | 289 | 0.02 | 54 | 2.7 |
| W-69 | <1 | 23 | 116 | <0.05 | <15 | 1.0 |
| W-70 | 437 | 77 | 591 | <0.05 | 3140 | 162 |
| W-71 | 1.0 | 2613 | 264 | 0.05 | <15 | 1.0 |
| W-72 | <1 | 274 | 145 | <0.05 | <15 | 2.1 |
| W-74 | 1565 | 69 | 630 | <0.05 | 2102 | 117 |
| W-76 | <1 | 189 | 109 | 0.13 | <15 | 1.0 |
| W-77 | 1.8 | 65 | 225 | 0.07 | <15 | 1.8 |
| W-80 | 12 | 4800 | 137 | <0.05 | <15 | 1.0 |
| W-81 | 1.0 | 100 | 159 | 0.05 | <15 | 1.0 |
| W-83 | 338 | 80 | 14 | <0.05 | 483 | 1.0 |
| W-84 | 233 | 32 | 629 | <0.05 | 14,290 | 11.0 |
| W-86 | 140 | 61 | 4.4 | <0.05 | 1464 | 1.3 |
| W-87 | 125 | n.d | 5.4 | 0.02 | 332 | 0.7 |
| W-88 | <1 | 8.00 | 216 | 0.18 | <15 | 1.9 |
| W-90 | <1 | 40 | 268 | 0.08 | <15 | 1.6 |
| W-91 | 521 | 19 | 15 | <0.05 | 94 | 1.1 |
| W-92 | 25 | 52 | 278 | <0.05 | 209 | 1.0 |
| W-94 | 25 | 60 | 273 | <0.05 | 218 | 1.0 |
| W-95 | 1.4 | <9 | 14.3 | 0.01 | 56 | 0.1 |
| W-96 | 3.6 | 13.5 | 239 | 0.13 | 79 | 1.2 |
| W-97 | 2.0 | 23 | 277.0 | 0.11 | 15.0 | 1.8 |
| W-98 | 0.2 | 39 | 97 | 0.09 | 62 | 1.8 |
| W-99 | 2.2 | 25 | 278 | 0.11 | <15 | 1.6 |
| W-102 | 117 | 2 | 47 | <0.05 | 1160 | 1.0 |
| W-103 | 2.1 | 32 | 74 | 0.03 | 59 | 0.3 |
| W-104 | 4.7 | 1434 | 127 | 0.13 | 169 | 1.0 |
| W-106 | <1 | 26 | 165 | <0.05 | <15 | 1.7 |
| W-108 | 4.2 | 66 | 120 | <0.05 | 53 | 1.3 |
| W-110 | 55 | 2195 | 108 | <0.05 | 17 | 1.0 |
| W-111 | 101 | 9.6 | 6.0 | 0.01 | 110 | 0.1 |
| W-114 | <1 | 35 | 109 | <0.05 | <15 | 1.0 |
| W-115 | <1 | 87 | 103 | 0.07 | <15 | 2.0 |
| W-116 | 0.18 | 10.7 | 115 | 0.14 | 51 | 1.9 |
| W-117 | 3.2 | 323 | 89 | <0.05 | <15 | 2.1 |
| W-118 | 3.6 | 203 | 113 | 0.05 | 15.0 | 1.3 |
| W-121 | <1 | 17 | 93 | 0.07 | 27 | 1.9 |
| W-122 | 13 | 115 | 110 | 0.1 | <15 | 2.2 |
| W-124 | 53 | 1.7 | 6.1 | <0.05 | 33 | 1.0 |
| W-126 | 132 | 5.1 | 183 | 0.05 | 297 | 15.0 |
| W-128 | <1 | 9.3 | 67 | 0.1 | <15 | 1.2 |
| W-129 | <1 | 17 | 75 | <0.05 | <15 | 1.2 |
| W-131 | 6.4 | 568 | 110 | <0.05 | 434 | 1.9 |
| W-132 | <1 | 8.2 | 139 | 0.15 | <15 | 2.4 |
| W-133 | 4.0 | 273 | 83 | 0.1 | 30 | 1.0 |
| W-134 | 2.5 | 6.6 | 104 | 0.14 | 48 | 2.0 |
| W-136 | 45 | 82 | 35 | <0.05 | 887 | 20.0 |
| W-138 | <1 | 53 | 79 | 0.11 | <15 | 1.2 |
| W-140 | 80 | 5.8 | 543 | <0.05 | 861 | 1.0 |
| W-142 | <1 | 43 | 246 | 0.07 | <15 | 1.0 |
| W-145 | <1 | 82 | 68 | <0.05 | <15 | 1.4 |
| W-146 | <1 | 145 | 40 | <0.05 | <15 | 1.0 |
| W-147 | <1 | 10.0 | 115 | 0.07 | 17 | 1.7 |
| W-149 | 109 | 7.6 | 384 | <0.05 | 160 | 1.0 |
| W-150 | <1 | 133 | 41 | <0.05 | 26 | 1.0 |
| W-152 | 1.2 | 538 | 108 | <0.05 | <15 | 1.0 |
| W-153 | 4.0 | 122 | 85 | 0.07 | 115 | 1.0 |
| W-155 | 109 | 76 | 384 | 0.05 | 160 | 1.0 |
| W-157 | 4.0 | 1477 | 263 | <0.05 | 283 | 1.9 |
| W-158 | 142 | 23 | 103 | <0.05 | 1730 | 1.0 |
| W-160 | <1 | 38 | 30 | 0.05 | <15 | 1.0 |
| W-161 | <1 | 301 | 73 | <0.05 | <15 | 1.7 |
| W-163 | 0.36 | n.d | 39 | 0.03 | 44 | 0.6 |
| W-164 | 4.1 | 35 | 40 | 0.05 | <15 | 1.0 |
| W-165 | 1.2 | 26 | 61 | 0.06 | 52 | 1.7 |
| W-166 | 3.5 | 18.9 | 99 | 0.1 | 75 | 2.9 |
| W-167 | 1.3 | 19.3 | 71 | 0.13 | 67 | 1.6 |
| W-168 | 16.0 | 12.0 | 60 | 0.05 | 162 | 1.0 |
| W-169 | 1.6 | n.d | 88 | 0.19 | 57 | 1.8 |
| W-170 | 1.4 | 27 | 30 | 0.05 | 46 | 1.0 |
| W-171 | 60 | 74 | 168 | 0.51 | 80 | 1.3 |
| W-172 | 8.6 | 695 | 177 | 0.63 | 59 | 2.9 |
| As (mg/kg) | Topsoil (0–20 cm) | Subsoil (80–100 cm) |
|---|---|---|
| Average | 38.5 | 46.4 |
| Median | 26.5 | 26.9 |
| Minimum | 6.3 | 8.6 |
| Maximum | 200 | 211 |
| Standard Error | 4.4 | 3.7 |
| Soil Ingestion | Dermal Contact | Inhalation Dust | HIi | Ri | Vegetables Ingestion (Tomato) | Vegetable Ingestion (Cabbage) | Sum of Soil Exposures | |
|---|---|---|---|---|---|---|---|---|
| HQ * | 1.19 (0.127) | 0.166 (2.54 × 10−2) | 2.05 × 10−5 | 1.36 (0.152) | 2.92 (0.625) | 0.213 (4.57 × 10−2) | ||
| R ** | 6.54 × 10−5 | 1.03 × 10−5 | 5.66 × 10−10 | 7.57 × 10−5 | 1.12 × 10−4 (9.64 × 10−5) | 8.22 × 10−6 (7.05 × 10−6) | ||
| SSL | 0.71 | 4.50 | 8.20 × 10−4 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghezzi, L.; Arrighi, S.; Petrini, R.; Bini, M.; Vittori Antisari, L.; Franceschini, F.; Franchi, M.L.; Giannecchini, R. Arsenic Contamination in Groundwater, Soil and the Food-Chain: Risk Management in a Densely Populated Area (Versilia Plain, Italy). Appl. Sci. 2023, 13, 5446. https://doi.org/10.3390/app13095446
Ghezzi L, Arrighi S, Petrini R, Bini M, Vittori Antisari L, Franceschini F, Franchi ML, Giannecchini R. Arsenic Contamination in Groundwater, Soil and the Food-Chain: Risk Management in a Densely Populated Area (Versilia Plain, Italy). Applied Sciences. 2023; 13(9):5446. https://doi.org/10.3390/app13095446
Chicago/Turabian StyleGhezzi, Lisa, Simone Arrighi, Riccardo Petrini, Monica Bini, Livia Vittori Antisari, Fabrizio Franceschini, Maria Letizia Franchi, and Roberto Giannecchini. 2023. "Arsenic Contamination in Groundwater, Soil and the Food-Chain: Risk Management in a Densely Populated Area (Versilia Plain, Italy)" Applied Sciences 13, no. 9: 5446. https://doi.org/10.3390/app13095446
APA StyleGhezzi, L., Arrighi, S., Petrini, R., Bini, M., Vittori Antisari, L., Franceschini, F., Franchi, M. L., & Giannecchini, R. (2023). Arsenic Contamination in Groundwater, Soil and the Food-Chain: Risk Management in a Densely Populated Area (Versilia Plain, Italy). Applied Sciences, 13(9), 5446. https://doi.org/10.3390/app13095446






