A Review of the Use of Near-Infrared Hyperspectral Imaging (NIR-HSI) Techniques for the Non-Destructive Quality Assessment of Root and Tuber Crops
Abstract
1. Introduction
2. Overview of Near-Infrared Reflectance Spectroscopy (NIRS)
2.1. Main Components and Modes of Operation of the NIRS
2.2. Constraints in the Application of NIRS
3. Overview of Hyperspectral Imaging Spectroscopy (HSI)
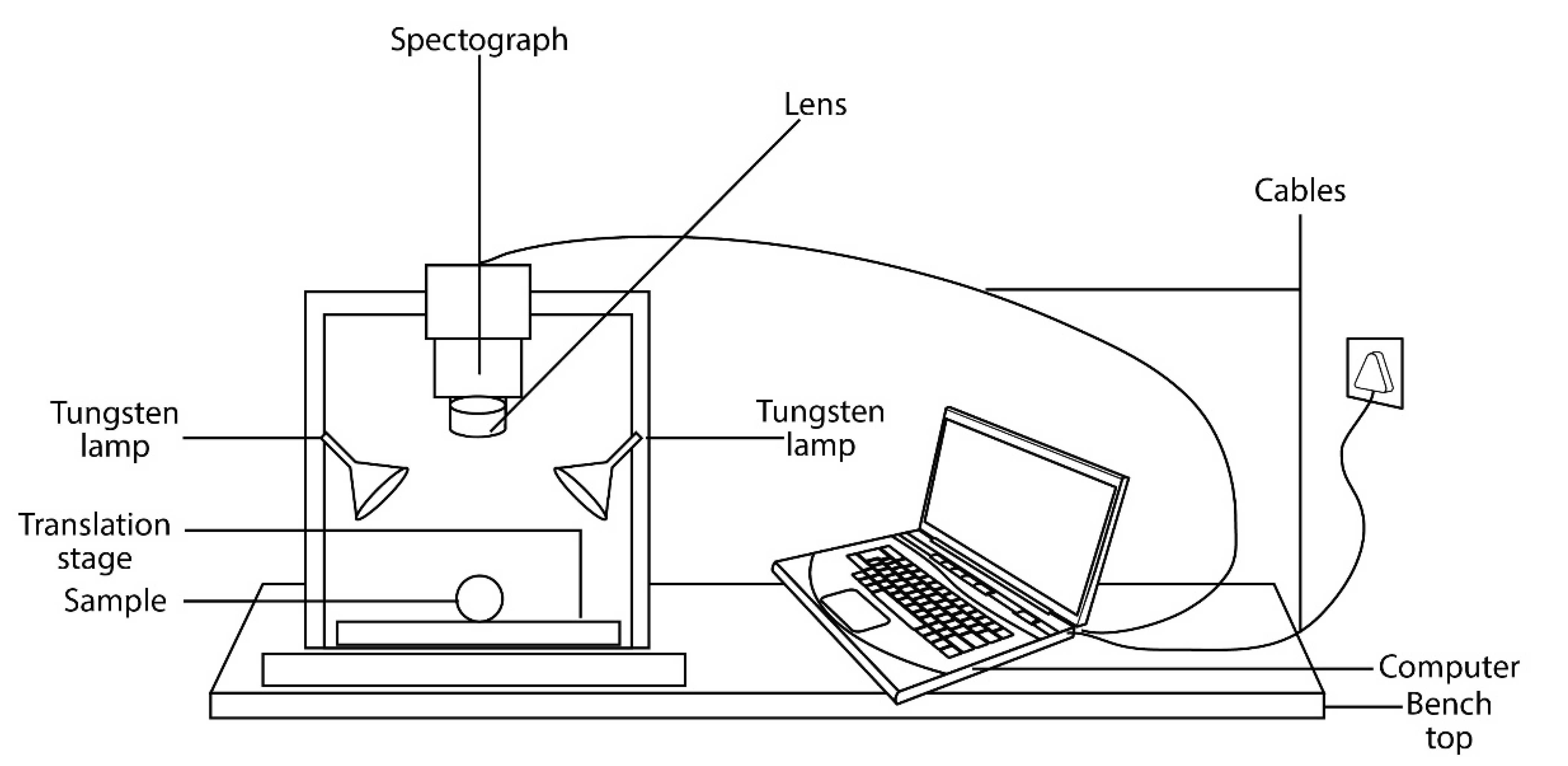
3.1. The Main Component of the Hyperspectral Imaging System
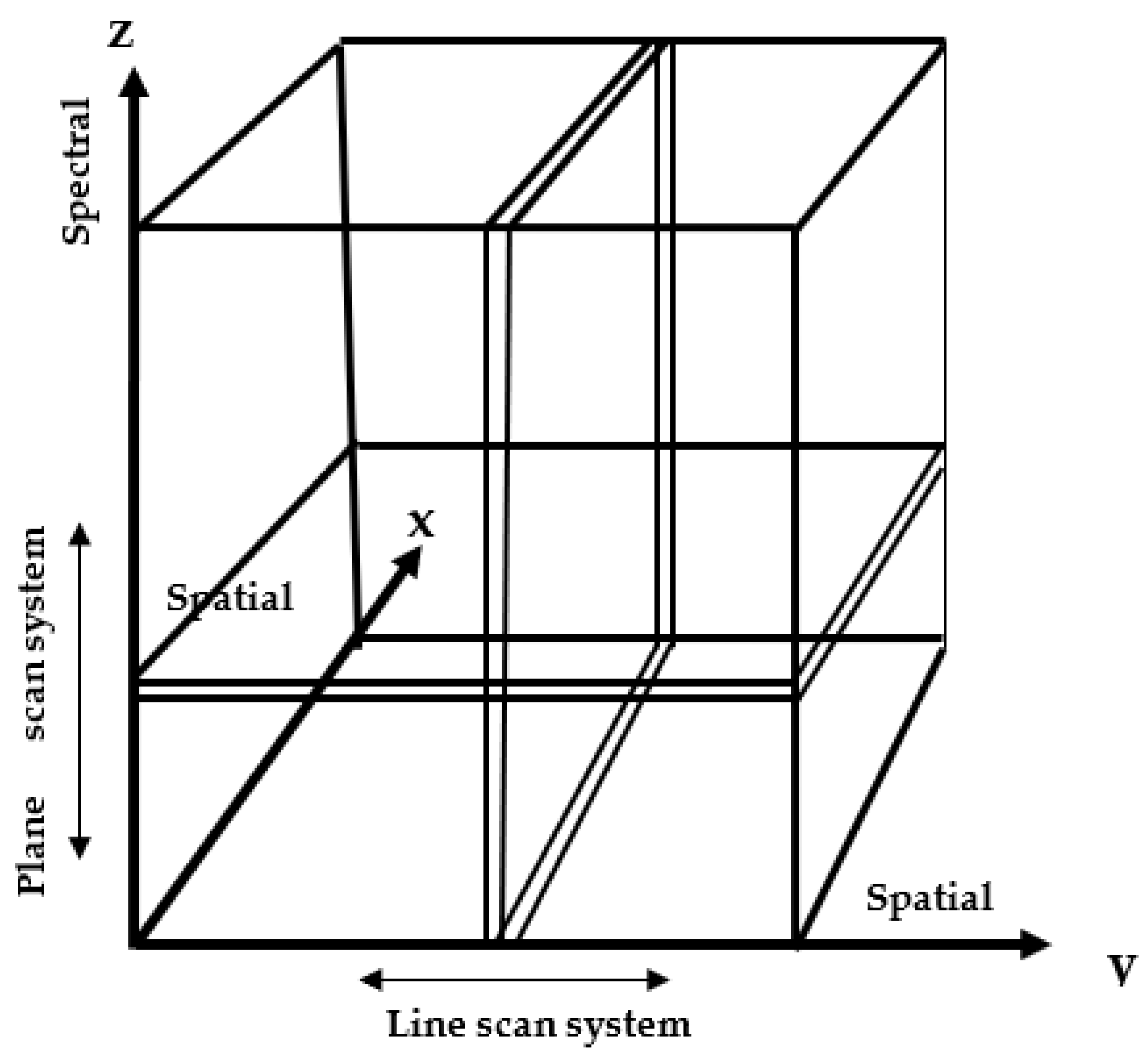
3.2. Hyperspectral Image Processing
3.3. Hyperspectral Imaging and Chemometrics
3.3.1. Chemometrics in NIR Imaging Processing
Pre-Processing
Multivariate Data Exploration
Model Development
Multivariate Image Analysis
4. NIR-Hyperspectral Imaging Spectroscopy for Yam and Cassava Food Quality
5. Quality Evaluation of Potatoes and Sweet Potatoes with NIR-Hyperspectral Imaging Techniques
Physical Parameters and Texture Analysis Using Hyperspectral Imaging
6. Limitation of NIR-HSI Spectroscopy
7. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abewoy, D. Review on postharvest handling practices of root and tuber crops. Int. J. Plant Breed. Crop Sci. 2021, 8, 992–1000. [Google Scholar]
- Scott, G.J. A review of root, tuber and banana crops in developing countries: Past, present and future. Int. J. Food Sci. Technol. 2021, 56, 1093–1114. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Piccirillo, C.; Tomlins, K.; Pintado, M.E. Cassava (Manihot esculenta Crantz) and Yam (Dioscorea spp.) Crops and Their Derived Foodstuffs: Safety, Security and Nutritional Value. Crit. Rev. Food Sci. Nutr. 2015, 56, 2714–2727. [Google Scholar] [CrossRef]
- Latif, S.; Müller, J. Potential of cassava leaves in human nutrition: A review. Trends Food Sci. Technol. 2015, 44, 147–158. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Akpabio, E.M. The geography of yam cultivation in southern Nigeria: Exploring its social meanings and cultural functions. J. Ethn. Foods 2017, 4, 28–35. [Google Scholar] [CrossRef]
- Belalcazar, J.; Dufour, D.; Andersson, M.S.; Pizarro, M.; Luna, J.; Londoño, L.; Morante, N.; Jaramillo, A.M.; Pino, L.; López-Lavalle, L.A.B.; et al. High-Throughput Phenotyping and Improvements in Breeding Cassava for Increased Carotenoids in the Roots. Crop. Sci. 2016, 56, 2916–2925. [Google Scholar] [CrossRef]
- Ikeogu, U.N.; Davrieux, F.; Dufour, D.; Ceballos, H.; Egesi, C.N.; Jannink, J.-L. Rapid analyses of dry matter content and carotenoids in fresh cassava roots using a portable visible and near infrared spectrometer (Vis/NIRS). PLoS ONE 2017, 12, e0188918. [Google Scholar] [CrossRef]
- Sanchez, T.; Ceballos, H.; Dufour, D.; Ortiz, D.; Morante, N.; Calle, F.; Zum Felde, T.; Dominguez, M.; Davrieux, F. Prediction of carotenoids, cyanide, and dry matter contents in fresh cassava root using NIRS and Hunter colour techniques. Food Chem. 2014, 151, 444–451. [Google Scholar] [CrossRef]
- Lebot, V.; Malapa, R. Application of near infrared reflectance spectroscopy for the evaluation of yam (Dioscorea alata) germplasm and breeding lines. J. Sci. Food Agric. 2012, 93, 1788–1797. [Google Scholar] [CrossRef]
- Davrieux, F.; Dufour, D.; Dardenne, P.; Belalcazar, J.; Pizarro, M.; Luna, J.; Londoño, L.; Jaramillo, A.; Sanchez, T.; Morante, N.; et al. LOCAL regression algorithm improves near-infrared spec-troscopy predictions when the target constituent evolves in breeding populations. J. Near Infrared Spectrosc. 2016, 24, 109–117. [Google Scholar] [CrossRef]
- Phambu, N.; Meya, A.S.; Djantou, E.B.; Phambu, E.N.; Kita-Phambu, P.; Anovitz, L.M. Direct Detection of Residual Cyanide in Cassava Using Spectroscopic Techniques. J. Agric. Food Chem. 2007, 55, 10135–10140. [Google Scholar] [CrossRef] [PubMed]
- Alamu, E.O.; Maziya-Dixon, B.; Felde, T.Z.; Kulakow, P.; Parkes, E. Application of near-infrared reflectance spectroscopy in the screening of fresh cassava (Manihot esculenta Crantz) storage roots for provitamin A carotenoids. In Proceedings of the 18th International Conference of Near-Infrared Spectroscopy; Engelsen, S., Sørensen, K., Berg, F., Eds.; IMPublications Open: Chichester, UK, 2019; pp. 91–97. [Google Scholar] [CrossRef]
- Lu, G.Q.; Huang, H.H.; Zhang, D.P. Prediction of sweet potato starch physiochemical quality and pasting properties using near-infrared reflectance spectroscopy. Food Chem. 2006, 94, 632–639. [Google Scholar] [CrossRef]
- Hong, J.; Ikeda, K.; Kreft, I.; Yasumoto, K. Near-infrared diffuse reflectance spectroscopic analysis of the amounts of moisture, protein, starch, amylose, and tannin in buckwheat flours. J. Nutr. Sci. Vitaminol. 1996, 42, 359–366. [Google Scholar] [CrossRef]
- Katayama, K.; Komaki, K.; Tamiya, S. Prediction of starch, moisture, and sugar in sweet potato by near-infrared transmittance. Hortic. Sci. 1996, 31, 1003–1006. [Google Scholar] [CrossRef]
- Lebot, V.; Malapa, R.; Jung, M. Use of NIRS for the rapid prediction of total N, minerals, sugars and starch in tropical root and tuber crops. N. Z. J. Crop Hortic. Sci. 2013, 41, 144–153. [Google Scholar] [CrossRef]
- Adebayo, S.E.; Hashim, N.; Abdan, K.; Hanafi, M. Application and potential of back-scattering imaging techniques in agricultural and food processing—A review. J. Food Eng. 2016, 169, 155–164. [Google Scholar] [CrossRef]
- Alamu, E.O.; Nuwamanya, E.; Cornet, D.; Meghar, K.; Adesokan, M.; Tran, T.; Belalcazar, J.; Desfontaines, L.; Davrieux, F. Near-Infrared spectroscopy (NIRS) applications for high throughput phenotyping (HTP) for cassava and yam: A review. Int. J. Food Sci. Technol. 2021, 56, 1491–1501. [Google Scholar] [CrossRef]
- ElMasry, G.M.; Nakauchi, S. Image analysis operations applied to hyperspectral images for non-invasive sensing of food quality—A comprehensive review. Biosyst. Eng. 2016, 142, 53–82. [Google Scholar] [CrossRef]
- Mahesh, S.; Manickavasagan, A.; Jayas, D.S.; Paliwal, J.; Whiteb, N.D.G. Feasibility of near-infrared hyperspectral imaging to differentiate Canadian wheat classes. Biosyst. Eng. 2008, 101, 50–57. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef] [PubMed]
- Peirs, A.; Scheerlinck, N.; Nicolai, B.M. Temperature compensation for near infrared reflectance measurement of apple fruit soluble solids contents. Postharvest Biol. And. Technol. 2003, 30, 233–248. [Google Scholar] [CrossRef]
- ElMasry, G.; Sun, D.W. Principles of hyperspectral imaging technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Academic Press: London, UK, 2010; pp. 3–43. [Google Scholar]
- Amjad, W.; Crichton SO, J.; Munir, A.; Hensel, O.; Sturm, B. Hyperspectral imaging for the determination of potato slice moisture content and chromaticity during the convective hot air-drying process. Biosyst. Eng. 2018, 166, 170–183. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, R.N.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 135, 726–779. [Google Scholar]
- Kjær, A.; Nielsen, G.; Stærke, S.; Clausen, M.R.; Edelenbos, M.; Jørgensen, B. Detection of Glycoalkaloids and Chlorophyll in Potatoes (Solanum tuberosum L.) by Hyperspectral Imaging. Am. J. Potato Res. 2017, 94, 573–582. [Google Scholar] [CrossRef]
- Do Trong, N.N.; Erkinbaev, C.; Nicolaï, B.; Saeys, W.; Tsuta, M.; De Baerdemaeker, J. Spatially resolved spectroscopy for nondestructive quality measurements of Braeburn apples cultivated in sub-fertilization condition. Sens. Technol. Biomat. Food Agric. 2013, 8881, 116–122. [Google Scholar]
- Su, W.H.; Sun, D.W. Advanced analysis of roots and tubers by hyperspectral techniques. Adv. Food Nutr. Res. 2019, 87, 255–303. [Google Scholar]
- Su, W.-H.; Bakalis, S.; Sun, D.-W. Fourier transform mid-infrared-attenuated total reflectance (FTMIR-ATR) micro spectroscopy for determining a textural property of microwave baked tuber. J. Food Eng. 2017, 218, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wu, H.F.; Huang, J.F. Application of neural networks to discriminate fungal infection levels in rice panicles using hyperspectral reflectance and principal components analysis. Comput. Electron. Agric. 2010, 72, 99–106. [Google Scholar] [CrossRef]
- Williams, P.; Geladi, P.; Fox, G.; Manley, M. Maize kernel hardness classification by near infrared (NIR) hyperspectral imaging and multivariate data analysis. Anal. Chim. Acta 2009, 653, 121–130. [Google Scholar] [CrossRef]
- Rady, A.; Guyer, D.; Lu, R. Evaluation of Sugar Content of Potatoes using Hyperspectral Imaging. Food Bioprocess Technol. 2015, 8, 995–1010. [Google Scholar] [CrossRef]
- Su, W.H.; Bakalis, S.; Sun, D.W. Chemometrics in tandem with near-infrared (NIR) hyperspectral imaging and Fourier transform mid-infrared (FT-MIR) microspectroscopy for variety identification and cooking loss determination of sweet potato. Biosyst. Eng. 2019, 180, 70–86. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Fourier Transform Infrared and Raman and Hyperspectral Imaging Techniques for Quality Determinations of Powdery Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 17, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Khamsopha, D.; Woranitta, S.; Teerachaichayut, S. Utilizing near-infrared hyperspectral imaging for quantitatively predicting adulteration in tapioca starch. Food Control 2021, 123, 107781. [Google Scholar] [CrossRef]
- Bock, J.E.; Connelly, R.K. Innovative Uses of Near-Infrared Spectroscopy in Food Processing. J. Food Sci. 2008, 73, R91–R98. [Google Scholar] [CrossRef] [PubMed]
- Badr, A. Near-infra-red Spectroscopy. In Wide Spectra of Quality Control; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Rathmell, C.; Bingemann, D.; Zieg, M.; Creasey, D. Portable Raman Spectroscopy: Instrumentation and Technology. In Portable Spectroscopy and Spectrometry; Wiley: Hoboken, NJ, USA, 2021; pp. 115–145. [Google Scholar]
- Tsenkova, R.; Atanassova, S.; Toyoda, K. Near-infrared spectroscopy for diagnosis: Influence of mammary gland inflammation on cow’s milk composition measurement. Near Infrared Anal. 2001, 2, 59–66. [Google Scholar]
- Corson, D.C.; Waghorn, G.C.; Ulyatt, M.J.; Lee, J. NIRS: Forage analysis and livestock feeding. In Proceedings of the New Zealand Grassland Association; New Zealand Grassland Association; Wellington, New Zealand, 1999; Volume 61, pp. 127–132. Available online: https://www.nzgajournal.org.nz/index.php/ProNZGA/article/view/2340 (accessed on 17 December 2022).
- Osborne, B.G. Near-infrared spectroscopy in food analysis. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 1–13. [Google Scholar]
- Restaino, E.A.; Fernández, E.G.; La Manna, A.; Cozzolino, D. Prediction of the nutritive value of pasture silage by near in-frared spectroscopy (Nirs). Chil. J. Agric. Resour. 2008, 69, 560–566. [Google Scholar]
- Huang, H.; Yu, H.; Xu, H.; Ying, Y. Near infrared spectroscopy for on/in-line monitoring of quality in foods and beverages: A review. J. Food Eng. 2008, 87, 303–313. [Google Scholar] [CrossRef]
- Choudhary, R.; Mahesh, S.; Paliwal, J.; Jayas, D.S. Identification of wheat classes using wavelet features from near-infrared hyperspectral images of bulk samples. Biosyst. Eng. 2009, 102, 115–127. [Google Scholar] [CrossRef]
- Sone, I.; Olsen, R.L.; Sivertsen, A.H.; Eilertsen, G.; Heia, K. Classification of fresh Atlantic salmon (Salmo salar L.) fillets stored under different atmospheres by hyperspectral imaging. J. Food Eng. 2012, 109, 482–489. [Google Scholar] [CrossRef]
- Barbin, D.F.; ElMasry, G.; Sun, D.-W.; Allen, P. Nondestructive determination of chemical composition in intact and minced pork using near-infrared hyperspectral imaging. Food Chem. 2013, 138, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Parsimonious model development for real-time monitoring of moisture in red meat using hyperspectral imaging. Food Chem. 2016, 196, 1084–1091. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Adhikari, B.; Guo, Z. Nondestructive Detection of Postharvest Quality of Cherry Tomatoes Using a Portable NIR Spectrometer and Chemometric Algorithms. Food Anal. Methods 2019, 12, 914–925. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Ariana, D.P.; Mendoza, F. Hyperspectral imaging-based classification and waveband selection for internal defect detection of pickling cucumbers. Food Bioprocess Technol. 2014, 7, 1689–1700. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W. Rapid and non-invasive detection of fish microbial spoilage by visible and near infrared hyperspectral imaging and multivariate analysis. LWT Food Sci. Technol. 2015, 62, 1060–1068. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Qu, J.-H.; Sun, D.-W.; Zeng, X.-A. Visible/near-infrared hyperspectral imaging prediction of textural firmness of grass carp (Ctenopharyngodon idella) as affected by frozen storage. Food Res. Int. 2014, 56, 190–198. [Google Scholar] [CrossRef]
- Gómez-Sanchís, J.; Lorente, D.; Soria-Olivas, E.; Aleixos, N.; Cubero, S.; Blasco, J. Development of a Hyperspectral Computer Vision System Based on Two Liquid Crystal Tuneable Filters for Fruit Inspection. Application to Detect Citrus Fruits Decay. Food Bioprocess Technol. 2014, 7, 1047–1056. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Potential of hyperspectral imaging for visual authentication of sliced organic potatoes from potato and sweet potato tubers and rapid grading of the tubers according to moisture proportion. Comput. Electron. Agric. 2016, 125, 113–124. [Google Scholar] [CrossRef]
- Gowen, A.; Odonnell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral imaging—An emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; ElMasry, G.; Sun, D.W.; Allen, P. Nondestructive assessment of instrumental and sensory tenderness of lamb meat using NIR hyperspectral imaging. Food Chem. 2013, 141, 389–396. [Google Scholar] [CrossRef]
- Sun, D.-W.; Brosnan, T. Pizza quality evaluation using computer vision—Part 2—Pizza topping analysis. J. Food Eng. 2003, 57, 91–95. [Google Scholar] [CrossRef]
- ElMasry, G.; Barbin, D.F.; Sun, D.W.; Allen, P. Meat quality evaluation by hyperspectral imaging technique: An overview. Crit. Rev. Food Sci. Nutr. 2012, 52, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Gowen, A.A.; O’Donnell, C.P. Comparison of hyperspectral imaging with conventional RGB imaging for quality evaluation of Agaricus bisporus mushrooms. Biosyst. Eng. 2011, 108, 191–194. [Google Scholar] [CrossRef]
- Ravikanth, L.; Jayas, D.S.; White, N.D.G.; Fields, P.G.; Sun, D.W. Extraction of spectral information from hyperspectral data and application of hyperspectral imaging for food and agricultural products. Food Bioprocess Technol. 2017, 10, 1–33. [Google Scholar] [CrossRef]
- Xiong, Z.; Xie, A.; Sun, D.W.; Zeng, X.A.; Liu, D. Applications of hyperspectral imaging in chicken meat safety and quality detection and evaluation: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-H.; Sun, D.-W.; He, J.-G.; Zhang, L.-B. Variation analysis in spectral indices of volatile chlorpyrifos and non-volatile imidacloprid in jujube (Ziziphus jujuba Mill.) using near-infrared hyperspectral imaging (NIR-HSI) and gas chromatography-mass spectrometry (GC–MS). Comput. Electron. Agric. 2017, 139, 41–55. [Google Scholar] [CrossRef]
- Tao, F.F.; Peng, Y.K. A nondestructive method for prediction of total viable count in pork meat by hyperspectral scattering imaging. Food Bioprocess Technol 2015, 8, 17–33. [Google Scholar] [CrossRef]
- Feng, Y.-Z.; Sun, D.-W. Application of hyperspectral imaging in food safety inspection and control: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 1039–1058. [Google Scholar] [CrossRef]
- Valous, N.A.; Mendoza, F.; Sun, D.-W.; Allen, P. Colour calibration of a laboratory computer vision system for quality evaluation of pre-sliced hams. Meat Sci. 2009, 81, 132–141. [Google Scholar] [CrossRef]
- Mehl, P.M.; Chen, Y.R.; Kim, M.S.; Chan, D.E. Development of hyperspectral imaging technique for the detection of apple surface defects and contaminations. J. Food Eng. 2004, 61, 67–81. [Google Scholar] [CrossRef]
- Kim, J.G.; Xia, M.; Liu, H. Extinction coefficients of hemoglobin for near-infrared spectroscopy of tissue. IEEE Eng. Med. Biol. Mag. 2005, 24, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Tallada, J.; Kobayashi, T. Bruise detection using NIR hyperspectral imaging for strawberry (Fragaria ananassa Duch.). Environ. Control Biol. 2006, 44, 133–142. [Google Scholar] [CrossRef]
- Zhu, H.; Gowen, A.; Feng, H.; Yu, K.; Xu, J.L. Deep spectral-spatial features of near infrared hyperspectral images for pixel-wise classification of food products. Sensors 2020, 20, 5322. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.F.; Geladi, P. Techniques and Applications of Hyperspectral Image Analysis; John Wiley & Sons Ltd.: West Sussex, UK, 2007; pp. 1–15. [Google Scholar]
- Li, X.L.; He, Y. Evaluation of least squares support vector machine regression and other multivariate calibrations in determination of internal attributes of tea beverages. Food Bioprocess Technol. 2010, 3, 651–661. [Google Scholar] [CrossRef]
- Lawrence, K.C.; Park, B.; Windham, W.R.; Mao., C. Calibration of A Pushbroom Hyperspectral Imaging System for Agricultural Inspection. Trans. ASAE 2003, 46, 513. [Google Scholar] [CrossRef]
- Burger, J.; Gowen, A. Data handling in hyperspectral image analysis. Chemom. Intell. Lab. Syst. 2011, 108, 13–22. [Google Scholar] [CrossRef]
- Su, W.-H.; Xue, H. Imaging Spectroscopy and Machine Learning for Intelligent Determination of Potato and Sweet Potato Quality. Foods 2021, 10, 2146. [Google Scholar] [CrossRef]
- Amigo, J.M. Practical issues of hyperspectral imaging analysis of solid dosage forms. Anal. Bioanal. Chem. 2010, 398, 93–109. [Google Scholar] [CrossRef]
- Amjad, W.; Hensel, O.; Munir, A.; Esper, A.; Sturm, B. Thermodynamic analysis of drying process in a diagonal-batch dryer developed for batch uniformity using potato slices. J. Food Eng. 2016, 169, 238–249. [Google Scholar] [CrossRef]
- Moscetti, R.; Haff, R.P.; Ferri, S.; Raponi, F.; Monarca, D.; Liang, P.; Massantini, R. Real-Time Monitoring of Organic Carrot (var. Romance) During Hot-Air Drying Using Near-Infrared Spectroscopy. Food Bioprocess Technol. 2017, 10, 2046–2059. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Comparative assessment of feature-wavelength eligibility for measurement of water binding capacity and specific gravity of tuber using diverse spectral indices stemmed from hyperspectral images. Comput. Electron. Agric. 2016, 130, 69–82. [Google Scholar] [CrossRef]
- Wang, S.; Tian, H.; Tian, S.; Yan, J.; Wang, Z.; Xu, H. Evaluation of dry matter content in intact potatoes using different optical sensing modes. J. Measure. Characterizat. 2022, 22, 1–6. [Google Scholar] [CrossRef]
- Pan, L.; Lu, R.; Zhu, Q.; McGrath, J.M.; Tu, K. Measurement of moisture, soluble solids, sucrose content and mechanical properties in sugar beet using portable visible and near-infrared spectroscopy. Postharvest Biol. Technol. 2015, 102, 42–50. [Google Scholar] [CrossRef]
- Tian, X.; Aheto, J.H.; Bai, J.; Dai, C.; Ren, Y.; Chang, X. Quantitative analysis and visualization of moisture and anthocyanins content in purple sweet potato by Vis–NIR hyperspectral imaging. J. Food Process. Preserv. 2020, 45, e15128. [Google Scholar] [CrossRef]
- Su, W.H.; Sun, D.W. Rapid visualization of moisture migration in tuber during dehydration using hyperspectral imaging. In Proceedings of the CIGR-AgEng Conference, Aarhus, Denmark, 26–29 June 2016; pp. 26–29. [Google Scholar]
- Su, W.-H.; Sun, D.-W. Evaluation of spectral imaging for inspection of adulterants in terms of common wheat flour, cassava flour and corn flour in organic Avatar wheat (Triticum spp.) flour. J. Food Eng. 2017, 200, 59–69. [Google Scholar] [CrossRef]
- Khamsopha, D.; Teerachaichayut, S. Detection of Adulteration of Tapioca Starch with Dolomite by near Infrared Hyperspectral Imaging. Key Eng. Mater. 2020, 862, 46–50. [Google Scholar] [CrossRef]
- Meghar, K. SOP for Hyperspectral Imaging Analysis of Fresh RTB Crops. High-Throughput Phenotyping Protocols (HTPP), WP3; RTBfoods Project-CIRAD: Montpellier, France, 2020. [Google Scholar]
- Qiao, J.; Wang, N.; Ngadi, M.O. Water content and weight estimation for potatoes using hyperspectral imaging. In Proceedings of the 2005 ASAE Annual Meeting, Tampa, FL, USA, 17–20 July 2005; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2005; p. 1. [Google Scholar]
- Su, W.H.; Sun, D.W. Chemical imaging for measuring the time series variations of tuber dry matter and starch concentration. Comput. Electron. Agric. 2017, 140, 361–373. [Google Scholar] [CrossRef]
- Su, W.H.; Bakalis, S.; Sun, D.W. Chemometric determination of time series moisture in both potato and sweet potato tubers during hot air and microwave drying using near/mid-infrared (NIR/MIR) hyperspectral techniques. Dry. Technol. 2020, 38, 806–823. [Google Scholar] [CrossRef]
- Su, W.H.; Sun, D.W. Rapid determination of starch content of potato and sweet potato by using NIR hyperspectral imaging. Hortscience 2019, 54, S38. [Google Scholar]
- Wang, F.; Wang, C.; Song, S. A study of starch content detection and the visualization of fresh-cut potato based on hyperspectral imaging. RSC Adv. 2021, 11, 13636–13643. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Chu, X.; Jiang, H.; Jia, B.; Yang, Y.; Kimuli, D.; Qin, H.; Dong, A. Rapid and nondestructive quantification of cassava starch adulterants in potato starch by using hyperspectral imaging. In Proceedings of the 2018 ASABE Annual International Meeting, St. Joseph, MI, USA, 15 February 2018; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2018; p. 1. [Google Scholar]
- Angel, D.-N.; Arno, F.; Pilar, C.; Esteban, V.-F.; Manuel, F.-D. Common Scab Detection on Potatoes Using an infrared hyperspectral imaging system. Image Anal. Process. 2011, 6979, 303–312. [Google Scholar]
- Angel, D.-N.; Arno, F.; Pilar, C.; Esteban, V.-F.; Manuel, F.-D. Non–destructive Detection of Hollow Heart in Potatoes Using Hyperspectral Imaging. Comput. Anal. Images Patterns 2011, 6855, 180–187. [Google Scholar]
- Evi Masithoh, R.; Amanah, H.Z.; Yoon, W.-S.; Joshi, R.; Cho, B.-K. Determination of protein and glucose of tuber and root flours using NIR and MIR spectroscopy. Infrared Phys. Technol. 2021, 113, 103577. [Google Scholar] [CrossRef]
- Heo, S.; Choi, J.-Y.; Kim, J.; Moon, K.-D. Prediction of moisture content in steamed and dried purple sweet potato using hyperspectral imaging analysis. Food Sci. Biotechnol. 2021, 30, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; He, Y.; Li, Q.; Jiao, W.; Zhu, Y.; Zhao, X. Non-destructive estimation of potato yield using relative variables derived from multi-period LAI and hyperspectral data based on weighted growth stage. Plant Methods 2020, 16, 150. [Google Scholar] [CrossRef]
- Rady, A.M.; Guyer, D.E.; Kirk, W.; Donis-González, I.R. The potential use of visible/near infrared spectroscopy and hyperspectral imaging to predict processing-related constituents of potatoes. J. Food Eng. 2014, 135, 11–25. [Google Scholar] [CrossRef]
- Sun, W.; Feng, L.; Zhang, Z.; Ma, Y.; Crosby, T.; Naber, M.; Wang, Y. Prediction of End-Of-Season Tuber Yield and Tuber Set in Potatoes Using In-Season UAV-Based Hyperspectral Imagery and Machine Learning. Sensors 2020, 20, 5293. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, Y.; Xuan, G.; Wang, Y.; Gao, Z.; Hu, Z.; Han, X.; Gao, C.; Wang, K. Application of hyperspectral imaging for spatial prediction of soluble solid content in sweet potato. RSC Adv. 2020, 10, 33148. [Google Scholar] [CrossRef]
- Somaratne, G.; Reis, M.M.; Ferrua, M.J.; Ye, A.; Nau, F.; Floury, J.; Dupont, D.; Singh, R.P.; Singh, J. Mapping the Spatiotemporal Distribution of Acid and Moisture in Food Structures during Gastric Juice Diffusion Using Hyperspectral Imaging. J. Agric. Food Chem. 2019, 67, 9399–9410. [Google Scholar] [CrossRef]
- Zhuang, H.; Ni, Y.; Kokot, S. A Comparison of Near- and Mid-Infrared Spectroscopic Methods for the Analysis of Several Nutritionally Important Chemical Substances in the Chinese Yam (Dioscorea opposita): Total Sugar, Polysaccharides, and Flavonoids. Appl. Spectrosc. 2015, 69, 488–495. [Google Scholar] [CrossRef]
- Do Trong, N.N.; Tsuta, M.; Nicolaï, B.M.; De Baerdemaeker, J.; Saeys, W. Prediction of optimal cooking time for boiled potatoes by hyperspectral imaging. J. Food Eng. 2011, 105, 617–624. [Google Scholar] [CrossRef]
- Ayvaz, H.; Rodriguez-Saona, L.E. Application of handheld and portable spectrometers for screening acrylamide content in commercial potato chips. Food Chem. 2015, 174, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Bozdogan, A.; Giusti, M.M.; Mortas, M.; Gomez, R.; Rodriguez-Saona, L.E. Improving the screening of potato breeding lines for specific nutritional traits using portable mid-infrared spectroscopy and multivariate analysis. Food Chem. 2016, 211, 374–382. [Google Scholar] [CrossRef] [PubMed]
- López-Maestresalas, A.; Keresztes, J.C.; Goodarzi, M.; Arazuri, S.; Jarén, C.; Saeys, W. Nondestructive detection of blackspot in potatoes by Vis-NIR and SWIR hyperspectral imaging. Food Control 2016, 70, 229–241. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Xie, A.; Yu, H.; Yin, Y.; Li, X.; Duan, X. Potential of Hyperspectral Imaging for Rapid Prediction of Anthocyanin Content of Purple-Fleshed Sweet Potato Slices During Drying Process. Food Anal. Methods 2017, 10, 3836–3846. [Google Scholar] [CrossRef]
- Moscetti, R.; Sturm, B.; Crichton, S.O.; Amjad, W. Massantini, R. Postharvest monitoring of organic potato (cv. Anuschka) during hot-air drying using visible-NIR hyperspectral imaging. J. Sci. Food Agric. 2017, 98, 2507–2517. [Google Scholar] [CrossRef]
- Teeken, B.; Agbona, A.; Bello, A.; Olaosebikan, O.; Alamu, E.; Adesokan, M.; Awoyale, W.; Madu, T.; Okoye, B.; Chijioke, U.; et al. Understanding cassava varietal preferences through pairwise ranking of gari-eba and fufu prepared by local farmer–processors. Int. J. Food Sci. Technol. 2021, 56, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Bai, X.; He, Y. Rapid screen of the color and water content of fresh-cut potato tuber slices using hyperspectral imaging coupled with multivariate analysis. Foods 2020, 1, 94. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Sanchez, P.D.; Hashim, N.; Shamsudin, R.; Nor, M.Z. Applications of imaging and spectroscopy techniques for non-destructive quality evaluation of potatoes and sweet potatoes: A review. Trends Food Sci. Technol. 2020, 96, 208–221. [Google Scholar] [CrossRef]
- Chen, L.; Opara, U.L. Texture measurement approaches in fresh and processed foods—A review. Food Res. Int. 2013, 51, 823–835. [Google Scholar] [CrossRef]
| S_N | Method | Trait | Product | Software | Equipment | References |
|---|---|---|---|---|---|---|
| 1 | NIR-HSI | Moisture and weight | Potatoes | ANN -MATLAB 7.0 | Image spectrometer (ImSpector V10E) with CMOS camera (BCi4-USB-M40LP) | [86] |
| 2 | NIR-HSI | Dry matter and starch | Potatoes and sweet potatoes | MLR, LWPLSR, PLSR -MATLAB R2016a | Push broom hyperspectral imaging system—Specim | [87] |
| 3 | NIR-HSI | Moisture content | Potatoes and sweet potatoes | PLSR, SVMR, LWPLSR, BPANN | Image spectrometer (ImSpector V10E) with CMOS camera (Xeva 992, Venix Infrared Solutions) | [88] |
| 4 | NIR-HSI | Starch content | Potatoes and sweet potatoes | PLSR, FMCIA | Push broom hyperspectral imaging system—Specim | [89] |
| 5 | Vis/NIR-HSI | Moisture and anthocyanin content | Purple sweet potato | PLSR -MATLAB 2014a- | CCD camera (V10EB1610) and spectrograph (ImSpector V10E2/3) | [81] |
| 6 | Vis/NIR-HSI | Fresh cut visualization and starch content | Potato tubers | PLSR -MATLAB 2014a- | Image spectrometer (ImSpector V10E) with CCD camera (IGVB1620) | [90] |
| 7 | NIR-HSI | Adulteration | Cassava starch | PLS | Push broom hyperspectral imaging system—Specim | [91] |
| 8 | NIR-HSI | Visual authentication and rapid classification of tubers using the moisture content | Sliced, oven-dried potatoes | PLS-DA -MATLAB 7.12 software- | Specim ImSpector N17E spectrograph | [79] |
| 9 | NIR-HSI | Scab disease detection | Potato tubers | Support Vector Machine -Not specified- | Xenics Xeva 1.7–320 camera with Specim Imspector—N17E spectrograph | [92] |
| 10 | NIR-HSI | Hollow heart disease detection | Potato tubers | Support Vector Machine | Xenics Xeva 1.7—320 camera with Specim Imspector—N17E spectrograph | [93] |
| 11 | MIR | Protein and glucose | Cassava, sweet potato, and taro flour | PCA and PLSR -Unscrambler®X (Version 10.5.1)- | FT-IR spectrometer—Nicolet 6700 | [94] |
| 12 | NIR-HSI | Moisture content | Steamed and dried sweet potato | PLS-DA -Unscrambler®X (Version 10.5.1)- | ImSpector N17E—Specim | [95] |
| 13 | NIR-HSI | Plant yield | Potato | Multi-period relative vegetation indices | USB 2000 spectrometer—Ocean Optics | [96] |
| 14 | VIS/NIR-HSI | Processing quality parameters | Potato tubers | PLSR -MATLAB 7.5.0.342 software- | CCD camera (C4880)—Hamamatsu Photonics | [97] |
| 15 | HSI | Tuber yield and tuber set | Potato tubers | OLS, PLSR, SVR, RF, AdaBoost -SpectralView software- | Headwall nano-hyper spec imager | [98] |
| 16 | Vis/NIR-HSI | Soluble solid content | Sliced sweet potato | PLSR, SVR, MLR -ENVI 4.6 and MATLAB 2011a- | Hyperspectral imager—GaiaField-V10E (Dualix Instruments) | [99] |
| 17 | HSI | Moisture and gastric acid distribution | Steamed and fried sweet potato | PLS -Prediktera Evince software 2.7.2- | VIS-InGaAs hyperspectral camera and a Headwall spectrograph (Model 1003B-10151) | [100] |
| 18 | NIR-HIS | Moisture migration during dehydration | Fresh potato tubers | PLSR -Matlab 7.12- | CCD camera (Xeva 992) and ImSpector N17E spectrograph (Specim) | [82] |
| 19 | NIR-HSI | Moisture content | Potato and sweet potato tubers | LWPLSR -Matlab R2017b- | CCD camera (Xeva 992) and ImSpector N17E spectrograph (Specim) | [29] |
| MIR-HSI | LUMOS FT-MIR (Bruker Optics) in ATR mode | |||||
| 20 | NIR-HSI | Variety identification and cooking loss determination | Sweet potato tubers | PLSR -Unscrambler 10.1 software and PLStoolbox v8.6 in Matlab R2017b software- | CCD camera (Xeva 992) and ImSpector N17E Spectrograph (Specim) | [34] |
| MIR-HSI | LUMOS FT-MIR (Bruker Optics) in ATR mode | |||||
| 21 | MIR | Total sugar, polysaccharides, and flavonoids | Chinese yam | PLS | Thermo Nicolet 380 Fourier transform (FT-IR) | [101] |
| 22 | HSI | Optimal cooking time | Potato tubers | PLS-DA -MATLAB 7.5- | CCD camera (KP-F120) with ImSpector V10 spectrograph | [102] |
| 23 | MIR | Acrylamide content | Potato chips | PLSR -Pirouette 4.0 software- | Excalibur 3500 Fourier-Transform IR spectrometer and Agilent FTIR spectrometer (Cary 630) | [103] |
| 24 | MIR | Nutritional traits | Freeze-dried potato flour | PLSR -Pirouette 4.0 software- | Agilent FT-IR spectrometer (Cary 630) | [104] |
| 25 | Vis-NIR/SWIR-HSI | Black spot detection | Potato tubers | PLS-DA -MATLAB R2014a- | CCD camera (TXG14) with ImSpector V10 spectrograph | [105] |
| 26 | Vis/NIR-HSI | Moisture content and chromaticity | Potato slices | PLS -MATLAB 2013b- | Schneider lens (Xenoplan 1.9/35) with ImSpector V10 spectrograph | [25] |
| 27 | Vis/NIR-HSI | Glycoalkaloids and chlorophyll | Potato | PLSR -R software- | TechSpec 25 mm with ImSpector V10 spectrograph | [27] |
| 28 | HSI | Anthocyanin content | Purple-fleshed sweet potato slices | PLSR, MLR, and LS-SVM -ENVI 5.1, LS-SVM v1.5 toolbox, and MATLAB R2013a- | Inno-Spec CCD camera (VRmC-9) with an Inno-Spec image spectrograph (Golden EYE/P 3810) | [106] |
| 29 | Vis/NIR-HSI | Postharvest monitoring during hot air drying | Organic potato | PLS -MATLAB R2015b, PLS_Toolbox software v8.1, and R v3.3.3- | Schneider lens (Xenoplan 1.9/35) with ImSpector V10E spectrograph | [107] |
| 30 | Vis/NIR-HSI | Sugar content | Potato slices | PLSR -MATLAB 7.5.0.342 software- | CCD camera (C4880)—Hamamatsu Photonics | [33] |
| Trait | Product | Software | Equipment | Accuracy | Reference |
|---|---|---|---|---|---|
| Adulteration | Cassava flour | FMCIA- PLS MATLAB-Mathworks | CCD camera Xeva 992—Xenics Infrared Solutions | (R2 = 0.98, SECV = 0.026) | [83] |
| Adulteration | Tapioca starch | PLSR | Specim Fx17, Spectral Imaging Ltd., Oulu, Finland | The calibration set’s total accuracy = 99.33%, prediction set’s absolute accuracy = 100%. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adesokan, M.; Alamu, E.O.; Otegbayo, B.; Maziya-Dixon, B. A Review of the Use of Near-Infrared Hyperspectral Imaging (NIR-HSI) Techniques for the Non-Destructive Quality Assessment of Root and Tuber Crops. Appl. Sci. 2023, 13, 5226. https://doi.org/10.3390/app13095226
Adesokan M, Alamu EO, Otegbayo B, Maziya-Dixon B. A Review of the Use of Near-Infrared Hyperspectral Imaging (NIR-HSI) Techniques for the Non-Destructive Quality Assessment of Root and Tuber Crops. Applied Sciences. 2023; 13(9):5226. https://doi.org/10.3390/app13095226
Chicago/Turabian StyleAdesokan, Michael, Emmanuel Oladeji Alamu, Bolanle Otegbayo, and Busie Maziya-Dixon. 2023. "A Review of the Use of Near-Infrared Hyperspectral Imaging (NIR-HSI) Techniques for the Non-Destructive Quality Assessment of Root and Tuber Crops" Applied Sciences 13, no. 9: 5226. https://doi.org/10.3390/app13095226
APA StyleAdesokan, M., Alamu, E. O., Otegbayo, B., & Maziya-Dixon, B. (2023). A Review of the Use of Near-Infrared Hyperspectral Imaging (NIR-HSI) Techniques for the Non-Destructive Quality Assessment of Root and Tuber Crops. Applied Sciences, 13(9), 5226. https://doi.org/10.3390/app13095226






